Abstract
This study used 16 h/day measurement of renal blood flow (RBF) and arterial pressure (AP) to determine the role of nitric oxide (NO) in mediating the renal vasodilation caused by onset of type 1 diabetes. The AP and RBF power spectra were used to determine the autoregulatory efficiency of the renal vasculature. Rats were instrumented with artery and vein catheters and a Transonic flow probe on the left renal artery and were divided randomly into four groups: control (C), diabetes (D), control plus nitro-l-arginine methyl ester (l-NAME; CL), and diabetes plus l-NAME (DL). Mean AP averaged 90 ± 1 and 121 ± 1 mmHg in the D and DL groups, respectively, during the control period, and RBF averaged 5.9 ± 1.2 and 5.7 ± 0.7 ml/min, respectively. Respective C and CL groups were not different. Onset of diabetes (streptozotocin 40 mg/kg iv) in D rats increased RBF gradually, but it averaged 55% above control by day 14. In DL rats, on the other hand, RBF remained essentially constant, tracking with RBF in the nondiabetic C and CL groups for the 2-wk period. Diabetes did not change mean AP in any group. Transfer function analysis revealed impaired dynamic autoregulation of RBF overall, including the frequency range of tubuloglomerular feedback (TGF), and l-NAME completely prevented those changes as well. These data strongly support a role for NO in causing renal vasodilation in diabetes and suggest that an effect of NO to blunt RBF autoregulation may play an important role.
Keywords: autoregulation, chronic animal models
the mechanism for renal vasodilation in early diabetes mellitus has generated considerable interest because of its effect to allow transmission of systemic arterial pressure into the glomerulus, thus facilitating hemodynamic-mediated renal injury (32, 33, 55). However, despite the general renal vasodilatory effect of nitric oxide (NO), there is evidence both for (5, 8, 12, 17, 28) and against (1, 37, 38, 42) NO as a primary determinant of the increased renal blood flow (RBF) and glomerular filtration rate (GFR) in early experimental diabetes. The explanation for why there are opposing findings is not clear, but it could be due to different time points in diabetes at which GFR is measured, the degree of hyperglycemia, anesthetized versus awake preparations, or the level of reactive oxygen species. Another potential explanation, however, could be the methods used to measure renal hemodynamics.
All studies to date, including many previous studies from our laboratory (5, 8, 17), have used acute measurements of RBF and GFR primarily with clearance techniques. One limitation is that those measurements are a snapshot of renal function over a period of minutes, and in a chronic disease such as diabetes, all the factors noted above can come into play and contribute to the potential for wide variation in results among studies that measure renal function from the first several days to several months after induction of diabetes. We recently developed the technique for measuring RBF and arterial pressure (AP) for up to 24 h/day in undisturbed conscious rats during control conditions and through the first 2 wk of type 1 diabetes (2). We measured a sustained increase in RBF immediately following induction of diabetes, and in the present study, we used that same technique to test the hypothesis that the increase in RBF is dependent on nitric oxide (NO).
In addition, we used transfer function analysis of the daily AP and RBF signals as a means to evaluate the role of NO in RBF autoregulation in this chronic experimental animal model. An advantage of using dynamic autoregulation to study autoregulation of RBF is that repeated measurements can be made in the same animal in the conscious, undisturbed state over the time course of the entire study (4, 18). Previously we used this method to show that dynamic autoregulation in the tubuloglomerular feedback (TGF) frequency range was impaired (2). This is a novel approach in a chronic experimental animal model for gaining insight into the potential intrarenal mechanisms through which NO may control RBF in diabetes, particularly because we analyzed 16-h RBF and AP records each day in each rat.
METHODS
The experiments were conducted in male Sprague-Dawley rats (350–375 g; Harlan Sprague-Dawley, Madison, WI), and protocols were approved by the Institutional Animal Care and Use Committee. Anesthesia was induced with pentobarbital sodium (50 mg/kg), and atropine was administered (40 μg ip per rat) to minimize airway secretions. Under aseptic conditions, a laparotomy was performed, and a 1-mm Transonic flow probe (Transonic Systems) was placed on the artery of the left kidney. Although care was taken to avoid denervation, fat was cleared from the renal artery using a cotton swab to enable an unimpeded ultrasonic signal. The right kidney was not removed. A nonocclusive polyvinyl catheter then was implanted in the abdominal aorta, below the renal artery, by inserting the catheter tip through a puncture wound in the aortic wall made with the tip of an L-shaped 18-gauge needle. The insertion point was sealed with cyanoacrylate adhesive, and the catheter and flow probe cable were exteriorized through the lateral abdominal wall. A femoral vein catheter was implanted through a separate incision. The flow probe leads and catheters were routed subcutaneously to the scapular region and exteriorized through a Dacron-covered plastic button sutured subcutaneously over the scapulae.
