Abstract
Background and purpose:
VGX-1027 is a novel, low molecular weight, immunomodulatory compound that has shown efficacy against a variety of immuno-inflammatory disease models in animals including autoimmune diabetes in NOD mice, collagen-induced arthritis and chemically induced inflammatory colitis. Here, we have studied the effects of VGX-1027 on the development of endotoxin-induced uveitis (EIU) in male Lewis rats, as a model of inflammatory ocular diseases in humans.
Experimental approach:
EIU was induced by a single footpad injection of 200 μg lipopolysaccharide (LPS). Groups of rats were treated with either VGX-1027 (25 mg kg−1) or its vehicle at different time points (30 min, 6 h or 12 h) after the challenge with LPS or, as positive control, with dexamethasone. The rats were killed within 16 h after LPS challenge, and the eyes and aqueous humor were collected to study serological, immunological and histological signs of EIU.
Key results:
The rats treated with VGX-1027 within 6 h after LPS challenge exhibited milder clinical, histological and laboratory signs of EIU than those treated with vehicle.
Conclusion and implications:
This study provides the first evidence that systemic treatment with VGX-1027 counteracts the uveitis-inducing effect of LPS in rats and suggests that this drug may have potential in the treatment of immuno-inflammatory conditions of the eye in humans.
Keywords: cytokines, immunotherapy, uveitis, VGX-1027
Introduction
A single intraplantar injection of endotoxin in susceptible strains of rodents provokes an acute immuno-inflammatory reaction in the eyes, endotoxin-induced uveitis (EIU) (reviewed by Smith et al., 1998). This occurs through an upregulated expression of adhesion molecules such as P-selectin, E-selectin and ICAM-1 in the iris ciliary bodies of the endotoxin-challenged rodents (Withcup et al., 1993, 1995; Suzuma et al., 1997; Whitcup et al., 1999) that allows the intraocular migration of leukocytes. Through their activation, by nuclear translocation of nuclear factor κ-beta (NF-κB), these cells subsequently produce at the ocular level large amounts of pro-inflammatory mediators such as tumour necrosis factor-α (TNF-α), interleukin-1 and interleukin-6 and nitric oxide (NO) (Planck et al., 1994; Goureau et al., 1995; Jacquemin et al., 1996; Suzuki et al., 2006).
Endotoxin-induced uveitis develops within 24 h of endotoxin challenge, with signs of uveitis that range from discrete dilatation of the iris and conjunctival vessels to intense iridal hyperaemia with flare in the anterior chamber, along with fibrinous exudate in the pupillary area. These clinical signs are accompanied by augmented content of proteins and inflammatory mediators such as interleukin-6, TNF-α and NO in the aqueous humor (Planck et al., 1994; Goureau et al., 1995; Jacquemin et al., 1996; Bucolo et al., 2003; Jin et al., 2006; Suzuki et al., 2006). A spontaneous regression of clinical, histological and seroimmunological signs of EIU can be observed starting 16–24 h after lipopolysaccharide (LPS) challenge, with marked amelioration being observable within 72 h after induction of the disease (Medeiros et al., 2008).
These clinical, biochemical, immunological and histological characteristics make rat EIU a suitable in vivo model to gain insights into the immunopathogenesis of, and evaluate novel therapeutic approaches for, the treatment of inflammatory ocular diseases in humans. Chronic intraocular inflammation denotes a heterogeneous group of diseases that is a leading cause of acquired blindness in adults (reviewed by Bora and Kaplan, 2007; Tellier, 2007). As the main anatomical site of inflammation is the uveal tract, which is the vascular organ of the eye, the term uveitis is used to describe intraocular inflammation more globally. Uveitis encompasses a wide range of underlying aetiologies. It may be idiopathic, that is, of unknown cause, or it may be associated with systemic immuno-inflammatory diseases such as Behçet's disease, ankylosing spondylitis, Reiter's syndrome and a variety of infectious agents. Uveitis is responsible for approximately 3% of blindness in the United States, and about 18% of active uveitis patients experience a transient or permanent loss of vision. Inflammation within the anterior segment of the eye, anterior uveitis, is the most common form characterized in its acute form by ciliary injection, keratitic precipitates, cells and protein flare in the aqueous liquor and meiosis with posterior synechiae. The conventional treatment of intraocular inflammation includes corticosteroids and immunosuppressive agents such as corticosteroids and cyclosporin A (Tellier, 2007). However, a large proportion of patients are resistant to steroids, and both corticosteroids and cyclosporin A have a wide range of significant side effects, such as cataract, increase of intraocular pressure, increased susceptibility to microbial infection for the former and nephrotoxicity for the latter (Tellier, 2007). Therefore, there is a need to evaluate novel and less toxic immunomodulatory and anti-inflammatory compounds that either alone or in combination with low doses of corticosteroids and cyclopsorin A may be considered for the treatment of patients suffering from acute anterior uveitis.
