Abstract
The db/db mouse is a genetic model of type 2 diabetes that exhibits progressive renal disease. Obesity, hyperglycemia, and albuminuria (822 ± 365 vs. 28 ± 8 μg/day) are evident in 18-wk-old db/db compared with db/m (lean littermate control) mice. Our goal was to determine the blood pressure (BP) phenotype of the db/db mouse. Mean arterial BP measured in conscious mice by radiotelemetry was not different between db/db (n = 9) and db/m (n = 12) mice, averaging 113 ± 3 and 112 ± 2 mmHg, respectively. The circadian BP profile of db/db mice was shifted to the left and exhibited a significant reduction in amplitude compared with db/m mice. Heart rate (487 ± 9 vs. 542 ± 7 beats/min; P < 0.05) and locomotor activity were significantly reduced in db/db compared with db/m mice. We tested the hypothesis that intact afferent arteriole (AA) responsiveness to increases in renal artery pressure (RAP) and angiotensin (ANG) II sensitivity contributes to normal BP in this diabetic model. AA diameters of in vitro blood-perfused juxtamedullary nephrons of db/db mice (15.7 ± 0.5 μm; n = 38) were significantly larger than those of db/m mice (12.5 ± 0.4 μm; n = 37). AA responses to increases in RAP and ANG II were not different between kidneys of db/db and db/m mice. Significant AA vasoconstriction to 1 nM ANG II was observed in kidneys of db/db mice (−11 ± 4%), while 10 nM ANG II decreased AA diameter in both groups [db/db, −20 ± 4%, (n = 12); db/m, −26 ± 4% (n = 12)]. In summary, AA responses to increases in renal perfusion pressure and ANG II remain intact in db/db mice. Diabetic renal disease occurs in db/db mice independently of elevated BP.
Keywords: radiotelemetry, juxtamedullary nephron, angiotensin II, hyperglycemia
type 2 diabetes mellitus affects 24 million Americans and 220 million people worldwide (1, 4) and is the sixth leading cause of death in the US. Obesity has been identified as the principal risk factor associated with the rising prevalence of type 2 diabetes (7). According to the American Diabetes Association, ∼73% of adults with diabetes are hypertensive; therefore, over one-quarter of patients are normotensive. Diabetic nephropathy is the most common cause of end-stage renal disease in the Western world. Diabetic nephropathy is characterized by progressive albuminuria, declining glomerular filtration rate (GFR), and increased risk for cardiovascular disease.
The db/db mouse was first identified at the Jackson Laboratory in 1966 (16). The diabetes mutation (db) is inherited as an autosomal recessive trait and resembles the metabolic disturbances in diabetes mellitus in humans. Thirty years later, it was discovered that the db mutation is a G-to-T point mutation in the gene encoding the leptin receptor. This mutation causes abnormal splicing and defective signaling of the adipocyte-derived hormone leptin (6, 19). The lack of leptin receptor signaling in the hypothalamus leads to persistent hyperphagia and obesity. The db/db mouse exhibits hyperleptinemia and hyperinsulinemia and develops hyperglycemia in association with insulin resistance. The db/db mouse exhibits metabolic disturbances of diabetes mellitus similar to the characteristics of humans, which makes this a valuable model of type 2 diabetic kidney disease (5, 16, 30). Most important, this model exhibits a robust increase in albuminuria, renal hypertrophy, glomerular hypertrophy associated with increased length and surface area of glomerular capillaries, mesangial matrix expansion, focal glomerular sclerosis, thickening of the glomerular basement membrane, large subepithelial nodular densities with foot process fusion, arteriolar hyalinosis, and tubulointerstitial accumulation of extracellular matrix proteins, which are all features of human type 2 diabetic nephropathy (5, 9, 16, 30). However, currently there is no evidence of advanced tubulointerstitial fibrosis (5). An assessment of the renal microvascular function of this model of type 2 diabetes has not been directly performed previously. In addition, contradictory accounts of the blood pressure (BP) phenotype of the db/db mouse have been reported. With the tail cuff method, a significant elevation (3) or no change (17, 35) in BP in db/db compared with db/m mice has been reported. BP is not different in db/db mice measured under anesthesia (20). Of interest, BP measured by indwelling catheters in the leptin-deficient ob/ob mouse is significantly decreased compared with lean controls (22). Currently there are no publications reporting the BP, heart rate (HR), and circadian profile in db/db and db/m mice by radiotelemetry methodology.
Glomerular hyperfiltration leads to glomerular injury, which eventually progresses to reduced renal function, hypofiltration, and the development of diabetic glomerulopathy. Ohishi et al. (25) have demonstrated that juxtamedullary single-nephron and whole kidney GFR are significantly elevated in streptozotocin-induced type 1 diabetic rats. This elevation in GFR was associated with significantly larger baseline afferent arteriole (AA) diameters (25). Glomerular hyperfiltration (20) and hypofiltration (8) have been described in the db/db mouse. The contribution of changes in the AA structure to the changes in GFR has not been previously assessed in this animal model.
Pharmacological drugs that inhibit the actions of angiotensin (ANG)-converting enzyme and the ANG type 1 receptor delay the onset and slow the progression of diabetic nephropathy in humans, indicating the importance of the renin-angiotensin system in diabetic renal disease. The vasoconstrictor response to ANG II is increased in the renal circulation of prediabetic obese Zucker rats compared with lean rats (31), although the influence of ANG II on juxtamedullary AA and efferent arterioles is similar in kidneys of type 1 diabetic and sham-treated rats (29). Interestingly, ANG II sensitivity of rabbit AA is enhanced after acute incubation in high-glucose medium (2). The vasoconstriction induced by ANG II in the renal arterioles of kidneys of type 2 diabetic mice has not been investigated previously.