The rats were allowed to recover from surgery and then were placed in individual metabolic cages in a quiet, air-conditioned room with a 12:12-h light-dark cycle. The catheters and flow probe leads were passed through a stainless steel spring that was attached to the button, and the opposite end of the spring was connected to a customized adapter on an electrical swivel (Airflyte Electronics) mounted above the cage. The four flow probe leads were soldered to the swivel wires, and the artery and vein catheters were passed through a hole in the center of the electrical swivel to a dual-channel hydraulic swivel (Instech) above. A customized connector joined the two swivels so that both would turn synchronously with rat movement. The venous catheter was connected, via the hydraulic swivel, to a syringe pump (Harvard Apparatus) that ran continuously throughout the study. All solutions were infused through a Millipore filter (0.22 μm; Cathivex; Millipore). The arterial catheter was filled with heparin solution (1,000 USP U/ml) and connected, also via the hydraulic swivel, to a pressure transducer for continuous measurement of arterial pressure. The flow probe connector was soldered to the electrical swivel wires and connected to a Transonic model T206 flowmeter (Transonic Systems) for continuous measurement of RBF. The pulsatile flow signals from the flowmeter and the amplified pulsatile arterial pressure signals (CB Sciences) were sampled at 100 Hz, 16 h/day throughout the experiment, using PowerLab and a Macintosh computer.
Total sodium intake throughout the experiment was maintained constant at ∼3.1 mmol/day by continuous intravenous infusion of 20 ml/day sterile 0.9% saline combined with sodium-deficient rat chow (0.006 mmol sodium/g; Teklad). A sodium-deficient diet ensured that the daily sodium intake could be controlled precisely at normal levels by the infusion. This infusion was begun immediately after placement of the rat in the metabolic cage, and 3–5 days were allowed for acclimation.
Experimental Protocol
After recovery from surgery and acclimation, the rats were divided randomly into four groups: control (C; n = 7), diabetes (D; n = 6), control plus nitro-l-arginine methyl ester (l-NAME; CL; n = 7), and diabetes plus l-NAME (DL; n = 8). After baseline measurements, l-NAME (10 μg·kg−1·min−1 iv) (5, 17, 21, 30) was added to the infusate of the CL and DL rats and maintained throughout the remainder of the study. Six days later, type 1 diabetes was induced in the two diabetic groups with streptozotocin (STZ; 40 mg/kg iv). The diabetes experimental period lasted for 2 wk. Insulin was added to the daily saline infusion syringe if needed to keep blood glucose from exceeding 450 mg/dl. On day 4 of the control period and once per week during the 2-wk diabetes period (days 3 or 4 and day 12), 1.3 ml of arterial blood was collected from the arterial catheter for measurement of GFR, plasma renin activity (PRA), hematocrit, glucose concentration, and plasma protein and electrolyte concentrations. Samples were replaced with an equal volume of 0.9% saline.
Analytical Methods
GFR was measured after a 24-h intravenous infusion of [125I]iothalamate (Glofil; ∼20 μCi). Because steady state is achieved during the 24-h infusion, the isotope infusion rate was substituted for urinary isotope excretion rate to calculate clearance (3). Urinary sodium and potassium concentrations were determined with ion-sensitive electrodes (Synchron El-ise; Beckman Coulter), and urine glucose was documented with a Bayer Clinitek 50 dipstick analyzer. PRA was measured by radioimmunoassay (Diasorin). Data were analyzed using a two-factor analysis of variance (ANOVA) with repeated measures, with Dunnett's test used for within-group comparisons over time. For between-group comparisons, completely randomized ANOVA tests were run for each day and supplemented with Scheffé's test to determine specific group differences. P < 0.05 was considered statistically significant, and data are means ± SE.
Transfer function analysis.
Daily data files covering the time period from ∼1300 to 0500 (16 h) were processed off-line using previously developed software routines (16) written for Matlab (version 7, release 14). Data from day 8 were used for the day 7 data in some cases if the day 7 file was truncated due to catheter clotting or probe malfunction. The 100-Hz data files were digitally low-pass filtered (3.5-Hz cut-off frequency, finite-impulse response, order 50) and decimated to 5 Hz. These 5-Hz data files were split into blocks of 4,096 data points, yielding a frequency discrimination of 0.001 Hz. Power spectral density of AP and RBF was calculated using Welch's method [with units of mmHg2/Hz and (ml/min)2/Hz, respectively]. The transfer function spectra were calculated from AP (input) and RBF (output). The transfer function was taken as the quotient of the cross spectrum of input and output divided by the power spectrum of the input. These algorithms involved mean detrending and a Hanning window with 50% overlap of the blocks. To permit comparison among rats, we normalized the transfer function gain (magnitude) values over the frequency range to the mean renal vascular conductance over the duration of the data set. After conversion of the normalized transfer function gain values into decibels [20 log(gain)], a mean spectrum was calculated from the consecutive spectra in each rat, and these were subsequently averaged for all rats. Curves were compared using ANOVA followed by unpaired t-tests.