VGX-1027 is a small molecule being developed for the treatment of human immuno-inflammatory diseases such as rheumatoid arthritis and type 1 diabetes and it is currently being evaluated in phase I studies. We have shown that VGX-1027 successfully counteracts immunopathogenic pathways leading to autoimmune diabetes in non-obese diabetic (NOD) mice and in mice made diabetic with multiple low doses of streptozotocin (Stosic-Grujicic et al., 2007). It also suppresses LPS-induced lethality, carrageenan-induced pleurisy, type II collagen-induced arthritis (Stojanovic et al., 2007) and dinitrobenzensulphonic acid-induced inflammatory colitis in mice (Mangano et al., 2008).
VGX-1027 appears to target the function of macrophages, as it inhibits LPS-induced NF-κB and p38 MAP kinase signalling pathways and TNF-α secretion from purified peritoneal macrophages (Stojanovic et al., 2007). VGX-1027 downregulates TNF-α production from macrophages in response to activators of both Toll-like receptor-4 (LPS) and Toll-like receptor-2/6 (zymosan), and the drug also inhibits LPS-induced secretion of interleukin-1β, TNF-α and interleukin-10 from peritoneal cells. VGX-1027 is effective in vivo both when given intraperitoneally and orally, and acute and subacute toxicological studies show no toxicity at pharmacological doses in these preclinical models.
The above observations prompted us to evaluate the effects of VGX-1027 in rat EIU. Thse data show that VGX-1027, administered intraperitoneally, markedly counteracted clinical, laboratory and histopathological signs of EIU. The effects were pronounced when the drug was administered within 6 h after the LPS challenge.
Materials and methods
Animal groups and EIU
All animal procedures and experiments were in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. Eight-week-old male Lewis rats (180–220 g) purchased from Harlan-Nossan (S Pietro al Natisone, Udine, Italy) were used. EIU was induced by injection into the footpad of 200 μg LPS from Salmonella typhimurium (Sigma-Aldrich, St Louis, MO, USA) that had been diluted in 0.2 mL of phosphate-buffered saline (PBS, pH 7.4). Different groups of rats were created for the experimental design: the active group was treated with VGX-1027, a control group with its vehicle, and a positive control group with 1 mg kg−1 dexamethasone 30 min before, simultaneously with and 30 min after LPS injection, as described by Jin et al., 2006. To evaluate the therapeutic effect of VGX-1027 on the development of uveitis, the rats were treated with VGX-1027 at 30 min, 6 h or 12 h after LPS injection and killed at 16 h. In addition, to evaluate whether VGX-1027 was able to accelerate the recovery phase of uveitis, the rats were treated with VGX-1027 at the expected peak of the disease, 16 h after LPS injection, and killed at 72 h. VGX-1027, dissolved in Na2HPO4/H2O was administered intraperitoneally at a dose of 25 mg kg−1 in a final volume of 200 μL. This dose of VGX-1027 was chosen on the basis of previous studies that indicated this dose to be a pharmacologically relevant dose in murine models of immuno-inflammation and autoimmune diseases (Stojanovic et al., 2007; Stosic-Grujicic et al., 2007; Mangano et al., 2008). An additional control group received sham-treatment for comparison.
Clinical grading of uveitis
After 16 or 72 h, the induction of uveitis, the eyes were examined by slit lamp by an observer unaware of the treatment. Uveitis in each eye was graded according to a previously reported scoring system (Behar-Cohen et al., 1998): (0) no inflammatory reaction; (1) discrete dilatation of the iris and conjunctival vessels; (2) moderate dilatation of the iris and conjunctival vessels; (3) intense iridal hyperaemia with flare in anterior chamber; and (4) same clinical signs as grade 3 plus the presence of fibrinous exudate in the pupillary area with intense flare in the anterior chamber. No signs of uveitis were observed in the animals at the beginning of each experiment.
Infiltrating cells, protein concentration, TNF-α and no levels in aqueous humor
Immediately after the slit lamp examination, the rats were killed and the aqueous humor was collected from both eyes by an anterior chamber puncture (15–20 μl per rat) using a 30-gauge needle under a surgical microscope. The aqueous humor from each eye of five rats from the same group was then pooled and diluted 10 times with PBS (pH 7.4).
