Abstract
Despite its long-standing reputation as a foul smelling and toxic gas that is associated with the decay of biological matter, hydrogen sulphide (H2S) has emerged as an important regulator of cardiovascular homoeostasis. H2S promotes a number of cellular signals that regulate metabolism, cardiac function and cell survival. Endogenous H2S bioavailability is regulated by several enzymes involved in the biosynthesis of cysteine. This study by Brancaleone et al. in the current issue of the British Journal of Pharmacology provides novel insights into the impairment of H2S biosynthesis in the setting of diabetes mellitus. The authors report that enzymic H2S biosynthesis is impaired in a murine model of type 1 diabetes and the attenuation in H2S bioavailability is associated with impaired vascular reactivity. This study has profound implications for the use of pharmacological agents to augment endogenous H2S synthesis or agents that release H2S to augment the levels of this gaseous signalling molecule in cardiovascular disease.
Keywords: diabetes mellitus, cardiovascular disease, risk factors, hydrogen sulphide, mouse models, metabolic syndrome, cardioprotection, hypertension, vascular pathology, vasodilation
Recent basic science studies have highlighted the importance of hydrogen sulphide (H2S) as a critical regulator of cardiovascular homoeostasis in the normal physiological state (Szabo, 2007). On account of its chemical nature and highly diverse signalling profile, H2S exerts a number of powerful effects on the heart, blood vessels and circulating blood elements. In this regard, H2S has been shown to modulate metabolic state (Blackstone et al., 2005), vascular reactivity and systemic blood pressure (Szabo, 2007), leukocyte–endothelial cell interactions (Zanardo, 2006), mitochondrial function, cellular redox status and apoptosis (Szabo, 2007). On account of the profound effects of H2S on cellular metabolism, early studies were focused on the ability of H2S to induce a state of suspended animation to promote survival (Blackstone et al., 2005; Szabo, 2007). More recently, investigators have begun to investigate the numerous effects of relatively low levels of H2S therapy on cardiovascular physiology and pharmacology in a number of in vitro and in vivo model systems. New studies are emerging at a rapid rate that underscore the importance of this novel, endogenous, gaseous signalling molecule synthesized by the actions of the two endogenous enzymes, cystathionine γ-lyase (CGL or CSE) and cystathionine β-synthase (CBS) (Szabo, 2007). These enzymes are two pyridoxal-5′-phosphate-dependent enzymes that are responsible for metabolizing homocysteine to cysteine in mammals (Szabo, 2007) (Figure 1).
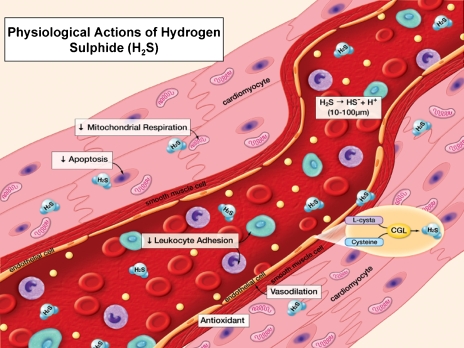
Figure 1.
Physiological actions of hydrogen sulphide (H2S). H2S is synthesized in the vasculature through the action of cystathionine γ-lyase (CGL), converting cysteine to H2S under normal physiological conditions. Normal circulating blood concentrations of H2S are believed to be in a range between 10–100 μM. H2S exerts a number of actions on the heart and circulation. (Modified from Lefer, 2007, with permission).
Previous studies have demonstrated that both CBS and CGL are critical for the maintenance of cardiovascular function and that treatment of animals with exogenous forms of hydrogen sulphide have demonstrated very robust protection of various organs during a number of cardiovascular diseases including: ischaemia-reperfusion injury, various forms of shock, stroke, inflammatory disorders and models of ischaemia-induced angiogenesis (Mok, 2004; Kimura et al., 2006; Elrod et al., 2007; Szabo, 2007, Jha et al., 2008). It is important to note that the protective actions of H2S therapy have been observed primarily at lower dosages or concentrations of H2S and that higher levels of H2S are clearly associated with significant toxicity that has been well characterized. Furthermore, the majority of studies evaluating the effects of pharmacological inhibition of CBS or CGL have demonstrated enhanced pathology of various diseases. Studies of gene-targeted mice support the concept that inhibition of H2S biosynthetic enzymes exacerbates cardiovascular disease whereas genetic overexpression attenuates disease severity (Elrod et al., 2007; Szabo, 2007).
