Abstract
A main mechanism of idiopathic hypercalciuria (IH) in calcium stone-forming patients (IHSF) is postprandial reduction of renal tubule calcium reabsorption that cannot be explained by selective reduction of serum parathyroid hormone levels; the nephron site(s) responsible are not as yet defined. Using fourteen 1-h measurements of the clearances of sodium, calcium, and endogenous lithium during a three-meal day in the University of Chicago General Clinical Research Center, we found reduced postprandial proximal tubule reabsorption of sodium and calcium in IHSF vs. normal subjects. The increased distal sodium delivery is matched by increased distal reabsorption so that urine sodium excretions do not differ, but distal calcium reabsorption does not increase enough to match increased calcium delivery, so hypercalciuria results. In fact, urine calcium excretion and overall renal fractional calcium reabsorption both are high in IHSF vs. normal when adjusted for distal calcium delivery, strongly suggesting a distal as well as proximal reduction of calcium reabsorption. The combination of reduced proximal tubule and distal nephron calcium reabsorption in IHSF is a new finding and indicates that IH involves a complex, presumably genetic, variation of nephron function. The increased calcium delivery into the later nephron may play a role in stone formation via deposition of papillary interstitial apatite plaque.
Keywords: nephrolithiasis, tubule reabsorption, lithium clearance, hypercalciuria, proximal tubule
idiopathic hypercalciuria (IH) is best described as a state of high urine calcium excretion commonly found in patients who form calcium renal stones and thought to play a causal role in such stones (22). Although gastrointestinal calcium absorption is above normal, offering an obvious mechanism for hypercalciuria, the kidney also participates. After eating common foods, patients with calcium stones and IH (IHSF) reduce their overall renal calcium reabsorption more than well-matched normal control subjects (23). This reduction permits high urine calcium losses despite unchanged serum calcium and calcium filtered load. Although serum parathyroid hormone decreases with meals, levels do so equally in IHSF patients and normal subjects, and therefore cannot be the main mechanism for the reduced tubule calcium reabsorption (23). Because the differential increase in urine calcium occurs without a corresponding difference of urine sodium between IHSF and normal, the cortical thick ascending limb of the loop of Henle was considered an attractive site of reduced calcium reabsorption (1) along with the distal convoluted tubule (8). Since filtered load of calcium did not differ between patients and normal subjects, hypercalciuria essentially arose from the change in tubule reabsorption.
Which nephron sites participate has clinical relevance because IHSF patients form interstitial papillary deposits of calcium—as apatite—that are thought to play a crucial role in stone pathogenesis (5, 9, 11, 12). These deposits, called Randall's plaque after the person who first described them, migrate from the site of their initial production in the basement membranes of the thin segments of the loop of Henle (6) through the interstitium to the suburothelial space, where they can be seen at surgery. We (10) found that plaque abundance, gauged with digital intraoperative imaging, was proportional to urine calcium excretion and inversely proportional to urine volume and pH.
The first of these relationships suggests that calcium movement through the nephron somehow fosters plaque. If only the cortical thick ascending limb of the loop played a main role, increased calcium delivery into the tubule would be limited to the collecting ducts, because filtered load of calcium is not increased in IHSF. The same would be true if the distal convoluted tubule were the main site of reduced reabsorption. If, however, the proximal tubule also reduced its reabsorption, calcium delivery could be increased in the entire loop. Under such a condition, we would have to presume that distal sites reabsorbed the additional sodium delivered out of the proximal tubule but not the calcium, so hypercalciuria would occur without natriuresis.
Our purpose here was to test the hypothesis that reduced proximal tubule calcium reabsorption, gauged by endogenous lithium clearance, might contribute to hypercalciuria in IHSF, and that therefore calcium delivery is increased throughout the loop as well as in the later nephron segments. This approach has validity because proximal tubule calcium reabsorption is predominantly paracellular and is driven by reabsorption of sodium and water, which lithium clearance tracks accurately under conditions of adequate sodium intake (16).
METHODS
Patients and Normal Subjects
Twelve IHSF patients were compared with 11 normal subjects (Table 1). Of the normal subjects, none had stones or a family history of stones, known illnesses, or chronic medications apart from daily vitamins and birth control. Of the patients, we excluded as causes for hypercalciuria sarcoidosis, vitamin D excess, malignant neoplasm, renal tubular acidosis, and calcium supplement use at the time of testing. All were normocalcemic. The protocol was approved by the Institutional Review Board, and informed consent was obtained. IHSF patients and normal subjects did not differ significantly in age, weight, or height (Table 1). Three 24-h urine samples had been obtained for each patient as part of their clinical evaluation before treatment for stone prevention, and all satisfied clinical criteria for hypercalciuria [>250 mg/24 h (women), >300 mg/24 h (men), and/or >140 mg/g urine creatinine (either sex)]. Of the present subjects, the first four normal subjects and first five IHSF patients in Table 1 were participants in our prior study (23).
Table 1.
