Abstract
Background and purpose:
Proteasome inhibitors exhibit cytotoxic against tumours of different histology. However, the mechanism of apoptosis induction by these compounds remains unclear and is likely to be a complex cascade of events. Bcl-2-associated athanogene (BAG) family proteins are characterized by their property of interaction with a variety of partners involved in modulating the proliferation/death balance, including heat shock proteins (HSP), Bcl-2, Raf-1. The role of BAG family proteins in proteasome inhibition has not been elucidated.
Experimental approach:
Effects of proteasome inhibitors on BAG2 expression were evaluated using real-time reverse transcription-polymerase chain reaction (RT-PCR). BAG2 expression was knocked down by small interfering RNAs (siRNA). Cell death was evaluated using Annexin V/propidium iodide staining and subsequent FACS.
Key results:
The proteasome inhibitors, MG132, PSI, lactacystin and epoxomicin, induced BAG2 at the transcriptional level. MG132-induced apoptosis was significantly suppressed by BAG2 knockdown using RNA interference.
Conclusions and implications:
Our results suggest that BAG2 is a novel molecule induced by proteasome inhibition, which exhibits a pro-apoptotic property in death of thyroid cancer cells induced by proteasome inhibition.
Keywords: BAG2, proteasome inhibitor, apoptosis, transcription, carcinoma
Introduction
Proteasome inhibitors constitute a novel class of compounds with promising antitumour activity. Exposure to proteasome inhibitors has been shown to result in growth arrest and apoptosis in tumours of different histologies, including solid and haematological malignancies (Adams and Cooper, 2007). Inspite of the exciting preclinical and clinical results obtained with proteasome inhibitors in cancer treatment, the mechanism of apoptosis induction by these compounds is still not fully understood and is likely to be the product of a complex cascade of events. Thus, a better understanding of the molecular mechanisms of action of these agents will aid in their optimal clinical application.
Co-chaperone proteins that share the Bcl-2-associated athanogene (BAG) domain possess general nucleotide exchange activities towards the heat shock protein, Hsc/Hsp70. Aside from the formation of the BAG–Hsp70 complex, BAG proteins functionally interact with a variety of binding partners and coordinate diverse cellular processes such as stress signalling, cell division, cell death and cell differentiation (Takayama et al., 1997, 1999; Takayama and Reed, 2001). There are currently six known human BAG family members (BAG1–6), and homologues have been identified in yeast, invertebrates, mammals and plants (Takayama and Reed, 2001). All BAG proteins contain a homologous BAG domain near their carboxy terminal, whereas their amino terminal regions are quite different (Takayama et al., 1997, 1999; Takayama and Reed, 2001). Recently, BAG2 has been shown to interact with the carboxyl terminus of HSP70-interacting protein (CHIP) (Arndt et al., 2005; Dai et al., 2005), an HSP70-associated ubiquitin ligase that participates in the ubiquitin–proteasome system by ubiquitylating misfolded proteins associated with cytoplasmic chaperones (Connell et al., 2001). BAG2 inhibits ubiquitylation of misfolded proteins by CHIP by a co-chaperone-dependent regulatory mechanism (Arndt et al., 2005; Dai et al., 2005), suggesting that BAG2 plays an endogenous role in regulating proteasome-mediated degradation of chaperone substrates.
In the present study, for the first time, we found that BAG2 was induced by proteasome inhibitors in cancer cell lines derived from different histology. Upregulation of BAG2 was more obvious in cells sensitive to proteasome inhibitors. We also found that prevention of BAG2 upregulation, using small interfering RNAs (siRNAs), inhibited cytotoxicity induced by MG132, suggesting that BAG2 might exhibit a pro-apoptotic action in response to proteasome inhibitors.
Materials and methods
Culture of multiple cancer cell lines
The undifferentiated thyroid cancer (ARO, FRO, KTC1, KTC2, KTC3, 8305C and 8505C), HCT116 colon cancer, HT1080 human fibrosarcoma, HeLa cervical cancer, MCF7 breast cancer, OVCAR8 ovarian cancer, SW1990 pancreatic cancer, Hep2 hepatic cancer, LK87 lung cancer, CaKi2 kidney cancer and SH-SY5Y neuroblastoma cell lines were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich, Saint Louis, MO, USA) supplemented with 10% foetal bovine serum (Sigma-Aldrich).
