Abstract
Unilateral ureteral obstruction (UUO) is characterized by decreases in renal function, increased interstitial fibrosis, tubular apoptosis, and cellular infiltration. It has been suggested that inhibition of tubular apoptosis may protect against renal damage in obstruction. We have recently developed a series of peptides which are concentrated in the inner mitochondrial membrane and prevent cell death. These peptides are also active in vivo, in myocardial infraction, ischemic brain injury, and amyotrophic lateral sclerosis models. We therefore used SS-31, a prototype of these peptides, and assessed its effects on renal damage and oxidative stress in a 14-day obstruction model. SS-31 (1 or 3 mg/kg) or saline was given 1 day before and throughout the 14 days of obstruction. Kidneys were harvested and assessed for apoptosis (terminal transferase-dUTP-nick-end labeling, caspase 3 expression), fibrosis (trichrome staining), macrophage infiltration, fibroblast expression (immunoperoxidase), and oxidative damage (8-OH deoxyguanosine and heme oxygenase-1 expression), cytokines, and signaling pathways (transforming growth factor-β, CCR-1, p38-MAPK, NF-κB). SS-31 significantly attenuated the effects of obstruction on all aspects of renal damage which were examined, with both the 1 and 3 mg/kg doses showing efficacy. We noted increased oxidative stress in obstruction, which was also attenuated by SS-31 treatment. Signaling via NF-κB and p38 MAPK pathways were both affected by SS-31 treatment. This study provides a proof of concept that peptides which protect mitochondria in vitro can provide protection from renal damage in a UUO model. The mechanism by which protection is afforded requires further studies both in vitro and in vivo.
Keywords: mitochondria, fibrosis, peptides
unilateral ureteral obstruction (UUO) is characterized by decreases in renal function, increased interstitial fibrosis, tubular apoptosis, and cellular infiltration (9, 17). Histological studies suggest that tubular dilation precedes the inflammatory response and interstitial fibrosis. Mechanical stretch of the tubular epithelium and oxidative stress are believed to be early stress factors leading to tubular cell injury and death. Following an initial increase in blood flow to the kidney, renal blood flow decreases markedly in chronic obstruction, and ischemia is likely to also contribute to tubular cell injury. Injured tubular cells release proinflammatory cytokines and chemokines, causing an inflammatory response with macrophage infiltration in UUO. The release of profibrotic cytokines, such as transforming growth factor (TGF)-β, is believed to be of importance in post-UUO fibrosis (9, 17).
We and others (15, 19, 21, 27) have demonstrated that stretch induces caspase-dependent apoptosis in tubular epithelial cells. Apoptosis can be triggered either by death signals acting on death receptors on the cell surface (extrinsic pathway) or by mitochondrial release of proapoptotic factors into the cytosol that leads to apoptosome assembly, activation of caspase-9, and the cleavage of effector caspases (intrinsic pathway) (8). There is evidence that this intrinsic mitochondrial pathway is involved in stretch-induced tubular cell apoptosis. Tubular BAX protein expression increased over time after UUO, while there was a decrease in Bcl-2 expression (41).
The intrinsic mitochondrial pathway to apoptosis is further enhanced by oxidative stress. Oxidative damage has been implicated previously in UUO. Ricardo et al. (30) demonstrated that there was an increase in superoxide anion and hydrogen peroxide release in kidney slices obtained from UUO kidneys, and a corresponding decrease in catalase and copper-zinc superoxide dismutase mRNA. Others have demonstrated increased 8-hydroxy-2′-deoxyguanosine (8-OHdG) and heme oxygenase-1 (HO-1) as markers of oxidative stress in UUO (14, 25), and upregulation of HO-1 provided protection against renal injury in UUO (16). In mice with a catalase mutation resulting in acatalsemia and severe reduction in functional renal catalase, Sunami and colleagues (36) have shown accelerated atrophy of renal tubules, enhanced tubular dilatation, interstitial fibrosis, lipid peroxidation, and increased tubular apoptosis in a UUO model. Stretch appears to play a direct role in oxidative stress in tubular cells, as decreased catalase mRNA was also found when tubular cells were subjected to cyclic mechanical stretch (30).
Thus it appears that stretch, ischemia, and oxidative stress are the primary factors behind tubular cell apoptosis in UUO, and it has been suggested that inhibition of tubular cell apoptosis may protect against macrophage infiltration and interstitial fibrosis in UUO (5). Neutralizing antibody to TGF-β1 inhibited stretch-induced tubular apoptosis and reduced the severity of interstitial fibrosis (20). The use of angiotensin-converting enzyme inhibitors also caused a reduction in early apoptosis and late fibrosis (7, 13, 32). Antioxidants, including quercetin, α-tocopherol, and fluvastatin, have shown some degree of renal protection in UUO, but only fluvastatin was shown to significantly reduce oxidative markers and tubular apoptosis (13, 18, 23, 25, 31).