Patients and normal subjects
| Age, yr | Sex | Weight, kg | Height, cm | Stone Type |
Fasting |
Fed
|
|||
|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | ||||||
| Normal | 50 | M | 76 | 183 | None | 121 | 76 | 134 | 77 |
| Normal | 24 | F | 71 | 178 | None | 135 | 80 | 129 | 73 |
| Normal | 28 | F | 62 | 161 | None | 113 | 72 | 112 | 73 |
| Normal | 37 | M | 90 | 189 | None | 117 | 67 | 131 | 70 |
| Normal | 47 | F | 86 | 160 | None | 104 | 68 | 111 | 69 |
| Normal | 55 | M | 88 | 181 | None | 131 | 81 | 133 | 79 |
| Normal | 55 | F | 67 | 169 | None | 152 | 98 | 157 | 90 |
| Normal | 26 | F | 68 | 168 | None | 119 | 64 | 111 | 62 |
| Normal | 44 | M | 85 | 178 | None | 139 | 88 | 132 | 82 |
| Normal | 28 | M | 66 | 163 | None | 105 | 63 | 110 | 62 |
| Normal | 44 | F | 55 | 158 | None | 111 | 67 | 106 | 67 |
| Mean | 40±4 | 74±4 | 172±3 | 122±4 | 75±3 | 124±5 | 73±3 | ||
| IHSF | 47 | F | 58 | 167 | CaP | 98 | 62 | 99 | 63 |
| IHSF | 46 | F | 95 | 167 | CaP | 137 | 75 | 136 | 69 |
| IHSF | 29 | M | 91 | 185 | CaOx | ||||
| IHSF | 47 | F | 79 | 158 | Ca | 109 | 63 | 94 | 52 |
| IHSF | 56 | M | 95 | 178 | CaP | 114 | 69 | 118 | 66 |
| IHSF | 45 | F | 57 | 164 | Ca | 107 | 71 | 107 | 69 |
| IHSF | 27 | M | 77 | 174 | CaP | 119 | 80 | 122 | 73 |
| IHSF | 48 | M | 102 | 185 | CaOx | 127 | 79 | 136 | 83 |
| IHSF | 28 | M | 75 | 182 | CaOx | 113 | 72 | 115 | 70 |
| IHSF | 43 | M | 91 | 181 | CaOx | 123 | 71 | 119 | 67 |
| IHSF | 52 | F | 61 | 158 | Ca | 118 | 76 | 121 | 74 |
| IHSF | 63 | M | 89 | 173 | CaOx | 118 | 69 | 121 | 72 |
| Mean | 44±3 | 81±4 | 173±3 | 117±3 | 72±2 | 117±4 | 69±2 | ||
Individual values and means ± SE are shown. Values of means did not differ significantly between stone formers with idiopathic hypercalciuria (IHSF) and normal subjects for any measurement shown. F, female; M male; Ca, stones reported as calcium stones by patient; CaOx, stone analysis shows calcium oxalate; CaP, stone analysis shows calcium phosphate; SBP and DBP, systolic and diastolic pressures (mmHg).
Experimental Design
Our primary comparison was distal calcium delivery from proximal tubule in IHSF patients vs. normal subjects in the postprandial state, since we already know that IHSF patients reduce their overall renal calcium reabsorption with meals. Endogenous lithium clearance was used to gauge proximal fractional tubule reabsorption of sodium and water, and distal delivery for sodium and calcium was calculated with filtered loads. Blood pressure was measured at the time each blood sample was drawn, from left or right arm in sitting position, with standard automated instruments (20 measurements/subject). One subject lacks blood pressure measurements, which were made and lost by the clinical research center (Table 1).
Our design has been detailed elsewhere (23). Briefly, we fed our subjects three meals during a single-day protocol in the University of Chicago General Clinical Research Center. All patients began their first meal between 7:30 and 8:30 AM. The basic study diet (1,800 calories) contained calcium (1,160 mg), phosphorus (1,240 mg), sodium (2,141 mg), and potassium (2,427 mg) as determined by analysis of frozen aliquots of whole meal homogenates (Covance, Madison, WI). The diet was scaled up by body weight in two levels of 2,100 or 2,400 calories with the Schofield equation (15) predicting basal metabolic rate; mineral contents were scaled accordingly. Meals were composed of common foods purchased from a chain grocer. Fourteen 1-h urine collections were obtained, 2 fasting and 12 fed (4 between breakfast and lunch, 5 between lunch and supper, and 3 after supper until the end of the protocol). Blood was drawn hourly at the end of each urine and also at the midpoint of the urines collected during the 2 h after each meal (20 samples in all). No caffeinated beverages were permitted; water was used as desired.
We instructed subjects to eat a diet at home that matched the study diet for the 4 days preceding the study day. Although our basal study diet provided 100 meq of sodium in its lowest version and 116 and 133 meq for the 2,100- and 2,400-calorie versions, our total average sodium excretions for IHSF patients and normal subjects—adding up all sodium for the 14 urines of the study + the overnight sample—was 178 and 166 meq for normal subjects and IHSF patients, respectively. Although both values exceed the sodium content of the prescribed diet, meaning incomplete adaptation to the diet at home, the values do not differ.
Laboratory Methods
Routine measurements.
Serum, serum ultrafiltrate (UF), urine calcium, creatinine, sodium, and potassium, and urine volume were measured as described elsewhere (13).
Lithium measurements.
