Abstract
Background and purpose:
Oxidative stress caused by cytokine exposure is a major cause of pancreatic islet death in vitro and of diabetogenesis. Antioxidant compounds may prevent cytokine-induced damage to islet cells. Hence, we studied the potential of curcumin, an antioxidant and anti-inflammatory compound, in vitro to protect islets against pro-inflammatory cytokines and in vivo to prevent the progression of diabetes induced by multiple low doses of streptozotocin (MLD-STZ).
Experimental approach:
Pancreatic islets from C57/BL6J mice were pretreated with curcumin (10 μM) and then exposed to a combination of cytokines. Islet viability, reactive oxygen species (ROS), NO, inducible NO synthase and NF-κB translocation were studied. Curcumin pretreated (7.5 mg kg−1 day−1) C57/BL6J mice were given MLD-STZ (40 mg kg−1), and various parameters of diabetes induction and progression were monitored.
Key results:
Curcumin protected islets from cytokine-induced islet death in vitro by scavenging ROS and normalized cytokine-induced NF-κB translocation by inhibiting phosphorylation of inhibitor of kappa B alpha (IκBα). In vivo, curcumin also prevented MLD-STZ, as revealed by sustained normoglycaemia, normal glucose clearance and maintained pancreatic GLUT2 levels. Pro-inflammatory cytokine concentrations in the serum and pancreas were raised in STZ-treated animals, but not in animals pretreated with curcumin before STZ.
Conclusions and implications:
Here, we have demonstrated for the first time that curcumin in vitro protects pancreatic islets against cytokine-induced death and dysfunction and in vivo prevents STZ-induced diabetes.
Keywords: curcumin, cytokines, islet death, MLD-STZ
Introduction
Pancreatic islet cell death is an orchestrated cellular event that occurs following oxidative and/or inflammatory stress, and treatment with antioxidants shows good results in cell rescue and survival (Bolaffi et al., 1986; Matthews et al., 2005). Cytokines such as tumour necrosis factor-α (TNFα), interleukin-1β (IL-1β) and interferon-γ (IFNγ) under in vitro conditions give rise to free radicals that cause expression of inducible nitric oxide synthase (iNOS) (Darville et al., 2004). This rise in iNOS causes the generation of NO and translocation of nuclear factor kappa B (NF-κB), a transcription factor that is normally sequestered in the cytoplasm, into the nucleus, which eventually leads to islet cell death (Kwon et al., 1995; Hayden and Ghosh, 2004). Hence, blockade of the islet cell death pathway at any of the checkpoints is a primary approach employed for the protection of islet cells from cytokine-induced death and dysfunction. This has been achieved with some success by pretreatment with various antioxidants and other compounds. Inhibition of iNOS expression by synthetic iNOS inhibitors such as 1400W, or even induction of the heat shock response, protects islets against cytokine insult in vitro (Scarim et al., 1998; Rydgren and Sandler, 2002). However, mixed results were observed on in vivo administration (Sternesjo et al., 1997). NF-κB inhibitors have also been employed to prevent islet cell death in vitro as well as in vivo (Mabely et al., 2003; Kim et al., 2004; Kwon et al., 2006). Vitamin metabolites such as [1,25-(OH) 2D3] and regulatory cytokine treatments such as IL-11 are also capable of blocking NF-κB activation, thus protecting islets and RINm5F cells against cytokine-induced cell death (Riachy et al., 2002; Lgssiar et al., 2004).
The other approach, tested by many groups with a limited degree of success, involves induction and overexpression of various enzymes scavenging reactive oxygen species (ROS) in islets, as these cells have inherently low levels of these enzymes (Mandrup-Poulsen et al., 1985; Pappacio et al., 2002; Chen et al., 2005).
A more comprehensive approach is to employ a compound capable of performing a multitude of these functions, such as free radical scavenging, inhibition of inflammatory responses, induction of cellular defence responses, cytoprotection and inhibition of important transcription factors, such as NF-κB, which contribute to initiation of cell death cascade. Matsuda et al. (2005) have tried such an approach by using silymarin, a flavonoid with antioxidant, anticarcinogenic, anti-inflammatory and cytoprotective activities. Silymarin protected human islets and RINm5F cells against IL-1β- and IFNγ-mediated toxicity by suppression of the c-Jun NH2-terminal kinase and JAK/STAT pathways. However, very few of these compounds have, on their own, been able to confer complete protection against cytokine-induced islet cell death along with maintenance of cellular homoeostasis and function, both in vitro and in vivo. Following their lead, we have tested the efficacy of curcumin, another polyphenolic flavonoid, for the protection of islets from the pro-inflammatory cytokines, TNFα, IL-1β and IFNγ. Curcumin, a component of turmeric, a widely used spice and food-colouring agent, has been shown to possess potent antioxidant, antitumour-promoting and anti-inflammatory properties in vitro as well as in vivo. Although the mechanisms of the differing effects manifested by curcumin are poorly understood, this compound is known to protect against oxidative stress and cause upregulation of cellular defence proteins such as haem oxygenase-1 along with inhibition of NF-κB (Motterlini et al., 2000; Fujisawa et al., 2004; Weber et al., 2006). We recently demonstrated that curcumin protects islets against streptozotocin (STZ)-induced death and dysfunction (Kanitkar et al., 2007). We have also demonstrated the efficacy of adding curcumin to the medium during cryopreservation of islets (Kanitkar and Bhonde, 2008). In spite of this, little is known about its ability to protect the islets against pro-inflammatory cytokine-induced death and/or dysfunction. Furthermore, limited information is available regarding its ability to prevent diabetogenesis in vivo. To fill this gap in our knowledge and to better understand the potential of curcumin as a β-cell protective agent against cytokine insults in vitro and in vivo, we tested its efficacy against a combination of cytokines (TNFα+IL-1β+IFNγ) and multiple low doses of STZ-induced (MLD-STZ) diabetogenesis in vivo. Our results demonstrate that curcumin prevents cytokine-induced islet cell death and dysfunction by promoting relocalization of NF-κB p65 into the cytoplasm. It also prevents MLD-STZ-induced diabetes in C57/BL6J mice.
Materials and methods
Test systems used
All animal procedures used complied with international guidelines governing animal experimentation. C57/BL6J male mice (6 to 8 weeks old) weighing at least 22 g were obtained from an inbred colony at the National Centre for Cell Science, India, and given free access to standard feed and water.
Experimental design
Live islets were aseptically isolated from C57/BL6J mice as described by Shewade et al. (1999) and randomly divided into four experimental groups, each consisting of at least 500 islets during each experiment. The first group was left untreated (untreated control); the second group was pretreated with 10 μM curcumin for 24 h (CUR control) and the third group was exposed to a combination of cytokines (TNFα (1000 U mL−1)+IL-1β (150 U mL−1)+IFNγ (1000 U mL−1)) for 48 h (cytokine), whereas the fourth group was first pretreated with curcumin and later exposed to cytokine combination for 48 h (CUR+cytokines). These islets were then harvested and used for further experimentation, either live or dead.
For detection of phosphorylated inhibitor of kappa B alpha (IκBα) levels and NF-κB translocation as well as NF-κB activity assay, islet samples were collected at 0 h and then exposed to cytokines for 48 h. Samples were collected at 12, 24, 36 and 48 h.
Determination of islet viability and insulin secretion in vitro and in vivo
Islets were incubated with Hoechst 33342 (10 μM) followed by fixing with 4% freshly prepared paraformaldehyde and then exposed to ethidium bromide (10 μM) for 10 min. Islets were then scanned using the LSM510 confocal laser scanning microscope (Zeiss, Jena, Germany), and image stacks were taken at 20-μm interval. These were used to quantitate the live (Hoechst positive) and dead (Hoechst and ethidium bromide positive) cells. At least three different preparations and 150 dead nuclei were counted per preparation.