We tested the hypothesis that intact AA responsiveness to increases in renal artery pressure (RAP) and ANG II sensitivity contributes to normal BP in this diabetic model.
METHODS
Animals
The procedures used in this study were approved by the Animal Care and Use Committee of Louisiana State University Health Sciences Center and conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were performed in 18-wk-old adult male db/db (n = 55 in total; Leprdb BKS.Cg-m +/+ Leprdb/J) and db/m (n = 58 in total; BKS.Cg-m +/+) mouse littermates (Jackson Laboratory, Bar Harbor, ME; JAX Mice Database-000642 BKS.Cg-m +/+ Leprdb/J). Sixty-nine adult male Sprague-Dawley rats (457 ± 6 g; Charles River Laboratories, Raleigh, NC) were used as blood donors. All animals were provided ad libitum access to food and water before the study, with the exception of food removal for fasting blood glucose measurements.
Assessment of Urinary Albumin Excretion
Urine was collected in a separate group of 18-wk-old db/db (n = 12) and db/m (n = 11) mice housed individually in metabolic cages. After a 3-day equilibration period, three consecutive 24-h urine samples were obtained in mice with free access to water and food. Urine albumin concentration was determined with a commercially available indirect competitive ELISA (Mouse Urine Albumin ELISA Kit no. 1011, Exocell/Albuwell M, Philadelphia, PA) according to the manufacturer's instructions.
Telemetry
Cardiovascular parameters were obtained in conscious, freely moving mice with radiotelemetry probes (TA11PA-C10, Data Sciences International, St. Paul, MN). Radiotelemetric probes were implanted into the left carotid artery of db/db (n = 9) and db/m (n = 12) mice at 15 wk of age as we have previously described (18). After 2-wk recovery from surgery, 24-h BP, HR, and activity were monitored in conscious, unrestrained mice in their home cages for 7 consecutive days. Values were obtained for a 10-s period once every 5 min over 24 h and averaged for each day in each animal. The average of the seven individual daily values was obtained for each mouse. At the conclusion of the telemetry study, blood glucose was measured after 6-h fasting of mice housed individually in metabolic cages.
Mouse in Vitro Blood-Perfused Juxtamedullary Nephron Technique
On the day of the microcirculation experiment, blood glucose was determined in conscious fed mice and anesthetized fed rats. The fed blood glucose values of the conscious mice were used to determine the glucose concentration used for the in vitro perfusion and superfusion solutions (30 mM). The fed blood glucose value for each individual anesthetized rat was used to calculate the amount of glucose that needed to be added to obtain a final blood glucose of 30 mM. AA diameters were assessed in kidneys obtained from adult male db/db (n = 34) and db/m (n = 35) mice at 18 wk of age. Experiments were conducted with the mouse in vitro blood-perfused juxtamedullary nephron technique as previously reported in detail (10, 11, 27). AA diameters were studied at a site 25–50% along the length from the glomerulus. Experimental protocols were begun after a 15-min stabilization period. In some kidneys, another AA was visualized, protocols were repeated, and images were recorded. Only the right kidney was studied from each animal.
Acute Renal Exposure to Euglycemic, Hyperglycemic, or Hyperosmotic Incubation Conditions
Kidneys were studied under euglycemic (NG, 5 mM = 110 mg/dl glucose), hyperglycemic (HG, 30 mM = 540 mg/dl glucose), or hyperosmotic (30 mM mannitol) incubation conditions. HG perfusion and superfusion solutions utilized for the in vitro conditions mimic the fed blood glucose values of db/db mice. NG conditions mimic the fed blood glucose values of db/m mice. Mannitol was added to the perfusion and superfusion solutions to control for the hyperosmolarity of the HG solutions. The 5% perfusion solution used during the mouse kidney microdissection, 1% albumin superfusion solution, and rat plasma were prepared in the NG, HG, and mannitol conditions (4-h total kidney incubation time).
Autoregulatory Responses to Increasing Pressure in Kidney of db/db and db/m Mice
AA diameters were monitored in response to elevations in renal perfusion pressure in kidneys from db/db and db/m mice with NG, HG, or mannitol in the perfusion and superfusion solutions. AA diameters were measured during a 6-min control period at 95 mmHg. Renal perfusion pressure was reduced to 75 mmHg and then increased in a stepwise fashion to 95, 115, 135, and 155 mmHg as we have previously described in rat (12, 32) and mouse (10) kidneys. Pressures were maintained at each level for 3 min and then returned to 95 mmHg for a 15-min recovery period. In a subset of mice, ANG II dose responses were determined after the recovery period, as described below.
AA Responses to ANG II in db/m and db/db Mice
AA diameters from db/db and db/m mice were measured during superfusion with ANG II (human ANG II, catalog no. 002-12, Phoenix Pharmaceuticals) and NG, HG, or mannitol in the perfusion and superfusion solutions. After a 5-min control period or the recovery from the change in perfusion pressure protocol, the kidneys were superfused sequentially with 0.1, 1.0, and 10 nM ANG II for a period of 5 min for each concentration. A recovery period of 10 min was then observed.