Lithium was measured in deproteinated serum and in urine by atomic emission (Instrumentation Laboratory model 951 AA/AE) with an air-acetylene-N2O flame at a wavelength of 670.8 nm and a bandwidth of 0.5 nm with background correction. Although the assay was linear to at least 25 μg/l, samples were diluted, if necessary, for analysis within a standard range of 0.1–5 μg/l. To correct for ionization effects, samples were assayed against lithium standards (Fisher Scientific) prepared in dilutions of physiological saline (140 mM Na+, 5 mM K+) that matched the sum of sodium and potassium in the samples. Statistical limits of the assay were determined with standards prepared in either 1:2 (70 mM Na+–2.5 mM K+) or 1:5 (28 mM Na+–1 mM K+) physiological saline. The detection limit of the assay was 0.035 μg/l. The quantitation limit (lower limit of the standard range) was 0.116 μg/l. Lithium recovery in the assay was 103 ± 0.8% for urine and 102.7 ± 3.2% for serum. Intra-assay variation was 3.7% for urine and 5.3% for serum. Interassay variation was 1.4% for urine and 4.1% for serum.
For serum samples, proteins were precipitated by the addition of 1 part 30% TCA to 5 parts serum. After being vortexed, the mixtures stood for 5 min and were centrifuged at 13,000 g for 10 min. Supernatants were diluted 1:1 in deionized water (total serum dilution was 1:2.4) for assay of lithium against lithium standards prepared in 1:2 physiological saline (70 mM Na+–2.5 mM K+, 2.5% TCA). Urine samples were diluted to a total of 29 mM (Na+ + K+), based on the measured concentrations of sodium and potassium in the urine samples. The urine was then assayed for lithium against a standard curve prepared in 1:5 physiological saline. In cases of high or low urine lithium, samples were rediluted and assayed against a standard curve prepared in either 1:2 or 1:10 (14 mM Na+–0.5 mM K+) physiological saline. Pooled serum and urine samples, assayed both by standard additions and against a standard curve, served as quality controls. Final lithium values are expressed in micromoles per liter.
Calculations
Clearances were calculated with the mean of serum or UF values at the beginning and end of each urine collection; when present, midpoint serum values were included in the mean. Creatinine clearances (CCr) and lithium clearances (CLi) were calculated conventionally in liters per hour with UF values for creatinine and serum lithium as measured by the method detailed above. In our prior work (23), we found that substitution of iothalamate clearance for creatinine clearance did not alter results. Distal delivery of calcium was calculated as
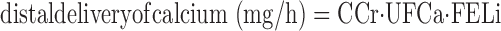 |
(1) |
where FELi is the fraction of filtered lithium excreted in the urine, UFCa is UF calcium concentration (mg/l), and CCr is in liters per hour. Since lithium reabsorption is considered predominantly or exclusively proximal and closely parallels sodium reabsorption, FELi gauges the fraction of filtered sodium and water delivered distally out of the proximal tubule. Since calcium reabsorption in the proximal tubule is predominantly paracellular and driven by sodium and water reabsorption, FELi and fraction of filtered calcium leaving the proximal tubule are equivalent. FELi is calculated simply as
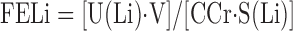 |
(2) |
where U(Li) and S(Li) are urine and serum lithium concentrations (μmol/l) and V is urine volume (l/h). This reduces Eq. 1 to
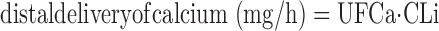 |
(3) |
For sodium delivery, Eq. 3 substitutes serum sodium for UFCa. Absolute distal reabsorption was calculated as distal delivery − urine excretion. Fraction of distal delivery excreted was calculated as excretion rate/distal delivery. Overall fractional excretions for sodium and calcium were calculated conventionally as excretion rate/filtered load.
Statistical Approach
General analytic approach.
Because the data consist of repeated measures on individuals over time, statistical methods for correlated data were used to analyze the mean difference in each laboratory measurement of interest between IHSF and normal. In particular, generalized estimating equations (4) utilizing compound symmetrical covariance structures were used to compare laboratory values between IHSF and normal. To determine whether the mean difference in each laboratory value comparing IHSF and normal changed with respect to mealtime, multiplicative interactions between the covariates were modeled. For simplicity, mealtimes were modeled as fasting vs. nonfasting. Interaction terms were tested with multivariate Wald tests. In all cases, empirical SE estimates (21) were used to guarantee consistent variance estimates.
Covariate adjustments.
Age, sex, height, and weight were all considered potential confounding factors, and hence we decided a priori to adjust for these covariates in all analyses. To further explore the independent effect of IHSF vs. normal status on calcium excretion, additional analyses adjusting for calcium delivery to the distal nephron were also conducted.