RESULTS
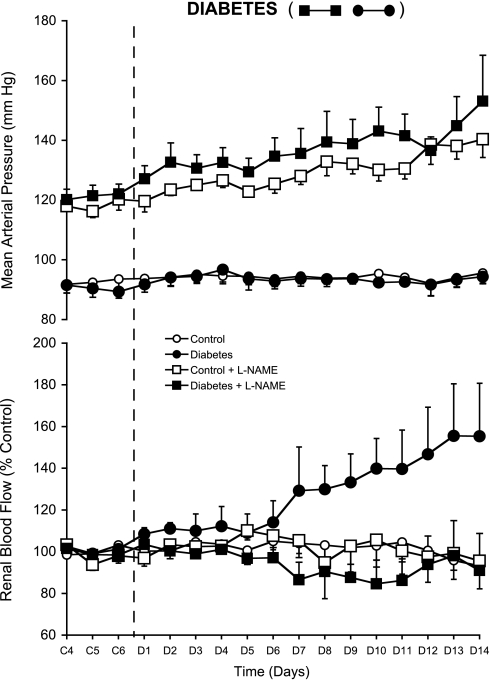
Renal blood flow averaged 6.8 ± 0.8 and 6.2 ± 0.8 ml/min in the L and DL groups, respectively, during baseline measurements made before beginning the control period (data not shown) and averaged 5.5 ± 0.7 and 5.7 ± 0.7 ml/min, respectively, under the influence of the chronic l-NAME infusion during the control period [days 4–6 of the control period (C4–C6) are plotted in Fig. 1 as a percentage of control]. RBF averaged 6.1 ± 0.7 and 5.9 ± 1.2 ml/min in the C and D groups, respectively, during the control period and also is plotted as percent control values for C4–C6 in Fig. 1. Mean arterial pressure (MAP) was higher during the control period in the l-NAME-infused rats, averaging 118 ± 1 and 121 ± 1 mmHg in CL and DL compared with 93 ± 1 and 90 ± 1 mmHg in the C and D groups, respectively (Fig. 1). Thus, although chronic l-NAME administration did not cause pronounced reductions in daily RBF, there were approximate 57 and 48% increases in renal vascular resistance caused by chronic l-NAME infusion in the L and DL groups, respectively, at baseline.
Fig. 1.
Mean arterial pressure (AP; top) and renal blood flow (RBF; bottom) in control (○), diabetic (•), control + nitro-l-arginine methyl ester (l-NAME; □), and diabetic + l-NAME rats (▪) during the last 3 control period days (C4–C6) and the 14-day diabetes period (D1–D14).
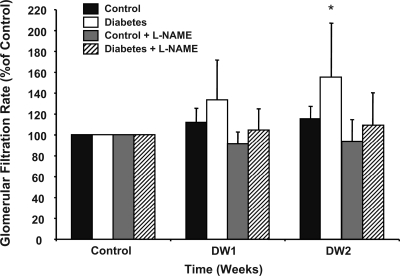
Administration of STZ increased blood glucose significantly in the D and DL groups (Table 1). RBF averaged 111 ± 3% of control in the D rats by the second day of diabetes, which, although not statistically significant, was similar to the initial response we reported previously with these methods (2). The plateau in RBF from days 3–6 of diabetes (D3–D6), however, differed from the continued rise we reported previously during week 1 (2), but RBF was increased significantly by D7 and was ∼55% above control levels by D14 (Fig. 1). In the DL rats, on the other hand, the renal vasodilatory response was eliminated completely, with RBF essentially remaining constant over the entire experimental period and tracking with RBF in the nondiabetic C and CL control groups (Table 1). Likewise, Fig. 2 shows that the increase in GFR caused by diabetes also was prevented in the DL rats, similar to our previous reports (5, 17). Diabetes did not change MAP in any group.
Table 1.
Blood glucose, PRA, hematocrit, plasma protein concentration, and urinary Na+ excretion during each study period in control, diabetic, control + l-NAME, and diabetes + l-NAME rats
| Variable/Group |
Experimental Period |
||
|---|---|---|---|
| Control | DW1 | DW2 | |
| Blood Glucose, mg/dl | |||
| C | 131±4.0 | 123±1.7 | 124±5.0 |
| D | 131±3.2 | 477±22.2*† | 475±17.5*† |
| CL | 123±2.8† | 132±9.1 | 124±1.8 |
| DL | 120±3.5† | 429±46.2*† | 391±47.1*† |
| PRA, ng ANG I·ml−1·h−1 | |||
| C | 1.6±0.7 | 1.1±0.7 | 1.2±0.1 |
| D | 0.8±0.4 | 1.5±1.2 | 2.1±0.8 |
| CL | 0.8±0.3 | 2.2±0.8* | 1.9±0.8 |
| DL | 0.6±0.2# | 1.8±1.2* | 2.0±1.7 |
| Hematocrit, % | |||
| C | 41±0.7 | 42±0.6 | 40±2.7 |
| D | 40±2.7 | 42±0.9 | 39±3.1 |
| CL | 42±0.8 | 42±0.9 | 42±0.7 |
| DL | 43±0.7 | 43±0.6 | 42±0.7 |
| Plasma Protein, g/dl | |||
| C | 6.8±0.4 | 6.8±0.2 | 6.7±0.1 |
| D | 6.4±0.2 | 7.5±0.4*† | 6.8±0.2 |
| CL | 6.7±0.1 | 6.4±0.1 | 7.0±0.2 |
| DL | 6.5±0.1 | 7.5±0.3*† | 6.7±0.2 |
| UNaV, mEq/day | |||
| C | 2.3±0.5 | 2.8±0.8 | 2.8±0.1 |
| D | 2.9±0.1 | 3.4±0.1*† | 3.6±0.1*† |
| CL | 2.4±0.2 | 2.6±0.1 | 2.7±0.1* |
| DL | 2.9±0.1 | 3.4±0.1*† | 3.3±0.2*† |
Values are means ± SE for blood glucose, plasma renin activity (PRA), hematocrit, plasma protein concentration, and urinary Na+ excretion (UNaV) in control (C), diabetic (D), control + nitro-l-arginine methyl ester (l-NAME; CL), and diabetic + l-NAME (DL) rats during control period and weeks 1 (DW1) and 2 (DW2) of diabetes period.