RNA isolation and real-time reverse transcription-PCR
RNA isolation and real-time reverse transcription-PCR were performed as previously reported (Wang et al., 2007). For BAG1, the forward primer was 5′-AGAAGATAGCTGACCAGCTG-3′ and the reverse was 5′-CAGGATCAGTGTGTCAATCTC-3′. For BAG2, the forward primer was 5′-CTTTGAGAGAAGCAGCAACTG-3′ and the reverse was 5′-TGACACTTCAACGGTGAGAG-3′. For BAG3, the forward primer was 5′-CATCCAGGAGTGCTGAAAGTG-3′ and the reverse was 5′-TCTGAACCTTCCTGACACCG-3′. For BAG4, the forward primer was 5′-AATACTGCCTCATACTCAGG-3′ and the reverse was 5′-TTCAGTCTGACAGTCCTGCTG-3′. For BAG5, the forward primer was 5′-ACGTCTTCTCAAAGAGTTGG-3′ and the reverse was 5′-CATGTGTCAGCCTCAGAATG-3′. For BAG6, the forward primer was 5′-ACCTTGGACTCTCAAACTCG-3′ and the reverse was 5′-TCATCTTGCAGAACTCGTCC-3′. For β-actin, the forward primer was 5′-GAGACCTTCAACACCCCAGCC-3′ and the reverse was 5′-GGATCTTCATGAGGTAGTCAG-3′. Results were normalized against those of β-actin.
Detection of cell death
For cell death assays, cells were analysed by fluorescence-activated cell scanner flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) after being labelled with Annexin V-FITC (Biovision, Mountainview, CA, USA) and propidium iodide (PI; Sigma-Aldrich) according to the manufacturer's instructions.
Caspase-3 activity assay
For caspase-3 enzymatic assays, 50 μg of whole cell extract was added to reaction buffer containing 25 mM HEPES (pH 7.5), 4 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 1 mM dithiothreitol, 1 mM phenylmethyl sulphonyl fluoride, 2 μg mL−1 aprotinin, 1 μg mL−1 leupeptin and 2 μg mL−1 pepstatin, to achieve a total reaction volume of 500 μL. Ac-DEVD-AMC (Ac-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin; Alexis Biochemicals, San Diego, CA, USA) was added to the mixture at a concentration of 100 μM and incubated for 1 h at 37 °C. Cleavage of the substrate was measured by fluorescence spectrometer (HTS 7000; Perkin Elmer, Boston, MA, USA) using an excitation and emission wavelength of 360 and 465 nm, respectively. The activities were expressed as fluorescence increase per μg of protein.
Small interfering RNA
The siRNA sequences used here were as follows: siRNA against BAG2 (siBAG2; CCAUCAAGCUAUUAGAGCAUU). The scramble nonsense siRNA (scramble; CCGUAUCGUAAGCAGUACU) that has no homology to any known genes was used as control. Transfection of siRNA oligonucleotide was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations.
Western blot analysis
Western immunoblotting was performed using primary antibodies against BAG1 (Abcam, Cambridge, UK), BAG2 (Abcam), BAG3 (Abcam) or γ-tubulin (Sigma-Aldrich), horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies (Amersham Biosciences, Buckinghamshire, UK) and ECL solutions (Amersham Biosciences).
Statistics
All experiments were repeated three times and data were expressed as the mean±s.d. from a representative experiment. The statistical significance of the difference between group means was analysed by ANOVA and post hoc Dunnett's test. Statistical significance was defined as P<0.05.
Chemicals
MG132, epoxomicin, PSI and lactacystin were purchased from Calbiochem (La Jolla, CA, USA). Actinomycin D and cycloheximide (CHX) were obtained from Sigma (Saint Louis, MO, USA).