For cell counting, the aqueous humor was suspended in an equal amount of Türk stain solution, and the cells were counted with a haemocytometer under a light microscope. The number of cells per field (an equivalent of 0.1 μL) was manually counted, and the number of cells per μL was obtained by averaging the results of four fields from each sample. The total protein concentration in the aqueous humor samples was measured with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA).
The levels of TNF-α in the aqueous humor were assessed with a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA). Intra- and interassay coefficients of variations were lower than 10%. The aqueous humor samples were in all cases stored in ice water until tested on the day of sample collection.
Measurement of intraocular levels of nuclear NF-κB/p65
Nuclear factor κ-beta p65 translocation into the nucleus, as an index of NF-κB activation, was measured using a sandwich ELISA (ActivELISA Imgenex, Analitica De Mori, Milano, Italy) according to the manufacturer's protocol. Soon after collection of aqueous humor, both eyes were enucleated. Ten eyes from five animals were pooled together so that each experimental group consisted of five samples. The eyes were cut into small pieces, washed with cold PBS, homogenized in hypotonic buffer and centrifuged for 10 min at 4500 g. The supernatants were used as cytoplasmic extract. Nuclear lysis buffer, 500 μL, was added to the pellet and centrifuged at 8800 g for 10 min at 4 °C. This supernatant was used as nuclear extract. The anti-NF-κB p65 antibody-coated plate was used to capture the nuclear or cytoplasmic NF-κB p65 in the respective samples (0.5–1 mg mL−1 of protein) and the amount of bound NF-κB p65 was detected by adding a secondary antibody followed by an alkaline phosphatase-conjugated antibody. The absorbance value for each well was determined at 405 nm using a microplate reader (Bio-Rad, Hercules, CA, USA). The relative ratio of nuclear-to-cytoplasmic NF-κB p65 was calculated from the respective absorbance values.
Histological examination
A separate set of rats treated in the same way as described earlier were used for the histological study. For microscopic histological evaluation, rat eyes were collected and fixed in a solution containing 0.1% glutaraldehyde (25%) and 4% paraformaldehyde for 24 h at room temperature, dehydrated by graded ethanol and embedded in paraffin (Paraplast; Sherwood Medical, Mahwah, NJ, USA). Tissue sections (7 μm), cut near the optic nerve (obtained from eight eyes of four rats in each group), were deparaffinized with xylene, stained with May–Grünwald–Giemsa and studied by light microscopy (Dialux 22; Leitz, Wetzlar, Germany).
Localization of ICAM-1, P-selectin and iNOSAQ4 by immunohistochemistry
Three groups of animals (sham-treated, controls and VGX-1027-treated) were used for the immunohistochemical study. Iris ciliary bodies were fixed in an immunofixation solution of 0.1% glutaraldehyde (25%) and 4% paraformaldehyde in phosphate buffer. Indirect immunofluorescence staining was performed on 7-μM-thick sections. After deparaffinization, the sections were hydrated (three times for 15 min in PBS) and permeabilized with 0.1% Triton X-100 in PBS for 20 min. Non-specific adsorption was minimized by incubating the section in 2% normal goat serum in PBS for 20 min. Endogenous biotin or avidin binding sites were blocked by sequential incubation for 15 min with avidin and biotin (DBA, Milan, Italy). Sections were incubated overnight with (1) anti-ICAM-1 polyclonal antibody (CD54) (1:500 in PBS, v/v) (DBA), (2) anti-P-selectin polyclonal antibody (1:100 in PBS, v/v) or (3) anti-iNOS polyclonal antibody (1:500 in PBS, v/v) (Santa Cruz, DBA). Specific labelling was detected with a biotin-conjugated goat anti-rabbit IgG, donkey anti-goat IgG or goat anti-mouse IgG and avidin–biotin peroxidase complex (DBA). To verify the binding specificity for ICAM-1, P-selectin, TNF-α and iNOS, some sections were also incubated with primary antibody only (no secondary antibody) or with secondary antibody only (no primary antibody). In these situations, no positive staining was found in the sections.
Statistical analysis
All values are expressed as mean±s.d. The results were analysed by one-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. P<0.05 was considered as significant. The figures from histological and immunohistochemical experiments are representative of at least three experiments (five slides for each eye from different animals) performed on different days.