For insulin secretion assay, 75 hand-picked islets of roughly 150 μm from each treatment group were incubated in Krebs Ringer bicarbonate HEPES buffer (KRBH) with the following composition (in mM): 129 NaCl, 5 NaHCO3, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2 and 10 HEPES at pH 7.4. The buffer was supplemented with 0.1% BSA and 5.5 mM glucose. Islets were incubated at 37 °C for 1 h and the supernatant was collected. The same islets were then incubated in KRBH containing higher glucose concentration (16.5 mM) and the supernatant was collected. All supernatants were stored at −80 °C until further use.
For serum preparation, blood was collected by retro-orbital bleeding and incubated at 37 °C for 30 min. It was later centrifuged at 3200 g for 30 min; clear serum was collected and stored at −80 °C. For pancreatic insulin content, pancreatic samples were collected in acid ethanol, homogenized and centrifuged at 3200 g for 30 min. The supernatant was collected and stored at −80 °C until further use. Insulin concentrations from in vivo and in vitro samples were estimated using mouse insulin ELISA kit (Mercodia, Uppsala, Sweden).
Estimation of NO from islets
Concentrations of NO in islets were estimated using the Griess reagent (0.1% naphthylethylenediamine dihydrochoride+1% sulphanilamide). Conditioned medium (100 μL) from treated and untreated islet groups was incubated with equal volume of freshly prepared Griess reagent and incubated in the dark for 30 min. Optical density was measured using spectrophotometer at 550 nm. Values were expressed as micromolar per 150 islets. NaNO2 was used as a standard.
Islet cell lysis and estimation of iNOS and NF-κB
Islet pellets were washed with cold phosphate-buffered saline, resuspended in cold lysis buffer (20 mM Tris-acetate, pH 7.0, 0.27 mM sucrose, 1 mM EDTA, 1% Triton-X 100, 1 × protease inhibitor cocktail) and centrifuged at 3200 g, and the supernatant was stored at −80 °C until use. Nuclear and cytoplasmic fractions for NF-κB translocation studies were prepared using the NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL, USA). Total protein was quantitated using the bioassay protein assay kit (Bio-Rad, Hercules, CA, USA), resolved on 10% SDS-polyacrylamide gel electrophoresis (PAGE), electroblotted onto polyvinylidene fluoride membranes (GE Healthcare, St Giles, Bucks, UK) and probed with primary antibodies against iNOS (1:2500; BD Transduction Laboratories, San Jose, CA, USA) and NF-κB p65 (1:1000; Santa Cruz, Santa Cruz, CA, USA). The secondary antibodies were used at 1:2000 (Santa Cruz) or 1:10 000 (Bio-Rad). Blots were visualized using the ECL- plus HRP-conjugate detection system (GE Healthcare). The subcellular fractionations and immunoblots demonstrated here are representative of at least three different preparations.
Estimation of NF-κB activity in nuclear and cytosolic fractions
Frozen islets were lysed, and the nuclear and cytosolic fractions were separated using the NE-PER nuclear and cytoplasmic extraction reagents (Pierce). Then the levels of NF-κB activity were assayed using the NF-κB p65, total bioassay ELISA kit (US Biological, Swampscott, MA, USA) and carried out according to the manufacturer's instructions. The levels of active NF-κB were normalized against total protein concentrations.
Measurement of intracellular ROS
For the estimation of ROS, 300 islets from all treatment groups were incubated in the presence of 10 μM dichlorofluorescein diacetate at 37 °C for 10 min and the resulting fluorescence was measured using Fluroskan Ascent FL fluorimeter (Thermo Electron Corporation, Milford, MA, USA). All values were corrected by subtracting auto-fluorescence for the respective groups. Values were reported as fluorescence intensity per 150 islets.
Determination of phosphorylated IκBα
Frozen islets were lysed and the levels of phosphorylated IκBα were estimated using the Bio-Plex phosphoprotein IκBα testing kit and the Bio-Plex protein array reader (Bio-Rad). The levels of phosphorylated protein were normalized against total protein levels.
RNA preparation and RT-PCR
Total RNA from islets and pancreatic samples was isolated using TRIZOL (Invitrogen Corporation, CA, USA) and cDNA was prepared from 5 μg of samples using the Superscript First Strand synthesis system (Invitrogen Corporation) as per the manufacturer's protocol. PCR amplification was carried out using Taq DNA polymerase (Invitrogen Corporation) and cycle numbers for amplification were limited to 35 for all primer sets used. The sequences of primers used are as follows: β-actin: F: TGGAATCCTGTGGCATCCA, R: TAACAGTCCGCCTAGAAGCA; insulin: F: CCC TGCTGCCCCTGCTCTT, R: AGGTCTGAACCTCACCTGCT; GLUT2: F: CCACCA GTTTACAAGCTC, R: TGTACGCAGTACGGG TCCTC; TNFα: F: CGAGGTGGAACTGGCAGAAG, R: GGTACAACCCATCGGCTGGCA; IL-1β: F: TCATGGGATGATGATGATAACCTGCT, R: CCCATACTTTAGGAAGACACGGATT. The annealing temperatures were: β-actin, insulin and GLUT2: 58 °C; TNFα: 68 °C; IL-1β: 60 °C. Products were resolved by electrophoresis using 1% agarose gel; bands were stained with ethidium bromide (0.1 μg mL−1) and visualized using UV transillumination (Ultra Violet Products Ltd, Upland, CA, USA).
In vivo experimental design
Animals were randomly distributed into four treatment groups: untreated animals (master control), mice treated with curcumin alone (7.5 mg kg−1 day−1) for five consecutive days (CUR control), mice treated with STZ alone (40 mg kg−1 for five consecutive days) (MLD-STZ) and mice pretreated with curcumin for 5 days before being exposed to MLD-STZ (CUR+MLD-STZ). A series of experiments were performed for the standardization of curcumin dosage, and the dose yielding optimal results was used for all further experiments. After treatments, 2 weeks were allowed for the development of diabetes. Mice consistently exhibiting fasting blood glucose concentrations higher than 11.1 mM, along with impaired glucose tolerance, were considered as diabetic and used for further studies. When animals were killed on days 4, 5, 6 and 7 of treatment, one representative mouse from each group was left until the conclusion of the experiment.
Solvent and route of administration
Stock of curcumin was prepared in absolute alcohol, which was later diluted using phosphate-buffered saline. This was then injected intraperitoneally into mice for the required number of days. STZ was dissolved in sodium citrate and also administered intraperitoneally.
Development of diabetes and intraperitoneal glucose tolerance test
Glucose tolerance of animals was determined by injecting 2 g kg−1 of glucose into animals after overnight fasting. Blood glucose levels were measured at 0, 30, 60 and 90 min after glucose injections.
Estimation of plasma glucose concentrations
Plasma glucose was estimated from the tail vein. A drop of blood extracted from the tail vein was inserted into a mechanized counter (Accu-Chek sensor comfort; Roche Diagnostics Pvt. Ltd, Basel, Switzerland) that provided digital readings in millimoles per litre.
Estimation of serum cytokine levels
Blood was collected on days 4, 5, 6 and 7 after commencement of MLD-STZ treatment and serum was stored at −80 °C until further use. TNFα and IL-1β levels in the same serum samples were estimated using the highly sensitive, customized mouse cytokine 5-plex assay (Bio-Rad).