Results
Elevation of BAG2 expression in thyroid carcinoma cells upon treatment with proteasome inhibitors
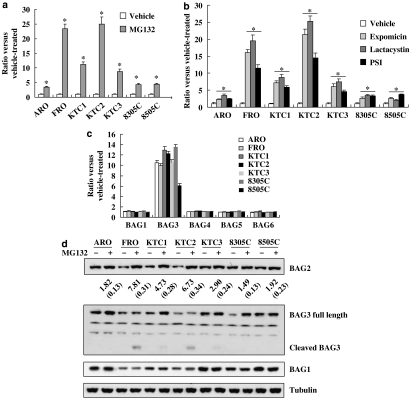
We have reported that a panel of thyroid cancer cells demonstrated different sensitivity to apoptosis induced by proteasome inhibitors: FRO and KTC2 cells were the most sensitive to proteasome inhibition, whereas ARO and 8305C cells were the most resistant (Wang et al., 2007). BAG2 mRNA expression was induced by the proteasome inhibitor MG132 (2 μM, 8 h) in the panel of thyroid cancer cells including both sensitive and insensitive cells (Figure 1a). FRO and KTC2 cells demonstrated 20- to 25-fold increase in BAG2 mRNA, whereas only 3- to 10-fold increase was observed in the other cells (Figure 1a), suggesting that BAG2 was more inducible in cells sensitive to proteasome inhibitors. Three other proteasome inhibitors, PSI, lactacystin and epoxomicin elevated BAG2 mRNA expression similarly (Figure 1b). Consistent with our previous report (Wang et al., 2008), proteasome inhibitors also induced BAG3 expression, but had no effect on the expression of BAG1, BAG4, BAG5, as well as BAG6 (Figure 1c).
Figure 1.
BAG2 transcripts were increased upon exposure to the proteasome inhibitor MG132. (a) A panel of thyroid cancer cells was treated with 2 μM MG132 for 8 h and real-time RT-PCR was performed. (b) Thyroid cancer cells were treated with different proteasome inhibitors and real-time RT-PCR was performed. (c) Thyroid cancer cells were treated with 2 μM MG132 for 8 h and BAG1 and BAG3–6 mRNA levels were analysed. (d) Representative images of BAG1, BAG2 and BAG3 immunoblotting from cell lysates treated with 2 μM MG132 for 24 h. γ-tubulin was used to ensure equal gel loading. The BAG2 protein levels, relative to those of vehicle-treated cells, are noted at the bottom of the blot. The data are presented as the mean (s.d.) of three repeated experiments. *P<0.01 versus vehicle-treated. RT-PCR, reverse transcription-polymerase chain reaction.
We then performed western blot analysis to determine if proteasome inhibition also altered BAG2 expression at the protein level. Consistent with the observation of differential induction of BAG2 mRNA, the expression of BAG2 protein was increased markedly in sensitive cell lines FRO and KTC2, but only slightly increased in insensitive cell lines ARO, 8305C and 8505C (Figure 1d). Increase of full-length BAG3 by MG132 was dramatic in insensitive cell lines, whereas in sensitive cell lines, this increase was minor with concurrent BAG3 cleavage, resulting in increase of truncated form of BAG3 (Figure 1d), consistent with our previous report (Du et al., 2008). MG132 had no effect on BAG1 protein levels in any investigated cell lines (Figure 1d).
Time course analysis of BAG2 induction by MG132
Time course experiments showed that the rise in the level of BAG2 started without significant time lag in the MG132-sensitive FRO cell, with a marked stimulation of BAG2 expression as early as 1 h with a peak at 8 h incubation (Figure 2a). The BAG2 expression decreased gradually after 16 h possibly because of a large amount of cell death (Figure 2a). In the MG132-insensitive ARO cells, there was a consistent 8–16 h lag before BAG2 started to accumulate. A significant stimulation of BAG2 was first observed at 8 h and more than 24 h elapsed before it reached its peak (Figure 2b). In addition, the maximum stimulation of BAG2 was smaller in ARO cells than that in FRO cells (Figures 2a and b).
Figure 2.