The relatively large body of recent publications demonstrating cytoprotective actions of H2S therapy in a number of disease conditions provide support for the development of novel H2S-based therapeutic agents. There appear to be several options for H2S therapy in cardiovascular disease including H2S gas, H2S donors or releasing compounds, and H2S pro-drugs that activate H2S-generating enzymes to increase circulating and tissue levels of H2S. During acute cardiovascular diseases (that is, shock states or acute myocardial infarction) rapidly acting compounds with shorter half-lives may be more appropriate. Under these conditions, H2S gas or H2S donors would be ideal candidates to evaluate. For more chronic cardiovascular disease states and chronic inflammatory states, the H2S pro-drugs such as S-allylcysteine, a compound derived from garlic, would be more suitable. It will also be important to design H2S therapeutics that can be administered by a number of routes including oral, intravenous and inhalation routes. One of the major challenges in the development of H2S therapy is the extremely short half-life of H2S in the circulation as it is very rapidly metabolized to inactive metabolites. Furthermore, there are also a number of challenges related to the accurate measurement of both circulating and tissue H2S levels. Finally, because of the extensive history of H2S as a highly toxic environmental hazard, extensive investigations into the potential toxicity of H2S are required (Szabo, 2007). The majority of information regarding H2S toxicity is derived from studies of sulphide gas inhalation. Future studies will be aimed at the investigation of H2S therapy administered through the oral route or following injection of H2S donors or pro-drugs.
To date, the vast majority of studies related to the molecular, biochemical or biological actions of H2S have been performed in healthy animals or tissues derived from normal animals. This is somewhat problematic in that persons suffering from cardiovascular disease typically present with a number of pre-existing risk factors, including hypertension, dyslipidaemia, and diabetes mellitus. It is well appreciated that these prevalent cardiovascular risk factors attenuate endothelial cell generation of another gaseous signalling molecule, nitric oxide, and result in a state referred to as ‘endothelial dysfunction' (Cai and Harrison, 2000). At present, very little is known regarding H2S bioavailability and vascular reactivity to H2S in the setting of established cardiovascular risk factors. The report by Brancaleone et al. (2008) in this issue of the British Journal of Pharmacology investigates the state of H2S biosynthesis in non-obese diabetic mice and takes an important first step towards defining the effects of diabetes on H2S synthesis. This elegant study by Brancaleone et al. clearly demonstrates that vascular reactivity, plasma H2S levels, and vascular H2S production all decline progressively as the severity of diabetes increases over time in the non-obese diabetic mouse model. These data support earlier observations that plasma levels are significantly reduced in patients with coronary heart disease and in hypertensive rats. The authors investigated three distinct stages of diabetes mellitus (mild to severe), as determined by both blood glucose and urine glucose levels, and found that vasorelaxation of isolated aortic segments was attenuated by up to 75–80% in the most severe diabetic state. Interestingly, the authors found that the diabetic vessels displayed normal or enhanced reactivity to exogenous H2S donors suggesting that H2S donor therapy may be efficacious in diabetic animals and patients. Clearly, additional studies investigating endogenous H2S biosynthesis and bioavailability in a number of cardiovascular disease states including hypertension, obesity and metabolic syndrome, diabetes and hyperlipidaemia are warranted. Furthermore, it is also important to examine the vascular reactivity of various vascular beds both in vitro and in vivo in the setting of cardiovascular disease in the future. The study by Brancaleone et al. (2008) provides an excellent starting point for future investigations into H2S biochemistry and physiology in the diabetic state as well as in other important diseases. It appears that investigation of hydrogen sulphide will provide exciting research opportunities for many years to come.
Abbreviations
- CBS
cystathionine β-synthase
- CGL
cystathionine γ-lyase
References
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- Brancaleone V, Roviezzo F, Vellecco V, De Gruttola L, Bucci M, Cirino G.Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice Br J Pharmacol 2008155673–680.(this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury via preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and anti-apoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci USA. 2007;104:17907–17908. doi: 10.1073/pnas.0709010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok YY. Role of hydrogen sulfide in haemorrhagic shock in the rat: Protective effect of inhibitors of hydrogen sulfide biosynthesis. Br J Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C. Hydrogen sulfide and its therapeutic potential. Nat Reviews. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Zanardo RC. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]