P < 0.05 for within-group comparisons vs. control period value.
P < 0.05 for between-group comparison with control group during that period.
Fig. 2.
Glomerular filtration rate (GFR) in control, diabetic, control + l-NAME, and diabetic + l-NAME rats during the control period and weeks 1 (DW1) and 2 (DW2) of the diabetes period. *P < 0.05 for within-group comparison vs. control.
Effects of Chronic NO Blockade on Spectral Analysis of AP and RBF
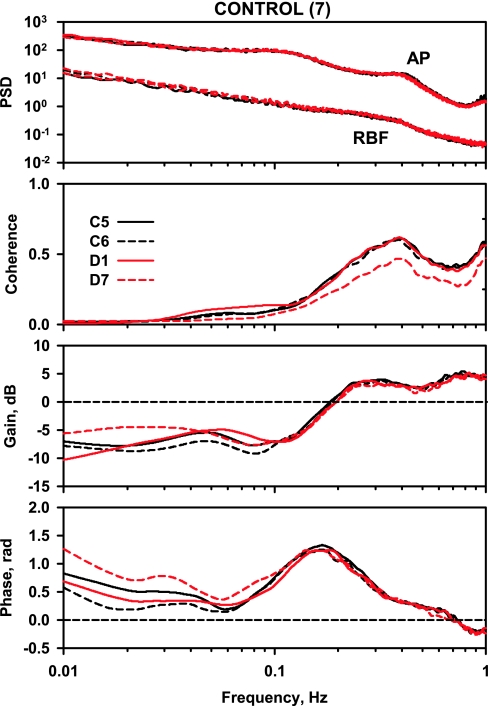
Power spectra, coherence, gain, and phase for the C group are shown in Fig. 3. Peak coherence occurred at 0.3–0.5 Hz, consistent with pressure-passive dynamics in this region of the spectrum, and coherence was reduced at frequencies below 0.2 and 0.04 Hz, consistent with operation of autoregulation. Gain was positive above 0.2 Hz due to renal arterial compliance. There was a discrete reduction in gain below 0.2 Hz and a local maximum in gain between 0.04 and 0.06 Hz. Phase approached zero at 0.3–0.5 Hz, and there was a strong positive phase peak coincident with the gain reduction below 0.2 Hz and another phase peak centered near 0.03 Hz. The pattern of gain reduction in association with a positive phase peak indicates an autoregulatory mechanism that operates to attenuate the AP-induced RBF fluctuations within its operating frequency band. A zero phase angle indicates simultaneous AP and RBF fluctuations so that a pressure-passive behavior of the vasculature is reflected by a slightly positive but near-zero phase angle.
Fig. 3.
Power spectra for arterial pressure (AP) and renal blood flow (RBF), coherence, gain, and phase in the control rats. In each panel, the solid black line represents day 5 of the control period (C5), the dashed black line represents day 6 of the control period (C6), the solid red line represents day 1 of the diabetes period (D1), and the dashed red line represents day 7 of the diabetes period (D7).
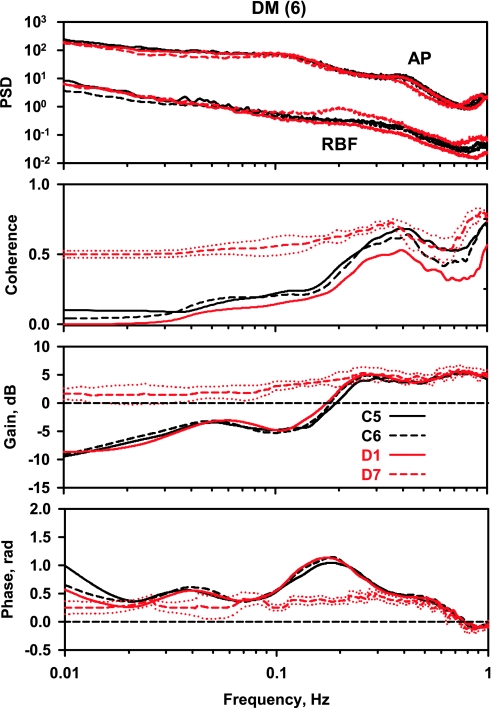
Effect of diabetes.