Data Analysis
Renal arterial perfusion pressure and vessel diameters were sampled at 1 Hz and converted to digital form with analog-to-digital data-acquisition and analysis software as we have previously described (11, 27, 34). AA luminal diameters were measured manually and continuously throughout the protocol at a single site along the length of the selected vessel with a digital image-shearing monitor on playback of the DVD. The average vessel diameters (μm) during the control (5 min), treatment, and recovery (final 5 min) periods were used for statistical analysis. The average diameter during the plateau phase (final 2 min of each period) of the stepwise pressure protocol and the average diameter during the ANG II dose (5 min) were utilized for statistical analysis. Statistical analyses (SigmaStat 3.5, Systat Sofware) were performed on the raw diameter data within each group by one-way repeated-measures ANOVA followed by Dunnett's test. Comparisons between groups were performed by two-way ANOVA. Because of the significant difference in baseline AA diameters between db/db-HG and db/m-NG mice and between db/db-NG and db/db-mannitol mice, two-way ANOVA was conducted on the Δpercent change from control diameter. Telemetry data, baseline AA diameters, and urinary parameters between the groups were analyzed by unpaired t-test. P < 0.05 was considered statistically significant. Values are means ± SE (n = number of arterioles or number of mice as appropriate).
RESULTS
db/db and db/m Animals
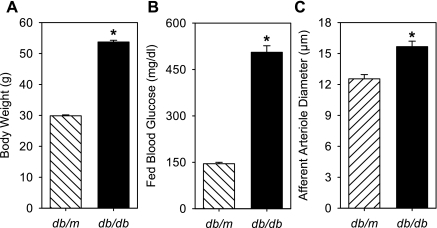
The body weights (Fig. 1A) of db/db mice were significantly greater than those of db/m mice [53.7 ± 0.6 g (n = 55) vs. 29.8 ± 0.3 g (n = 58); P < 0.05].
Fig. 1.
Body weight (A), fed blood glucose (B), and baseline renal afferent arteriole (AA) diameter (C) in 18-wk-old db/m and db/db mice. Body weight, fed blood glucose, and AA diameter of db/db mice (n = 55, 22, 38) were significantly larger than those of db/m mice (n = 58, 26, 37). *P < 0.05, db/m vs. db/db.
Urinary albumin excretion experiments.
Urine volume was significantly higher in db/db (n = 12) compared with db/m (n = 11) mice, averaging 5.1 ± 1.0 and 1.1 ± 0.2 ml/day, respectively. The db/db mice exhibited elevated urinary albumin excretion averaging 822 ± 365 μg/day compared with 28 ± 8 μg/day in db/m mice (P < 0.05).
Telemetry experiments.
Body weight at the time of probe implantation of 15-wk-old db/db mice (n = 9) was significantly higher than that of db/m mice (n = 12), averaging 52.9 ± 1.0 and 27.6 ± 6 g, respectively. Body weight was significantly reduced 14 days after surgery in both groups [44.5 ± 0.9 g (−16 ± 1%) and 26.0 ± 0.4 g (−5 ± 2%)], while the reduction was significantly greater in the db/db mice. The body weight of the db/db mice remained significantly greater than that of the db/m mice after surgery. Fasted blood glucose was significantly higher in conscious db/db mice (340 ± 64 mg/dl; n = 4) compared with db/m mice (123 ± 4 mg/dl; n = 12).
Microcirculation experiments.
Conscious blood glucose levels in the fed state (Fig. 1B) were significantly higher in db/db (506 ± 21 mg/dl; n = 22) compared with db/m (145 ± 4 mg/dl; n = 26) mice measured on the morning of the microcirculation experiments. Blood glucose values in the anesthetized fed rats averaged 160 ± 10 mg/dl (n = 24). Rat blood glucose after removal of blood from the rat, centrifugation, washing, resuspension, and spiking with glucose averaged 550 ± 9 mg/dl (30 mM; n = 24).
Measurement of BP, HR, and Locomotor Activity by Radiotelemetry
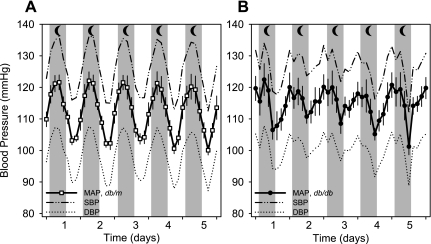
The mean arterial (MAP), systolic (SBP), diastolic, and pulse pressures measured continuously for 7 days in db/db mice (n = 9) were not different from those in db/m mice (n = 12) when averaged over the entire 24-h period, the 12-h period during daytime, or the 12-h period during nighttime (Table 1). BP averaged over 3-h periods beginning at 6:00 PM over 5 days for db/m and db/db mice is plotted in Fig. 2. Peak BP occurred at 12:00–3:00 AM, while trough BP occurred at 12:00–3:00 PM in db/m mice (Fig. 2A). The circadian rhythm was slightly shifted to the left in db/db mice, in which peak BP occurred at 6:00–9:00 PM, while trough BP occurred at 6:00–9:00 AM. The Δpeak-to-trough MAP for db/m mice was 19.5 ± 2.3 mmHg, which was significantly higher than in db/db mice, averaging 12.3 ± 2.4 mmHg. The Δpeak-to-trough MAP for db/db mice calculated at the same time points as for db/m mice averaged only 6.1 ± 2.3 mmHg. Interestingly, MAP measured between 12:00 PM and 3:00 PM was significantly higher in db/db than in db/m mice (111.1 ± 2.6 vs. 101.5 ± 1.0 mmHg). Both db/db and db/m mice demonstrated significantly higher BP, HR, and activity during nighttime compared with daytime. The ratios of daytime to nighttime MAP and SBP were significantly higher in db/db compared with db/m mice (MAP: 0.96 ± 0.01 and 0.91 ± 0.02 mmHg; SBP: 0.96 ± 0.01 and 0.91 ± 0.01 mmHg, respectively), suggestive of a nondipper pattern of BP in the db/db mice. HR and locomotor activity of db/db mice were significantly lower than in db/m mice at all times (Table 1).