The disappointing outcome with antioxidant treatments may be explained by their limited distribution to mitochondria, the primary source of intracellular reactive oxygen species (ROS). We have developed a series of water-soluble small peptides (SS-peptides) that are cell permeable and selectively target and concentrate 1,000-fold in the inner mitochondrial membrane (42, 44). SS-31, a tetrapeptide of the structure d-Arg-Dmt-Lys-Phe-NH2 (where Dmt is dimethyltyrosine), which is a prototype of the SS-peptide group, has been shown to concentrate 5,000-fold in mitochondria. The SS-peptides are extraordinarily potent in preventing cell death caused by prooxidants (44) and are also active in models of ischemia-reperfusion, myocardial infarction, ischemic brain injury, and amyotrophic lateral sclerosis (2, 3, 26, 40). Recently, SS-31 was shown to improve the viability of pancreatic islet cells during isolation for transplantation and significantly improved graft survival and reduced insulin requirements in the recipients (38). Therefore, we sought to determine whether targeting mitochondria with antioxidants would be an appropriate strategy in protecting the kidney from damage in UUO. In the present experiments, we utilized a 14-day UUO model in rats to examine the effect of SS-31 on renal damage in UUO. We examined several parameters including fibrosis, apoptosis, macrophage infiltration, tubular proliferation, cytokines, and signaling pathways and markers of oxidative damage including 8–0HdG and HO-1.
MATERIALS AND METHODS
Materials
d-Arg-2′,6-dimethyltyrosine-Lys-Phe-NH2 (SS-31) was prepared by solid-phase synthesis and provided by Dr. Peter W. Schiller (Clinical Research Institute of Montreal, Quebec, Canada) (38).
In Vivo UUO
Sprague-Dawley rats underwent unilateral ureteral ligation with 4-0 silk suture through a midline abdominal incision under sterile conditions as routinely carried out in our laboratory (10). SS-31 (1 or 3 mg/kg; n = 8) was administered intraperitoneally 1 day before UUO and continuing for 14 days. A separate group of animals was given saline, as a control (n = 16). Animal treatment adhered to approved institutional guidelines.
Renal Histology
Trichrome sections of paraffin-embedded specimens were examined by a board-certified pathologist (S. V. Seshan, renal pathology specialist), and fibrosis was scored on a scale of 0 to +++.
Immunohistochemical Analysis
Immunohistochemical analyses for macrophages was carried out using a monoclonal antibody to ED-1 (Serotec) as previously described (10). Macrophages were counted in 10 high-power fields (HPF; ×400) by two different independent investigators in a blinded fashion. A terminal transferase-dUTP-nick-end labeling (TUNEL) assay was performed as previously described (20), and TUNEL-positive apoptotic renal tubules were quantitated as above.
We also analyzed tissue for the presence of fibroblasts using immunohistochemistry, as previously described (10). The antibody utilized was DAKO S100-A4 (1:100 dilution). The S100-A4 antigen is also known as fibroblast-specific protein (FSP)-1 (1, 35). The antigen was retrieved by incubating cells with proteinase K for 20 min in an oven. The remaining immunoperoxidase protocol was carried out as routinely done in our laboratory. Staining for S100-A4 was found in spindle-shaped interstitial cells, and also in cells which were round, and were identified as inflammatory cells by the pathologist. Only spindle-shaped cells were included in the counts. Samples incubated without primary antibody exhibited no staining. 8-OH dG staining was carried using proteinase K for antigen retrieval. The antibody used was from the Japan Institute Control of Aging and was used at a dilution of 1:200- 1:500. The TGF-β antibody was obtained from R&D Systems.
PCR Analysis
PCR for HO-1, TGF-β1, and NF-κB was performed as follows. Rat kidneys were harvested and were kept at −80°C until use. Total RNA was extracted using the TRIzol-chloroform extraction procedure. mRNA was purified using a Oligotex mRNA extraction kit (Qiagen, Valencia, CA) according to manufacturer's instructions. mRNA concentration and purity were determined by measuring absorbance at 260 nm. RT-PCR was preformed using a Qiagen One-step PCR kit (Qiagen). PCR was performed in an automated Thermal Cycler ThermoHybrid PX2 with an initial activation step for 15 min at 95°C followed by 35 cycles of denaturation for 45 s at 94°C, annealing for 30 s at 60°C, and extension for 60 s at 72°C. PCR products were separated by 2% agarose gel electrophoresis. Bands on gels were visualized by ethidium bromide staining and analyzed using Image J densitometric analysis software.
Real-Time PCR
We carried out real-time PCR using the TaqMan system from Applied Biosystems. All real-time PCR was carried out in the core facility at Weill Cornell Medical College using standard assays. We used real-time PCR to examine mRNA expression of CCR-1, caspase-3, HO-1, and p38 MAPK. CT2ΔΔ was calculated to demonstrate the fold-change of expression of the individual genes; gene expression was indexed to GAPDH, and contralateral kidney values were set to 1.0.
ELISA for p65 Subunit of NF-κB
Extracts were made from renal tissue from control and SS-31-treated kidneys. Equal amounts of protein were analyzed for the presence of the p65 subunit of NF-κB using transAM p65 ELISA kits from Active Motif (catalog no. 40096, Carlsbad, CA). Each sample was assayed in triplicate.
ELISA for TGF-β
Extracts were made from renal tissue from control and SS-31-treated kidneys. Equal amounts of protein were analyzed for the presence of TGF-β using a TGF-β ELISA kit from R&D Systems (catalog no. DB100B). Each sample was assayed in duplicate.
