Abstract
PIP4Ks (type II phosphatidylinositol 4-phosphate kinases) are phosphatidylinositol 5-phosphate (PtdIns5P) 4-kinases, believed primarily to regulate cellular PtdIns5P levels. In this study, we investigated the expression, localization, and associated biological activity of the least-studied PIP4K isoform, PIP4Kγ. Quantitative RT-PCR and in situ hybridization revealed that compared with PIP4Kα and PIP4Kβ, PIP4Kγ is expressed at exceptionally high levels in the kidney, especially the cortex and outer medulla. A specific antibody was raised to PIP4Kγ, and immunohistochemistry with this and with antibodies to specific kidney cell markers showed a restricted expression, primarily distributed in epithelial cells in the thick ascending limb and in the intercalated cells of the collecting duct. In these cells, PIP4Kγ had a vesicular appearance, and transfection of kidney cell lines revealed a partial Golgi localization (primarily the matrix of the cis-Golgi) with an additional presence in an unidentified vesicular compartment. In contrast to PIP4Kα, bacterially expressed recombinant PIP4Kγ was completely inactive but did have the ability to associate with active PIP4Kα in vitro. Overall our data suggest that PIP4Kγ may have a function in the regulation of vesicular transport in specialized kidney epithelial cells.
Keywords: phosphoinositide, phosphatidylinositol 5-phosphate 4-kinase, collecting duct, Golgi apparatus
polyphosphoinositides are quantitatively minor lipid components of cells and are increasingly being recognized as making diverse and important contributions to many aspects of cell physiology, for example, in ion channel regulation, membrane trafficking, cell proliferation, and cytoskeletal rearrangement (for reviews see Refs. 10, 18, 32, 40, 46). Phosphatidylinositol 5-phosphate (PtdIns5P) is the most recent polyphosphoinositide to be found, first identified as the primary substrate for the type II PtdInsP kinases (33). Here these enzymes are referred to as PIP4Ks (consistent with their substrate specificity as PtdIns5P 4-kinases), although it should be noted that gene nomenclature still refers to them as PIP5K2s. As a minor component of cell polyphosphoinositides, it is suggested that PtdIns5P either provides a specific lipid pool for a (minor) localized production of PtdIns(4,5)P2 or that it is a signaling molecule in its own right. Suggested functions include nuclear responses (9, 13), insulin signaling, and protein kinase regulation (5, 22, 31, 38) and phosphatase activation (39).
PtdIns5P can be synthesized from PtdIns by a PtdIns 5-kinase (37) or by dephosphorylation from PtdIns(3,5)P2 or PtdIns(4,5)P2 (28, 41–43). The main route of PtdIns5P metabolism is via PIP4Ks, which are represented in vertebrates by three characterized isoforms, α, β, and γ. PIP4Kα is found predominantly in the cytosol and can be recruited to the plasma membrane (15, 45). PIP4Kβ is localized to the nucleus (3, 7, 36), where there is evidence that it regulates PtdIns5P levels (21), probably in concert with type I PtdIns(4,5)P2 4-phosphatase (47), although it has also been seen to associate with the cytosolic TNF receptor (6). As cellular PtdIns5P levels are modified in response to stress (21, 47), to bacterial infection (28), to receptor-mediated signaling (26, 45), and during cell cycle progression (8), the ability of the PIP4Ks to localize to specific compartments, or to shuttle between them, may be intrinsic to their function.
The third isoform, PIP4Kγ (19), has not yet been associated with a cellular function, either related to production of PtdIns(4,5)P2 or to the attenuation of PtdIns5P. In the present study, we have extensively characterized the tissue distribution of all of the PIP4K isoforms and found an exceptionally high level of PIP4Kγ expression in the kidney. We have further investigated the localization within this organ of PIP4Kγ, using a specific antibody to this isoform, and found that it is remarkably confined to specific cell populations, where it is localized to vesicular structures. Transfection experiments with PIP4Kγ suggest that these may be derived from the Golgi apparatus. Our results suggest that PIP4Kγ may be involved in vesicle trafficking in specific kidney cells and that this would imply a specialized function for this enzyme.
MATERIALS AND METHODS
PIP4K cloning.
PIP5K2 genes were amplified from a whole human brain marathon-ready cDNA library (Clontech Laboratories, Mountain View, CA) using gene-specific primers (PIP5K2A forward: 5′-ATGGCGACCCCCGGCAACCTAGGGTC-3′ and reverse: 5′-TTACGTCAAGATGTGGCCAATAAAGTC-3′; PIP5K2B forward: 5′-ATGTCGTCCAACTGCACCAGCACCAC-3′ and reverse: 5′-CTACGTCAGGATGTTGGACATAAAC-3′; and PIP5K2C forward: 5′-ATGGCGTCCTCCTCGGTCCCACCAG-3′ and reverse: 5′-TTAGGCAAAGATGTTGGTAATAAAATC-3′). PCR products were cloned, via incorporated HindIII and BamHI restriction sites, into appropriate plasmid vectors. Recombinant protein expressed from Escherichia coli harboring PIP5K2s in pET-32a (Novagen, Madison, WI) was purified using TALON metal affinity resin (Clontech) and cleaved with enterokinase (New England Biolabs, Ipswich, MA). Endotoxin-free plasmid from bacterial clones harboring PIP5K2s in pEGFP-C1 (Clontech) was prepared (DNA extraction kit; Qiagen, Huntsville, AL). All constructs were confirmed by sequencing.
Cell culture and transfection.
HeLa, HEK 293, COS-7, and NRK cells were maintained in DMEM (GIBCO, Paisley, UK) supplemented with 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were transiently transfected with plasmid constructs for 24 h with TransFectin reagent (Bio-Rad, Hercules, CA), using the manufacturer's protocol. Cell lysates for immunoprecipitation were made by suspending cells in cold lysis buffer (PBS with 1% Triton X-100, 5 mM EDTA, 5 mM EGTA, and 100 μl/ml Sigma P8340 protease inhibitor cocktail) and centrifuging at 10,000 g for 10 min at 4°C.
Tissue sample preparation.