Haematoxylin and eosin staining of paraffin-embedded sections of the pancreas
Sections were de-waxed with xylene (three washes—each for 5 min). The sections were rehydrated by passing them through alcohol grades, namely 100, 90, 70, 50 and 30%. After a last dip in the water, the sections were incubated in haematoxylin stain (Sigma-Aldrich Reagents, St Louis, MO, USA) for 2 min. Alkaline water (0.3% NH4OH) treatment was given to the sections for 5–10 min till the appearance of intense blue colour. The sections were partially dehydrated and incubated with eosin stain (Sigma-Aldrich Reagents) for 5 min. Dehydration steps were continued up to 100% alcohol. Slides were dipped once in xylene, air-dried and mounted with DPX (a mixture of Distyrene, a plasticizer, and xylene).
Data analysis and statistical procedures
Statistical comparisons were made between groups using one-way ANOVA. Significant differences between groups were determined by the Tukey post hoc analysis.
Drugs, chemical reagents and materials
Recombinant mouse cytokines, TNFα, IL-1β and IFNγ, were purchased from BD Biosciences PharMingen (San Diego, CA, USA). Collagenase, curcumin, dichlorofluorescein diacetate and STZ were purchased from Sigma-Aldrich Reagents. RPMI 1640 with HGB and foetal calf serum were purchased from Invitrogen, and all plastic ware were from Nunc (Thermo Fischer Scientific, Rochester, NY, USA).
Results
Curcumin pretreatment prevents cytokine-induced islet cell death and maintains partial functionality
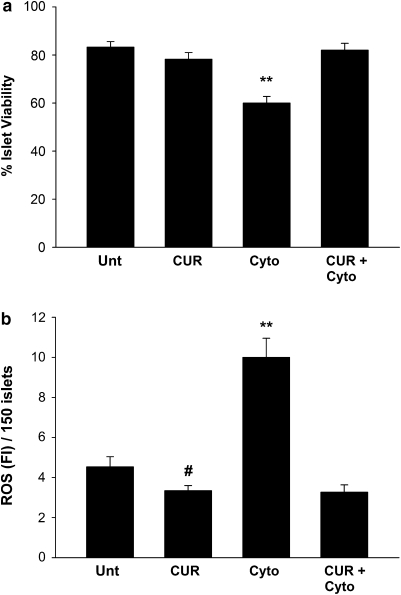
Our objective was to test the efficacy of curcumin as a protective agent against cytokine-induced islet cell death. Islet viability in untreated control islets and curcumin-treated control islets did not differ significantly (Figure 1a). Exposure for 48 h to a combination of pro-inflammatory cytokines decreased viability, whereas curcumin pretreatment rescued islets from this cytokine-induced increase in islet cell death, with viability back to control levels. Concentration–response and time course studies revealed that curcumin pretreatment at higher concentrations (20, 40 and 80 μM) and incubation periods (24, 48 and 72 h) also effectively rescued islets from cytokine-induced cell death (data not shown). However, as pretreatment with 10 μM curcumin for 24 h resulted in maximum protection against cytokine insult, all further studies were carried out under these conditions.
Figure 1.
Curcumin totally protects against cytokine-induced decrease in islet viability and scavenges concomitant increases of islet reactive oxygen species (ROS). (a) Percentage viability in curcumin-treated (CUR) and untreated (unt) islets. Islet viability was detected by Hoechst 33342 and ethidium bromide staining. Hoechst-stained nuclei were considered live, whereas nuclei showing dual (positive for Hoechst as well as ethidium bromide) were considered negative. At least 150 nuclei per preparation were screened and each experiment was repeated at least three times. (b) ROS scavenging in curcumin-treated and untreated islets. Live islets were loaded with dichlorofluorescein diacetate (DCFH-DA) for 10 min and values are reported as fluorescent intensity per 150 islets. At least 150 islets were screened per preparation and each experiment was repeated three times. Correlation coefficient between ROS and percentage viability=−0.936 when P=0.0060 (Pearson correlation coefficient.) **Values differ from all other groups; #value differs significantly from ROS in untreated islets (P<0.050).
As ROS has an important function in cytokine-induced islet cell death, we estimated ROS levels in islets. Live islet ROS in control untreated and control curcumin-treated islets did not differ significantly from one another (Figure 1b). However, exposure to pro-inflammatory cytokine combination caused 2.2-fold increase in ROS with a simultaneous decrease in viability. Curcumin pretreatment rescued islets from this cytokine-induced increase in ROS. Negative correlation coefficients between these groups indicated that cytokine-induced cell death may contribute to the increase in cellular ROS. Although ROS may not be the only causative agent for islet cell death, its contribution towards that effect cannot be ruled out.
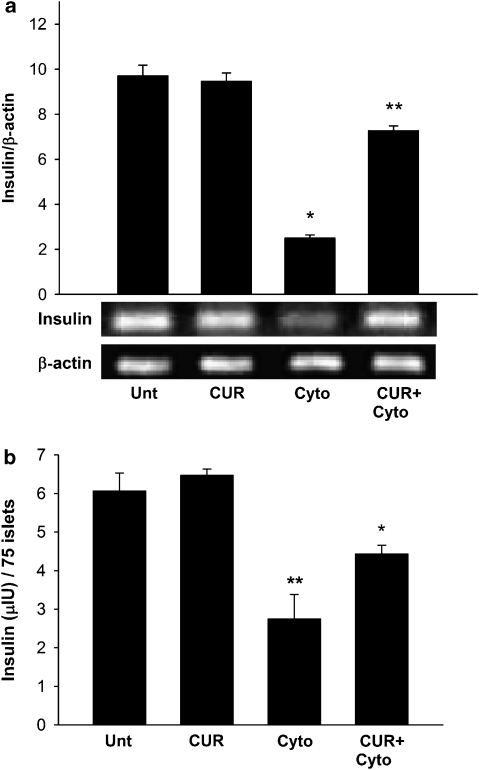
As exposure to cytokine combination is known to cause dysfunction in mouse islets, we next estimated the levels of insulin mRNA and secreted insulin to determine the functionality of control and experimental islets. As expected, cytokine exposure caused 4.28-fold depletion in the levels of insulin mRNA as compared with untreated islets (Figure 2a). Curcumin pretreatment partially rescued the islets from cytokine-induced dysfunction with a much reduced decrease (1.43-fold) in the insulin mRNA levels compared with control untreated islets. Insulin secretion data from the same experimental sets corroborated these findings (Figure 2b). Cytokine exposure caused a nearly 2.2-fold reduction in the concentration of secreted insulin (insulin secreted at 16.5 mM glucose concentration), whereas curcumin pretreatment partially rescued cytokine exposed islets from dysfunction. However, insulin concentrations in both these groups remained lower than those in untreated islets or curcumin controls, which did not vary significantly. The treatments did not affect the stimulation indices of these islets, which remained constant around two for all groups. Curcumin control islets showed some reduction in the levels of insulin mRNA; however, this was not statistically significant. These results indicated that curcumin rescued the islets completely from cytokine-induced death and partially from dysfunction.
Figure 2.
Insulin secretion and mRNA expression in curcumin- and cytokine-treated islets. (a) Secreted insulin content was determined from 75 hand-picked islets of roughly the same size. Stimulated insulin content was determined from at least three independent islet preparations, and values are shown as micro-international units per 75 islets. (b) Total mRNA was isolated from cultured islets and PCR was quantified using densitometry. Insulin expression was normalized against that of β-actin. Data are the means±s.d. for three independent islet preparations. *Values differ significantly from curcumin and untreated controls (P<0.001); **values differ from cytokine-treated islets (P<0.01).