RESULTS
Lithium
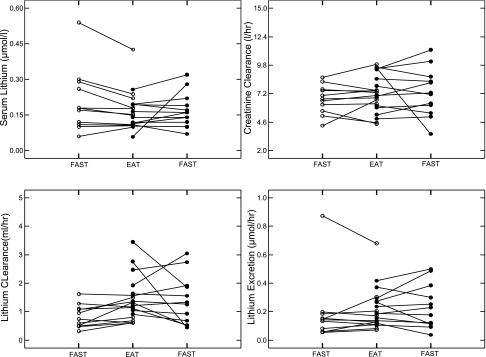
Serum lithium and creatinine clearance values (Table 2, Fig. 1, top) and lithium filtered load (Table 2) did not differ (Table 3) between IHSF and normal, fasting or fed. Lithium clearances (Fig. 1, bottom left) and fractional lithium excretions (Table 2) differed between groups, fed and fasting (Table 3), IHSF being higher than normal. Lithium excretions (Fig. 1, bottom right) did not differ between groups. A reason that lithium clearances differ and urine lithium excretions do not differ is that one normal subject had high urine and serum lithium levels; clearance for that subject was unremarkable and in the general range for normal subjects.
Table 2.
Values by meal period
|
Fasting |
Fed
|
|||
|---|---|---|---|---|
| Normal | IHSF | Normal | IHSF | |
| Serum Li, μmol/l | 0.21±0.05 | 0.17±0.02 | 0.18±0.03 | 0.15±0.02 |
| CCr, l/h | 6±0.38 | 7±0.6 | 7±0.34 | 8±0.5 |
| Lithium FL, μmol/h | 1±0.31 | 1±0.23 | 1±0.22 | 1±0.15 |
| CLi, l/h | 0.9±0.12 | 1±0.2 | 1.1±0.11 | 2±0.22 |
| FELi, % | 15±2 | 19±2 | 16±2 | 24±2 |
| Li excretion, μmol/h | 0.21±0.08 | 0.23±0.05 | 0.20±0.06 | 0.23±0.03 |
| Serum sodium, meq/l | 139±0.47 | 139±0.58 | 139±0.56 | 138±0.58 |
| Sodium FL, meq/h | 954±62 | 993±59 | 975±62 | 1022±59 |
| Distal Na delivery, meq/h | 125±17 | 183±28 | 148±15 | 245±31 |
| Distal Na reabsorption, meq/h | 116±17 | 176±27 | 138±15 | 236±31 |
| Distal FENa, % | 9.3±2 | 5±1 | 9.1±2 | 4.9±1 |
| Sodium excretion, meq/h | 8±1.6 | 7±1.3 | 10±1.5 | 10±1.2 |
| FENa, % | 0.9±0.14 | 0.69±0.08 | 1.0±0.14 | 0.92±0.09 |
| Serum potassium, meq/l | 4±0.1 | 3.8±0.1 | 4±0.1 | 3.7±0.1 |
| Potassium excretion, meq/h | 4.1±0.5 | 3.4±0.5 | 3.2±0.5 | 2.9±0.5 |
| UF calcium, mg/dl | 5±0.06 | 5±0.08 | 5±0.04 | 5±0.06 |
| Calcium FL, mg/h | 340±21 | 361±33 | 350±18 | 401±28 |
| Distal Ca delivery, mg/h | 48±7 | 68±11 | 57±6 | 95±12 |
| Distal Ca reabsorption, mg/h | 42±7 | 59±11 | 47±6 | 76±11 |
| Distal FECa, % | 16±3 | 17±3 | 22±4 | 26±4 |
| Calcium excretion, mg/h | 6±0.43 | 9±1.2 | 9±1 | 19±2.5 |
| FECa, % | 2±0.12 | 2±0.23 | 3±0.31 | 5±0.41 |
Values are means ± SE. SEs are adjusted for within-subject correlation stemming from multiple measurements within subject. Li, lithium; CCr, creatinine clearance; CLi, clearance of lithium; FL, filtered load; FENa and FECa, fractional excretion of sodium and calcium; distal FENa and distal FECa, fractional excretion of distally delivered sodium and calcium.
Fig. 1.
Renal lithium handling in individual subjects. Serum lithium values (top left) and creatinine clearances (top right) did not differ between normal subjects (○) and idiopathic hypercalciuria with calcium stone (IHSF) patients (•), fasting or fed. Lithium clearance values (bottom left) of IHSF patients were above those of normal subjects, on average, in the fed state. Lithium excretion (bottom right) of IHSF patients and normal subjects did not differ on average; note that the one very high excretion value was from the subject with high serum lithium, so clearance values for this person were in the middle of the normal range.
Table 3.