Protein and mRNA samples were prepared from adult mouse tissues obtained post mortem and immediately frozen on dry ice after collection. Tissues (50 mg) for mRNA extraction were pulsed in lysing matrix D tubes (Qbiogene) with 1 ml Tri-Reagent (Sigma-Aldrich, Poole, UK) on a Hybaid Ribolyser. Samples were purified (RNeasy kit; Qiagen, and Turbo DNase; Ambion, Austin, TX), and cDNA libraries were constructed by RT-PCR (Sprint Powerscript kit; Clontech). Protein lysates were prepared in RIPA buffer (150 mM sodium chloride, 50 mM Tris pH 7.4, 1 mM EDTA, 1% Triton X-100, 1% deoxycholic acid, and 0.1% SDS) with 10 mM tetrasodium pyrophosphate, 10 mM sodium fluoride, 17.5 mM β-glycerophosphate, and 100 μl/ml Sigma P8340 protease inhibitor cocktail, using 10–15 strokes in a 5 ml dounce homogenizer. Cell debris was cleared by centrifugation at 2,900 g for 20 min at 4°C, and lysates were clarified by further centrifugation at 29,000 g for 45 min at 4°C. Kidney tissue was dissected under a binocular microscope to produce cortical and medullary samples. Tissues for immunochemistry from perfused mice were fixed in 4% paraformaldehyde, sectioned on a Leica cryostat at −20°C, mounted on slides, and stored at −80°C.
Quantitative PCR.
Singleplex quantitative PCR was carried out using specific primers for each PIP5K2 gene, designed to the 3′-untranslated region (PIP5K2A forward: 5′-AAGAGTCTGATGCCAAGAACCTGT-3′ and reverse: 5′-TGCAGTGCAACTTAAGGATGGTAA-3′; PIP5K2B forward: 5′-CATCCTCACAGAAGAACATGGC-3′ and reverse: 5′-CCTGGTCATTCACCGTCTCA-3′; and PIP5K2C forward: 5′-CATCTTCCACTGCTAATGTGTCTCC-5′ and reverse: 5′-TTGAGTTATGGCTCTGACTCCTCTCT-3′). Three cDNA libraries were constructed for each tissue tested and analyzed in triplicate. PCR amplification was performed in a 96-well plate using SYBR Green I master mix (Applied Biosystems, Warrington, UK), following the manufacturer's protocol. Reactions were heated (50°C for 2 min, 95°C for 10 min) and cycled 40 times (95°C for 15 sec, 60°C for 1 min) on an ABI Prism 7700 sequence detection system (Applied Biosystems). Dissociation curves for each primer set indicated a single product, and no-template controls were negative after 40 cycles. Reactions using RNA as template were negative, showing that the preparations were free from genomic DNA contamination. Primer concentrations were chosen to give threshold cycle (CT) values of 20–25. Validation experiments showed equivalent relative amplification efficiencies between each PIP5K2 gene and a primer set for mouse β-actin (forward: 5′-GACGATATCGCTGCGCTGGT-3′ and reverse: 5′-CCACGATGGAGGGGAATA-3′), and ΔCT values were analyzed using the comparative CT method (23).
In situ hybridization.
Two oligonucleotide probes were designed to the PIP5K2C sequence, one in the 3′-untranslated region (5′-GACTGGGTGGATTGAGTTATGGCTCTGACTCCTCT-3′) and one in the coding sequence (5′-ATAGGAGATAAGGAAACGGCCATCACTGCCTTCAG-3′), which only identified PIP5K2C when BLAT searched against the European Molecular Biology Laboratory mouse database. Probes were 3′-tail-labeled with [35S]dATP (NEN, Hounslow, UK), hybridized with 20-μm mouse tissue sections and autoradiographed for 5 wk, as described previously (12). Slides of interest were dipped in autoradiographic emulsion and after development (12 wk) were counterstained with hematoxylin and eosin, and images were captured using an Axioskop II light microscope.
Antibody analysis and Western blotting.
A peptide (amino acids 333–352), unique to the variable region of the mouse PIP4Kγ sequence, was used to raise a custom polyclonal antibody (NeoMPS, San Diego, CA), which was subsequently purified from rabbit serum by affinity matrix chromatography. Surface plasmon resonance analysis was carried out on a Biacore 3000 instrument (GE Healthcare Life Sciences, Bucks, UK) using two optical biosensor chips: carboxymethylated dextran preimmobilized with nitrilotriacetic acid (NTA) or streptavidin (SA). Purified PIP4K recombinant protein and control 6xHis tag protein were adsorbed to the NTA chip at concentrations of 1–9 ng/mm2. Anti-PIP4Kγ antibody was passed over the chip at 10 μg/ml to detect specific interaction, and data were normalized to the nonspecific control. Biotinylated anti-PIP4Kγ and control antibodies were bound to the SA chip, to which PIP4Kγ was then applied at saturating levels, to show specific antibody-binding interaction.
Protein samples (50 μg) were resolved on 10% polyacrylamide gels, and Western blots were carried out as described previously (16), using anti-PIP4Kγ at 0.5 μg/ml. Anti-PIP4Kγ was neutralized by saturating with excess of antigenic peptide for 30 min. Other antibodies used in this study were diluted to the manufacturers recommendations: goat polyclonal antibodies to aquaporin 1 (AQP1), aquaporin 2 (AQP2) and Tamm-Horsfall protein, and polyclonal rabbit antibodies to lgp110 (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal antibodies to TGN38 were a gift from G. Banting; mouse Mabs to EEA1, BiP, and GM130, and rabbit polyclonal antibodies to p115 (BD Transduction Laboratories, Oxford, UK); rabbit polyclonal antibodies to calreticulin (Calbiochem, La Jolla, CA); mouse Mabs to tubulin (Sigma-Aldrich); and goat polyclonal antibodies to golgin160 and rabbit polyclonal antibodies to catalase, p58K, mannose 6-phosphate receptor, and mannosidase II (Abcam, Cambridge, UK). Alexa Fluor 568 phalloidin and TO-PRO-3 iodide were used to directly stain actin and DNA (Molecular Probes, Paisley, UK). Horseradish peroxidase-conjugated secondary antibodies and SuperSignal West Dura substrate were used for Western blotting (Pierce Protein Research Products, Rockford, IL), and Alexa dye-conjugated secondary antibodies were used for fluorescence microscopy (Molecular Probes). Immunoprecipitation with anti-PIP4Kγ rat Mabs (16) and protein G-Sepharose (GE Healthcare) was carried out for 16 h at 4°C. Beads were washed in cold PBS and used directly for Western blotting or kinase assay.