Curcumin pretreatment prevents NO generation by inhibition of iNOS expression
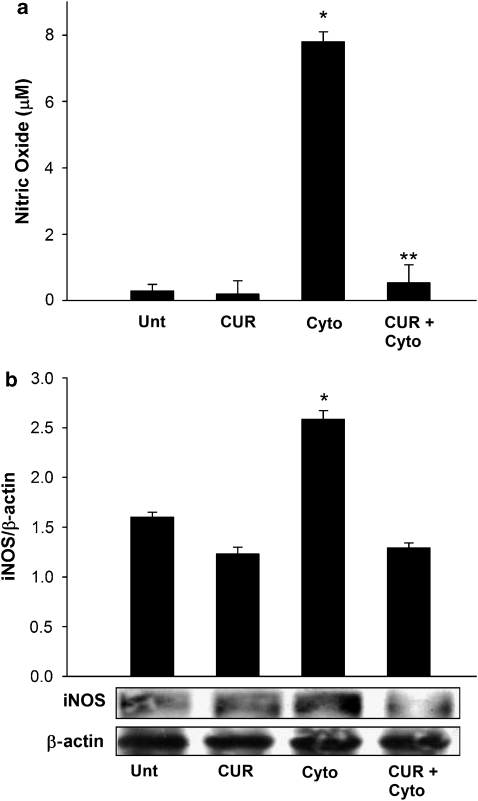
NO is a primary cell death effector molecule in cytokine-induced islet cell death, and the NO levels in control and experimental islets were estimated to assess the effects of curcumin pretreatment on these levels. Cytokine exposure caused a 27-fold increase in NO levels as compared with untreated islets, and pretreatment with curcumin almost abolished this cytokine-induced increase in NO formation (Figure 3a). Curcumin also caused marginal (0.6825-fold; P<0.001) reduction in the NO levels in islets without cytokine stimulation compared with untreated controls, indicating that curcumin treatment marginally inhibited the basal, unstimulated generation of NO in the preparation of islets.
Figure 3.
Curcumin prevents cytokine-induced increase in islet NO and inducible nitric oxide synthase (iNOS) expression. (a) NO in treated and untreated islets after exposure to cytokines. Levels of NO (micromolar) were estimated using the Greiss reagent. *Value differs significantly from all other values; **value differs significantly from the cytokine group. (b) Representative immunoblot of iNOS expression in control and experimental islets. The same islet samples were used for the determination of NO and iNOS. Levels were normalized against β-actin. Data are means±s.d. for three independent experiments. *iNOS values differ significantly from all other groups.
The cytokine-induced increased NO generation in islets, which was prevented by curcumin pretreatment, is attributed to an increase of iNOS expression. We therefore analysed the effect of curcumin pretreatment on cytokine-induced expression of iNOS in vitro. Cytokine exposure caused 1.3-fold increase in islets, whereas curcumin-treated islets, on exposure to cytokines, did not show increased levels of iNOS (Figure 3b). Control untreated islets and control curcumin-treated islets neither showed overexpression of iNOS and nor varied significantly from one another. Hence, it can be concluded that curcumin pretreatment of islets prevents cytokine-induced iNOS overexpression and NO generation, which may contribute to protection against cytokine-induced islet cell death and dysfunction.
Curcumin normalizes cytokine-induced activation of NF-κB
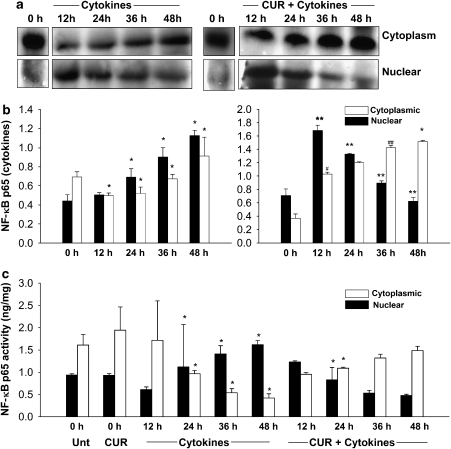
Exposure of islets to pro-inflammatory cytokines caused non-oscillatory, sustained NF-κB activation along with initiation of islet cell death (Figure 4a). Our observations also reveal an increase in the total amount of nuclear and cytoplasmic NF-κB p65 in islets treated with cytokines (Figure 4b). Islets pretreated with curcumin on exposure to cytokines showed an initial translocation of NF-κB into the nucleus till 12 h and then a gradual decrease in the levels of nuclear NF-κB, with negligible levels of NF-κB p65 sequestered in the nucleus by the end of the 48-h period. Curcumin also partially inhibited the concentrations of translocated NF-κB p65, and higher levels of the same remain sequestered in the cytoplasm. To further corroborate our immunoblotting data, we assayed the NF-κB p65 activity assay in the nuclear and cytosolic fractions (Figure 4c). Cytokine exposure caused, from 12 to 48 h, a progressive increase in the concentrations of nuclear NF-κB, with a concomitant decrease in its cytosolic concentrations. However, islets pretreated with curcumin did not show such a translocation pattern. Although a rise in translocated NF-κB was observed at 12 h, a progressive decrease in nuclear NF-κB was observed later, at the 24, 36 and 48 h time points. Concomitantly, a rise in the levels of cytosolic NF-κB was observed, which indicated that curcumin pretreatment normalized the translocation pattern, that is, curcumin did not completely prevent the translocation of NF-κB but reversed cytokine-induced sustained and non-oscillatory NF-κB translocation.
Figure 4.
Curcumin normalizes cytokine-induced, sustained, non-oscillatory nuclear factor kappa B (NF-κB) translocation into the nucleus. (a) Representative immunoblot of NF-κB p65 expression in the cytoplasmic and nuclear fractions of control and experimental islets. Islets were sampled at 0 h and then exposed to cytokine combination. Samples were collected at 12, 24, 36 and 48 h. (b) Cytoplasmic NF-κB p65 was normalized against β-actin, and nuclear NF-κB p65 was normalized against poly(ADP-ribose) polymerase (PARP). Data are means±s.d. for three independent experiments. *Value is significantly different from the value at an earlier time point (P<0.001); **values significantly differ from the value at an earlier time point (P<0.01); (#value differs significantly from 0 h time point (P<0.050); ##value differs significantly from 24 h time point (P<0.50). (c) NF-κB p65 activity assay in the nuclear and cytoplasmic samples of treated and untreated islets. Data are mean activity levels±s.d. for three independent experiments. *Values differ from all other groups.
Curcumin prevents cytokine-induced phosphorylation of IκBα
IκBα phosphorylation is directly correlated with NF-κB translocation. Compounds such as vitamin C are effective in inhibiting cytokine-induced NF-κB translocation by preventing the phosphorylation of IκBα (Carcamo et al., 2002). Estimation of phospho-IκBα in control and experimental islets revealed that levels in cytokine-treated islets gradually increase until 48 h (Table 1). Islets pretreated with curcumin and then exposed to cytokines showed a small increase in the levels of phospho-IκBα during the first 24 h followed by a decrease till they returned to basal levels by 48 h, indicating that curcumin may prevent phosphorylation of IκBα. This observation coincides with our NF-κB translocation data, and the reductions in the levels of phospho-IκBα after 24 h coincide with reversal of NF-κB translocation (Figures 4a–c). These observations, in conjunction with the NF-κB data, suggest that pretreatment with curcumin may retard the initiation of the death cascade in islet cells, by normalizing the translocation of NF-κB, presumably by preventing phosphorylation of IκBα.
Table 1.