Drugs and chemicals
VGX-1027 was provided by VGX Pharmaceuticals (VGXP, Blue Bell, PA, USA). Dexamethasone (Soldesan) was purchased from a local pharmacy. Primary anti-P-selectin, anti-ICAM-1, anti-nitrotyrosine and anti-poly(ADP-ribose) synthetase antibodies were from DBA. All other reagents were purchased from Sigma Chemical Co. (Milan, Italy).
Results
Inhibition of clinical signs of EIU by VGX-1027
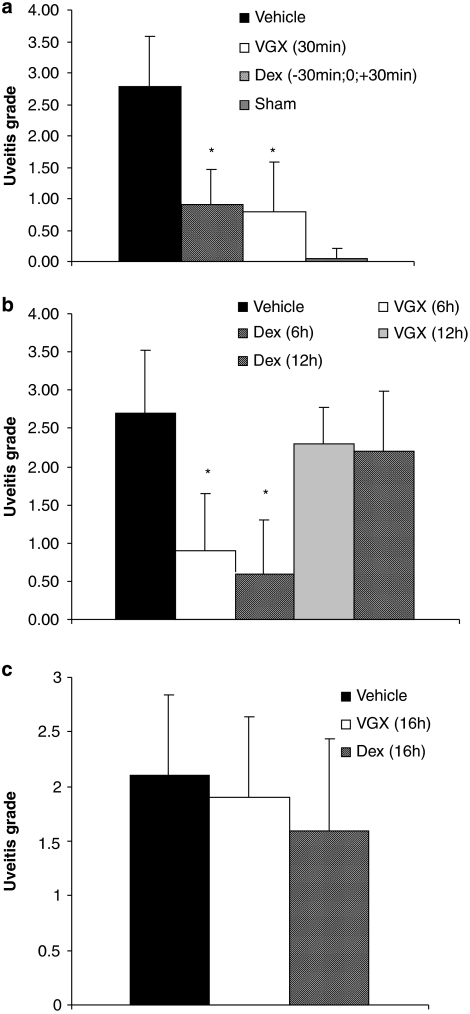
As shown in Figures 1a and b, the clinical inflammation score, which was determined 16 h after injection of LPS, was significantly (P<0.0001) lower in the group of rats treated with VGX-1027 either 30 min or 6 h after LPS challenge compared with the negative (untreated) controls. The effect of VGX-1027 was comparable with that of the positive control group receiving dexamethasone (Figures 1a and b). However, neither VGX-1027 nor dexamethasone was capable of significantly inhibiting the clinical development of EIU when first administered 12 h after LPS injection (Figure 1b). In addition, when administered to rats with fully established uveitis, 16 h after LPS challenge, VGX-1027 treatment was not able to significantly accelerate the spontaneous clinical amelioration observed in the vehicle-treated controls (Figure 1c). A more pronounced trend towards faster recovery that also did not reach statistical significance was observed in the rats treated with dexamethasone (Figure 1c).
Figure 1.
The effects of VGX-1027 on clinical development of EIU. The rats were treated with VGX-1027 at 30 min (a), 6 and 12 h (b) and 16 h (c) after lipopolysaccharide (LPS) injection or with dexamethasone (Dex) 30 min before, simultaneously with and 30 min after LPS (a) 6 h and 12 h (b) and 16 h (c) after LPS injection, and were evaluated 16 h (a and b) and 72 h (c) after LPS challenge. The EIU score is the mean±s.d.; 10 animals were examined in each group (20 eyes). *P<0.0001 vs vehicle.