Immunocytochemistry and immunohistochemistry.
Mammalian cells expressing green fluorescent protein-tagged constructs were fixed in 4% paraformaldehyde for 30 min on ice. Cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min and blocked with 4% fish skin gelatin (Sigma-Aldrich) in PBS for 30 min and then incubated for 60 min with primary and secondary antibodies (2% gelatin in PBS) with PBS washes between and after incubation. Slides were mounted using ProLong Gold antifade reagent (Molecular Probes), and images were taken on a Leica TCS SP5 laser scanning confocal microscope running LAS AF software (Leica Microsystems, Wetzlar, Germany). Golgi dissociation was achieved by treatment with Brefeldin A (10 μg/ml in DMEM) for 30 min at 37°C.
Sagittal and horizontal tissue sections for fluorescent labeling were pretreated to reduce autofluorescence (20 min in 1 mg/ml sodium borohydride), blocked for 2 h (1% fish skin gelatin, 0.3% Triton X-100 in PBS), exposed to primary antibody (24 h at 4°C), and fluorophore-labeled secondary antibody (4 h) before being mounted as described previously. Confocal images were spectrally separated by LAS AF software using reference spectra obtained from unstained tissue and cells overexpressing green fluorescent protein. Tissue sections for chemical staining were pretreated for 20 min (20% methanol and 5% hydrogen peroxide), blocked, and incubated with primary antibody as described, and then they were incubated with biotinylated anti-rabbit IgG antibodies before visualization using Vectastain ABC and DAB substrate kits (Vector Laboratories, Burlingame, CA). Slides were treated with Histoclear and mounted with DPX reagent (Sigma-Aldrich) before light microscopy.
Lipid kinase assay.
PIP4K assays were carried out as described previously (16) with slight adaptation. Substrate lipid (6 μM PtdIns5P) was dried under vacuum, and micelles were made by sonication in kinase buffer (50 mM Tris pH 7.4, 10 mM MgCl2, 80 mM KCl, and 2 mM EGTA). Recombinant PIP4K was added to the reaction mixture with 10μCi [γ-32P]ATP for 90 min at 30°C. Lipids were extracted and separated by silica-gel thin layer chromatography (16), and results were obtained by autoradiography.
RESULTS
PIP5K2 isoforms have different expression profiles.
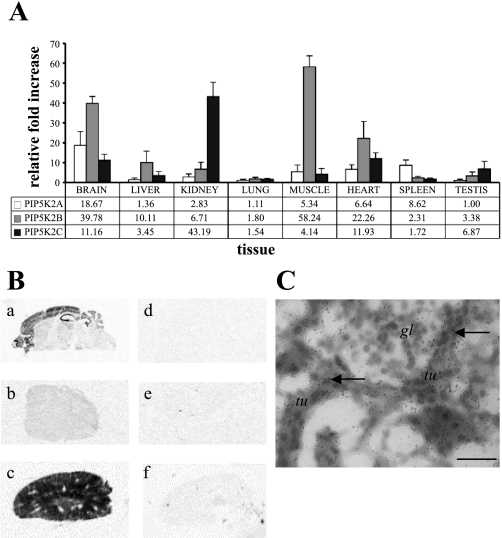
Expression studies using RT-PCR have previously suggested that levels of PIP5K2 transcription are high in different tissues (1, 19). Using a series of isoform-specific PCR primer pairs for PIP5K2A, PIP5K2B, and PIP5K2C, we quantitatively assessed the expressed levels of these transcripts in mRNA isolated from a range of mouse tissues (Fig. 1). Automated quantitative PCR of synthesized cDNA libraries was completed using three biological replicates for each tissue. Pooled data were analyzed by the Livak method (23), normalizing PIP5K2 to the housekeeping gene β-actin, and values were calculated as the relative fold increase above the lowest observed tissue expression (Fig. 1A). All three isoforms were expressed at higher levels in the brain, with PIP5K2A expression increased in spleen and PIP5K2B in muscle. PIP5K2C was especially high in kidney, as originally reported by Itoh et al. (19). Tissue analysis by in situ hybridization with a range of PIP5K2C probes confirmed that transcription of this isoform was upregulated in brain and kidney, compared with a control tissue, and that the expression was localized to discrete regions of these organs (Fig. 1B). Silver grain labeling of PIP5K2C mRNA in the kidney suggested that expression was confined to segments of the nephron within the cortical and medullary regions and was not present in the kidney vasculature (Fig. 1C).
Fig. 1.
PIP5K2C is differentially expressed in adult mouse tissues. A: expression of PIP5K2A, PIP5K2B, and PIP5K2C determined by quantitative PCR using the comparative threshold cycle (CT) method. Normalized expression is presented as relative fold increase above PIP5K2A in testis (n = 9). B: in situ hybridization using PIP5K2C-specific probes on thin sections of adult mouse brain (a), liver (b), and kidney (c; not to scale). Control incubations using an excess of cold probe indicate nonspecific binding (d–f). C: autoradiographic emulsion staining of mouse kidney cortex tubules, silver grains (arrows) indicating PIP5K2C mRNA expression (tu, tubule; gl, glomerulus). Scale bar = 20 μm.
Endogenous PIP4Kγ is differentially expressed in mouse tissues.