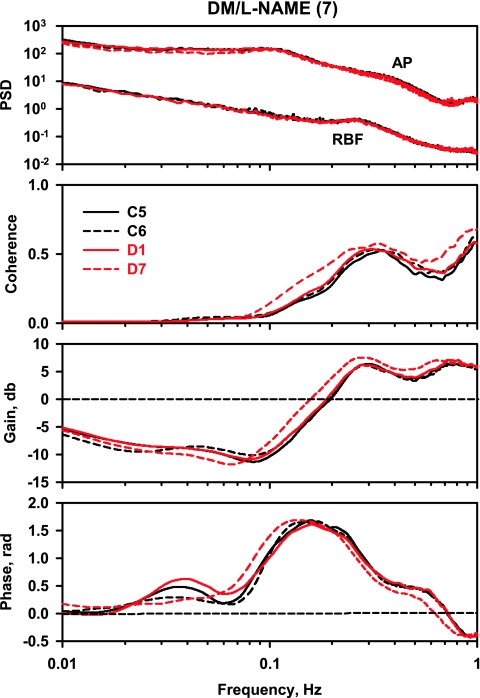
After STZ administration, neither AP nor RBF were significantly affected on D1 (Fig. 1), and the spectral parameters were unchanged from those observed on C5 and C6 (Fig. 4). On D7, RBF was increased significantly and RBF power near 0.2 Hz was approximately twice as large as on D1; however, that difference did not achieve statistical significance. Instead of the normal decrease in coherence below 0.3 Hz seen on C5, C6, and D1, coherence remained above 0.5 Hz below 0.3 Hz; this close relationship between AP and RBF fluctuations results from the absence of autoregulation. In the gain spectrum, instead of the steep reversal from positive to negative gain values between 0.1 and 0.3 Hz (myogenic) as well as the local gain maximum at 0.02–0.05 Hz (TGF) observed on C5, C6, and D1, gain remained positive and declined slowly to zero, reflecting a pressure-passive behavior dependent on arterial compliance (i.e., lack of autoregulatory behavior). In contrast to the normal presence of positive phase peaks at frequencies where gain reduction is maximal (0.1–0.3 and 0.02–0.06 Hz) observed on C5, C6, and D1, phase was significantly different for D7, remaining at low positive values consistent with the absence of autoregulatory behavior.
Fig. 4.
Power spectra for AP and RBF, coherence, gain, and phase in the diabetes mellitus (DM) rats.
Effect of l-NAME.
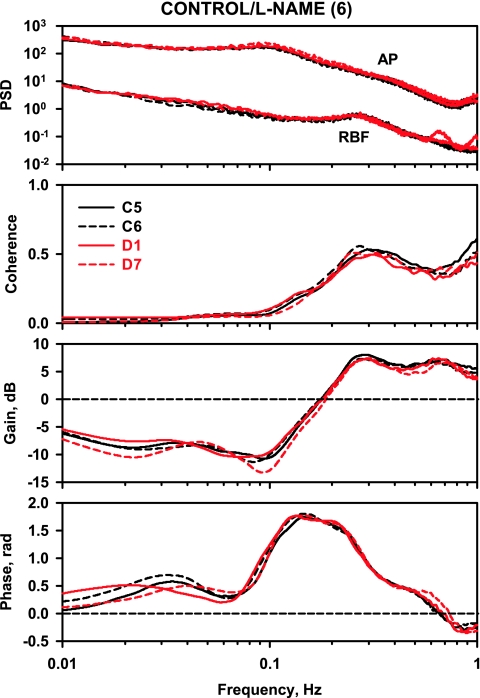
Commensurate with the hypertension, l-NAME increased AP spectral power in both L and DL rats (from 0.001 to 0.01 Hz; data not shown). In addition, compared with control rats (Fig. 3), gain above 0.2 Hz was increased in control/l-NAME rats (Fig. 5), and gain below 0.2 Hz was significantly more negative compared with control over the frequency range 0.04–0.1 Hz. Phase peak from 0.08 to 0.3 Hz also increased. Similar observations have been made in previous transfer function analysis studies (26, 53) and are taken to indicate a strengthening of the myogenic mechanism following NO synthase (NOS) inhibition. When diabetes was induced in l-NAME-treated rats, not only was the increase in RBF prevented but also the abnormal coherence, gain, and phase spectra measured on D7 in the diabetic rats (Fig. 4) were eliminated (Fig. 6).
Fig. 5.
Power spectra for AP and RBF, coherence, gain, and phase in the control/l-NAME rats.
Fig. 6.
Power spectra for AP and RBF, coherence, gain, and phase in the DM/l-NAME rats.
DISCUSSION
The main finding from this study is that the increase in RBF caused by the onset of diabetes was prevented by chronic blockade of NO synthesis. In addition, blocking NO synthesis prevented diabetes from impairing dynamic autoregulation of RBF. These results not only strongly suggest that NO is required for diabetes-induced renal vasodilation but also suggest that the mechanism may involve attenuation of RBF autoregulation.
Understanding the mechanism for renal vasodilation early in diabetes is important because of its effect to allow transmission of systemic arterial pressure into the glomerulus, thus facilitating hemodynamic-mediated renal injury (32, 33, 55). NO is an attractive candidate to mediate the increase in GFR, but its potential role in that regard has been questioned. For example, Pflueger et al. (37, 38) reported that NO-dependent renal vasodilation actually was defective in STZ diabetic rats, and blockade of NO synthesis has been reported to have similar effects on GFR in diabetic and normal rats (1, 42). In addition, Keynan et al. (27) reported decreased neuronal NOS (nNOS) activity and protein in the renal cortex with no change in endothelial cell NOS (eNOS) activity and protein during the first week of STZ diabetes in rats. Thus there is evidence against NO-mediated renal vasodilation in diabetes, which has been attributed to decreased, or no change in, NOS activity or expression.
On the other hand, there is evidence that NO may play a significant role in mediating diabetic hyperfiltration and renal vasodilation. Increased NO metabolites have been reported in diabetic animals (12, 28, 31, 45, 50) and in human subjects (11, 20), and inhibition of NO synthesis has been reported to selectively block or attenuate diabetic hyperfiltration (12, 15, 28, 31, 45, 50). The reason for the disparate findings is not known, but it could be due to factors such as the level of hyperglycemia (44), the stage of diabetes at which measurements are made (39), or the relative balance between superoxide (13, 19, 34, 35, 41, 46) and NO under various experimental conditions. An alternative, but related, explanation could be the method for evaluating renal hemodynamics.