Statistics
Samples were analyzed by one-way ANOVA, and differences were considered significant at P < 0.05 using the least significant difference test.
RESULTS
Fourteen-day UUO produces a characteristic set of changes in the kidney, including increased interstitial fibrosis, tubular apoptosis, macrophage infiltration, and tubular proliferation.
Interstitial Fibrosis
Analysis of trichrome slides.
The contralateral kidney (CK) showed very little, if any, inflammation or fibrosis in tubules, glomeruli, or interstitium. The obstructed kidney (OK) of the control group showed moderate (1–2+) medullary trichrome staining, along with areas of focal peripelvic 1+ staining. The cortex showed less fibrosis than the medulla. The OK also showed moderate inflammation, generally scored as 1+ in the cortex and 2+ in the medulla. SS-31-treated kidneys showed significantly less trichrome staining, being 0 to a trace in the cortex and a trace to 1+ in the medulla. When interstitial volume was measured, medullary interstitial volume in the control OK was 69.2 ± 2.1% compared with 0.5 ± 0.1% in the CK. Treatment with 1 mg/kg SS-31 modulated the increase in interstitial volume to 54.9 ± 2.3%; a higher dose of SS-31 was also effective (Fig. 1).
Fig. 1.
SS-1 decreases medullary fibrosis in a 14-day unilateral ureteral obstruction (UUO) model. Animals were pretreated with SS-31 1 day before UUO and daily for 14 days. Control animals received saline only throughout the period of UUO. In these trichrome-stained slides, the contralateral kidney (CK) shows almost no fibrosis, whereas the control obstructed kidney (OK) exhibits significant fibrosis. SS-31 treatment attenuated fibrosis. Interstitial volume (as measured in materials and methods) is depicted graphically.
Fibroblast-specific protein expression.
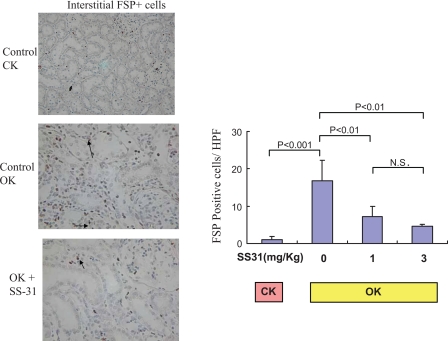
Increased expression of FSP-1 was also found in the OK. There were a small number of interstitial fibroblasts present in CK (Fig. 2; 1.1 ± 0.3 FSP+ cells/HPF). 14-day UUO increased this number to 16.9 ± 2.3 cells/HPF in the OK. SS-31 (1 mg/kg) significantly decreased the amount of fibroblast infiltration in the OK to 43.4% of control; SS-31 (3 mg/kg ) treatment further decreased the amount of fibroblast infiltration in the OK to 28.0% of the untreated OK.
Fig. 2.
SS-31 decreases fibroblast expression in a 14-day UUO model. Animals were pretreated with SS-31 1 day before UUO and daily for 14 days. Control animals received saline only throughout the period of UUO. Fibroblasts were visualized by immunoperoxidase for FSP (see materials and methods). Control CK, control OK, and SS-31-treated OK (1 mg/kg) are shown, as in Fig. 1. Representative FSP+ cells are shown by arrows, and counts of FSP+ cells are shown on the right.
Renal Tubular Apoptosis
In the untreated OK, 2 wk of UUO resulted in a significant increase in apoptotic tubular cells compared with the CK (Fig. 3). SS-31 at 1 mg/kg significantly decreased tubular apoptosis from 15.1 ± 3.1 to 5.1 ± 0.5 cells/HPF (P < 0.05); SS-31 at 3 mg/kg caused a further significant decrease in renal tubular apoptosis (3.0 ± 0.3 apoptotic cells/HPF). Using real-time PCR, we examined caspase 3 expression. As shown in Table 1, caspase 3 was increased ∼10-fold in the obstructed kidney, but this was significantly reduced with SS-31 treatment.
Fig. 3.
SS-31 decreases tubular apoptosis in a 14-day UUO model. Animals were pretreated with SS-31 1 day before UUO and daily for 14 days. Control animals received saline only throughout the period of UUO. Apoptotic cells were visualized by use of the terminal transferase-dUTP-nick-end labeling (TUNEL) assay (see materials and methods). Control CK, control OK, and SS-31-treated OK (1 mg/kg) are shown, as in Fig. 1. Representative apoptotic cells are shown by arrows, and counts of TUNEL-positive cells are shown on the right.
Table 1.
Fold-increase in mRNA expression of various genes in the obstructed kidney
| CLK | OK | CLK-SS-31 | OK-SS-31 | |
|---|---|---|---|---|
| Caspase-3 | 1.0±0.1 | 9.6±1.4* | 1.0±0.1 | 5.4±1.3† |
| HO-1 | 1.0±0.1 | 17.9±7.9* | 1.0±0.1 | 6.9±2.9† |
| CCR-1 | 1.0±0 | 4.7±1.3* | 1.0±0.1 | 5.12±1.5 |
| P38-MAPK | 1.0±0.1 | 3.6±0.5* | 1.0±0 | 2.9±0.7† |
Values (means ± SE) listed are CT2ΔΔ for the obstructed kidney (OK) compared with the contralateral kidney (CLK) set as 1.0. Real-time PCR was carried out as in materials and methods. HO, heme oxygenase. SS-31 are kidneys treated with 1 mg/kg SS-31 for 14 days.