Table 1.
Telemetry data for conscious 18-wk-old db/db and db/m mice
| Genotype | MAP, mmHg | SBP, mmHg | DBP, mmHg | PP, mmHg | HR, bpm | Activity, AU | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h (6 PM–6 PM) | ||||||||||||
| db/m | 112.3±2.0 | 125.8±2.4 | 98.4±2.1 | 27.3±2.6 | 542±7 | 7.5±0.8 | ||||||
| db/db | 113.3±2.9 | 126.2±3.4 | 100.7±3.3 | 25.3±3.1 | 487±9* | 2.6±0.3* | ||||||
| Daytime (6 AM–6 PM) | ||||||||||||
| db/m | 106.3±1.3 | 119.6±2.0 | 92.8±1.5 | 26.7±2.6 | 500±6 | 4.4±0.8 | ||||||
| db/db | 110.5±2.4 | 123.1±3.1 | 98.2±2.8 | 24.8±3.2 | 468±9* | 2.0±0.3* | ||||||
| Nighttime (6 PM–6 AM) | ||||||||||||
| db/m | 117.5±2.9† | 131.3±3.1† | 103.4±3.0† | 27.9±2.6† | 579±11† | 10.0±1.0† | ||||||
| db/db | 115.9±3.5† | 129.0±3.9† | 103.0±3.8† | 25.8±3.0† | 503±12*† | 3.6±0.5*† | ||||||
Values are means ± SE. Data represent the average of the daily 24-h and daytime and nighttime 12-h means for 7 consecutive days in each animal; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; HR, heart rate; bpm, beats/min; AU, arbitrary units.
P < 0.05, db/m (n = 12) vs. db/db (n = 9);
P < 0.05, daytime vs. nighttime.
Fig. 2.
Average blood pressure (BP) of conscious 18-wk-old db/m (A) and db/db (B) mice measured by radiotelemetry. Mean arterial BP (MAP), systolic BP (SBP), and diastolic BP (DBP) are plotted as the 3-h average over 24 h for a period of 5 days. BP was not different between db/m (n = 12) and db/db (n = 9) mice. BP traces demonstrate a prominent, large-amplitude circadian pattern in db/m mice, which is significantly less prominent in db/db mice. ΔPeak-to-trough MAP was significantly lower in db/db compared with db/m mice. Nighttime (moon), 6 PM–6 AM.
Baseline AA Diameters of db/db and db/m Mice
Baseline AA diameters measured during the initial control period (95 mmHg perfusion pressure) in db/db mice (15.7 ± 0.5 μm, n = 38) were significantly larger than in db/m mice (12.5 ± 0.4 μm, n = 36) (Fig. 1C). Baseline AA diameters of kidneys from db/db mice incubated with hyperosmotic (30 mM mannitol) solutions demonstrated a significantly larger diameter than db/db kidneys incubated in NG solutions [18.8 ± 0.9 (n = 10) and 14.3 ± 0.7 μm (n = 16), respectively]. However, baseline AA diameters of kidneys from db/db mice incubated with HG (30 mM glucose) solutions were not different from those of db/db kidneys incubated in NG solutions (14.9 ± 0.8 μm; n = 12). Baseline AA diameters of kidneys from db/m mice incubated in NG, HG, and mannitol were not different [13.2 ± 0.8 (n = 15), 12.1 ± 0.5 (n = 13), and 12.1 ± 0.9 μm (n = 9), respectively].
Intact AA Renal Autoregulatory Responses of db/db and db/m Mice
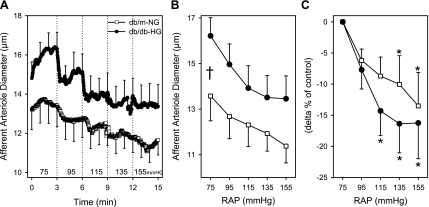
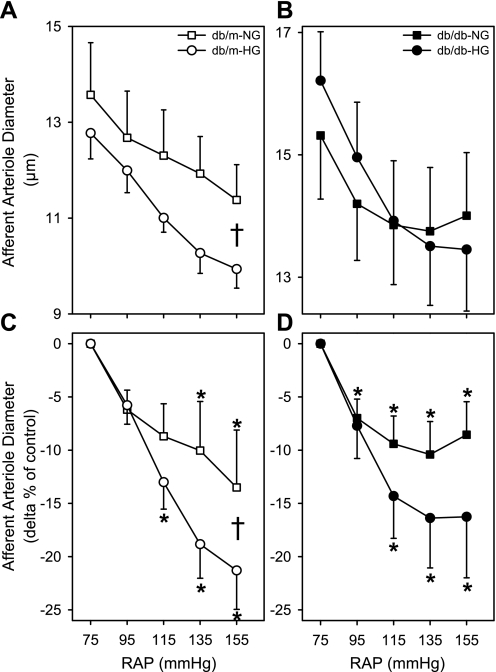
AA from db/db and db/m mice incubated in NG or HG solutions exhibited significant autoregulatory responses (P < 0.05); however, incubation in mannitol solutions blocked these responses. There was no significant difference in AA autoregulatory responsiveness between db/db-HG and db/m-NG (Fig. 3). After stepwise increases in renal perfusion pressure to 135 and 155 mmHg, AA diameters of db/m-NG decreased significantly by 10 ± 5% and 14 ± 5% (Fig. 3C). In kidneys of db/db-HG, AA diameter decreased significantly by 14 ± 4%, 16 ± 5%, and 16 ± 6% at 115, 135, and 155 mmHg, respectively (Fig. 3C). AA autoregulatory responses of db/m-NG mice were significantly enhanced by acute HG (Fig. 4, A and C) but not by mannitol exposure. After stepwise increases in renal perfusion pressure to 115, 135, and 155 mmHg, AA diameters of db/m-HG decreased by 13 ± 3%, 19 ± 3%, and 21 ± 4% relative to the diameter at 75 mmHg (Fig. 4C). After stepwise increases in renal perfusion pressure to 95, 115, 135, and 155 mmHg, AA diameters of db/db-NG were decreased by 7 ± 2%, 9 ± 3%, 10 ± 3%, and 9 ± 3% relative to the diameter at 75 mmHg (Fig. 4, B and D). AA diameters of db/m-mannitol averaged 12.4 ± 0.1, 11.8 ± 0.8, 11.3 ± 0.0, 11.2 ± 0.8, and 11.2 ± 0.8 μm (P > 0.05) at renal perfusion pressures of 75, 95, 115, 135, and 155 mmHg, respectively. AA diameters of db/db-mannitol averaged 19.0 ± 1.0, 18.2 ± 1.1, 17.6 ± 1.3, 17.4 ± 1.4, and 17.3 ± 1.5 μm (P > 0.05) at renal perfusion pressures of 75, 95, 115, 135, and 155 mmHg, respectively. There was no significant difference in the AA responses to increases in RAP between db/db-NG and db/m-NG. AA diameters were not significantly different during the recovery period compared with the initial baseline diameters for all groups.
