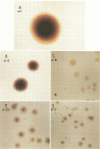
Abstract
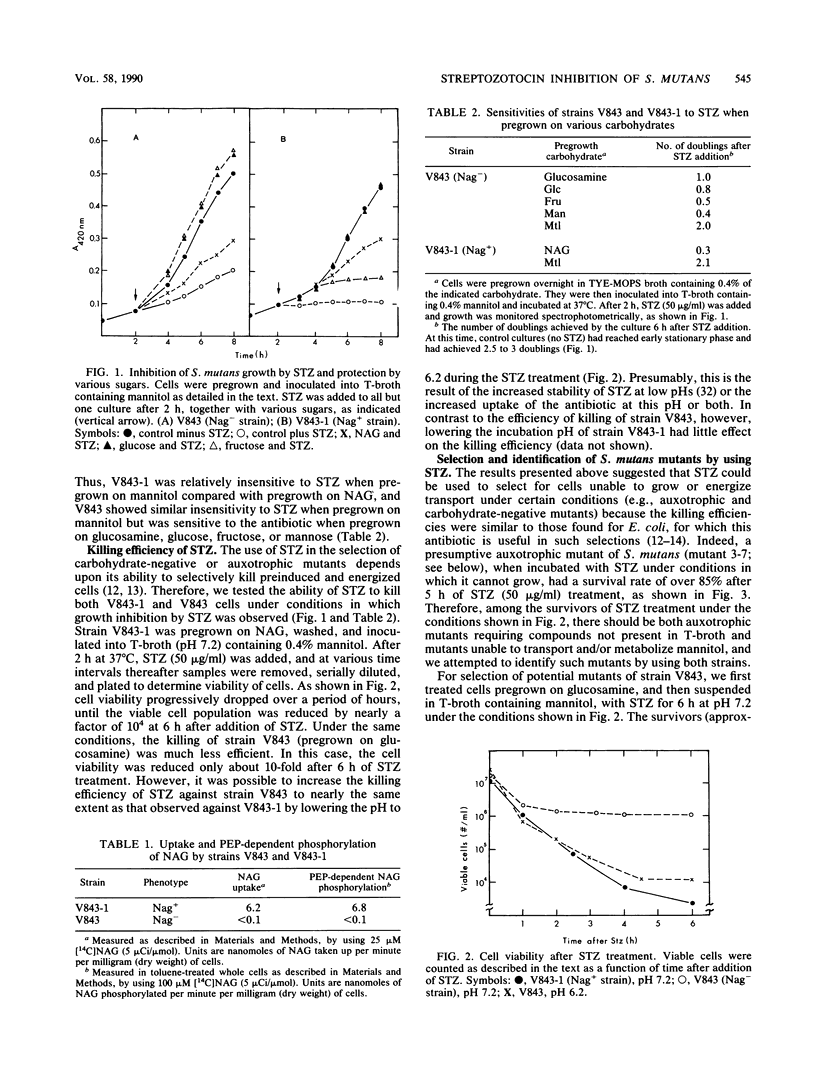
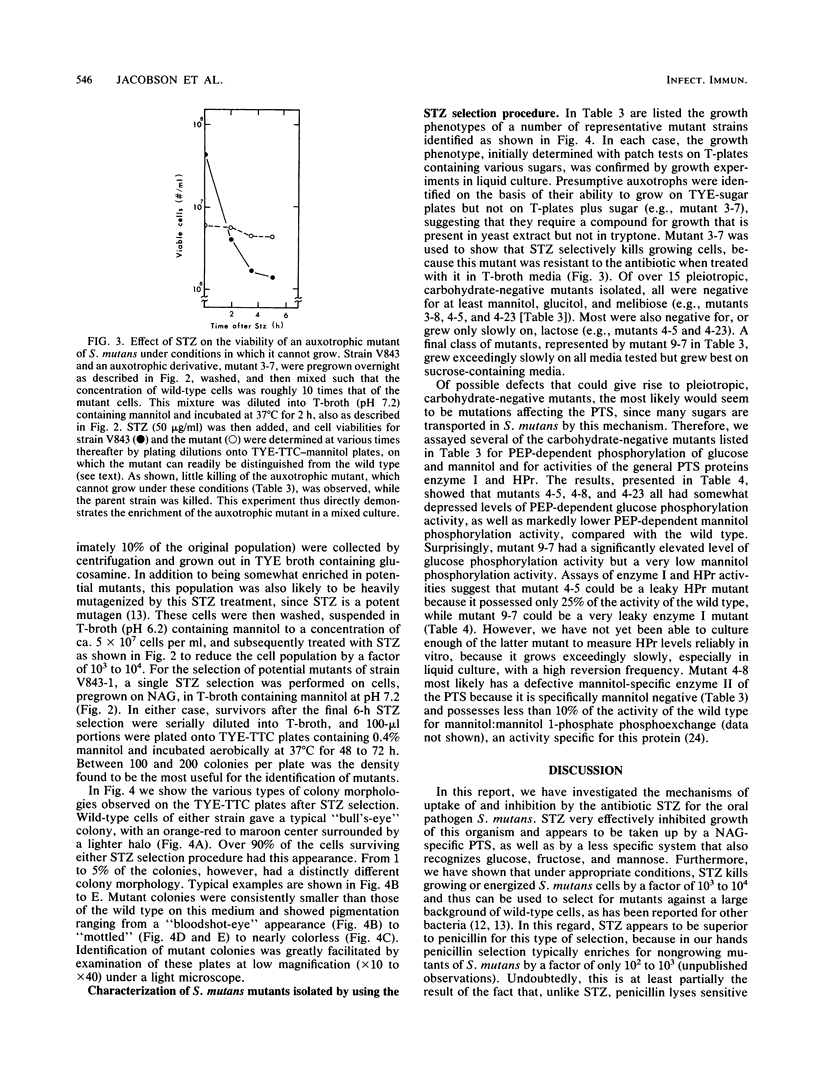
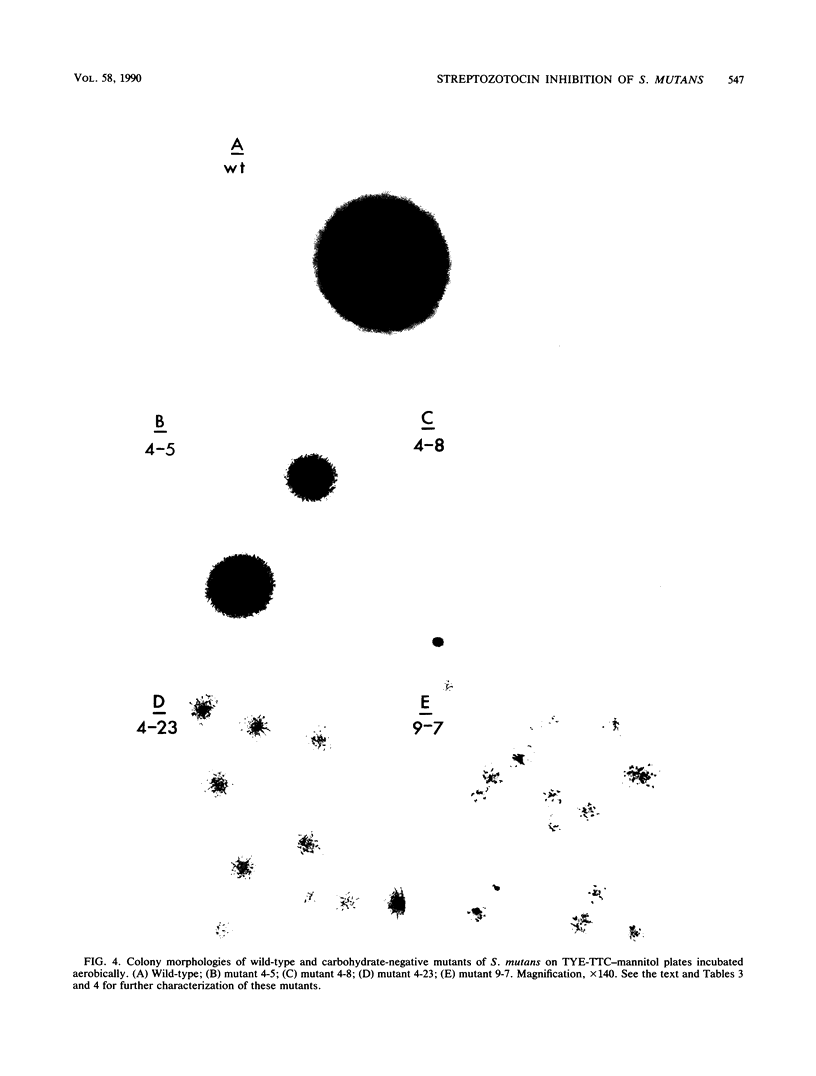
The antibiotic streptozotocin [2-deoxy-2-(3-methyl-3-nitrosoureido)-D-glucopyranoside], an analog of N-acetylglucosamine (NAG), has been shown to be useful for the selection of carbohydrate-negative and auxotrophic bacterial mutants. We have adapted this method for use with the oral pathogen Streptococcus mutans, a gram-positive, aerotolerant anaerobe that uses predominantly carbohydrates as carbon sources for growth. Streptozotocin selectively kills growing cells of S. mutans GS-5, and under appropriate conditions it can reduce the number of viable cells in actively growing cultures by a factor of 10(3) to 10(4). However, unlike in enteric bacteria, which take up this antibiotic by a single NAG-specific transport system, streptozotocin appears to be taken up in S. mutans by both a NAG-specific system and a relatively nonspecific system that is also involved in glucose, fructose, and mannose uptake. Combining streptozotocin selection and a screening procedure involving indicator plates containing triphenyl-tetrazolium chloride, we developed a general method for the isolation of carbohydrate-negative and auxotrophic mutants of S. mutans. A preliminary characterization of both pleiotropic and specific carbohydrate-negative mutants isolated by using this procedure is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. T., Wittenberger C. L. Mannitol and sorbitol catabolism in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):117–126. doi: 10.1016/0003-9969(73)90026-5. [DOI] [PubMed] [Google Scholar]