P < 0.05 compared with CLK.
P < 0.05 compared with untreated OK.
Macrophage Infiltration
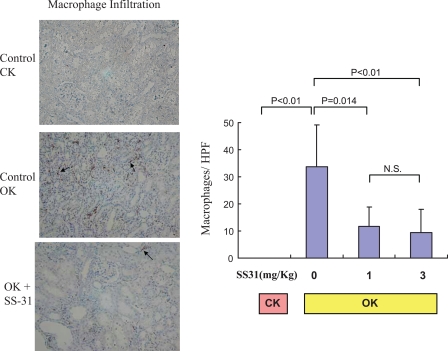
Similarly, there was a significant increase in macrophage infiltration into the OK compared with the CK after 2 wk of UUO (33.8 ± 6.3 vs. 0.04 ± 0.03 cells/HPF; Fig. 4). Both the 1 and 3 mg/kg doses of SS-31 significantly decreased macrophage infiltration into the OK. (Fig. 4).
Fig. 4.
SS-31 decreases macrophage expression in a 14-day UUO model. Animals were pretreated with SS-31 1 day before UUO and daily for 14 days. Control animals received saline only throughout the period of UUO. Macrophages were visualized by immunoperoxidase for ED-1 (see materials and methods). Control CK, control OK, and SS-31-treated OK (1 mg/kg) are shown, as in Fig. 1. Representative macrophages are shown by arrows, and counts of macrophages are shown on the right.
Renal Tubular Proliferation
The obstructed kidney is associated with increased proliferation of renal tubular cells (Fig. 5). SS-31 caused a significant increase in renal tubular proliferation in the OK. Tubular proliferation was increased 2-fold at the 1 mg/kg dose and 3.5-fold at the 3 mg/kg dose.
Fig. 5.
SS-31 increases tubular proliferation in a 14-day UUO model. Animals were pretreated with SS-31 1 day before UUO and daily for 14 days. Control animals received saline only throughout the period of UUO. Proliferating cells were visualized by immunoperoxidase for PCNA (see materials and methods). Control CK, control OK, and SS-31-treated OK (1 mg/kg) are shown, as in Fig. 1. Representative proliferating tubular cells are shown by arrows, and counts of PCNA-positive cells are shown on the right.
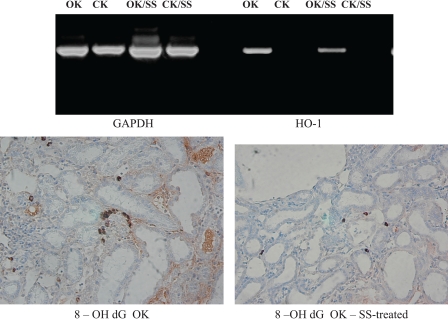
Renal Oxidative Damage
Renal oxidative damage was assessed by examining renal HO-1 expression using RT-PCR and real-time PCR, and immunohistochemistry for 8-OH dG. RT-PCR and real-time PCR were performed using GAPDH as an internal control and using the primers described in materials and methods. PCR analysis revealed that UUO was associated with an increase in HO-1 expression (Fig. 6) (HO-1/GAPDH ratio 0.07 CK vs. 1.09 control OK). With SS-31 treatment, HO-1 expression decreased (HO-1/GAPDH ratio in SS-31-treated animals, 0.58). These results were confirmed using real-time PCR as shown in Table 1. HO-1 was significantly increased in the OK, and the increase was attenuated with SS-31. Using immunohistochemistry, we noted that 8-OH dG staining was detected in both tubular and interstitial compartments of the OK. The number of 8-OH dG-positive cells/HPF was significantly increased in the OK compared with the CK (5.0 ± 1.4 vs. 1.4 ± 0.1 cells/HPF). Treatment with SS-31 significantly decreased the number of 8-OH dG positive cells in the OK (Fig. 6).
Fig. 6.
SS-31 decreases oxidative damage in a 14-day UUO model. Animals were pretreated with SS-31 1 day before UUO and daily for 14 days. Control animals received saline only throughout the period of UUO. Oxidative damage was assessed through expression of either heme oxygenase-1 (HO-1; PCR, top) or 8-OH deoxyguanosine (8-OH dG; immunoperoxidase; bottom). SS, animal treated with SS-31.
Renal Cytokines and Signaling Pathways
TGF-β has been implicated in the fibrosis which results from UUO. We studied the effect of SS-31 on TGF-β expression. Using RT- PCR, we demonstrated that TGF-β expression was increased in the obstructed kidney; surprisingly, SS-31 did not affect TGF-β expression (Fig. 7, top). Immunohistochemical analysis of TGF-β confirmed this (not shown), as did TGF-β ELISA. TGF-β was increased 4.3-fold in the control OK and 3.7-fold in the SS-31-treated kidneys (Table 2). Both of these are significantly different from their respective controls, and not from each other. Chemokine receptor CCR-1 has been shown to be increased in UUO (6). We confirmed by real-time PCR that CCR-1 was increased in UUO, but its expression was not affected by SS-31 treatment. As shown in Table 1, CCR-1 was increased four- to fivefold in either control of SS-31-treated OK compared with their contralateral controls.