Fig. 3.
AA diameter responses to elevations in renal arterial perfusion pressure (RAP) in kidneys of db/m and db/db mice. Kidneys from db/m mice (db/m-NG; n = 12) were incubated in euglycemic (5 mM glucose; NG) perfusion and superfusion solutions, while kidneys from db/db mice (db/db-HG; n = 11) were incubated in hyperglycemic (30 mM glucose; HG) solutions. AA diameter responses to stepwise increases in RAP are plotted as the time course (A), average in micrometers (B), and Δ% of control (C). AA from db/m-NG and db/db-HG kidneys responded to increases in perfusion pressure with a significant reduction in diameter (P < 0.05). The magnitude of the AA vasoconstrictor responses (Δ% of control) was not significantly different between db/m-NG and db/db-HG kidneys. Data are means ± SE. *P < 0.05 vs. diameter at 75 mmHg; †P < 0.05 db/m-NG vs. db/db-HG.
Fig. 4.
AA diameter responses to elevations in RAP in kidneys of db/m and db/db mice. Kidneys from db/m and db/db mice were incubated in euglycemic (NG, 5 mM glucose) and hyperglycemic (HG, 30 mM glucose) perfusion and superfusion solutions. A and C: AA diameter (μm, A; Δ% of control, C) responses to stepwise increases in RAP for db/m-NG (n = 12) and db/m-HG (n = 13) kidneys. B and D: AA diameter (μm, B; Δ% of control, D) responses to stepwise increases in RAP for db/db-NG (n = 12) and db/db-HG (n = 12) kidneys. AA from db/m-NG, db/m-HG and db/db-NG, db/db-HG responded to increases in perfusion pressure with a significant reduction in diameter. The magnitude of the AA vasoconstrictor responses was not significantly different between db/m-NG and db/db-NG kidneys or between db/db-NG and db/db-HG kidneys. Data are means ± SE. *P < 0.05 vs. baseline diameter at 75 mmHg; †P < 0.05 db/m-NG vs. db/m-HG.
AA Responses to ANG II in db/db and db/m Mice
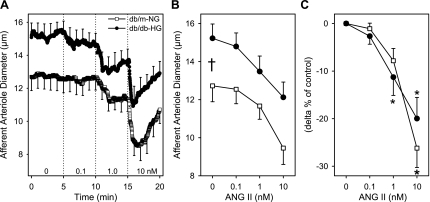
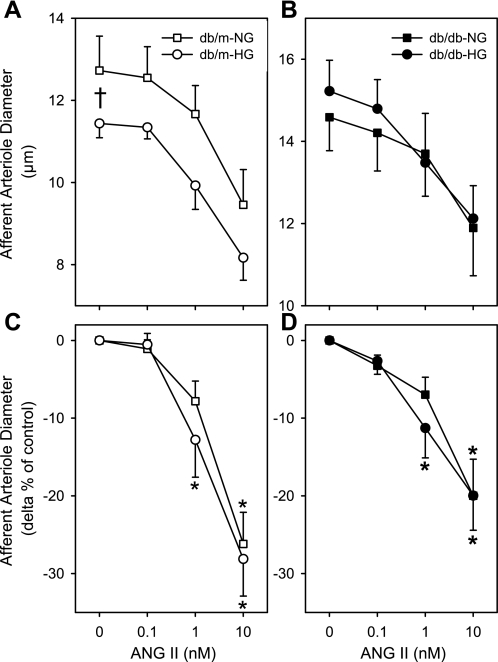
ANG II produced dose-dependent vasoconstriction of AA diameter in kidneys of db/m-NG and db/db-HG mice (Fig. 5). AA diameters from db/m-NG mice vasoconstricted to 1 and 10 nM ANG II by 8 ± 3% and 26 ± 4%, respectively, while reductions in diameter of arterioles from db/db-HG were 11 ± 4% and 20 ± 4%, respectively (Fig. 5). There was no significant difference between db/db-HG and db/m-NG responses to ANG II (Fig. 5C). AA diameters contracted significantly to 1 nM ANG II in db/db-HG (−11 ± 4%) and db/m-HG (−13 ± 5%) (Fig. 6, C and D). AA diameters from db/m-NG, db/m-HG, db/db-NG, db/db-HG, and mannitol exhibited significant vasoconstriction in response to ANG II (P < 0.05; Fig. 6); however, incubation in mannitol solutions blocked the response in kidneys of db/m mice. AA diameters of db/m-mannitol averaged 12.1 ± 0.6, 11.9 ± 0.5, 11.7 ± 0.6, and 10.9 ± 0.7 μm (P > 0.05) at 0, 0.1, 1, and 10 nM ANG II, respectively. AA diameters of db/db-mannitol averaged 18.0 ± 1.2, 17.9 ± 1.1, 17.2 ± 1.3, and 15.5 ± 1.2 μm (P < 0.05) at 0, 0.1, 1, and 10 nM ANG II, respectively. Acute incubation of kidneys from db/m mice in HG conditions resulted in a significant enhancement in the AA response to ANG II (Fig. 6, A and C). Significant vasoconstriction to 10 nM ANG II was observed in db/m-HG (−28 ± 5%), db/db-NG (−20 ± 5%) (Fig. 6), and db/db-mannitol (−13 ± 4%). There was no significant difference in the AA Δpercent responses to ANG II between db/db-NG and db/m-NG. AA diameters were not significantly different during the recovery period compared with baseline diameters for all groups.
Fig. 5.