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Seno S. Regulation of hexitol catabolism in Streptococcus mutans. J Bacteriol. 1983 Feb;153(2):861–866. doi: 10.1128/jb.153.2.861-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G., Funk C., Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981 Nov;6(3):270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feary T. W., Mayo J. A. Detection of streptococcal mutants presumed to be defective in sugar catabolism. Appl Environ Microbiol. 1984 Jun;47(6):1348–1351. doi: 10.1128/aem.47.6.1348-1351.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L., Mayrand D., Vadeboncoeur C. Isolation of a novel protein involved in the transport of fructose by an inducible phosphoenolpyruvate fructose phosphotransferase system in Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):755–763. doi: 10.1128/jb.160.2.755-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L., Vadeboncoeur C., Mayrand D. Loss of sensitivity to xylitol by Streptococcus mutans LG-1. Caries Res. 1984;18(4):289–295. doi: 10.1159/000260779. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson G. R., Lodge J., Poy F. Carbohydrate uptake in the oral pathogen Streptococcus mutans: mechanisms and regulation by protein phosphorylation. Biochimie. 1989 Sep-Oct;71(9-10):997–1004. doi: 10.1016/0300-9084(89)90103-x. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Williamson M. I., Marsh P. D., Ellwood D. C. Evidence that glucose and sucrose uptake in oral streptococcal bacteria involves independent phosphotransferase and proton-motive force-mediated mechanisms. Arch Oral Biol. 1984;29(11):871–878. doi: 10.1016/0003-9969(84)90085-2. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Analysis of the physiological effects of the antibiotic streptozotocin on Escherichia coli K 12 and other sensitive bacteria. Arch Microbiol. 1980 Dec;128(2):196–203. doi: 10.1007/BF00406158. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Characterisation of mutants of Escherichia coli K12, selected by resistance to streptozotocin. Mol Gen Genet. 1980;179(1):49–54. doi: 10.1007/BF00268445. [DOI] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Role of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Streptococcus mutans GS5 in the regulation of lactose uptake. Infect Immun. 1984 Feb;43(2):536–542. doi: 10.1128/iai.43.2.536-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge J., Jacobson G. R. Starvation-induced stimulation of sugar uptake in Streptococcus mutans is due to an effect on the activities of preexisting proteins of the phosphotransferase system. Infect Immun. 1988 Oct;56(10):2594–2600. doi: 10.1128/iai.56.10.2594-2600.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura C. S., Eisenberg L. B., Jacobson G. R. Resolution of the phosphotransferase enzymes of Streptococcus mutans: purification and preliminary characterization of a heat-stable phosphocarrier protein. Infect Immun. 1984 Jun;44(3):708–715. doi: 10.1128/iai.44.3.708-715.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura C. S., Poy F., Jacobson G. R. ATP-dependent protein kinase activities in the oral pathogen Streptococcus mutans. J Cell Biochem. 1987 Mar;33(3):161–171. doi: 10.1002/jcb.240330303. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T., Lolkema J. S. Enzymes II of the phosphoenolpyruvate-dependent sugar transport systems: a review of their structure and mechanism of sugar transport. Biochim Biophys Acta. 1988 Oct 11;947(3):493–519. doi: 10.1016/0304-4157(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Lin P. Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Kinetic characterization. J Biol Chem. 1980 Sep 25;255(18):8579–8584. [PubMed] [Google Scholar]