Effects of 48 h cytokine exposure on the levels of phosphorylated IκBα in curcumin-pretreated and untreated islets
|
Cytokine treatment (h) |
|||||
|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 36 h | 48 h | |
| Untreated (pg per mg protein) | 61.33±16.0 | 145.66±4.3* | 245.83±25* | 393.0±174* | 1946.0±146* |
| Curcumin pretreated (pg per mg protein) | 56.8±16.7 | 84.33±7.2** | 110.83±52** | 76.83±25.44** | 73.25±17.98** |
Abbreviation: IκBα, inhibitor of kappa B alpha.
*Values differ significantly from that of the previous time point (P⩽0.050).
**Values differ significantly from corresponding time points of untreated islets exposed to cytokines (P⩽0.050).
Data are means±s.d. for three or four experiments and expressed as pg per mg protein. Curcumin-pretreated or untreated islets were sampled at 0 h and then exposed to cytokine combination for 48 h. Samples were taken at 12, 24, 36 and 48 h. The levels of phosphorylated IκBα were estimated, as given in the Materials and methods, for each time point and normalized against total IκBα levels in the same islet samples. Data were analysed by ANOVA (Tukey's test).
Curcumin pretreatment prevents necrosis and diabetogenesis in MLD-STZ-treated mice
To confirm the accuracy of our in vitro results, we tested our hypothesis in the MLD-STZ model of diabetogenesis. We monitored the incidence of MLD-STZ-induced diabetogenesis in control and experimental groups, and our results confirm that curcumin pretreatment prevents diabetogenesis and resultant hyperglycaemia in 62% of experimental mice. Animals exposed to MLD-STZ alone developed marked hyperglycaemia and diabetes. The intraperitoneal glucose tolerance test in control and experimental animals revealed that curcumin+MLD-STZ-exposed mice showed normal glucose clearance, whereas mice exposed to MLD-STZ alone had abnormal glucose clearance, along with consistent hyperglycaemia (Table 2). Untreated controls, curcumin-treated controls and curcumin+MLD-STZ-treated groups were not different from each other. We also tested the total insulin production capacity by estimating the serum and pancreatic insulin concentrations in treated and untreated animals (Table 3). Exposure to MLD-STZ caused development of diabetes and thereby decreased insulin production, that is, significantly reduced the serum and pancreatic insulin concentrations. On the other hand, mice pretreated with curcumin did not show a decrease in pancreatic and serum insulin concentrations induced by MLD-STZ. These mice were comparable to the untreated mice. It must also be noted that curcumin control mice did not show any change in insulin levels, relative to those in the untreated animals, indicating that curcumin did not affect the normal insulin secretion in vivo. This means that curcumin prevents islet death and hence prevents a reduction in the insulin production ability of the pancreas.
Table 2.
Intraperitoneal glucose tolerance test in animals pretreated with curcumin or left untreated on exposure to MLD-STZ
| 0 min | 30 min | 60 min | 90 min | |
|---|---|---|---|---|
| Untreated control | 6.05±2.02 | 11.92±2.03 | 5.71±1.25 | 6.16±1.2 |
| CUR control | 5.77±1.23 | 9.85±2.07 | 7.05±1.3 | 5.09±3.2 |
| MLD-STZ | 16.98±1.5* | 26.37±2.4* | 21.5±3.5* | 22.0±1.5* |
| CUR+MLD-STZ | 6.16±1.11 | 12.54±2.1 | 10.0±1.4 | 6.83±1.6 |
Abbreviations: CUR, curcumin; MLD-STZ, multiple low doses of streptozotocin.
*Values differ from all other groups at respective time points (P⩽0.001).
Data indicate the mean blood glucose levels (mM)±s.d. for three experiments. Each group consisted of 8–9 animals. Plasma glucose was estimated at 0 min and i.p. glucose load of 2 g kg−1 was administered intraperitoneally. Blood glucose was estimated at 30-min intervals thereafter.
Table 3.
Serum and pancreatic insulin concentrations in experimental and control groups
| Groups | Serum insulin (μIU mL−1) | Pancreatic insulin (μIU mL−1) |
|---|---|---|
| Untreated mice | 18.37±0.73 | 72.05±3.26 |
| Curcumin control | 18.25±0.64 | 63.48±2.62 |
| MLD-STZ | ND | 19.50±0.96* |
| CUR+MLD-STZ | 33.18±5.05 | 56.92±2.58 |
Abbreviations: CUR, curcumin; MLD-STZ, multiple low doses of streptozotocin; ND, not detectable.
*Values differ with respect to all other groups.
Data indicate mean insulin concentrations (μIU mL−1)±s.d. for three independent experiments Serum and pancreatic samples were collected after a 4-week follow-up period. The number of animals used was 8–10 per group.
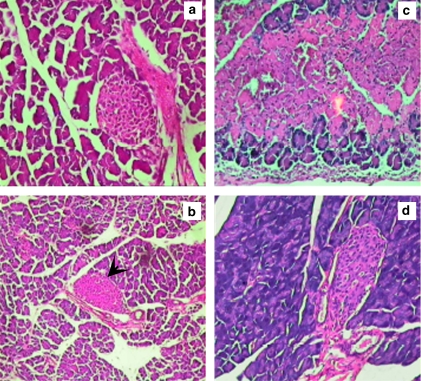
Inflammation and necrosis of the pancreas are indicators of early stages of diabetogenesis. We found inflammation and necrosis in the MLD-STZ-treated mice pancreas, whereas curcumin-treated animals did not show inflammation and necrosis of the pancreas, further confirming that curcumin pretreatment completely prevents MLD-STZ diabetogenesis (Figure 6).
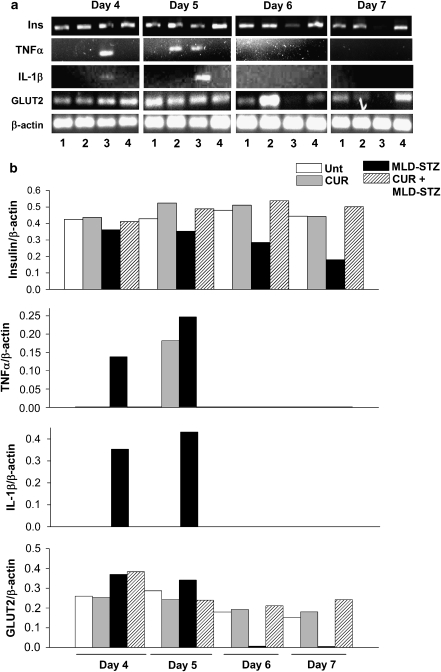
Curcumin pretreatment prevents a reduction in the pancreatic GLUT2 and insulin levels and an increase in the pancreatic and serum cytokine expression caused due to MLD-STZ
Levels of GLUT2 are clear indicators of the progression and development of diabetes in the MLD-STZ model, and a significant reduction in GLUT2 mRNA has been reported from day 4 onwards of MLD-STZ injection (Wang and Gleichmann, 1998). Hence, we have tested the extent of protection provided by curcumin in vivo by estimating the pancreatic GLUT2 mRNA levels on days 4, 5, 6 and 7. We observed that the GLUT2 levels in MLD-STZ decreased from day 6 onwards (Figures 5a and b). Curcumin-pretreated mice on exposure to MLD-STZ did not show such reduction in GLUT2 mRNA levels. MLD-STZ causes a rise in the pancreatic pro-inflammatory cytokine levels leading to diabetogenesis (Freisen et al., 2004). In our model, pancreatic mRNA levels of TNFα and IL-1β were increased on days 4 and 5, prime days of diabetes progression, whereas no expression was observed on days 6 and 7 (Figures 5a and b). Untreated, curcumin-treated control and curcumin-pretreated mice exposed to MLD-STZ did not show an increase in pro-inflammatory cytokine levels on days 4 or 5 as seen in mice treated with MLD-STZ alone.