Fig. 7.
Effect of SS-31 on TGF-β1 and NF-κB-p65 subunit mRNA expression in UUO. Animals were pretreated with SS-31 1 day before UUO and daily for 14 days. Control animals received saline only throughout the period of UUO. Top: RT-PCR for TGF-β1. Bottom: RT-PCR for NF-κB-p65 subunit A representative figure is shown. SS-OK, OK from animal treated with SS-31; SS-CK, CK from animal treated with SS-31.
Table 2.
Effect of SS-31 treatment on TGF-β and NF-κB expression in the obstructed kidney
| CLK | OK | CLK-SS-31 | OK-SS-31 | |
|---|---|---|---|---|
| TGF-β | 0.31±0.03 | 1.34±0.17* | 0.34±0.04 | 1.26±0.11* |
| NF-κB | 0.59±0.09 | 0.90±0.08* | 0.54±0.04 | 0.74±0.05† |
Values are means ± SE. ELISA for either transforming growth factor (TGF)-β or NF-κB was carried out as described in materials and methods.
P < 0.05 compared with respective control.
P < 0.05 compared with control OK.
Signaling pathways including the MAPKs or NF-κB may mediate the effects of SS-31. Therefore, we examined whether these pathways were affected by SS-31 treatment. Using real-time PCR, we demonstrated that p38 MAPK was increased in UUO (Table 1). This increase was attenuated by SS-31 treatment. We also assayed for the p65 subunit of NF-κB. A representative gel is shown in Fig. 7, bottom. As can be seen, there was increased expression of the′ p65 subunit in the obstructed kidney, which was attenuated by SS-31 treatment. We further assayed for the p65 subunit of NF-κB by ELISA (Table 2). In the control CK, p65 expression was 0.59 ± 0.09 units. This was significantly increased to 0.90 ± 0.08 units in the control OK. In the SS-31-treated kidneys, the increase was attenuated (0.54 ± .04 units in the CK and 0.74 ± .05 units in the SS-31/OK).
DISCUSSION
Ureteral obstruction leads to renal damage via a bimodal process, with tubular apoptosis occurring as an early event followed by the release of cytokines and chemokines from the injured tubular cell, resulting in inflammatory reaction and interstitial fibrosis. Thus it has been suggested that inhibition of tubular apoptosis may protect against subsequent inflammatory response and interstitial fibrosis (5). Treatments targeting various mediators of apoptosis and inflammation, such as neutralizing antibodies of TGF-β1 and angiotensin-converting enzyme (ACE) inhibitors, have reduced early apoptosis and late fibrosis. Oxidative stress resulting from mechanical stretch and ischemia also play an important role in tubular apoptosis, and antioxidants may be useful therapeutic agents for UUO. However, previous attempts to limit tissue damage by targeting ROS with antioxidants have met with limited success (13, 18, 22, 25, 31). In this study, we provide a proof of concept that using a mitochondria-targeted antioxidant significantly decreases renal apoptosis and that subsequent fibrosis, macrophage infiltration, and fibroblast expression are greatly reduced.
Given the extensive evidence for oxidative damage in UUO, the less than optimal outcome of previous studies using antioxidants may have resulted from their poor distribution to the target site. Mitochondria are the major source of intracellular ROS, and oxidative damage to cardiolipin on the inner mitochondrial membrane is known to promote mitochondrial permeability transition and cytochrome c release. Thus it is imperative to deliver the antioxidant to mitochondria to prevent the onset of mitochondria-initiated apoptosis. Many natural antioxidants, including vitamin E and coenzyme Q, are highly lipophilic and tend to be sequestered in the plasma membrane. There have been several attempts to develop antioxidants that target mitochondria (for review, see Refs. 24 and 33). The primary approach has been conjugation of conventional antioxidants to a lipophilic cation, thus allowing them to penetrate into the mitochondrial matrix in a potential-dependent manner. Murphy and colleagues (24) conjugated triphenylphosphonium ion to coenzyme Q (MitoQ) and vitamin E (MitoVitE), while Sheu and colleagues (33) synthesized choline esters of glutathione (MitoGSH) and NAC (MitoNAC) to create mitochondria-targeted antioxidants. These methods allow improved delivery of these conventional antioxidants into the mitochondrial matrix, and MitoVitE was 350-fold more potent than vitamin E in protecting against oxidant-induced cell death in fibroblast cells from Freidrich ataxia patients (12). Both MitoVitE and MitoQ inhibit oxidant-induced cell death at 1 μM; however, they are cytotoxic at concentrations of 25–50 μM due to mitochondrial depolarization. The success of these mitochondria-targeted antioxidants in intact animals is less certain. A recent paper reported that MitoVitE was not neuroprotective in a model of hypoxic-ischemia striatal injury in neonatal rats even though the compound was administered via continuous infusion into the striatum (4). The failure of MitoVitE, as well as an earlier report on MitoQ (32), in this disease model may reflect the limitation of potential-driven mitochondrial uptake in pathological conditions with mitochondrial depolarization.