Time course of induction of BAG2 transcripts by MG132. (a) FRO cells were treated with 2 μM MG132 for the indicated time and BAG2 mRNA levels were analysed using real-time RT-PCR. (b) ARO cells were treated as in (a) and BAG2 levels were analysed. RT-PCR, reverse transcription-polymerase chain reaction.
Stimulation of BAG2 expression in various types of carcinoma cells
To investigate whether BAG2 is stimulated by proteasome inhibitors in other cells, several lines of carcinoma cells were checked. The stimulatory effects were also observed in other cell types derived from a variety of tumours, including HT1080 (fibrosarcoma), MCF7 (breast cancer), HeLa (cervical cancer), OVACR8 (ovarian cancer), SW1990 (pancreatic cancer), Hep2 (hepatic cancer), LK87 (lung cancer), CaKi2 (kidney cancer) and SH-SY5Y (neuroblastoma) cells (Figure 3a). We then performed western blot analysis to determine if MG132 also altered BAG2 expression at the protein level. Consistent with the observation of increased BAG2 mRNA, the expression level of BAG2 protein was increased during MG132 treatment (Figure 3b).
Figure 3.
Upregulation of BAG2 in various types of cancer cells treated with MG132. (a) A range of cancer cells was treated with 2 μM MG132 for 8 h and BAG2 mRNA levels were analysed. (b) Cancer cells were treated with 2 μM MG132 for 24 h and western blots were performed.
The elevation of BAG2 mRNA upon proteasome inhibition requires de novo RNA synthesis
To determine whether de novo RNA or protein synthesis is required for the elevation of BAG2, FRO cells were pretreated with actinomycin D and CHX, inhibitors of RNA and protein synthesis, respectively, and then exposed to MG132 for 8 h. Actinomycin D significantly suppressed the elevation of BAG2 mRNA induced by MG132 (Figure 4a), on the other hand, CHX had no effect on MG132-stimulated BAG2 expression (Figure 4b), indicating that the upregulation of BAG2 by MG132 requires de novo RNA synthesis, but de novo protein synthesis is not necessary for its stimulation.
Figure 4.
BAG2 induction by MG132 required de novo RNA synthesis. (a) FRO cells were pretreated with actinomycin D, then treated with 2 μM MG132 for 8 h and BAG2 mRNA was analysed. (b) FRO cells were pretreated with cycloheximide, then treated with 2 μM MG132 for 8 h and BAG2 mRNA levels were analysed. (c) FRO cells were pretreated with actinomycin D or cycloheximide, then treated with 2 μM MG132 for 24 h and western blot analysis was performed. γ-tubulin was used to ensure equal gel loading. *P<0.01. NS, not significant.
Western blot analysis was then performed to determine if induction of BAG2 mRNA and de novo protein synthesis are necessary for upregulation of its protein. Actinomycin D completely inhibited MG132-mediated increase in BAG2 protein (Figure 4c); in addition, CHX also completely suppressed upregulation of BAG2 protein (Figure 4c), indicating that increase in BAG2 protein is due to the upregulation of its mRNA expression and subsequent nascent BAG2 protein synthesis, but not due to the alteration of BAG2 protein stability.
Downregulation of BAG2 inhibits thyroid cancer cell death induced by proteasome inhibitors
To evaluate the effect of BAG2 elevation upon exposure to proteasome inhibitors, siRNA against BAG2 was used to prevent the elevation of BAG2. Cells were transfected with scrambled or BAG2 siRNAs as described and then challenged with MG132 for 24 h. Transfection of siBAG2, but not scrambled siRNA, resulted in a marked suppression of BAG2 expression induced by MG132 in FRO cells (Figure 5a). Modulation of BAG2 levels did not affect basal cell survival (Figure 5b). MG132-induced cell death was unaffected in cells transfected with scrambled siRNAs. Conversely, in FRO cells transfected with siBAG2, MG132-induced apoptosis was delayed and partially inhibited (Figure 5b). The effects of siRNAs were confirmed by assessment of caspase-3 activity measured 2, 4, 8, 12 and 24 h after MG132 treatment (Figure 5c). Again, transfection with scrambled siRNAs had no effect on caspase-3 activity at all times. On the other hand, MG132-induced caspase-3 activity was partially reduced in FRO cells transfected with siBAG2 at all times investigated (Figure 5c). The suppression effects of MG132-mediated cell death and caspase-3 activation by siBAG2 were more obvious during early stage of MG132 treatment (Figures 5b and c).