Figure 5.
Curcumin prevents multiple low doses of streptozotocin (MLD-STZ)-induced depletion in pancreatic mRNA levels of GLUT2 and insulin along with increase in tumour necrosis factor-α (TNFα) and interleukin-1β (IL-1β). (a) Representative pancreatic GLUT2, insulin, TNFα and IL-1β mRNA expression in control and experimental animals. Total pancreatic RNA was isolated from one control and experimental animal per day (days 4, 5, 6 and 7) of MLD-STZ exposure. Each experiment was repeated at least three times. (1) Untreated animals; (2) curcumin control group; (3) MLD-STZ group and (4) curcumin+MLD-STZ. (b) The mRNA expression levels of GLUT2, insulin, TNFα and IL-1β was normalized against that of β-actin. Data are shown for untreated (unt), curcumin control (CUR), MLD-STZ-treated and curcumin+MLD-STZ-treated groups. The levels shown here represent densitometric profiles of depicted blots and hence are not shown with standard deviations. Each experiment was conducted at least three times and data from at least 3–5 mice from each group were used for the analysis.
A similar expression profile was observed for insulin on days 4, 5, 6 and 7. Along with a decrease in GLUT2 (which is an indicator of total β-cell mass), the total amount of insulin mRNA also progressively reduced from day 5 onwards to day 7—where limited expression of insulin mRNA is observed (Figures 5a and b). This indicated that after MLD-STZ injections on days 4 and 5, a substantial β-cell loss occurred in the pancreas as indicated by the reduction in GLUT2 expression. This reduction in β-cell mass caused a simultaneous decrease in insulin mRNA expression, that is, further decrease in secreted insulin—both of which are markers of progressing diabetogenesis. This phenomenon was completely prevented in the animals treated with curcumin prior to MLD-STZ exposure, indicating that curcumin prevented islet death and loss of pancreatic β-cell mass in vivo (Figure 6).
Figure 6.
Curcumin prevents necrosis and inflammation of pancreatic tissue on multiple low doses of streptozotocin (MLD-STZ) exposure. Data are representative of three independent experiments. At least 10 different mice were screened for each group. (a) Pancreatic sections from untreated animal. In (b), a necrosed pancreatic section from an MLD-STZ-treated mouse is shown; the arrowhead indicates a necrosed islet of Langerhans. (c) Pancreas of curcumin-treated control mice. In (d), a pancreatic section of mice treated with curcumin prior to MLD-STZ exposure (CUR+MLD-STZ), devoid of necrosis and inflammation, is shown.
Curcumin is a known anti-inflammatory compound, and this property may reflect inhibition of the expression of pro-inflammatory cytokines. We therefore also estimated the concentrations of these cytokines in the serum of our experimental animals (Table 4). Serum cytokine (IL-1β and TNFα) levels were markedly increased in mice treated with MLD-STZ, whereas control untreated, control curcumin-treated and curcumin+MLD-STZ-treated animals did not show any major change. This indicates that curcumin pretreatment inhibits the expression of these cytokines in the serum as well as the pancreas.
Table 4.
Serum IL-1β and TNFα concentrations (pg mL−1) in animals treated with curcumin or left untreated on exposure to MLD-STZ
| Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|
| IL-1β | ||||
| Untreated control | 23.0±8.46 | 34.8±17.0 | 37.79±10.6 | 44.91±18.15 |
| CUR control | 52.96±26.6 | 51.77±26.1 | 62.4±38.8 | 51.08±24.05 |
| MLD-STZ | 230.5±15* | 191.2±97.2* | 5321.5±285* | 738±61.4* |
| CUR+MLD-STZ | 76.46±12.6 | 71.62±12.8 | 63.61±29.3 | 76.6±55 |
| TNFα | ||||
| Untreated | 25.8±17.3 | 82.24±26.6 | 59.32±24.7 | 35.23±7.6 |
| CUR | 76.0±17.7 | 86.6±34.0 | 34.1±9.7 | 52.1±16.6 |
| MLD-STZ | 2215±232** | 5235±382.2* | 4843±490.4* | 1864.1±171* |
| CUR+MLD-STZ | 39.5±13.2 | 116.0±32.0 | 162.8±94.8 | 40±14.9 |
Abbreviations: CUR, curcumin; MLD-STZ, multiple low doses of streptozotocin; IL-1β, interleukin-1β; TNFα, tumour necrosis factor-α.
*Values differ from all other groups at the respective time points (P⩽0.050).
**Values differ from all other groups at the respective time points (P⩽0.01).
Mean serum cytokine, IL-1β and TNFα concentrations (pg mL−1)±s.d., respectively, for three independent experiments are given. Animals were sampled on days 4, 5, 6 and 7 and the same samples were used for the estimation of both cytokines, which were estimated using the highly sensitive multiplex assay system. Each group consisted of 8–9 animals.
Discussion
Curcumin is an antioxidant, free radical scavenging, anti-inflammatory and anticancer compound (Grandjean-Laquerriere et al., 2002; Aggarwal et al., 2003; Fujisawa et al., 2004). Its cytoprotective abilities against oxidative stress have also been demonstrated in various in vitro systems (Phan et al., 2001). However, its efficacy as an antidiabetic agent has been researched with limited success (Babu and Srinivasan, 1995; Srinivasan et al., 2003). Although these studies highlighted the effectiveness of curcumin in reducing secondary diabetic complications in vivo, they did not show the efficacy of curcumin used prophylactically for the prevention of β-cell death and diabetes progression. In our analyses, we have demonstrated the efficacy of curcumin in protecting islets against STZ-induced death and dysfunction (Kanitkar et al., 2007) by retarding the generation of islet ROS along with inhibition of poly(ADP-ribose) polymerase-1 activation (thereby preventing DNA damage and necrosis) and preventing the decrease in the cellular levels of free radical scavenging enzymes such as Cu/Zn superoxide dismutase. We have also shown that inclusion of curcumin in islet cryopreservation medium enhances islet viability after thawing and maintains islet functionality in culture (Kanitkar and Bhonde, 2008). Very recently, another study also reports that dietary curcumin ameliorated diabetes in high-fat diet induced obese and leptin-deficient ob/ob male C57BL/6J mice. Curcumin treatment also significantly reduced macrophage infiltration of white adipose tissue, increased adipose tissue adiponectin production and decreased hepatic nuclear NF-κB activity, hepatomegaly and markers of hepatic inflammation (Weisberg et al., 2008).
In this study, we demonstrate for the first time that pretreatment with curcumin totally protects isolated mouse islets against cytotoxicity induced by pro-inflammatory cytokines and partially prevents dysfunction in vitro. We also demonstrate that intraperitoneal administration of curcumin protects islets from MLD-STZ-induced death in vivo and prevents MLD-STZ-induced type I diabetogenesis. This is the first report indicating the benefit of the prophylactic use of curcumin and its efficacy in preventing islet cell death in vivo.
Cytokine-induced ROS has an important function in islet death, and curcumin scavenges the cellular ROS and contributes to islet cell rescue. Similarly, NO is a primary effector molecule that is augmented by an increase in iNOS expression and causes islet cell death. Curcumin prevents increase in NO concentrations by the inhibition of iNOS expression in islets exposed to cytokines, but not in unexposed islets, indicating that curcumin prevents excessive increase in iNOS levels but does not affect the basal levels of iNOS expression. Such an effect has also been shown with other flavonoids, such as epicathechin, which prevents IL-1β-induced islet cell death (Kwon et al., 1995).