Recently, a new series of mitochondria-targeted antioxidants (SS peptides) was described whose uptake is not dependent on mitochondrial potential. The SS peptides are tetrapeptides with alternating aromatic residues and basic amino acids (aromatic-cationic peptides) (for a review, see Ref. 37). The incorporation of a tyrosine or modified tyrosine provides radical scavenging ability. Tyrosine can scavenge oxyradicals forming relatively unreactive tyrosyl radicals, which can be followed by radical-radical coupling to give dityrosine, or react with superoxide to form tyrosine hydroperoxide. These peptides readily penetrate the cell membrane in a concentration-dependent manner (43). A distinct advantage of the SS peptides is their ability to concentrate >1,000-fold in the inner mitochondrial membrane (42–44). Contrary to MitoQ and MitoVitE, the uptake of these aromatic-cationic peptides into mitochondria is not dependent on mitochondrial potential and their uptake is therefore not self-limiting. Furthermore, because these peptides are not delivered into the mitochondrial matrix, they do not cause mitochondrial depolarization even at very high concentrations (44). The SS peptides have demonstrated efficacy and lack of toxicity in several animal models of oxidative damage, including ischemia-reperfusion, myocardial infarction, acute cerebral ischemia-reperfusion, amyotrophic lateral sclerosis, and pancreatic islet cell transplantation (2, 3, 26, 38, 40). In this study, we demonstrated that SS-31 significantly decreased tubular apoptosis, macrophage infiltration, fibroblast expression, and fibrosis, while significantly increasing tubular proliferation. The mechanism by which the SS peptides protect mitochondria is currently under investigation and, therefore, more detailed mechanistic studies in the UUO model would be premature.
SS-31 was extremely effective in reducing tubular apoptosis in UUO, with apoptosis almost completely prevented at a dose of 3 mg/kg. We also demonstrated that caspase 3 was activated in the OK and that SS-31 attenuated this increase. The increase in caspase 3 confirms previous studies by Truong and colleagues (39). It has been suggested that inhibition of tubular cell apoptosis may protect against macrophage infiltration and interstitial fibrosis in UUO (5 ). Neutralizing antibody to TGF-β1 inhibited stretch-induced tubular apoptosis and reduced the severity of interstitial fibrosis (20). The use of ACE inhibitors also caused a reduction in early apoptosis and late fibrosis (7, 13, 32). Previous studies with TGF-β antibody also demonstrated increased tubular proliferation concomitant with decreased apoptosis; the present study confirmed this finding, as well. Increased proliferation may represent an attempt by the kidney to undergo repair, which may be enhanced when apoptosis is decreased. Our findings suggest that by preventing the intrinsic mitochondrial apoptotic pathway, SS-31 significantly reduced macrophage infiltration and interstitial fibrosis and increased tubular proliferation. Surprisingly, despite its effects on fibrosis, SS-31 showed little effect on either TGF-β1 or CCR1. Effects of SS-31 on TGF-β were confirmed using PCR, immunohistochemistry, and ELISA. In previous studies, inhibition of TGF-β1 with monoclonal antibody (20) or knockout of CCR-1 (6) decreased fibrosis in the UUO models. This may suggest that SS-31 is targeting further downstream of either of these mediators. We demonstrated that there is an attenuation of the increase in the p65 subunit of NF-κB in the obstructed kidney, assaying for both mRNA and protein. Miyajima et al. (22) have demonstrated activation of NF-κB in UUO using EMSA. Furthermore, they demonstrated that an inhibitor of NF-κB activation also decreased renal tubular apoptosis and fibrosis in UUO. We also demonstrated an attenuation of the increase in p38 MAPK in the OK with SS-31 treatment. Thus our studies demonstrate that SS-31 decreases caspase-3 expression, which may account for its effects on tubular apoptosis. Downstream mediators NF-κB and p38 MAPK may also be involved in the effects of SS-31, but their exact role remains to be determined.
Apart from their role in the apoptotic pathway, ROS have been directly implicated in both fibrosis and epithelial-mesenchymal transition (EMT), the process of transition of epithelial cells to fibroblasts, a more fibrotic phenotype. Therefore, decreasing ROS would have additional beneficial effects on renal damage in UUO. Iwano et al. (11) demonstrated that fibroblasts in the OK can be derived both from bone marrow and from EMT. Both populations can express collagen I and proliferate during fibrosis. Using FSP expression, we have confirmed that the UUO model is characterized by increased fibroblast expression. ROS have been shown to mediate EMT. For example, Rhyu et al. (29) have shown that in TGF-β-induced EMT in NRK-52E cells antioxidants decreased EMT; H2O2 reproduced the effects of TGF-β. Others have shown that matrix metalloproteases induced EMT (28) through ROS intermediates. In this study, we examined the expression of 8-OH dG and HO-1, widely used as markers of oxidative DNA damage. Both 8-OH dG and HO-1 have been found to be increased in UUO by previous investigators (14, 25). We confirmed these findings in the present study. We showed 8-OH dG staining in both tubular and interstitial compartments of the kidney. SS-31 treatment significantly reduced renal expression of 8-OH dG, and this was associated with reduced presence of fibroblasts and decreased EMT. Furthermore, increased HO-1 was found, as demonstrated previously, and HO-1 expression was also decreased by SS-31. These findings confirm the role of oxidative damage in fibrosis and EMT.