Difference (IHSF-normal), fasting and fed
| Fasting | Fed | |
|---|---|---|
| Serum Li, μmol/l | −0.06 (−0.1, 0.03) | −0.05 (−0.1, 0.02) |
| CCr, l/h | 0.26 (−1.004, 1.52) | 0.42 (−0.325, 1.17) |
| Lithium FL, μmol/h | −0.33 (−0.94, 0.29) | −0.25 (−0.7, 0.2) |
| CLi, l/h | 0.59 (0.031, 1.15) | 0.65 (0.158, 1.13) |
| FELi, % | 6.9 (0.13, 14) | 7.8 (1.98, 14) |
| Li excretion, μmol/h | 0.014 (−0.16, 0.19) | 0.012 (−0.12, 0.14) |
| Serum sodium, meq/l | 0.026 (−1.20, 1.249) | −0.793 (−2.16, 0.569)* |
| Sodium FL, meq/h | 36.5 (−139.9, 213) | 53.8 (−48.5, 156) |
| Distal Na delivery, meq/h | 82.2 (4.4, 160) | 88.9 (21.3, 157) |
| Distal Na reabsorption, meq/h | 83.7 (7.04, 160) | 89.7 (22.23, 157) |
| Distal FENa, % | −5.5 (−10, −0.54) | −5.3 (−10, −0.69) |
| Sodium excretion, meq/h | −1.44 (−5.07, 2.19) | −0.84 (−3.61, 1.93) |
| FENa, % | −0.22 (−0.53, 0.081) | −0.14 (−0.43, 0.151) |
| Serum potassium, meq/l | −0.48 (−2.03, 1.066) | −0.41 (−1.25, 0.419) |
| Potassium excretion, meq/h | −0.055 (−0.10, −0.0054) | −0.053 (−0.10, −0.0069) |
| UF calcium, mg/dl | −0.139 (−0.34, 0.066) | 0.019 (−0.12, 0.160) |
| Calcium FL, mg/h | 4.81 (−69.6, 79.2) | 25.16 (−20.7, 71.0) |
| Distal Ca delivery, mg/h | 29.5 (−1.14, 60.2) | 34.7 (7.58, 61.7) |
| Distal Ca reabsorption, mg/h | 27.7 (−2.0622, 57.5) | 26.8 (0.0931, 53.6) |
| Distal FECa, % | −4.2 (−16, 7.4) | 0.093 (−12, 12.2) |
| Calcium excretion, mg/h | 1.89 (−1.83, 5.61) | 7.83 (4.17, 11.50)* |
| FECa, % | 0.4 (−0.27, 1.07) | 1.7 (0.82, 2.55)* |
Values are estimated adjusted differences between IHSF and normal subjects, with 95% confidence intervals in parentheses. Abbreviations are as in Table 2. Estimates are adjusted for age, sex, weight, and height. Significant differences vs. 0 are in bold.
Test of the interaction between IHSF vs. normal and fasting period, P < 0.05; a significant effect means that the difference between IHSF and normal differs between fasting and fed.
Sodium, Potassium, and Blood Pressure
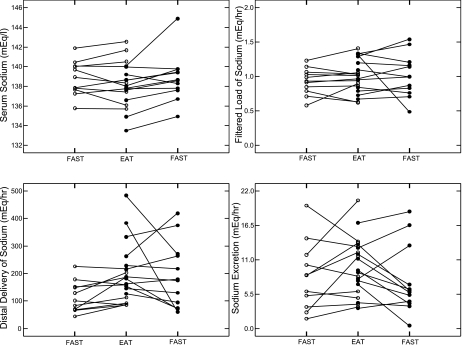
Serum sodium and sodium filtered load (Fig. 2, top; Table 2) did not differ between IHSF and normal (Table 3), although the difference in serum sodium between IHSF and normal increased with feeding (Table 3). Distal sodium delivery of IHSF exceeded normal both fasting and fed (Fig. 2, bottom left; Tables 2 and 3), as did distal absolute sodium reabsorption (Tables 2 and 3), but distal fractional excretion of sodium by normal subjects exceeded that by IHSF patients both fasting and fed (Tables 2 and 3) to the extent that neither total nor fractional sodium excretions differed between IHSF and normal, fasting or fed (Fig. 2, bottom right; Tables 2 and 3). Overall, despite a higher distal sodium delivery in IHSF vs. normal, final sodium excretion rates were the same, reflecting augmented distal sodium reabsorption in IHSF vs. normal. Although serum potassium values did not differ, potassium excretion values of IHSF patients were below those of normal subjects, fasting and fed (Tables 2 and 3), suggesting that the increase in sodium reabsorption was not occurring at a site of sodium/potassium exchange.
Fig. 2.
Renal sodium handling in individual subjects. Symbols as in Fig. 1. Only distal sodium delivery (bottom left) differed between IHSF patients and normal subjects. Serum sodium and sodium filtered load (top) and sodium excretion (bottom right) did not differ between IHSF patients and normal subjects.
Despite what appears to be a greater avidity for distal sodium reabsorption in IHSF, mean unadjusted systolic and diastolic blood pressures did not differ between IHSF and normal (Table 1). After adjustment for age, sex, height and weight, we estimated that nonfasting mean systolic blood pressure was 9.44 mmHg lower among IHSF patients compared with normal subjects (95% CI: −18.8, −0.10). A similar, though not statistically significant, difference was also observed for diastolic blood pressure (estimated difference of 5.1 mmHg; 95% CI: −11.0, 0.34). Addition of distal sodium delivery to the regression did not change results significantly.
Calcium
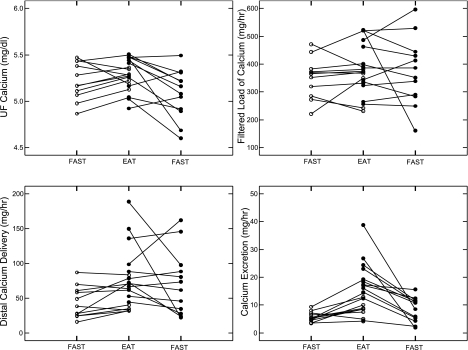
UF calcium concentration and calcium filtered load (Fig. 3, top) did not differ between fasting and fed periods or between IHSF and normal (Tables 2 and 3). With food, but not fasting, distal calcium delivery of IHSF exceeded normal (Fig. 3, bottom left; Tables 2 and 3), as did distal absolute calcium reabsorption. The fraction of distally delivered calcium excreted did not differ between IHSF and normal (Table 3). As expected, fractional and total calcium excretion rose with eating in IHSF and exceeded normal (Fig. 3, bottom right; Table 3). The increased distal calcium delivery of IHSF is the outcome of our primary comparison and the main result of this research.