AA diameter responses to angiotensin (ANG) II (0.1, 1, 10 nM) in kidneys of db/m and db/db mice. Kidneys from db/m mice (db/m-NG; n = 12) were incubated in euglycemic (5 mM glucose) perfusion and superfusion solutions, while kidneys from db/db mice (db/db-HG; n = 12) were incubated in hyperglycemic (30 mM glucose) solutions. AA diameter responses to ANG II are plotted as the time course (A), average in micrometers (B), and Δ% of control (C). Afferent arterioles from db/m-NG and db/db-HG kidneys responded to increasing doses of ANG II with a significant reduction in diameter (P < 0.05). The magnitude of the AA vasoconstrictor responses was not significantly different between db/m-NG and db/db-HG mice. Data are means ± SE. *P < 0.05 vs. baseline diameter; †P < 0.05 db/m-NG vs. db/db-HG.
Fig. 6.
AA diameter responses to ANG II (0.1, 1, 10 nM) in kidneys of db/m and db/db mice. Kidneys from db/m and db/db mice were incubated in euglycemic (NG, 5 mM glucose) and hyperglycemic (HG, 30 mM glucose) perfusion and superfusion solutions. A and C: AA diameter (μm, A; Δ% of control, C) responses to increasing doses of ANG II for db/m-NG (n = 12) and db/m-HG (n = 13) kidneys. B and D: AA diameter (μm, B; Δ% of control, D) responses to increasing doses of ANG II for db/db-NG (n = 16) and db/db-HG (n = 12) kidneys. AA from db/m-NG, db/m-HG and db/db-NG, db/db-HG responded to increases in ANG II with a significant reduction in diameter. The magnitude of the AA vasoconstrictor responses was not significantly different between db/m-NG and db/db-NG kidneys or between db/db-NG and db/db-HG kidneys. Data are means ± SE. *P < 0.05 vs. baseline diameter; †P < 0.05 db/m-NG vs. db/m-HG.
DISCUSSION
Mouse models of type 2 diabetes that result from loss of leptin signaling have been reported as hypotensive (22), normotensive (20, 33, 35), or hypertensive (3). BP measured by indwelling catheters in the leptin-deficient ob/ob mouse (14 wk old) is significantly decreased compared with lean control animals (22). There was no significant difference in 24-h MAP between wild-type and melanocortin 4 receptor-null mice (MC4R−/−, 22 wk old), which lack the ability to elicit some of the cardiovascular and metabolic actions of leptin (33). Tail cuff systolic BP measured in db/db mice was significantly greater than in db/m mice (12–14 wk old) (3). MAP was not different between db/db and db/m mice (12 wk old) measured under anesthesia (20). Systolic BP measured by tail cuff was not different between db/db and db/m mice (26 wk old), although BP was significantly elevated in db/db/endothelial nitric oxide synthase (eNOS)−/− mice (24–26 wk old) (35). To ensure that BP was not elevated because of the stress of surgery in the diabetic mice, we extended the recovery time after implantation of the radiotelemeters to 2 wk. BP was not different between db/db and db/m mice at 18 wk of age when calculated over 24 h or during nighttime or daytime. BP was significantly higher during nighttime compared with daytime in both db/db and db/m mice. The ratios of daytime to nighttime MAP and SBP were significantly higher in db/db compared with db/m mice, which is suggestive of a nondipper BP pattern (24). Normotensive type 1 diabetic human subjects who exhibit the nondipper pattern of BP have a worse prognosis for cardiovascular events and a higher risk to develop diabetic nephropathy (28). In humans with type 1 diabetes, an abnormal pattern of nighttime BP (described as a ratio of nighttime to daytime BP that is higher than 0.9) precedes the development of microalbuminuria (21). Therefore, even though the db/db mouse is normotensive, the lack of a dipping BP profile may contribute to the development of diabetic renal disease. At 18 wk of age, the db/db mouse exhibits significant urinary excretion of albumin, indicative of compromised renal function. Interestingly, MAP measured between 12:00 PM and 3:00 PM was significantly higher in db/db compared with db/m mice in our study. This may be due to the reduced amplitude of the circadian BP observed in db/db compared with db/m mice. The significantly higher BP observed in db/db mice compared with db/m mice at this time point may explain results obtained by other investigators in which BP was significantly higher in db/db mice that were measured only during daytime. Alternatively, the BP profile of the db/db mouse may change as a function of age and progression of the disease.