In summary, these results demonstrate that pretreatment of rats with SS-31, which in vitro is a mitochondrial protectant, decreased renal damage and oxidative stress in a model of UUO. Unlike other models of renal disease, wherein renal function can be monitored easily using urine and blood sampling, analysis of renal function in UUO requires complex split-function measurements. The present study, therefore, did not address the functional effects of SS-31 treatment. These will be done in future experiments, along with interventional studies designed to test whether SS-31 treatment can decrease already existing damage in response to UUO. Nevertheless, the present study provides a proof of concept that peptides which are targeted to and concentrated in the inner mitochondrial membrane in vitro can protect the kidney from damage elicited by UUO.
GRANTS
This work was supported in part by awards from the National Institutes of Health: R-01-DK-58355 (D. Felsen), P01-DA-08924 (H. H. Szeto), and RO1-DA-073595 (H. H. Szeto).
DISCLOSURES
Patent applications have been filed by the Cornell Research Foundation, Inc. (CRF) for the technology (SS peptides) described in this article. H. H. Szeto is the inventor. CRF, on behalf of Cornell University, has licensed the technology for further research and development to a commercial enterprise in which CRF and Dr. Szeto have financial interests.
Acknowledgments
We thank Dr. Peter Schiller (Clinical Research Institute of Montreal, Montreal, Quebec, Canada) for providing us with SS-31.
A portion of this work was presented at the American Society of Nephrology meeting in 2005.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barraclough R Calcium-binding protein S100A4 in health and disease. Biochim Biophys Acta 1448: 190–199, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Cho J, Won K, Soong Y, Liu S, Szeto HH, Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coronary Artery Dis 18: 215–220, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Cho S, Szeto HH, Kim Pinto JT HJ. A novel cell permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem 282: 4634–4642, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Covey MV, Murphy MP, Hobbs CE, Smith RA, Oorschot DE. Effect of the mitochondrial antioxidant, Mito Vitamin E, on hypoxic-ischemic striatal injury in neonatal rats: a dose-response and stereological study. Exp Neurol 199: 513–519, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Docherty NG, O'Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290: F4–F13, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Eis V, Luckow B, Vielhauer V, Siveke JT, Linde Y, Segerer S, Perez De Lema G, Cohen CD, Kretzler M, Mack M, Horuk R, Murphy PM, Gao JL, Hudkins KL, Alpers CE, Grone HJ, Schlondorff D, Anders HJ. Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J Am Soc Nephrol 15: 337–47, 2004. [DOI] [PubMed] [Google Scholar]
- 7.El Chaar M, Chen J, Seshan SV, Jha S, Richardson I, Ledbetter S, Vaughan, ED Jr, Poppas DP, Felsen D. The effect of combination therapy with enalapril and the TGF-β antagonist 1D11 in unilateral ureteral obstruction. Am J Physiol Renal Physiol 292: F1291–F1301, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Gulmi F, Felsen D, Vaughan ED Jr. Pathophysiology of urinary tract obstruction. In: Campbell's Urology (8th ed.,) edited by Walsh P, Retik A, Vaughan ED, Jr., and Wein A. Philadelphia, PA: Saunders, 2002.
- 10.Ito K, Chen J, Seshan S, Khodadadian J, Gallagher R, Chaar M, Vaughan E, Poppas D, Felsen D. Dietary arginine supplementation attenuates renal damage after relief of unilateral ureteral obstruction in rats. Kidney Int 68: 515–528, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Iwano M, Plieth D, Danoff T, Xue C, Okada H, Neilson E. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jauslin M, Meier T, Smith R, Murphy M. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J 17: 1972–1974, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Jones E, Shahed A, Shoskes D. Modulation of apoptotic and inflammatory genes by bioflavonoids and angiotensin II inhibition in ureteral obstruction. Urology 56: 346–351, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Kawada N, Moriyama T, Ando A, Fukunaga M, Miyata T, Kurokawa K, Imai E, Hori M. Increased oxidative stress in mouse kidneys with unilateral ureteral obstruction. Kidney Int 56: 1004–1013, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Kiley S, Thornhill B, Tang S, Ingelfinger J, Chevalier R. Growth factor-mediated phosphorylation of proapoptotic BAD reduces tubule cell death in vitro and in vivo. Kidney Int 63: 33–42, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Yang JI, Jung MH, Hwa JS, Kang KR, Park DJ, Roh GS, Cho GJ, Choi WS, Chang SH. Heme oxygenase-1 protects rat kidney from ureteral obstruction via an antiapoptotic pathway. J Am Soc Nephrol 17: 1373–1381, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Kuemmerle N, Brandt R, Chan W, Krieg R, Chan J. Inhibition of transforming growth factor beta 1 induction by dietary vitamin E in unilateral ureteral obstruction in rats. Biochem Mol Med 61: 82–86, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Miyajima A, Chen J, Kirman I, Poppas DP, Darracott Vaughan ED Jr, Felsen D. Interaction of nitric oxide and transforming growth factor-beta1 induced by angiotensin II and mechanical stretch in rat renal tubular epithelial cells. J Urol 164: 1729–1734, 2000. [PubMed] [Google Scholar]