Fig. 3.
Renal calcium handling in individual subjects. Symbols as in Fig. 1. Distal calcium delivery and calcium excretion (bottom) were both higher in IHSF patients than in normal subjects during the fed state. Heterogeneity of IHSF is notable. Ultrafiltrate (UF) calcium and calcium filtered load (top) did not differ between IHSF and normal.
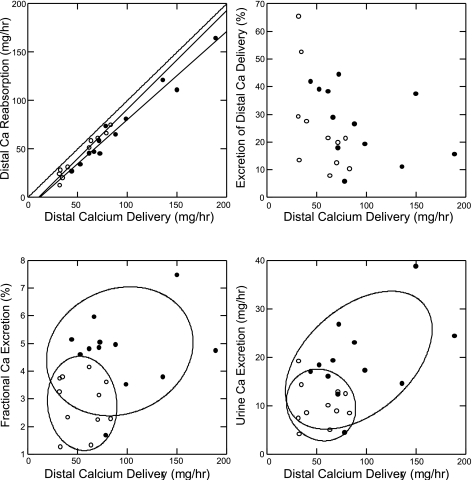
On the surface, these data suggest that hypercalciuria arises from increased distal delivery, but we believe an abnormal distal response is also present in the fed state. Absolute distal calcium reabsorption (Fig. 4, top left) is, of course, below the line of identity (highest diagonal line) in both normal subjects and IHSF patients, being the difference between distal delivery (x-axis) and urine excretion. Even though absolute calcium reabsorption is higher in IHSF than normal (Tables 2 and 3) IHSF points lie below the normal points, meaning that at comparable distal deliveries less calcium is reabsorbed. The fractions of distally delivered calcium excreted (Fig. 4, top right) by IHSF patients and normal subjects overlap (y-axis), but at comparable distal deliveries the fraction is higher in IHSF. These findings suggest a component of reduced distal reabsorption in IHSF vs. normal.
Fig. 4.
Relationship between distal calcium reabsorption, renal calcium excretion, and distal calcium delivery. Symbols as in Fig. 1. Distal calcium reabsorption (top left) rises with calcium delivery in normal subjects and IHSF patients. Values for both lie beneath the uppermost diagonal line of identity. Many IHSF reabsorption values lie beneath normal at overlapping values of distal calcium delivery, indicating a reduced fractional reabsorption of distally delivered calcium. The actual fraction (%) of distally delivered calcium excreted (top right) fell with increasing distal delivery, and points from many IHSF patients lie above normal points at comparable distal delivery; this panel visually amplifies the findings of panel at top left. Fractional and total calcium excretions, likewise, were high in many IHSF patients vs. normal subjects at comparable deliveries (bottom). Statistical analyses are in Tables 3 and 4.
Renal calcium fractional excretion (Fig. 4, bottom left) is higher in IHSF patients than in normal subjects at comparable distal calcium deliveries, which also argues for reduced distal reabsorption. As Fig. 4 suggests, fractional excretion of distally delivered calcium (FECa) of IHSF exceeds normal even when adjusted for distal delivery (Table 4). In addition, when calcium excretion is adjusted for distal calcium delivery (Table 4), values of IHSF clearly exceed normal as implied by Fig. 4, bottom right.
Table 4.
FECa and calcium excretion adjusted for delivery
|
Difference (IHSF-Normal) (95% CI) |
P Value* | ||
|---|---|---|---|
| Fasting | Fed | ||
| Calcium excretion, mg/h | 0.96 (−2.76, 4.68) | 6.74 (2.41, 11.06) | 0.004 |
| FECa, % | 0.41 (−0.27, 1.09) | 1.70 (0.77, 2.62) | 0.003 |
All analyses are adjusted for age, sex, weight, height, and distal Ca delivery; 95% confidence intervals (CI) are in parentheses.
Test of the interaction between IHSF vs. normal and fasting period, P < 0.05; a significant effect means that the difference between IHSF and normal differs between fasting and fed. Significant differences vs. 0 are bold.
In all, these results are most compatible with a combined increase of proximal delivery to the distal nephron coupled with a reduction of distal reabsorption in IHSF. Our figures also suggest possible heterogeneity among IHSF patients, some of whom have extremely high distal deliveries that perhaps account for much of their hypercalciuria.
DISCUSSION
Proximal Tubule Sodium and Calcium Reabsorption Are Reduced in IHSF
Although we selected our patients because of calcium renal stones and high urine calcium excretion, we found they harbor a distinctive abnormality of proximal tubule sodium reabsorption that is almost certain to increase calcium delivery out of the proximal tubule and foster both hypercalciuria and, perhaps, interstitial apatite plaque. Because of this abnormality, their distal sodium and calcium deliveries are increased. All of the “extra” sodium is reabsorbed so that urine sodium excretion matches that of our normal subjects. Of the distally delivered “extra” calcium, much appears in the final urine. To our knowledge these are new abnormalities in stone disease and hypercalciuria, and they suggest that IHSF patients differ from normal subjects in far more complex ways than has hitherto been understood. Furthermore, the increase of calcium delivery into the loop of Henle and distal nephron segments could help to foster deposition of plaque. Our conclusions rest on a series of measurements and calculations that each need separate analysis.