Figure 5.
Downregulation of BAG2 delayed and partially suppressed MG132-induced cell death. (a) FRO cells were transfected with scramble or siRNA against BAG2 and western blot was performed. (b) Twenty four hours after transfection with scrambled or siRNA against BAG2, FRO cells were treated with MG132 for the indicated time and cell death was analysed. (c) Cells were treated as in (b) and caspase-3 activity was measured. *P<0.05, **P<0.01. siRNA, small interfering RNA.
Discussion
Co-chaperone proteins that share BAG domains can interact with various HSPs, some of which have been reported to interfere with apoptotic events (Beere, 2001). Little is known of the induction of BAG2 and no exact function, except for inhibitory regulation of CHIP, has been assigned to it. In the current study, we reported for the first time that BAG2 was transcriptionally induced by proteasome inhibitors. We also report for the first time that proteasome inhibitors are able to upregulate BAG2 expression in various carcinoma cells. The time course of the upregulation of BAG2 expression by MG132 correlated well with the stimulation of both caspase-3 activity and cell death caused by MG132. Furthermore, the inhibition of BAG2 expression by siRNA leads to a partial, but statistically significant, reduction of MG132-induced apoptosis, suggesting that BAG2 expression may contribute to the pro-apoptotic effects of MG132. The upregulation of BAG2 expression by proteasome inhibitors may be due to transcriptional activation. The mechanism(s) of BAG2 gene transcriptional activation by MG132 are currently under investigation in our laboratory.
Interestingly, we have also demonstrated that proteasome inhibition transcriptionally induced another BAG family member, BAG3, which appeared to function as an antiapoptotic factor suppressing proteasome inhibition-induced apoptosis (Wang et al., 2008). BAG family members show close interactions with the ubiquitin–proteasome system. For example, BAG1 and BAG5 have been known to interact and interfere with the RING finger E3 ligases Siah-1 and parkin, respectively (Takayama and Reed, 2001; Kalia et al., 2004); BAG2 interacts with E3 ligase CHIP and suppresses its activity (Arndt et al., 2005). We therefore extensively examined the effect of proteasome inhibition on the expression of BAG family genes. However, no modifications were found except for BAG2 and BAG3.
Previous studies demonstrated that most of the BAG family proteins play important roles in the regulation of apoptosis, cell survival and stress response. Many of the BAG domain proteins possess antiapoptotic activities, although presumably through non-overlapping mechanisms. For example, the first family member, BAG1, was identified initially as a Bcl-2-binding protein with anti-cell death activity (Takayama and Reed, 2001), and the expression of BAG3 was elevated in stress stimuli and demonstrated synergism with Bcl-2 in preventing cell death (Liao et al., 2001; Lee et al., 2002; Romano et al., 2003). BAG4 (SODD) was also identified as a binding partner for tumour necrosis factor receptor-1, death receptor-3 and Bcl-2 and was shown to act in an antiapoptotic manner (Jiang et al., 1999; Antoku et al., 2001). In contrast, BAG5 was identified as a gene that was upregulated in dopaminergic neurons following medial forebrain bundle transfection and functioned as a pro-apoptotic factor enhancing dopaminergic neuron degeneration, via suppression of the E3 ligase activity of parkin (Kalia et al., 2004). In this study, for the first time we have shown a pro-apoptotic property of BAG2, following proteasome inhibition in thyroid cancer cells. Further investigation on the mechanism underlying pro-apoptotic effects of BAG2 might advance our understanding of the BAG family proteins.
Acknowledgments
We thank Dr Junichi Kurebayashi (Kawasaki Medical University, Japan) for generously providing KTC1, KTC2 and KTC3 cell lines. This work was partially supported by National Natural Science Foundation of China to H-Q Wang (30740086).