- Sato Y., Poy F., Jacobson G. R., Kuramitsu H. K. Characterization and sequence analysis of the scrA gene encoding enzyme IIScr of the Streptococcus mutans phosphoenolpyruvate-dependent sucrose phosphotransferase system. J Bacteriol. 1989 Jan;171(1):263–271. doi: 10.1128/jb.171.1.263-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Effect of growth conditions on sucrose phosphotransferase activity of Streptococcus mutans. Infect Immun. 1980 Mar;27(3):922–927. doi: 10.1128/iai.27.3.922-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Sucrose transport by Streptococcus mutans. Evidence for multiple transport systems. Biochim Biophys Acta. 1982 Nov 22;692(3):415–424. doi: 10.1016/0005-2736(82)90392-3. [DOI] [PubMed] [Google Scholar]
- Thibault L., Vadeboncoeur C. Phosphoenolpyruvate-sugar phosphotransferase transport system of Streptococcus mutans: purification of HPr and enzyme I and determination of their intracellular concentrations by rocket immunoelectrophoresis. Infect Immun. 1985 Dec;50(3):817–825. doi: 10.1128/iai.50.3.817-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAVRA J. J., DEBOER C., DIETZ A., HANKA L. J., SOKOLSKI W. T. Streptozotocin, a new antibacterial antibiotic. Antibiot Annu. 1959;7:230–235. [PubMed] [Google Scholar]
- Vadeboncoeur C., Proulx M. Lactose transport in Streptococcus mutans: isolation and characterization of factor IIIlac, a specific protein component of the phosphoenolpyruvate-lactose phosphotransferase system. Infect Immun. 1984 Oct;46(1):213–219. doi: 10.1128/iai.46.1.213-219.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C. Structure and properties of the phosphoenolpyruvate: glucose phosphotransferase system of oral streptococci. Can J Microbiol. 1984 Apr;30(4):495–502. doi: 10.1139/m84-073. [DOI] [PubMed] [Google Scholar]