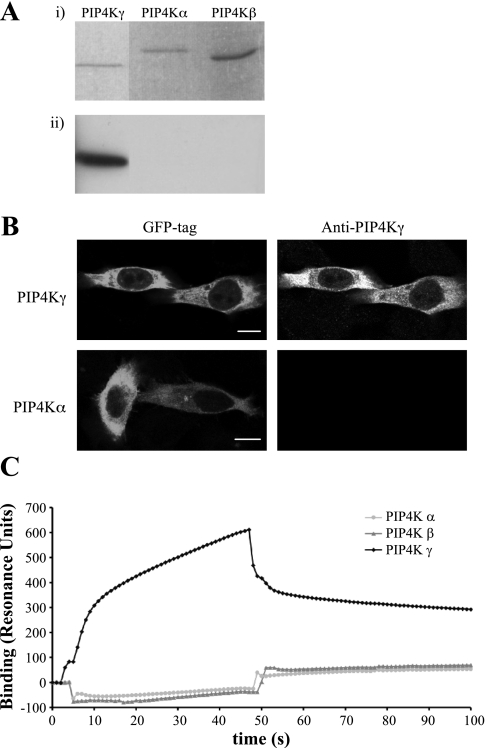
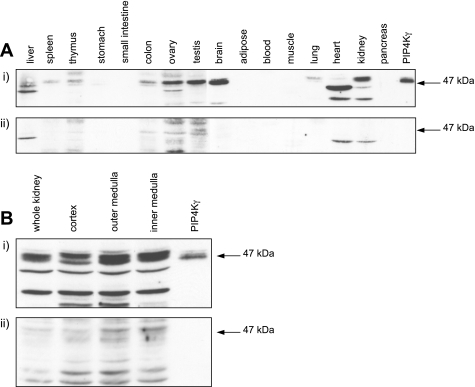
A polyclonal peptide antibody to the variable region of PIP4Kγ was raised and purified. Due to the similarity of the sequence of the PIP4K isoforms at the protein level, the specificity of the antibody for PIP4Kγ was determined (Fig. 2). The anti-PIP4Kγ antibody has no cross-reactivity with PIP4Kα or PIP4Kβ by Western blot (Fig. 2A) or by immunocytochemistry (Fig. 2B). Purified recombinant PIP4Ks were bound to an NTA sensor chip by 6xHis tag, and surface plasmon resonance analysis of the binding of anti-PIP4Kγ antibody indicated specificity for PIP4Kγ compared with PIP4Kα and PIP4Kβ (Fig. 2C). This was confirmed by binding biotinylated anti-PIP4Kγ antibody to an SA sensor chip and detecting specific binding to PIP4Kγ (data not shown). Direct Western blotting of a bank of tissue lysates confirmed the presence of significant endogenous levels of PIP4Kγ in brain, kidney, ovary, and testis (Fig. 3A, i). Other tissues had little or no detectable levels of PIP4Kγ, assuming representative expression based on equivalent loading of total lysate protein. Endogenous PIP4Kγ was detected as two bands of very similar molecular mass of ∼47 kDa, the larger band presumably representing the phosphorylated form of mature PIP4Kγ (19). Crude kidney fractionation and subsequent Western blotting of protein lysates indicated that PIP4Kγ was present throughout this organ, but comparatively higher levels were seen in the medulla (Fig. 3B, i). Nonspecific bands were visualized using neutralized antibody as control, and lower molecular mass immunoreactive bands were presumed to be products of proteolytic cleavage (Fig. 3, ii). Interestingly, PIP4Kγ in heart ran predominantly as a 40-kDa band, suggesting that processing of this isoform may be occurring (Fig. 3A).
Fig. 2.
Specificity of anti-PIP4Kγ antibody. Detection of PIP4Kγ, but not the PIP4Kα or PIP4Kβ isoforms, was established in various procedures. A: equivalent amounts of purified recombinant protein of each isoform were visualized on SDS-PAGE (i) and detected by Western blotting (ii). B: HeLa cells overexpressing green fluorescent protein (GFP)-tagged PIP4Kγ or PIP4Kα were fixed and stained with anti-PIP4Kγ antibody. Scale bar = 10 μm. C: Surface plasmon resonance analysis using PIP4Kα, PIP4Kβ, and PIP4Kγ adsorbed to an nitrilotriacetic acid biosensor. Anti-PIP4Kγ antibody was passed over the biosensor at 0 s and exchanged for wash buffer at 45 s. Data were normalized to 6xHis tag control protein binding.
Fig. 3.
PIP4Kγ is expressed in adult mouse tissues. Lysates were probed with PIP4Kγ-specific antibody (i) and with neutralized antibody after saturation with immunizing peptide (ii). Whole tissue lysates (A) and protein preparations from different kidney regions (B) were probed with the same antibody. Equivalent protein loading was based on total lysate concentration. Purified recombinant PIP4Kγ was used to identify endogenous PIP4Kγ at the correct molecular mass (arrow).
PIP4Kγ expression in kidney is localized to specific cells.
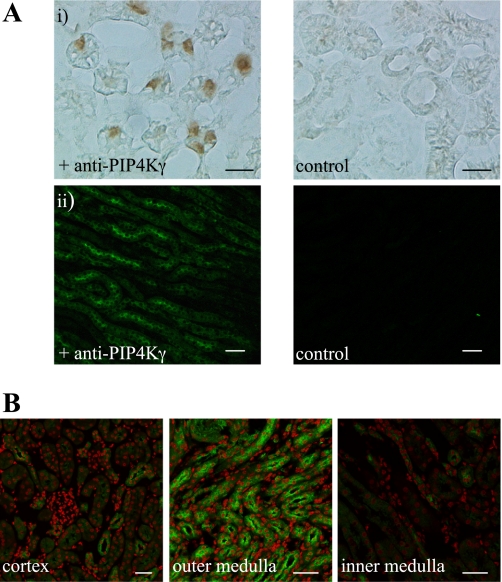
Identification of PIP4Kγ at a cellular level was shown to be specific by the use of peptide-saturated primary antibody controls, which demonstrated an absence of signal due to nonspecific binding of primary or secondary antibodies in tissue immunohistochemistry. Spectral separation was also utilized to remove significant autofluorescence from the kidney tissue in immunofluorescence experiments (Fig. 4A). Analysis of whole kidney sections confirmed that PIP4Kγ was expressed throughout this organ (data not shown). Detailed examination of representative regions of the kidney at high resolution indicated that the positive signal was seen to be concentrated in the outer medulla (Fig. 4B), confirming the results obtained for transcript expression (Fig. 1B), with much less signal being observed in the cortical labyrinth. This signal was restricted to whole regions of specific tubules, which could be visualized using sequential confocal imaging at 2-μm intervals throughout a 20-μm cryosectioned tissue slice (data not shown). The PIP4Kγ-expressing tubules were present in both the cortical medullary rays and the inner and outer stripe of the outer medulla but were absent from the tubules of the inner medulla (Fig. 4B). However, it was possible to observe single PIP4Kγ-positive cells within tubules in the cortex and inner medulla (Fig. 4B).