Inhibition of NF-κB translocation is a contributory factor in curcumin-induced islet rescue (Masamune et al., 2006; Weber et al., 2006). In contrast, reports also reveal that curcumin rescues pancreatic stellate cells from cytokine-induced damage without inhibition of NF-κB (Babu and Srinivasan, 1995). It was therefore necessary to assess whether curcumin-induced cytoprotection involved inhibition of NF-κB translocation. Exposure to cytokines causes sustained, non-oscillatory translocation of NF-κB into the nucleus (Ortis et al., 2006). We have shown here that this non-oscillatory translocation of NF-κB was prevented by pretreatment with curcumin, which normalized the translocation pattern and reversed the translocation after 12 h. The levels of phosphorylated IκBα corroborated these findings. Inhibition of NF-κB by the prevention of IκBα phosphorylation has been demonstrated earlier by Deeb et al. (2004). However, the involvement of curcumin-induced prevention of IκBα phosphorylation leading to prevention of islet cell death had not been reported earlier. Our immunoblotting data suggest that cytokine insult also leads to an overall increase in the levels of cellular NF-κB. To confirm these observations, we assayed the nuclear and cytosolic NF-κB activity in control and experimental islets. Pieper et al. (2004) have previously shown increases in the total levels of NF-κB in the nuclear as well as cytoplasmic fractions in the process of diabetogenesis in vivo, whereas there are almost no reports of such changes in vitro. In this context, we did not observe any increase in the overall cellular levels of NF-κB, but there was some decrease in the levels of active NF-κB in the curcumin+cytokine-treated groups. Although all of these subsidiary observations are of importance in their own right, they do not affect the conclusion that curcumin normalized the NF-κB translocation pattern and thereby contributed to islet cell rescue in vitro. We report here a unique activity of curcumin in this respect. However, further studies will have to be conducted before conclusive statements can be made.
The majority of the studies conducted so far have demonstrated the benefits of oral administration of curcumin and its analogues in reducing secondary complications in diabetes (Babu and Srinivasan, 1995; Srinivasan et al., 2003; Mabely et al., 2004). Some studies also highlight its benefit in the reduction of hyperglycaemia (Nishizono et al., 2000). Oral administration of many other antioxidants have also protected against MLD-STZ treatment (Ohly et al., 2000; Chen et al., 2005). However, the primary limitation of oral or i.v administration is that the bioavailability of curcumin in vivo is extremely low (Sharma et al., 2005). Our experiments in which curcumin was orally administered were inconclusive (data not shown). Also, curcumin and similar dietary substances are of prophylactic significance and are preferentially used prior to insult rather than after it (Sharma et al., 2005). Hence, we administered curcumin through i.p. injections prior to the MLD-STZ insult, which resulted in optimal protection against diabetogenesis.
Many compounds with higher potential for ROS scavenging or capable of inducing cell defence responses or inhibiting cell death cascade at different checkpoints have been tested for their efficacy against diabetes. However, diabetes progression includes a variety of cellular responses and, using a compound capable of inhibiting any one particular checkpoint may not be sufficient to completely retard or prevent the activation of all the other inter-related cascades. Hence curcumin, which by itself manifests a wide range of effects, may be effective where other compounds have yielded limited success. Our findings demonstrate, for the first time, the efficacy of curcumin in preventing cytokine-induced islet cell damage in vitro and MLD-STZ diabetes in vivo. Our findings also demonstrate that curcumin prevented the loss of β-cell mass and islet cell death on MLD-STZ exposure. Curcumin was also effective in maintaining the insulin production capacity of these β cells and thus protected them completely from the deleterious effects of MLD-STZ administration. This increases its desirability as a prophylactic antidiabetic compound.
Although the efficacy of curcumin in diabetes and other systems is now well established, there are many hurdles to overcome before the complete potential of this molecule may be harnessed for human use against diabetes. The first hurdle is the low bioavailability, which has to be substantially increased. This hurdle may be reduced by ingesting large amounts of curcumin daily. Also, the mode of delivery used here––i.p.––may be efficacious in an experimental system, it would be difficult on a daily basis in humans. Non-invasive routes of administration and increase in system bioavailability will have to be extensively researched before its full potential as a putative agent for preventing diabetes may be harnessed. However, its efficacy in islet transplantation systems may be exploited sooner, as its role in inhibiting pro-inflammatory cytokine-induced islet cell damage has been demonstrated clearly in this study.
Acknowledgments
We acknowledge Amita Limaye for help with Bioplex analysis, Dr GC Mishra for institutional facilities, Dr YS Shouche for laboratory facilities and Dr Anandwardhan Hardikar for critical review of the paper.
Abbreviations
- iNOS
inducible nitric oxide synthase
- IκB
inhibitor of kappa B
- NF-κB
nuclear factor kappa B
- ROS
reactive oxygen species
- STZ
streptozotocin
Conflict of interest
The authors state no conflict of interest.
References
- Aggarwal BB, Kumar A, Bharati AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- Babu PS, Srinivasan K. Influence of dietary curcumin and cholesterol on the progression of experimentally induced diabetes in albino rat. Mol Cell Biochem. 1995;152:13–21. doi: 10.1007/BF01076459. [DOI] [PubMed] [Google Scholar]
- Bolaffi JL, Nowlain RE, Cruz L, Grodsky GM. Progressive damage of cultured pancreatic islets after single early exposure to streptozotocin. Diabetes. 1986;35:1027–1033. doi: 10.2337/diab.35.9.1027. [DOI] [PubMed] [Google Scholar]
- Carcamo JM, Pedraza A, Borquez-Ojeda O, Golde DW. Vitamin C suppresses TNF alpha-induced NF kappa B activation by inhibiting I kappa B alpha phosphorylation. Biochemistry. 2002;41:12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- Chen H, Li X, Epstein PN. MnSOD and catalase transgenes demonstrate that protection of islets from oxidative stress does not alter cytokine toxicity. Diabetes. 2005;54:1437–1446. doi: 10.2337/diabetes.54.5.1437. [DOI] [PubMed] [Google Scholar]
- Darville MI, Terryn S, Eizirik DL. An octamer motif is required for activation of the inducible nitric oxide synthase promoter in pancreatic beta cells. Endocrinology. 2004;145:1130–1136. doi: 10.1210/en.2003-1200. [DOI] [PubMed] [Google Scholar]
- Deeb D, Jiang H, Gao XL, Hafner MS, Wong H, Divine G, et al. Curcumin sensitizes prostrate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. 2004;3:803–812. [PubMed] [Google Scholar]
- Freisen NT, Buchau AS, Schott-Ohly P, Lgssiar A, Gleichmann H. Generation of hydrogen peroxide and failure of anti-oxidative responses in pancreatic islets of male C57/BL6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2004;47:676–685. doi: 10.1007/s00125-004-1367-x. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Atsumi T, Ishihara M, Kadoma Y. Cytotoxicity, ROS-generation activity and radical scavenging activity of curcumin and related compounds. Anticancer Res. 2004;24:562–570. [PubMed] [Google Scholar]