HR and activity were significantly lower in db/db compared with db/m mice at all times in the present study. Both were significantly higher during nighttime compared with daytime in db/db and db/m mice, although only nighttime HR was significantly lower in MC4R−/− mice compared with wild-type mice (33). HR, physical activity, and body temperature measured by radiotelemetry declined rapidly 3–5 days after administration of streptozotocin in rats (15). Reduced physical activity and/or body temperature may partly underlie this reduction in HR (15). Human leptin deficiency caused by a missense mutation is associated with decreased sympathetic system dysfunction and low sympathetic tone (26). Therefore, there appear to be similarities in the sympathetic control of HR in humans and animal models of type 1 and type 2 diabetes.
Levine et al. (20) reported that single-nephron and whole kidney GFR are significantly elevated in db/db compared with db/m mice (12 wk old). However, a 50% reduction in GFR has also been reported in 16-wk-old db/db compared with db/m mice (8). In the present study, juxtamedullary AA of kidneys from db/db mice were significantly larger than those from db/m mice, suggestive of a state of hyperfiltration. Of interest, although AA of type 1 diabetic rats are significantly dilated, efferent arteriole diameters are not different from sham-treated rats (25). In contrast, an enhanced arteriolar tone has been demonstrated in the isolated, pressurized gracilis skeletal muscle arteriole of db/db mice compared with db/m mice (3). At this time, an assessment of the efferent arteriolar diameter of kidneys from db/db and db/m mice has not been made.
We found that AA autoregulatory responses were not different between db/db and db/m mice. Interestingly, the AA response to increasing RAP was enhanced in kidneys of db/m mice incubated in HG compared with NG perfusion and superfusion solutions. Enhanced responses to increasing RAP were not found in kidneys of db/m mice incubated in mannitol, suggesting that glucose and not the hyperosmolarity per se is responsible for the enhanced AA responsiveness to increasing RAP, although an attenuation of the AA diameter responses to increases in RAP was not found in kidneys of db/db mice incubated in NG perfusion and superfusion solutions. It is possible that the changes that occur in the renal microvasculature in the db/db mice exposed to lifetime elevation in HG are not altered after acute NG conditions. Studies in isolated, perfused hydronephrotic kidneys indicate that elevated RAP elicits myogenic vasoconstriction of AA in both obese and lean Zucker rats, although the responses were attenuated in vessels of obese rats (14). Similarly, studies of isolated, perfused hydronephrotic kidneys from streptozotocin-induced type 1 diabetic rats indicate that elevated RAP elicited myogenic vasoconstriction of AA diameter, although the responses were significantly attenuated in the diabetic kidneys (13). Although the streptozotocin-treated rats were HG, the kidneys were perfused with NG perfusion medium (13). In isolated, pressurized gracilis muscle arterioles from db/db mice, stepwise increases in intraluminal pressure elicited a greater reduction in diameter than in control vessels at each pressure step (3). Therefore, there appears to be heterogeneity in the peripheral and renal vascular responses to changes in pressure in the db/db mouse.
There were no significant differences in AA responses to ANG II between kidneys obtained from db/db and db/m mice. We found that the greatest sensitivity to ANG II (lowest dose of ANG II that produces a significant vasoconstriction) was observed in kidneys of db/db and db/m mice that were incubated in HG perfusion and superfusion solutions. The AA vasoconstriction in response to 10 nM ANG II was similar in both groups under all incubation conditions. The vasoconstrictor reactivity to ANG II is specifically increased in the renal circulation of prediabetic obese Zucker rats compared with lean rats (31). ANG II-stimulated forearm vasoconstriction is increased in viscerally obese normotensive men compared with nonobese men (23). Incubation of rabbit AA in HG medium (30 mM) for 3 h enhanced the sensitivity to ANG II (2). Inhibition of nitric oxide synthesis with nitro-l-arginine methyl ester (l-NAME) abolished the difference in sensitivity to ANG II between rabbit AA studied in NG and HG conditions, suggesting impaired nitric oxide synthesis by HG (2). Additionally, pretreatment with l-arginine abolished the HG-induced augmentation of ANG II action in rabbit AA, suggesting that the effect of HG on AA is mediated by basal nitric oxide synthesis inhibition (2). Juxtamedullary AA of type 1 diabetic rat kidneys display suppressed endogenous nitric oxide modulation of ANG II contraction (29). This effect is largely due to nitric oxide degradation via reaction with superoxide anion (29). The enhanced AA contractile sensitivity to ANG II that we have observed in our study in db/m mice during incubation in HG solutions may be a result of a reduced buffering effect of nitric oxide under these conditions.