Until our recent report (2), all measurements of RBF and/or GFR in diabetes were acute measurements, made at isolated time points, primarily using clearance techniques, in either the awake or anesthetized state. However, we measured RBF 18 h/day using a Transonic flow probe and recorded RBF during control conditions continuing through the first 2 wk of diabetes in the same animal (2). That study revealed a rapid increase in RBF when diabetes was initiated that was sustained for the 2-wk study period and was accompanied by significant increases in GFR (2). The present study used the same experimental approach, confirmed the renal vasodilatory response to onset of diabetes, and, in addition, showed that the renal vasodilatory response was blocked completely when diabetes was induced in rats with chronic blockade of NO synthesis. The increase in GFR also was blocked. Continuous measurement of RBF in conscious, unrestrained, and undisturbed animals during control and experimental conditions is the most accurate and sensitive method for determining true minute-to-minute RBF throughout the day and from day to day. It removes questions related to the potential influence of anesthesia, stress from transport to the laboratory for measurements, and time of day at which measurements are made (most acute laboratory studies in rats are conducted during the time when rats normally would be asleep). In addition, by measuring RBF continuously for 2 wk, the method addresses questions about whether experiments at the 2-wk time point reflect renal function during the first several days of hyperglycemia, and vice versa. Thus the methods used in the present study provide very strong evidence that NO synthesis is required for diabetes to cause renal vasodilation.
One potential mechanism for that relationship could be through interaction of NO with reactive oxygen species. Reactive oxygen species are increased in diabetes and have been shown to decrease NO levels and impair NO activity in the kidney (13, 19, 34, 35, 41, 46). On the other hand, an important action of NO may be to blunt the increase in superoxide and thereby protect against its potential vasoconstrictor influence (5). Another mechanism through which NO could induce, or mediate, renal vasodilation in diabetes could be through direct effects on renal blood vessels. Although some studies have not measured increases in renal eNOS in diabetes (23, 42), others have measured increased eNOS in pre- (15, 36, 45) and postglomerular vessels (15, 36) from diabetic animals and in human glomerular endothelial cells from type 2 diabetic patients with increased GFR (20). However, the effect of nonspecific NOS inhibition with l-NAME to block increases in RBF and GFR in diabetes, as shown in the present study and previously (5, 8, 12, 17, 28), is not necessarily limited to effects on eNOS, because there also is strong evidence that nNOS may play a major role in controlling renal vascular resistance and GFR in diabetes (24, 29, 47).
Expression of nNOS is increased in the macula densa in diabetes (29), and selective nNOS inhibition has been shown to blunt the increase in GFR in diabetes (24, 29, 47). A particularly intriguing aspect of an nNOS effect on GFR and RBF is the likely involvement of TGF (47). However, the mechanism linking NO and TGF to the control of renal vascular resistance in diabetes is not clear. One hypothesis is that glucose-driven sodium transport in the proximal tubule essentially causes the macula densa to sense decreased sodium concentration despite an increase in total distal delivery, thus initiating an nNOS-dependent signal in the macula densa cells that decreases TGF-mediated vasoconstriction and thereby causes afferent arteriolar dilation (48, 51). That hypothesis invokes a normal TGF reflex that responds to increases and decreases in sodium chloride sensing by the macula densa, with respective increases and decreases in afferent arteriolar resistance. However, an alternative hypothesis invokes the reported effect of nNOS-derived NO to blunt the sensitivity of TGF (22, 49, 52, 54). Blunted TGF sensitivity means that there is less afferent arteriolar resistance for any given level of distal sodium chloride delivery, thus leading to increased RBF and GFR. Our dynamic autoregulation analyses reported previously (2) and in the present study suggest that the TGF reflex is blunted at the onset of diabetes, and our present data with l-NAME suggest that NO mediates that effect.
In addition, there is evidence that NO strongly modulates myogenic autoregulation and that TGF is involved in that modulation (26, 43), and an apparent effect of diabetes shown in Fig. 4 is the increase in gain over the TGF and myogenic frequency ranges. That effect was measured on D7, but not D1, of diabetes, and it is interesting that RBF was not increased significantly until D7. In our previous study (2), increased gain was measured on D1, and RBF also increased much sooner. The explanation for the different RBF responses is not apparent, but the delayed vasodilation that occurred in the present study actually strengthens the associations between increased RBF in diabetes and impairment in dynamic autoregulation, because it shows that one response is not present without the other. Moreover, Figs. 2 and 6 show that when NO synthesis was inhibited, none of the effects of diabetes on RBF or dynamic autoregulation occurred.
Because there are no other reports on the effect of chronic NO synthesis inhibition on daily RBF and dynamic autoregulation, it is important that the effect of l-NAME alone to enhance the myogenic mechanism that we measured (gain plots in Figs. 5 and 6) is similar to what has been reported in acute dynamic autoregulation studies (26, 53). This builds confidence in our findings on the effect of NO on dynamic autoregulation in diabetes. In addition, our transfer function data in this study were normalized to mean renal vascular conductance, rather than the value at 0-Hz frequency as in our previous study (2). Both methods revealed impaired dynamic autoregulation of daily RBF in diabetes, which strengthens that fundamental observation, but normalizing by the more conventional method using renal vascular conductance (4, 14, 18, 25, 53) in the present study also builds confidence in the new findings regarding the role of NO.