- 20.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Miyajima A, Chen J, Poppas DP, Vaughan ED Jr, Felsen D. Role of nitric oxide in renal tubular apoptosis of unilateral ureteral obstruction. Kidney Int 59: 1290–1303, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Miyajima A, Kosaka T, Seta K, Asano T, Umezawa K, Hayakawa M. Novel nuclear factor kappa B activation inhibitor prevents inflammatory injury in unilateral ureteral obstruction. J Urol 169: 1559–1563, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Moriyama T, Kawada N, Nagatoya K, Takeji M, Horio M, Ando A, Imai E, Hori M. Fluvastatin suppresses oxidative stress and fibrosis in the interstitium of mouse kidneys with unilateral ureteral obstruction. Kidney Int 59: 2095–2103, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol Epub October 2, 2006. [DOI] [PubMed]
- 25.Pat B, Yang T, Kong C, Watters D, Johnson D, Gobe G. Activation of ERK in renal fibrosis after unilateral ureteral obstruction: modulation by antioxidants. Kidney Int 67: 931–943, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Petrie S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem 98: 1141–1149, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Power R, Doyle B, Higgins D, Brady H, Fitzpatrick J, Watson R. Mechanical deformation induced apoptosis in human proximal renal tubular epithelial cells is caspase dependent. J Urol 171: 457–461, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Radisky D, Levy D, Littlepage L, Liu H, Nelson C, Fata J, Leake D, Godden E, Albertson D, Nieto M, Werb Z, Bissell M. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436: 123–127, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhyu D, Yang Y, Ha H, Lee G, Song J, Uh S, Lee H. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 16: 667–675, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Ricardo S, Ding G, Eufemio M, Diamond J. Antioxidant expression in experimental hydronephrosis: role of mechanical stretch and growth factors. Am J Physiol Renal Physiol 272: F789–F798, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Saborio P, Krieg R, Kuemmerle N, Norkus E, Schwartz C, Chan J. Alpha-tocopherol modulates lipoprotein cytotoxicity in obstructive nephropathy. Pediatr Nephrol 14: 740–746, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Satoh M, Kashihara N, Yamasaki Y, Maruyama K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K, Makino H. Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 12: 317–325, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Sheu S, Lemasters J. Special issue: mitochondria in diseases and therapeutics. Biochim Biophys Acta 1762: 139, 2006. [Google Scholar]
- 34.Stone CE, Murphy MP, Smith RAJ, Oorschot DE. Are the free radical scavengers mitoquinol or N-tert-butyl-(2-sulfophenyl)-nitrone (S-PBN) protective for striatal medium-spiny neurons following an acute hypoxic- ischemic insult to the immature rat brain? Pedr Res 51: 444A–445A, 2002. [Google Scholar]
- 35.Strutz F, Okada H, Lo C, Danoff T, Carone R, Tomaszewski J, Neilson E. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sunami R, Sugiyama H, Wang D, Kobayashi M, Maeshima Y, Yamasaki Y, Masuoka N, Ogawa N, Kira S, Makino H. Acatalasemia sensitizes renal tubular epithelial cells to apoptosis and exacerbates renal fibrosis after unilateral ureteral obstruction. Am J Physiol Renal Physiol 286: F1030–F1038, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Szeto HH Cell-permeable mitochondria-targeted, peptide antioxidants. AAPS J 8: E521–E530, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas D, Zhao K, Stauffer C, Yang H, Sharma VJ, Szeto HH, Suthanthiran M. Mitochondrial targeting of islets with the cell permeant anti-oxidant peptide SS-31 reduces islet cell apoptosis and improves post-transplant function. J Am Soc Nephrol 18: 213–222, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Truong LD, Choi YJ, Tsao CC, Ayala G, Sheikh-Hamad D, Nassar G, Suki WN. Renal cell apoptosis in chronic obstructive uropathy: the roles of caspases. Kidney Int 60: 924–934, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Wu D, Soong Y, Zhao G, Szeto HH. A highly potent peptide analgesic that protects against ischemia-reperfusion-induced myocardial stunning. Am J Physiol Heart Circ Physiol 283: H783–H791, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Zhang G, Oldroyd SD, Huang LH, Yang B, Li Y, Ye R, El Nahas AM. Role of apoptosis and Bcl-2/Bax in the development of tubulointerstitial fibrosis during experimental obstructive nephropathy. Exp Nephrol 9: 71–80, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol 70: 1796–1806, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Zhao K, Luo G, Zhao GM, Schiller PW, Szeto HH. Transcellular transport of a highly polar 3+ net charge opioid tetrapeptide. J Pharmacol Exp Ther 304: 425–432, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Zhao K, Zhao G, Wu D, Soong Y, Birk A, Schiller P, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004. [DOI] [PubMed] [Google Scholar]