We show that adult male db/db mice exhibit albuminuria. Db/db mice are normotensive and display reduced HR and locomotor activity by radiotelemetric methodology. The circadian profile of MAP of db/db mice is significantly attenuated compared with the db/m mouse, which is suggestive of impaired BP control. A significant finding of the present study is that AA diameter is larger at baseline in kidneys of db/db compared with db/m mice, which may contribute to enhanced glomerular capillary pressure and renal damage. There is a significant vasoconstrictor response of AA diameter to increases in RAP in db/db and db/m kidneys, indicating an intact autoregulatory mechanism. The maintenance of renal microvascular contractile responsiveness to ANG II together with an intact autoregulatory mechanism in the kidneys of db/db mice may contribute to their ability to preserve normal BP at 18 wk of life. In addition, albuminuria occurs in db/db mice via mechanisms that are independent of an elevated systemic BP. A dilation of the AA could contribute to enhanced glomerular capillary pressure and renal injury in this model.
GRANTS
Support for this study was provided by National Institutes of Health Grants DK-62003 (L. M. Harrison-Bernard) and P20-RR-018766 (L. M. Harrison-Bernard, E. Lazartigues, Y. Feng).
Acknowledgments
Portions of this work have been published in abstract form (FASEB J 21: 892.1, 2007; J Am Soc Nephrol 18: 167A, 2007; FASEB J 22: 944.1, 2008).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anonymous. National Diabetes Statistics, 2007. Bethesda, MD: National Diabetes Information Clearinghouse, NIDDK, NIH, 2007.
- 2.Arima S, Ito S, Omata K, Takeuchi K, Abe K. High glucose augments angiotensin II action by inhibiting NO synthesis in in vitro microperfused rabbit afferent arterioles. Kidney Int 48: 683–689, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25: 1610–1616, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bays HE, Bazata DD, Clark NG, Gavin JR III, Green AJ, Lewis SJ, Reed ML, Stewart W, Chapman RH, Fox KM, Grandy S. Prevalence of self-reported diagnosis of diabetes mellitus and associated risk factors in a national survey in the US population: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes). BMC Public Health 7: 277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breyer MD, Bottinger E, Brosius FC III, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287: 356–359, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Gartner K Glomerular hyperfiltration during the onset of diabetes mellitus in two strains of diabetic mice (c57bl/6j db/db and c57bl/ksj db/db). Diabetologia 15: 59–63, 1978. [DOI] [PubMed] [Google Scholar]
- 9.Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J Anat 207: 813–821, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison-Bernard LM, Cook AK, Oliverio MI, Coffman TM. Renal segmental microvascular responses to ANG II in AT1A receptor null mice. Am J Physiol Renal Physiol 284: F538–F545, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Harrison-Bernard LM, Monjure CJ, Bivona BJ. Efferent arterioles exclusively express the subtype 1A angiotensin receptor: functional insights from genetic mouse models. Am J Physiol Renal Physiol 290: F1177–F1186, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Harrison-Bernard LM, Navar LG. Renal cortical and medullary microvascular blood flow autoregulation in rat. Kidney Int Suppl 57: S23–S29, 1996. [PubMed] [Google Scholar]
- 13.Hayashi K, Epstein M, Loutzenhiser R, Forster H. Impaired myogenic responsiveness of the afferent arteriole in streptozotocin-induced diabetic rats: role of eicosanoid derangements. J Am Soc Nephrol 2: 1578–1586, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Kanda T, Homma K, Tokuyama H, Okubo K, Takamatsu I, Tatematsu S, Kumagai H, Saruta T. Altered renal microvascular response in Zucker obese rats. Metabolism 51: 1553–1561, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Howarth FC, Jacobson M, Shafiullah M, Adeghate E. Long-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol 90: 827–835, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science 153: 1127–1128, 1966. [DOI] [PubMed] [Google Scholar]
- 17.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J 14: 439–447, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Lazartigues E, Lawrence AJ, Lamb FS, Davisson RL. Renovascular hypertension in mice with brain-selective overexpression of AT1a receptors is buffered by increased nitric oxide production in the periphery. Circ Res 95: 523–531, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Levine DZ, Iacovitti M, Robertson SJ, Mokhtar GA. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 290: R975–R981, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 347: 797–805, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens 17: 1949–1953, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen S, Halliwill JR, Joyner MJ, Jensen MD. Vascular response to angiotensin II in upper body obesity. Hypertension 44: 435–441, 2004. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien E, Sheridan J, O'Malley K. Dippers and non-dippers. Lancet 2: 397, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Ohishi K, Okwueze MI, Vari RC, Carmines PK. Juxtamedullary microvascular dysfunction during the hyperfiltration stage of diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 267: F99–F105, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 84: 3686–3695, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Park S, Bivona BJ, Harrison-Bernard LM. Compromised renal microvascular reactivity of angiotensin type 1 double null mice. Am J Physiol Renal Physiol 293: F60–F67, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Redon J, Lurbe E. Nocturnal blood pressure versus nondipping pattern: what do they mean? Hypertension 51: 41–42, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Schoonmaker GC, Fallet RW, Carmines PK. Superoxide anion curbs nitric oxide modulation of afferent arteriolar ANG II responsiveness in diabetes mellitus. Am J Physiol Renal Physiol 278: F302–F309, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Sharma K, McCue P, Dunn S. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol 284: F1138–F1144, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Stepp DW, Boesen EI, Sullivan JC, Mintz JD, Hair CD, Pollock DM. Obesity augments vasoconstrictor reactivity to angiotensin II in the renal circulation of the Zucker rat. Am J Physiol Heart Circ Physiol 293: H2537–H2542, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamedullary nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 267: F879–F887, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 48: 58–64, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Walker M, Harrison-Bernard LM, Cook AK, Navar LG. Dynamic interaction between myogenic and TGF mechanisms in afferent arteriolar blood flow autoregulation. Am J Physiol Renal Physiol 279: F858–F865, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]