One effect of NO synthesis inhibition in this study that would seem to contradict our previous reports (5, 6, 9, 17) is the finding that MAP did not increase in the DL rats, even though the increase in GFR was prevented. However, that hypertensive response is critically dependent on increased renin-angiotensin system activity (6, 10, 40), and there was not a marked or sustained increase in PRA in the DL rats in this study. These results are, however, consistent with previous studies that have shown an effect of diabetes to increase RBF and GFR, and the use of 16 h/day measurement of RBF strongly supports studies that suggest NO is required for that effect. In addition, our dynamic autoregulation analysis suggests that an effect of NO to blunt RBF autoregulation may contribute to the renal vasodilatory action. The unique roles of TGF and nNOS in RBF control may explain why RBF is increased in diabetes at the same time that skeletal muscle flow is decreased (7). However, although the links among NOS inhibition, RBF, and the TGF mechanism in this study are consistent with a predominant nNOS effect, neither the expression or activity of those NOS isoforms were measured in this study.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-56259 and HL-75625. G. F. DiBona was supported by research grants from the Adlerbertska Forskningsstiftelsen, The Royal Society of Arts and Sciences, Göteborg, Sweden, and Karolinska Institute, Stockholm, Sweden.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bank N, Aynedjian HS. Role of EDRF (nitric oxide) in diabetic renal hyperfiltration. Kidney Int 43: 1306–1312, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Bell TD, DiBona GF, Wang Y, Brands MW. Mechanisms for renal blood flow control early in diabetes as revealed by chronic flow measurement and transfer function analysis. J Am Soc Nephrol 17: 2184–2192, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Berger EY, Farber SJ, Earle DP Jr. Comparison of the constant infusion and urine collection techniques for the measurement of renal function. J Clin Invest 27: 710–719, 1948. [PubMed] [Google Scholar]
- 4.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. “Step” vs. “dynamic” autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol 285: F113–F120, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brands MW, Bell TD, Gibson B. Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension 43: 57–63, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Brands MW, Cloud LJ. Control of arterial pressure by angiotensin II and nitric oxide at the onset of diabetes. Am J Hypertens 16: 600–603, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Brands MW, Fitzgerald SM. Acute endothelium-mediated vasodilation is not impaired at the onset of diabetes. Hypertension 32: 541–547, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Brands MW, Fitzgerald SM, Hewitt WH, Hailman AE. Decreased cardiac output at the onset of diabetes: renal mechanisms and peripheral vasoconstriction. Am J Physiol Endocrinol Metab 278: E917–E924, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Brands MW, Fleming C, Bell TD, Sturgis LC, Janardhanan R, Labazi H. Lack of blood pressure salt-sensitivity supports afferent arteriolar action of nitric oxide in type I diabetes. Clin Exp Pharmacol Physiol 34: 475–479, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Brands MW, Labazi H. The role of glomerular filtration rate in controlling blood pressure early in diabetes. Hypertension 52: 188–194, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiarelli F, Cipollone F, Romano F, Tumini S, Costantini F, di Ricco L, Pomilio M, Pierdomenico SD, Marini M, Cuccurullo F, Mezzetti A. Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: relation to glomerular hyperfiltration. Diabetes 49: 1258–1263, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Choi KC, Lee SC, Kim SW, Kim NH, Lee JU, Kang YJ. Role of nitric oxide in the pathogenesis of diabetic nephropathy in streptozotocin-induced diabetic rats. Korean J Intern Med 14: 32–41, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 96: 25–28, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol Renal Physiol 292: F1105–F1123, 2007. [DOI] [PubMed] [Google Scholar]
- 15.De Vriese AS, Stoenoiu MS, Elger M, Devuyst O, Vanholder R, Kriz W, Lameire NH. Diabetes-induced microvascular dysfunction in the hydronephrotic kidney: role of nitric oxide. Kidney Int 60: 202–210, 2001. [DOI] [PubMed] [Google Scholar]
- 16.DiBona GF, Sawin LL. Effect of renal denervation on dynamic autoregulation of renal blood flow. Am J Physiol Renal Physiol 286: F1209–F1218, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald SM, Brands MW. Nitric oxide may be required to prevent hypertension at the onset of diabetes. Am J Physiol Endocrinol Metab 279: E762–E768, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK. Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol Renal Physiol 286: F1136–F1143, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14–E22, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hiragushi K, Sugimoto H, Shikata K, Yamashita T, Miyatake N, Shikata Y, Wada J, Kumagai I, Fukushima M, Makino H. Nitric oxide system is involved in glomerular hyperfiltration in Japanese normo- and micro-albuminuric patients with type 2 diabetes. Diabetes Res Clin Pract 53: 149–159, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Hu L, Manning RDJ, Brands MW. Long-term cardiovascular role of nitric oxide in conscious rats. Hypertension 23: 185–194, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Ichihara A, Navar LG. Neuronal NOS contributes to biphasic autoregulatory response during enhanced TGF activity. Am J Physiol Renal Physiol 277: F113–F120, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Ishii N, Patel KP, Lane PH, Taylor T, Bian K, Murad F, Pollock JS, Carmines PK. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol 12: 1630–1639, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Ito A, Uriu K, Inada Y, Qie YL, Takagi I, Ikeda M, Hashimoto O, Suzuka K, Eto S, Tanaka Y, Kaizu K. Inhibition of neuronal nitric oxide synthase ameliorates renal hyperfiltration in streptozotocin-induced diabetic rat. J Lab Clin Med 138: 177–185, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol 285: R619–R631, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Just A, Arendshorst WJ. Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback. J Physiol 569: 959–974, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keynan S, Hirshberg B, Levin-Iaina N, Wexler ID, Dahan R, Reinhartz E, Ovadia H, Wollman Y, Chernihovskey T, Iaina A, Raz I. Renal nitric oxide production during the early phase of experimental diabetes mellitus. Kidney Int 58: 740–747, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Komers R, Allen TJ, Cooper ME. Role of endothelium-derived nitric oxide in the pathogenesis of the renal hemodynamic changes of experimental diabetes. Diabetes 43: 1190–1197, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Komers R, Lindsley JN, Oyama TT, Allison KM, Anderson S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am J Physiol Renal Physiol 279: F573–F583, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Manning RDJ, Hu L, Mizelle HL, Montani JP, Norton MW. Cardiovascular responses to long-term blockade of nitric oxide synthesis. Hypertension 22: 40–48, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Mattar AL, Fujihara CK, Ribeiro MO, De Nucci G, Zatz R. Renal effects of acute and chronic nitric oxide inhibition in experimental diabetes. Nephron 74: 136–143, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Mogensen CE The effect of blood pressure intervention on renal function in insulin-dependent diabetes. Diabetes Metab 15: 343–351, 1989. [PubMed] [Google Scholar]
- 33.O'Bryan GT, Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol 17: 93–100, 1997. [PubMed] [Google Scholar]
- 34.O'Byrne S, Forte P, Roberts L, Morrow JD, Johnston A, Anggard E, Leslie RD, Benjamin N. Nitric oxide synthesis and isoprostane production in subjects with type 1 diabetes and normal urinary albumin excretion. Diabetes 49: 857–862, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Ohishi K, Carmines PK. Superoxide dismutase restores the influence of nitric oxide on renal arterioles in diabetes mellitus. J Am Soc Nephrol 5: 1559–1566, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int 61: 186–194, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Pflueger AC, Larson TS, Hagl S, Knox FG. Role of nitric oxide in intrarenal hemodynamics in experimental diabetes mellitus in rats. Am J Physiol Regul Integr Comp Physiol 277: R725–R733, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Pflueger AC, Osswald H, Knox FG. Adenosine-induced renal vasoconstriction in diabetes mellitus rats: role of nitric oxide. Am J Physiol Renal Physiol 276: F340–F346, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Pieper GM Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia 42: 204–213, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Rojas M, Bell TD, Sturgis LC, Janardhanan R, Fleming C, Brands MW. Blood pressure control early in diabetes requires a balance between glomerular filtration rate and the renin-angiotensin system. Am J Hypertens 19: 1249–1255, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Schnackenberg CG, Wilcox CS. The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int 59: 1859–1864, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz D, Schwartz IF, Blantz RC. An analysis of renal nitric oxide contribution to hyperfiltration in diabetic rats. J Lab Clin Med 137: 107–114, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Wang X, Chon KH, Cupples WA. Tubuloglomerular feedback-dependent modulation of renal myogenic autoregulation by nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R982–R991, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Suanarunsawat T, Klongpanichapak S, Chaiyabutr N. Role of nitric oxide in renal function in rats with short and prolonged periods of streptozotocin-induced diabetes. Diabetes Obes Metab 1: 339–346, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto HS, K/Matsuda M/Kushiro M/Hayashi Y/Hiragushi K/Wada J/Makino H. Increased expression of endothelial cell nitric oxide synthase (ecNOS) in afferent and glomerular endothelial cells is involved in glomerular hyperfiltration of diabetic nephropathy. Diabetologia 41: 1426–1434, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol Heart Circ Physiol 263: H321–H326, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Thomson SC, Deng A, Komine N, Hammes JS, Blantz RC, Gabbai FB. Early diabetes as a model for testing the regulation of juxtaglomerular NOS I. Am J Physiol Renal Physiol 287: F732–F738, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 286: F8–F15, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Thorup C, Persson AEG. Macula densa derived nitric oxide in regulation of glomerular capillary pressure. Kidney Int 49: 430–436, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Tolins JP, Shultz PJ, Raij L, Brown DM, Mauer SM. Abnormal renal hemodynamic response to reduced renal perfusion pressure in diabetic rats: role of NO. Am J Physiol Renal Fluid Electrolyte Physiol 265: F886–F895, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early type 1 diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol 14: 530–537, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Carretero OA, Garvin JL. Nitric oxide produced by THAL nitric oxide synthase inhibits TGF. Hypertension 39: 662–666, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Cupples WA. Interaction between nitric oxide and renal myogenic autoregulation in normotensive and hypertensive rats. Can J Physiol Pharmacol 79: 238–245, 2001. [PubMed] [Google Scholar]
- 54.Wilcox CS, Welch WJ. Macula densa nitric oxide synthase: expression, regulation, and function. Kidney Int 54, Suppl 67: S-53–S-57, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest 77: 1925–1930, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]