Abbreviations
- BAG
Bcl-2-associated athanogene
- CHIP
carboxyl terminus of HSP70-interacting protein
- CHX
cycloheximide
- HSP
heat shock protein
- PI
propidium iodide
- RT-PCR
reverse transcription-polymerase chain reaction
- siBAG2
siRNA against BAG2
- siRNA
small interfering RNA
- TNFR1
tumor necrosis factor receptor-1
- UPS
ubiquitin-proteasome system
Conflict of interest
The authors state no conflict of interest.
References
- Adams KW, Cooper GM. Rapid turnover of mcl-1 couples translation to cell survival and apoptosis. J Biol Chem. 2007;282:6192–6200. doi: 10.1074/jbc.M610643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoku K, Maser RS, Scully WJ, Jr, Delach SM, Johnson DE. Isolation of Bcl-2 binding proteins that exhibit homology with BAG-1 and suppressor of death domains protein. Biochem Biophys Res Commun. 2001;286:1003–1010. doi: 10.1006/bbrc.2001.5512. [DOI] [PubMed] [Google Scholar]
- Arndt V, Daniel C, Nastainczyk W, Alberti S, Hohfeld J. BAG-2 acts as an inhibitor of the chaperone-associated ubiquitin ligase CHIP. Mol Biol Cell. 2005;16:5891–5900. doi: 10.1091/mbc.E05-07-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM. Stressed to death: regulation of apoptotic signaling pathways by the heat shock proteins. Sci STKE. 2001;2001:RE1. doi: 10.1126/stke.2001.93.re1. [DOI] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- Dai Q, Qian SB, Li HH, McDonough H, Borchers C, Huang D, et al. Regulation of the cytoplasmic quality control protein degradation pathway by BAG2. J Biol Chem. 2005;280:38673–38681. doi: 10.1074/jbc.M507986200. [DOI] [PubMed] [Google Scholar]
- Du ZX, Meng X, Zhang HY, Guan Y, Wang HQ. Caspase-dependent cleavage of BAG3 in proteasome inhibitors-induced apoptosis in thyroid cancer cells. Biochem Biophys Res Commun. 2008;369:894–898. doi: 10.1016/j.bbrc.2008.02.112. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283:543–546. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- Kalia SK, Lee S, Smith PD, Liu L, Crocker SJ, Thorarinsdottir TE, et al. BAG5 inhibits parkin and enhances dopaminergic neuron degeneration. Neuron. 2004;44:931–945. doi: 10.1016/j.neuron.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Lee MY, Kim SY, Shin SL, Choi YS, Lee JH, Tsujimoto Y, et al. Reactive astrocytes express bis, a bcl-2-binding protein, after transient forebrain ischemia. Exp Neurol. 2002;175:338–346. doi: 10.1006/exnr.2002.7903. [DOI] [PubMed] [Google Scholar]
- Liao Q, Ozawa F, Friess H, Zimmermann A, Takayama S, Reed JC, et al. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001;503:151–157. doi: 10.1016/s0014-5793(01)02728-4. [DOI] [PubMed] [Google Scholar]
- Romano MF, Festa M, Pagliuca G, Lerose R, Bisogni R, Chiurazzi F, et al. BAG3 protein controls B-chronic lymphocytic leukaemia cell apoptosis. Cell Death Differ. 2003;10:383–385. doi: 10.1038/sj.cdd.4401167. [DOI] [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, et al. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Du ZX, Zhang HY, Gao DX. Different induction of GRP78 and CHOP as a predictor of sensitivity to proteasome inhibitors in thyroid cancer cells. Endocrinology. 2007;148:3258–3270. doi: 10.1210/en.2006-1564. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Liu HM, Zhang HY, Guan Y, Du ZX. Transcriptional upregulation of BAG3 upon proteasome inhibition. Biochem Biophys Res Commun. 2008;365:381–385. doi: 10.1016/j.bbrc.2007.11.001. [DOI] [PubMed] [Google Scholar]