Sodium
Endogenous lithium clearance should gauge sodium handling in our subjects.
Endogenous lithium clearance is a well-established marker of proximal tubule sodium and water reabsorption and widely used in clinical investigations (14, 16, 17). Multiple animal studies document a reasonable estimate of proximal tubule reabsorption with lithium even though, in rats, there exists some evidence for distal reabsorption as well (18, 20). Distal lithium reabsorption appears to predominate mainly when sodium intake is low; in our work here, diet sodium was set at 100 meq/day, an ample supply. Overall, our finding of increased fractional excretion of lithium can therefore be taken as reasonable evidence that in IHSF proximal sodium and water reabsorption are reduced compared with normal. Since total mean 24-h sodium excretions of IHSF patients did not exceed those of normal subjects (166 vs. 178 meq/day, detailed in methods), diet sodium was fixed at 100–133 meq/day, and excretion rates of IHSF patients and normal subjects were not different during the 14 clearance periods (Tables 2 and 3) we cannot ascribe the decreased proximal lithium reabsorption in IHSF to a difference in extracellular fluid volume.
Increased distal delivery is balanced by increased distal reabsorption.
Reduced lithium reabsorption implies increased distal delivery of sodium and water out of the proximal tubule, but sodium excretion of IHSF patients is the same as that of normal subjects, at least on our diet, so calculated absolute and fractional distal tubule sodium reabsorption by IHSF exceeds normal, permitting overall fractional excretion of distally delivered sodium to be equal between IHSF and normal (Tables 2 and 3). Possibly, IHSF patients have increased distal sodium avidity and balance their urine sodium excretion against sodium intake via reduced proximal tubule reabsorption. Alternatively, decreased proximal sodium reabsorption is a primary abnormality and the distal increase of reabsorption is an adaptation to it. We cannot distinguish these alternatives.
Blood pressure and sodium handling in IHSF.
Our results echo and contradict observations made in studies of hypertension, in which blood pressure was higher in subjects with lower FELi. Chiolero et al. (3) noted that normal control subjects had higher FELi than their hypertensive subjects and that among the hypertensive subjects those with the least tendency to increase their blood pressure with salt loading had higher FELi than those whose blood pressures were more salt sensitive. In metabolic syndrome, which includes hypertension, FELi is reduced compared with normal subjects (19). In one large population study gene polymorphisms associated with hypertension were also associated with decreased FELi. In a detailed study of hypertensive people, increased proximal sodium reabsorption—reduced FELi—was observed (2). By contrast, our IHSF patients had higher FELi than our normal subjects, and yet a lower average systolic blood pressure, although both groups were normotensive (Table 1). Further study of the relationships between proximal sodium handling and blood pressure in IHSF are warranted.
Calcium
Because proximal tubule calcium reabsorption follows that of sodium and water we can gauge distal calcium delivery with FELi and calcium filtered load.
Although calcium reabsorption in the proximal tubule includes an element of active transepithelial transport, it proceeds mostly via passive reabsorption driven by diffusion and solvent drag (7). For this reason, proximal tubule fluid-to-UF calcium concentration ratios hover very close to 1 when measured by micropuncture. Given that this is true in humans—not as yet a proven fact—and given that FELi is a reasonable gauge of proximal tubule sodium and water reabsorption in our subjects, an increase of FELi must reflect a corresponding proportional increase in distal delivery of calcium out of the proximal tubule, the proportionality constant being the product of UF calcium concentration and glomerular filtration rate, the latter gauged here by creatinine clearance.
One might wonder, if this were true, why in the fasting state distal sodium delivery can be high yet distal calcium delivery is not high; linkage should make both high at once. In fact, they are both high, but the smaller number of observations during the fasting state reduces statistical power. Mean difference for calcium delivery (IHSF-normal) is 29.5 mg/h in fasting vs. 34.7 mg/h in the fed state, but the 95% CI is much larger fasting than fed, so statistical significance is not reached.
Distal nephron calcium reabsorption also appears reduced in IHSF.
Distal handling of calcium differs from that of sodium, in that the “extra” calcium delivered distally is not reabsorbed completely, but at least in part is excreted in the urine. When calcium excretion is adjusted for calcium delivery to the distal nephron, values in IHSF clearly exceed normal, supporting a reduction in distal calcium reabsorption (Table 4). This point is illustrated in Fig. 4, bottom. In some of our IHSF patients, overall renal FECa, and calcium excretion, exceed normal even at overlapping values of distal calcium delivery; other IHSF patients seem to achieve high FECa, and hypercalciuria, mainly via very high delivery. The weight of evidence therefore suggests that a combination of increased distal calcium delivery and reduced distal nephron calcium reabsorption occurs in IHSF, and also suggests heterogeneity among IHSF patients in regard to the balance between proximal and distal tubule responses.