- Grandjean-Laquerriere A, Gangloff SC, Le Naour R, Trentesaux C, Hornebeck W, Guenounou M. Relative contribution of NF-κB and AP-1 in the modulation by curcumin and pyrrolidine dithiocarbamate of the UVB-induced cytokine expression by kertinocytes. Cytokine. 2002;18:168–177. doi: 10.1006/cyto.2002.0888. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Kanitkar M, Bhonde R. Curcumin treatment enhances islet recovery by induction of heat shock response proteins, Hsp70 and heme-oxygenase-1, during cryopreservation. Life Sci. 2008;82:182–189. doi: 10.1016/j.lfs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Kanitkar M, Galande S, Bhonde R. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007;577:183–191. doi: 10.1016/j.ejphar.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Ryu GR, Kang JH, Sim SS, Min DS, Rhie DJ, et al. Inhibitory effects of epicatechin on interlukin-1beta-induced inducible nitric oxide synthase expression in RINm5F cells and rat pancreatic islets by down regulation of NF-kappaB activation. Biochem Pharmacol. 2004;68:1775–1785. doi: 10.1016/j.bcp.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Kwon G, Corbett JA, Rodi CP, Sullivan P, McDaniel ML. Interleukin-1β-induced nitric oxide synthase expression by rat pancreatic β-cells: evidence for the involvement of nuclear factor κB in the signaling mechanism. Endocrinology. 1995;136:4790–4795. doi: 10.1210/endo.136.11.7588208. [DOI] [PubMed] [Google Scholar]
- Kwon KB, Kim EK, Jeong ES, Lee YH, Lee YR, Park JW, et al. Cortex cinnamomi extract prevents streptozotocin-and cytokine-induced beta-cell damage by inhibiting NF kappaB. World J Gastroenterol. 2006;12:4331–4337. doi: 10.3748/wjg.v12.i27.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lgssiar A, Hassan M, Schott-Ohly P, Freisen N, Nicoletti F, Trepicchio WL, et al. Interleukin-11 inhibits NF-κB and AP-1 activation in islets and prevents diabetes induced with streptozotocin in mice. Exp Biol Med. 2004;229:425–436. doi: 10.1177/153537020422900511. [DOI] [PubMed] [Google Scholar]
- Mabely JG, Rabonovitch A, Suarez-Pinzon W, Hasko G, Pacher P, Power R, et al. Inosine protects against the development of diabetes in multiple-low-dose-streptozotocin and nonobese diabetic mouse models of type 1 diabetes. Mol Med. 2003;9:96–104. doi: 10.2119/2003-00016.mabley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabely JG, Southan GJ, Salzman AL, Szabo C. The combined inducible nitric oxide synthase inhibitor and free radical scavenger guanidinoethyldisulfide prevents multiple low-dose streptozotocin-induced diabetes in vivo and interleukin-1beta-induced suppression of islet insulin secretion in vitro. Pancreas. 2004;28:E39–E44. doi: 10.1097/00006676-200403000-00018. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T, Bendtzen K, Neilsen JH, Bendixen G, Nerup J. Cytokines causes functional and structural damage to isolated islets of Langerhans. Allergy. 1985;40:424–429. doi: 10.1111/j.1398-9995.1985.tb02681.x. [DOI] [PubMed] [Google Scholar]
- Masamune A, Suzuki N, Kikuta K, Satoh M, Satoh K, Shimoseqawa T. Curcumin blocks activation of pancreatic stellate cells. J Cell Biochem. 2006;97:1080–1093. doi: 10.1002/jcb.20698. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, et al. Silymarin protects pancreatic beta cells against cytokine mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology. 2005;146:175–185. doi: 10.1210/en.2004-0850. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Suarez–Pinzon WL, Baust JJ, Strynadka K, Leiter EH, Rabinovitch A. Mechanisms underlying resistance of pancreatic islets from ALR/Lt mice to cytokine-induced destruction. J Immunol. 2005;175:1248–1256. doi: 10.4049/jimmunol.175.2.1248. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an anti-oxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Nishizono S, Hayami T, Ikeda I, Imaizumi K. Protection against the diabetogenic effect of feeding tert-butylhydroquinone to rats prior to the administration of streptozotocin. Biosci Biotechnol Biochem. 2000;64:1153–1158. doi: 10.1271/bbb.64.1153. [DOI] [PubMed] [Google Scholar]
- Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2000;43:1020–1030. doi: 10.1007/s001250050009. [DOI] [PubMed] [Google Scholar]
- Ortis F, Cardozo AK, Crispim D, Storling J, Mandrup-Poulsen T, Eizirik DL. Cytokine-induced pro-apoptotic gene expression in insulin-producing cells is related to rapid, sustained, and non-oscillatory nuclear factor κB activation. Mol Endocrinol. 2006;20:1867–1879. doi: 10.1210/me.2005-0268. [DOI] [PubMed] [Google Scholar]
- Pappacio G, Nicoletti F, Pisanti FA, Galderi M, Bendtzen K. An imidazoline compound completely counteracts interleukin-1 [beta] toxic effects to rat pancreatic islets [beta] cells. Mol Med. 2002;8:536–545. [PMC free article] [PubMed] [Google Scholar]
- Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implications for wound healing. J Trauma. 2001;51:927–931. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Roza AM, Henderson JD, Jr, Zhu YR, Lai CS. Spatial distribution and temporal onset of NF-κB activation and inducible nitric oxide synthase within the pancreatic islets in the pre-diabetic stage of genetic, diabetic prone BB rats: attenuation by drug intervention decreases inflammatory cell infiltration and incidence of diabetes. Inflamm Res. 2004;53:22–30. doi: 10.1007/s00011-003-1223-3. [DOI] [PubMed] [Google Scholar]
- Riachy R, Vandewalle B, Kerr Conte J, Moerman E, Sacchetti P, Lukowiak B, et al. 1, 25-Dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the anti-apoptotic protein A20. Endocrinology. 2002;143:4809–4819. doi: 10.1210/en.2002-220449. [DOI] [PubMed] [Google Scholar]
- Rydgren T, Sandler S. Efficacy of 1400 W, a novel inhibitor of inducible nitric oxide synthase, in preventing interleukin-1β-induced suppression of pancreatic islet function in vitro and multiple low-dose streptozotocin-induced diabetes in vivo. Eur J Endocrinol. 2002;147:543–551. doi: 10.1530/eje.0.1470543. [DOI] [PubMed] [Google Scholar]
- Scarim AL, Heitmeir MR, Corbett JA. Heat shock inhibits cytokine-induced nitric oxide synthase expression by rat and human islets. Endocrinology. 1998;139:5050–5057. doi: 10.1210/endo.139.12.6366. [DOI] [PubMed] [Google Scholar]
- Sharma R, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Shewade YM, Umrani M, Bhonde R. Large-scale isolation of islets by tissue culture of adult mouse pancreas. Transplant Proc. 1999;31:1721–1723. doi: 10.1016/s0041-1345(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Menon VP, Periaswamy V, Rajasekaran KN. Protection of pancreatic beta cell by the potential anti-oxidant bis-o-hydroxycinnmoyl methane, analogue of natural curcuminoid in experimental diabetes. J Pharm Pharm Sci. 2003;6:327–333. [PubMed] [Google Scholar]
- Sternesjo J, Welsh N, Sandler S. S-Methyl-L-thiocitrulline counteracts interleukin1beta induced suppression of islet function in vitro, but does not protect against multiple low-dose streptozotocin-induced diabetes in vivo. Cytokine. 1997;9:352–359. doi: 10.1006/cyto.1996.0176. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gleichmann H. GLUT2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47:50–56. doi: 10.2337/diab.47.1.50. [DOI] [PubMed] [Google Scholar]
- Weber WM, Hunsaker LA, Roybal CN, Borovnikova-Marjon EV, Abcouwer SF, Royer RE, et al. Activation of NF kappaB is inhibited by curcumin and related enones. Bioorg Med Chem. 2006;14:2450–2461. doi: 10.1016/j.bmc.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]