Fig. 4.
PIP4Kγ expression in the adult mouse kidney. Tissue immunohistochemistry using antibody specific to PIP4Kγ identified the expression of endogenous protein in kidney sections. A: positive PIP4Kγ signal detected by reporter enzyme (i: scale bar = 10 μm) or epifluorescence (ii; scale bar = 40 μm). Negative control reactions are also shown (primary antibody preincubated with antigenic peptide). B: high resolution imaging indicated that PIP4Kγ (green) was differentially distributed in the representative kidney regions indicated after removal of autofluorescence. Nuclei (red) are counterstained as contrast. Scale bar = 40 μm.
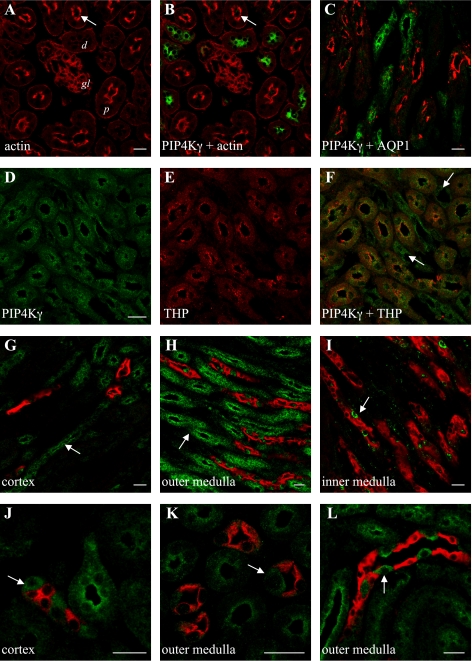
Costaining sections with selective markers for different nephron regions allowed accurate determination of the tubules and cells that expressed PIP4Kγ (Fig. 5). The absence of PIP4Kγ in cortical tubules with a significant brush-border lumen lining (Fig. 5, A and B) or coincident with the water channel AQP1 (Fig. 5C), a marker for the proximal convoluted tubule and thin descending limb of the loop of Henle (29), suggested that expression was restricted to the distal part of the nephron. PIP4Kγ-positive tubules also expressed Tamm-Horsfall protein in the outer medulla (Fig. 5, D–F), which suggested that PIP4Kγ is localized to cells in the thick ascending limb (TAL) of the loop of Henle (2). The presence of PIP4Kγ-positive tubules in the cortex and outer medulla (Fig. 5, G and H), but not in the inner medulla (Fig. 5I), and distinct from tubules expressing the collecting duct marker AQP2 (24), confirmed that PIP4Kγ was mainly expressed in TAL. Interestingly, isolated PIP4Kγ-positive cells were also localized to the collecting duct but were spatially differentiated from AQP2, which selectively stained principal cells (24). This suggested that PIP4Kγ was also localized to intercalated cells in cortical and medullary collecting ducts (Fig. 5, J–L).
Fig. 5.
Endogenous PIP4Kγ is differentially expressed in the adult mouse nephron. Actin (red) staining of cortical regions (A) identified luminal brush borders (arrow) in proximal tubules (d; distal tubule, p; proximal tubule), and localization of PIP4Kγ (green) in distal tubules (B). PIP4Kγ was not coincident with AQP1 (red) in the cortex (C) but was present in Tamm-Horsfall protein (THP; red) positive tubules in the outer medulla (D–F). PIP4Kγ was also seen in THP-negative tubules in this region (F; arrows). Double staining for PIP4Kγ (green) and AQP2 (red) in different kidney regions (G–L) revealed tubules positive for PIP4Kγ but negative for AQP2 in cortex and outer medulla (G, H; arrows). Isolated cells in AQP2-positive tubules also expressed PIP4Kγ (I–L; arrows). A, B, D–F, and K: images from horizontal sections. C, G–J, and L: images from sagittal sections. Scale bars = 20 μm.
PIP4Kγ has a distinct subcellular compartmentalization.