Relationship of Our New Findings to Formation of Interstitial Plaque
This study was engendered by our desire to better understand the mechanisms that could produce interstitial papillary apatite plaque in IHSF. One possibility was that increased amounts of calcium delivered into the loop of Henle could promote plaque. Although the proximal tubule fluid-to-UF calcium concentration ratio would be expected to approximate 1 (7), water extraction along the descending limb could increase final calcium concentration at the bend of the loop above normal in IHSF given more calcium availability. As well, delivery of more calcium to the thick ascending limb could increase absolute calcium reabsorption there, raising medullary interstitial calcium concentration and therefore the concentration of calcium in the descending vas recta. Since plaque originates in the basement membranes of thin limbs in the papillum (6), both loop fluid and vas calcium concentrations are relevant. Our work cannot further explore these or other alternative mechanisms, but adds the crucial fact that the reduced postprandial calcium reabsorption of IHSF is not confined to the distal nephron but involves the proximal tubule as well.
GRANTS
This work was supported by National Institutes of Health Grants DK-P01-56788 and M01-00055.
Acknowledgments
The authors thank the patients and normal subjects for participating and the nursing staff of the University of Chicago General Clinical Research Center for expert assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ba J, Friedman PA. Calcium-sensing receptor regulation of renal mineral ion transport. Cell Calcium 35: 229–237, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Burnier M, Biollaz J, Magnin JL, Bidlingmeyer M, Brunner HR. Renal sodium handling in patients with untreated hypertension and white coat hypertension. Hypertension 23: 496–502, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M. Proximal sodium reabsorption: an independent determinant of blood pressure response to salt. Hypertension 36: 631–637, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. Oxford: Oxford University Press, 2002.
- 5.Evan AP, Coe FL, Lingeman JE, Shao Y, Sommer AJ, Bledsoe SB, Anderson JC, Worcester EM. Mechanism of formation of human calcium oxalate renal stones on Randall's plaque. Anat Rec (Hoboken) 290: 1315–1323, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 607–616, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman PA In: Seldin and Giebisch's The Kidney, edited by Alpern RJ, Hebert SC. Amsterdam: Academic, 2008, p. 1851–1890.
- 8.Friedman PA Mechanisms of renal calcium transport. Exp Nephrol 8: 343–350, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Kim SC, Coe FL, Tinmouth WW, Kuo RL, Paterson RF, Parks JH, Munch LC, Evan AP, Lingeman JE. Stone formation is proportional to papillary surface coverage by Randall's plaque. J Urol 173: 117–119, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Kuo RL, Lingeman JE, Evan AP, Paterson RF, Parks JH, Bledsoe SB, Munch LC, Coe FL. Urine calcium and volume predict coverage of renal papilla by Randall's plaque. Kidney Int 64: 2150–2154, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Matlaga BR, Coe FL, Evan AP, Lingeman JE. The role of Randall's plaques in the pathogenesis of calcium stones. J Urol 177: 31–38, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Matlaga BR, Williams JC Jr, Kim SC, Kuo RL, Evan AP, Bledsoe SB, Coe FL, Worcester EM, Munch LC, Lingeman JE. Endoscopic evidence of calculus attachment to Randall's plaque. J Urol 175: 1720–1724, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Parks JH, Coward M, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int 51: 894–900, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Pechere-Bertschi A, Sunaric-Megevand G, Haefliger I, Panarello F, Maillard M, Burnier M. Renal sodium handling in patients with normal pressure glaucoma. Clin Sci (Lond) 112: 337–344, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Schofield WN Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39, Suppl 1: 5–41, 1985. [PubMed] [Google Scholar]
- 16.Seidlerova J, Staessen JA, Maillard M, Nawrot T, Zhang H, Bochud M, Kuznetsova T, Richart T, Van Bortel LM, Struijker-Boudier HA, Manunta P, Burnier M, Fagard R, Filipovsky J. Association between arterial properties and renal sodium handling in a general population. Hypertension 48: 609–615, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Shirley DG, Walter SJ, Noormohamed FH. Natriuretic effect of caffeine: assessment of segmental sodium reabsorption in humans. Clin Sci (Lond) 103: 461–466, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Shirley DG, Walter SJ, Sampson B. A micropuncture study of renal lithium reabsorption: effects of amiloride and furosemide. Am J Physiol Renal Fluid Electrolyte Physiol 263: F1128–F1133, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Strazzullo P, Barbato A, Galletti F, Barba G, Siani A, Iacone R, D'Elia L, Russo O, Versiero M, Farinaro E, Cappuccio FP. Abnormalities of renal sodium handling in the metabolic syndrome. Results of the Olivetti Heart Study. J Hypertens 24: 1633–1639, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen K, Shirley DG. The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron 77: 125–138, 1997. [DOI] [PubMed] [Google Scholar]
- 21.White H A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 48: 817–830, 1980. [Google Scholar]
- 22.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol 28: 120–132, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worcester EM, Gillen DL, Evan AP, Parks JH, Wright K, Trumbore L, Nakagawa Y, Coe FL. Evidence that postprandial reduction of renal calcium reabsorption mediates hypercalciuria of patients with calcium nephrolithiasis. Am J Physiol Renal Physiol 292: F66–F75, 2007. [DOI] [PubMed] [Google Scholar]