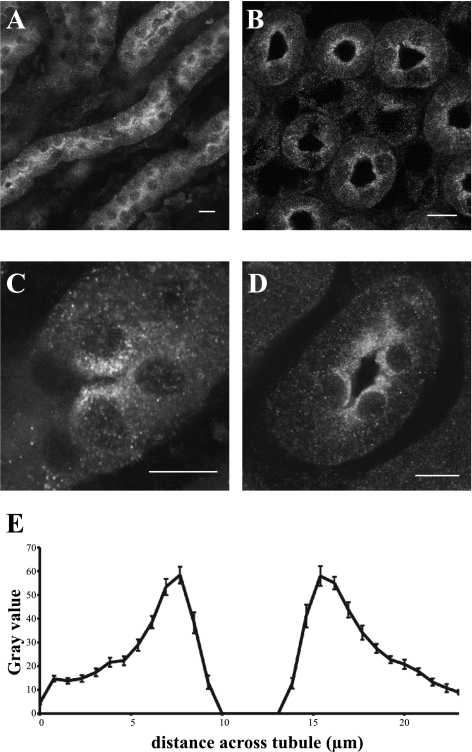
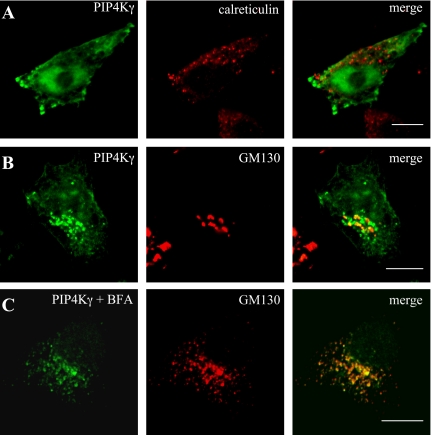
Tubule sections of medullary TAL showed a distinct concentration of PIP4Kγ around the lumen (Fig. 6, A–D). Analysis of a pool of cross-sectioned tubules with a diameter of 23–25 μm gave an average fluorescence profile that indicated a sixfold increase of signal within 3 μm of the apical membrane of tubule cells, compared with the basolateral membrane (Fig. 6E). Higher magnification of these cells suggested that PIP4Kγ might be present in a vesicular compartment (Fig. 6, C and D). To further investigate this compartmentalization, PIP4Kγ expression was studied in kidney-derived cell lines, but because endogenous levels of enzyme were not visible by immunocytochemistry in these cells, overexpressed protein levels were required for colocalization experiments. Cells expressing green fluorescent protein-tagged PIP4Kγ were stained with antibodies against different cellular markers (Fig. 7). PIP4Kγ was not seen to associate with markers for defined vesicular compartments such as peroxisomes (catalase), endosomes (EEA1, mannose 6-phosphate receptor), or lysosomes (lgp110) or with markers for structural components such as tubulin or actin (data not shown). In our experiments, PIP4Kγ was also not seen to associate with endoplasmic reticulum markers [calreticulin (Fig. 7A) and BiP] in contrast with the suggested endoplasmic reticulum localization seen by Itoh et al. (19). PIP4Kγ did, however, show a partial colocalization with a number of Golgi apparatus markers (golgin160, TGN38, p115, and p58K), most notably the cis-Golgi marker GM130 (Fig. 7B). Golgi dispersal by treatment of cells with Brefeldin A still retained partial colocalization of GM130 with the PIP4Kγ signal (Fig. 7C) but not with mannosidase II, a Golgi lumen protein, suggesting that the association was with the matrix component of this organelle (data not shown).
Fig. 6.
Distribution of PIP4Kγ in kidney thick ascending limb (TAL) cells in situ. Endogenous PIP4Kγ was detected in thin sections of the outer medulla region of adult mouse kidney. Saggital kidney section shows longitudinal TAL tubule sections (A, C), and horizontal kidney section shows TAL tubule cross-sections (B and D). Scale bars = 10 μm. Statistical analysis of PIP4Kγ distribution across TAL tubule cross-sections (such as in B), by fluorescence intensity, indicated peaks in the luminal region (E; n = 25).
Fig. 7.
Overexpressed PIP4Kγ localizes to a specific cellular compartment. Transiently transfected cells, expressing GFP-tagged PIP4Kγ (green), were costained with antibodies to specific markers for different cellular compartments (red). No specific colocalization was observed with the endoplasmic reticulum marker calreticulin (A). Partial colocalization was observed with the cis-Golgi marker GM130 (B),and this juxtaposed labeling was maintained during Golgi dissociation by treatment with Brefeldin A (C). Scale bars = 10 μm.
Lipid kinase activity of PIP4Kγ.
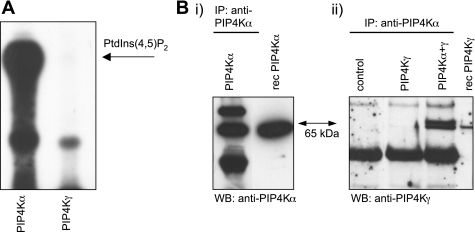
PIP4K, overexpressed in E. coli cells and purified by metal-affinity resin chromatography, was used as a source of enzyme for in vitro kinase assays. PIP4K activity was only observed using the PIP4Kα isoform as the recombinant PIP4Kγ was inactive (Fig. 8A). Using immunoprecipitation experiments with recombinant protein, we were able to show that PIP4Kγ can associate with PIP4Kα strongly enough to be selectively purified with a PIP4Kα-specific antibody in vitro (Fig. 8B).
Fig. 8.
Activity and binding characteristics of recombinant PIP4Ks. PIP4Kα and PIP4Kγ recombinant protein was purified from a bacterial source. A: PIP4K assay using equivalent amounts of recombinant protein from each PIP4K, showing generation of PtdIns(4,5)P2. B: immunoprecipitation (IP) of recombinant PIP4K protein using PIP4Kα-specific antibody. IPs are from equivalent amounts of PIP4Kα, PIP4Kγ, or a mixture of the 2 isoforms, in buffer. Control IP contains no recombinant protein. Western blots (WB) were probed with either anti-PIP4Kα (i) or anti-PIP4Kγ (ii). Recombinant protein markers (rec) indicate PIP4K-positive bands (65 kDa).
DICUSSION
In this study, we investigated the expression, localization and associated biological activity of PIP4Kγ. We discovered a unique and restricted localization for this PIP4K in kidney tissue and suggest that this has implications for the physiological function of this isoform.
We have shown that the comparative mRNA expression levels of all three isoforms are significant in brain, where they have a different spatial distribution (1). PIP4Kα is the most active of the three isoforms in vitro but has a comparably low mRNA expression in most of the tissues that we tested and is the most abundant isoform in the spleen, probably reflecting its role in hematocytes (17, 26). The PIP4Kβ isoform mRNA is highly expressed in heart and skeletal muscle cells, consistent with initial observations (6) and providing a link to insulin resistance (22). We have confirmed the original observation that PIP4Kγ is highly expressed in kidney (19) and also observe high mRNA expression levels in brain, heart, and testis compared with other tissues. Our detection of endogenous PIP4Kγ protein in tissues is consistent with these transcription levels, also suggesting that PIP4Kγ is abundant in the ovary and may be processed in heart tissue. The distribution of the PIP4Ks across a range of different tissues, and the observed differences in subcellular localization and intrinsic activity (36, 45), would suggest that specialized functions could be attributed to each and hence to the role of PtdIns(5)P in these locations.
Peptide analysis of the sequence of PIP4Kγ predicts that this protein would not be targeted to the endoplasmic reticulum or plasma membrane due to the absence of a recognized signal peptide, which is consistent with our observation that PIP4Kγ, when overexpressed in cells, is partially colocalized to the structural component of the Golgi apparatus. Roles for PtdIns3P and PtdIns4P in membrane trafficking are established (10, 32), but the recent study (25) of a Golgi-localized phospholipid-inositol phosphatase with a substrate preference for PtdIns5P presents the intriguing possibility that this phosphoinositide is also present in cellular vesicles. PIP4Kγ also has a role in actin remodeling during endocytic transport (30), and PtdIns5P levels have been associated with this and with vesicle translocation to the plasma membrane (38). PIP4Kγ could be recruited to the external surface of a specific microsomal compartment to modify the PtdIns5P signal or to synthesize PtdIns(4,5)P2.
The restricted expression of PIP4Kγ within the kidney may be significant within the context of the specialized function of different regions of the nephron. Our experiments indicate that PIP4Kγ is present in cells constituting the TAL and is also restricted to intercalated cells in the collecting duct and appears to have a similar subcellular localization in these cell types as that recently observed for members of the Arf GTPase family (11), which have known roles in membrane trafficking (34). These regions are predominantly concerned with homeostasis by active ion transport and pH regulation and contain a large number of channels and transporters specific to these tasks (for reviews see Refs. 20, 27). Phosphoinositide regulation of both the trafficking to the plasma membrane and the activity of various collecting duct-localized channels has been reported (14, 44), but a specific role for PIP4Kγ in this process has yet to be established.
PIP4Kγ has been shown to have PtdIns5P 4-kinase activity when immunoprecipitated from mammalian cells (19), and the recombinant protein, purified from E. coli, is inactive, suggesting that a eukaryotic modification to PIP4Kγ is required for kinase activation. However, due to the ability of the PIP4Ks to dimerize (4, 16, 35) in vivo, we also cannot rule out the possibility that the observed activity associated to PIP4Kγ is attributable to PIP4K heterodimers. This raises the possibility that PIP4Kγ is able to recruit PtdIns5P 4-kinase activity (in the form of PIP4Kα) to specific compartments, based on the potential ability to act as a scaffolding protein.
The roles of PtdIns5P, PtdIns(4,5)P2, and hence the PIP4Ks, in kidney function are unknown. We have shown that PIP4Kγ is the predominant PIP4K in the kidney, and we suggest that its role may be related to its specific localization in this organ.
GRANTS
This study was supported by a Programme Grant (WT063581) from the Wellcome Trust and the Biotechnology and Biological Sciences Research Council.
Acknowledgments
We thank Dr. Patrick Lynch for practical advice, Søren Nielsen for helpful suggestions, and Dr. Mihriban Tuna for technical expertise and analysis of biacore experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akiba Y, Suzuki R, Saito-Saino S, Owada Y, Sakagami H, Watanabe M, Kondo H. Localization of mRNAs for phosphatidylinositol phosphate kinases in the mouse brain during development. Brain Res Gene Expr Patterns 1: 123–133, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, Luft FC, Alenina N, Bader M, Thiele BJ, Prasadan K, Raffi HS, Kumar S. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 288: F559–F567, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bunce MW, Boronenkov IV, Anderson RA. Coordinated activation of the nuclear ubiquitin ligase Cul3-SPOP by the generation of phosphatidylinositol 5-phosphate. J Biol Chem 283: 8678–8686, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Burden LM, Rao VD, Murray D, Ghirlando R, Doughman SD, Anderson RA, Hurley JH. The flattened face of type II beta phosphatidylinositol phosphate kinase binds acidic phospholipid membranes. Biochemistry 38: 15141–15149. 1999. [DOI] [PubMed] [Google Scholar]
- 5.Carricaburu V, Lamia KA, Lo E, Favereaux L, Payrastre B, Cantley LC, Rameh LE. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc Natl Acad Sci USA 100: 9867–9872, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castellino AM, Parker GJ, Boronenkov IV, Anderson RA, Chao MV. A novel interaction between the juxtamembrane region of the p55 tumor necrosis factor receptor and phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem 272: 5861–5870, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Ciruela A, Hinchliffe KA, Divecha N, Irvine RF. Nuclear targeting of the beta isoform of type II phosphatidylinositol phosphate kinase (phosphatidylinositol 5-phosphate 4-kinase) by its alpha-helix 7. Biochem J 346: 587–591, 2000. [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke JH, Letcher AJ, D'Santos CS, Halstead JR, Irvine RF, Divecha N. Inositol lipids are regulated during cell cycle progression in the nuclei of murine erythroleukaemia cells. Biochem J 357: 905–910, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Lello P, Nguyen BD, Jones TN, Potempa K, Kobor MS, Legault P, Omichinski JG. NMR structure of the amino-terminal domain from the Tfb1 subunit of TFIIH and characterization of its phosphoinositide and VP16 binding sites. Biochemistry 44: 7678–7686, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657, 2006. [DOI] [PubMed] [Google Scholar]
- 11.El-Annan J, Brown D, Breton S, Bourgoin S, Ausiello DA, Marshansky V. Differential expression and targeting of endogenous Arf1 and Arf6 small GTPases in kidney epithelial cells in situ. Am J Physiol Cell Physiol 286: C768–C778, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Giudici ML, Emson PC, Irvine RF. A novel neuronal-specific splice variant of type I phosphatidylinositol 4-phosphate 5-kinase isoform gamma. Biochem J 379: 489–496, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Lugovskoy AA, Baird CL, Zhu H, Field SJ, Lessnick SL, Villasenor J, Mehrotra B, Chen J, Rao VR, Brugge JS, Ferguson CG, Payrastre B, Myszka DG, Cantley LC, Wagner G, Divecha N, Prestwich GD, Yuan J. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 114: 99–111, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with gamma-ENaC. J Biol Chem 280: 40885–40891, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hinchliffe KA, Ciruela A, Letcher AJ, Divecha N, Irvine RF. Regulation of type IIalpha phosphatidylinositol phosphate kinase localisation by the protein kinase CK2. Curr Biol 9: 983–986, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hinchliffe KA, Giudici ML, Letcher AJ, Irvine RF. Type IIalpha phosphatidylinositol phosphate kinase associates with the plasma membrane via interaction with type I isoforms. Biochem J 363: 563–570, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinchliffe KA, Irvine RF, Divecha N. Regulation of PtdIns4P 5-kinase C by thrombin-stimulated changes in its phosphorylation state in human platelets. Biochem J 329: 115–119, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irvine RF Nuclear lipid signaling. Nat Rev Mol Cell Biol 4: 349–360, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Itoh T, Ijuin T, Takenawa T. A novel phosphatidylinositol-5-phosphate 4-kinase (phosphatidylinositol-phosphate kinase IIgamma) is phosphorylated in the endoplasmic reticulum in response to mitogenic signals. J Biol Chem 273: 20292–20299, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Jentsch TJ, Hubner CA, Fuhrmann JC. Ion channels: function unravelled by dysfunction. Nat Cell Biol 6: 1039–1047, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Jones DR, Bultsma Y, Keune WJ, Halstead JR, Elouarrat D, Mohammed S, Heck AJ, D'Santos CS, Divecha N. Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kbeta. Mol Cell 23: 685–695, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Lamia KA, Peroni OD, Kim YB, Rameh LE, Kahn BB, Cantley LC. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta-/- mice. Mol Cell Biol 24: 5080–5087, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the [-Delta Delta C(T)] Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Marples D, Knepper MA, Christensen EI, Nielsen S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol Cell Physiol 269: C655–C664, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Merlot S, Meili R, Pagliarini DJ, Maehama T, Dixon JE, Firtel RA. A PTEN-related 5-phosphatidylinositol phosphatase localized in the Golgi. J Biol Chem 278: 39866–39873, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Morris JB, Hinchliffe KA, Ciruela A, Letcher AJ, Irvine RF. Thrombin stimulation of platelets causes an increase in phosphatidylinositol 5-phosphate revealed by mass assay. FEBS Lett 475: 57–60, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Mullins LJ, Bailey MA, Mullins JJ. Hypertension, kidney, and transgenics: a fresh perspective. Physiol Rev 86: 709–746, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Niebuhr K, Giuriato S, Pedron T, Philpott DJ, Gaits F, Sable J, Sheetz MP, Parsot C, Sansonetti PJ, Payrastre B. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S. flexneri effector IpgD reorganizes host cell morphology. EMBO J 21: 5069–5078, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol 120: 371–383, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436: 78–86, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Pendaries C, Tronchere H, Arbibe L, Mounier J, Gozani O, Cantley L, Fry MJ, Gaits-Iacovoni F, Sansonetti PJ, Payrastre B. PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J 25: 1024–1034, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pendaries C, Tronchere H, Racaud-Sultan C, Gaits-Iacovoni F, Coronas S, Manenti S, Gratacap MP, Plantavid M, Payrastre B. Emerging roles of phosphatidylinositol monophosphates in cellular signaling and trafficking. Adv Enzyme Regul 45: 201–214, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 390: 192–196, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Randazzo PA, Yang YC, Rulka C, Kahn RA. Activation of ADP-ribosylation factor by Golgi membranes. Evidence for a brefeldin A- and protease-sensitive activating factor on Golgi membranes. J Biol Chem 268: 9555–9563, 1993. [PubMed] [Google Scholar]
- 35.Rao VD, Misra S, Boronenkov IV, Anderson RA, Hurley JH. Structure of type IIbeta phosphatidylinositol phosphate kinase: a protein kinase fold flattened for interfacial phosphorylation. Cell 94: 829–839, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Richardson JP, Wang M, Clarke JH, Patel KJ, Irvine RF. Genomic tagging of endogenous type IIbeta phosphatidylinositol 5-phosphate 4-kinase in DT40 cells reveals a nuclear localisation. Cell Signal 19: 1309–1314, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sbrissa D, Ikonomov OC, Deeb R, Shisheva A. Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J Biol Chem 277: 47276–47284, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Sbrissa D, Ikonomov OC, Strakova J, Shisheva A. Role for a novel signaling intermediate, phosphatidylinositol 5-phosphate, in insulin-regulated F-actin stress fiber breakdown and GLUT4 translocation. Endocrinology 145: 4853–4865, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Schaletzky J, Dove SK, Short B, Lorenzo O, Clague MJ, Barr FA. Phosphatidylinositol-5-phosphate activation and conserved substrate specificity of the myotubularin phosphatidylinositol 3-phosphatases. Curr Biol 13: 504–509, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol 15: 370–378, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Tronchere H, Laporte J, Pendaries C, Chaussade C, Liaubet L, Pirola L, Mandel JL, Payrastre B. Production of phosphatidylinositol 5-phosphate by the phosphoinositide 3-phosphatase myotubularin in mammalian cells. J Biol Chem 279: 7304–7312, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Ungewickell A, Hugge C, Kisseleva M, Chang SC, Zou J, Feng Y, Galyov EE, Wilson M, Majerus PW. The identification and characterization of two phosphatidylinositol-4,5-bisphosphate 4-phosphatases. Proc Natl Acad Sci USA 102: 18854–18859, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker DM, Urbe S, Dove SK, Tenza D, Raposo G, Clague MJ. Characterization of MTMR3 an inositol lipid 3-phosphatase with novel substrate specificity. Curr Biol 11: 1600–1605, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Weixel KM, Edinger RS, Kester L, Guerriero CJ, Wang H, Fang L, Kleyman TR, Welling PA, Weisz OA, Johnson JP. Phosphatidylinositol 4-phosphate 5-kinase reduces cell surface expression of the epithelial sodium channel (ENaC) in cultured collecting duct cells. J Biol Chem 282: 36534–36542, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox A, Hinchliffe KA. Regulation of extranuclear PtdIns5P production by phosphatidylinositol phosphate 4-kinase 2alpha. FEBS Lett 582: 1391–1394, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol 65: 761–789, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Zou J, Marjanovic J, Kisseleva MV, Wilson M, Majerus PW. Type I phosphatidylinositol-4,5-bisphosphate 4-phosphatase regulates stress-induced apoptosis. Proc Natl Acad Sci USA 104: 16834–16839, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]