VGX-1027 reduces the LPS-induced increase in aqueous humor proteins, TNF-α, NO and the number of infiltrating cells
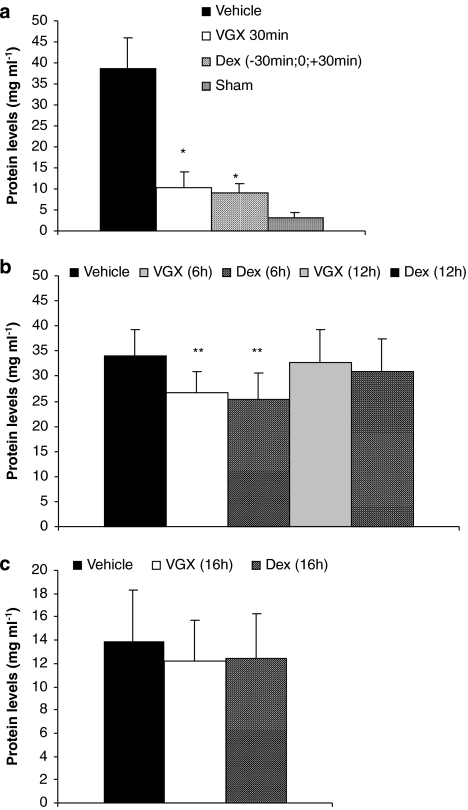
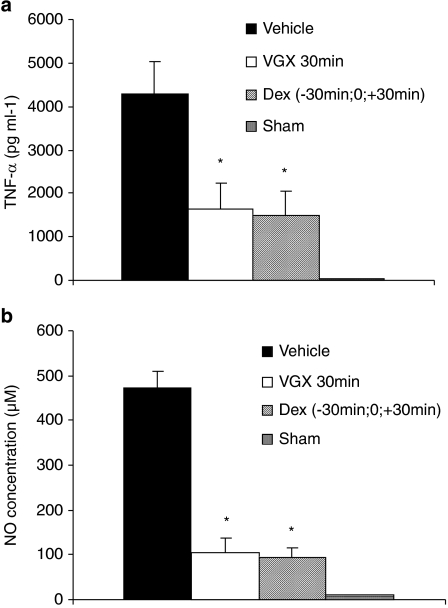
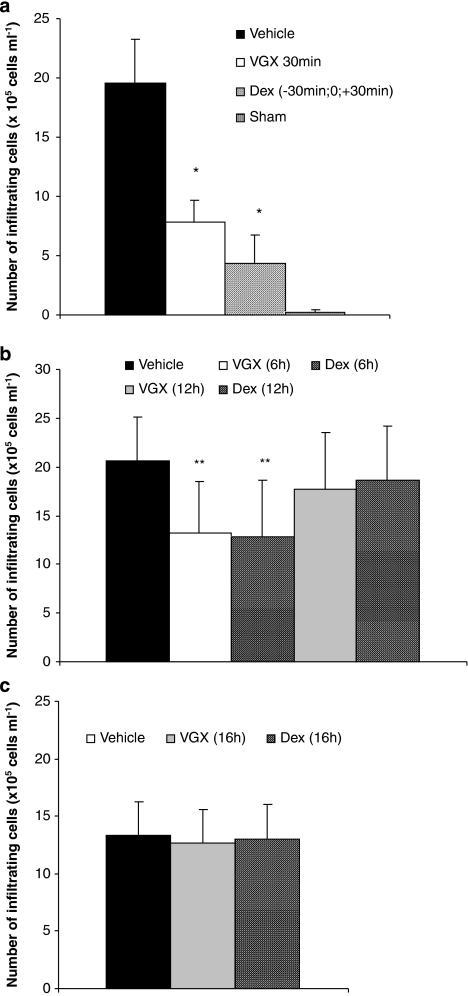
As reported previously (Bucolo et al., 2003; Jin et al., 2006), the aqueous humor of rats challenged with LPS contained significantly larger amounts of proteins, TNF-α, NO and inflammatory cells than those found in the group of sham-treated rats that were not challenged with LPS (Figures 2, 3 and 4). Relative to vehicle-treated rats, the LPS-induced elevation of proteins and increased number of inflammatory cells were significantly reduced by treatment with VGX-1027 when administered during the early onset of disease—within 6 h after challenge with LPS. The effect was comparable with that achieved with dexamethasone (Figures 2a, b and 4a, b). In addition, selected experiments carried out in rats treated with VGX-1027, 30 min after LPS challenge, also showed that the so-treated rats had lower levels of TNF-α and NO compared with vehicle-treated controls (Figures 3a and b). The effect was comparable with that observed with dexamethasone (Figures 3a and b). On the other hand, in agreement with the clinical findings, the treatment with either VGX-1027 or dexamethasone commenced 12 or 16 h after LPS challenge was unable to reduce the levels of proteins and inflammatory cells, as compared with vehicle-treated rats when assessed at 72 h after LPS (Figures 2b, c and 4b, c).
Figure 2.
The effects of VGX-1027 on protein levels in the aqueous humor of Lewis rats during development of EIU. The rats were treated with VGX-1027 at 30 min (a), 6 h and 12 h (b) and 16 h (c) after lipopolysaccharide (LPS) injection or with dexamethasone (Dex) 30 min before, simultaneously with and 30 min after LPS (a) 6 h, 12 h (b) and 16 h (c) after LPS injection. The aqueous humor was collected from individual rats 16 h (a and b) or 72 h (c) after LPS injection. Data are expressed as mean±s.d. (five animals for each group, n=10 eyes). *P<0.0001; **P<0.01 vs vehicle.
Figure 3.
The effects of VGX-1027 on TNF-α (a) and NO (b) levels in the aqueous humor of Lewis rats during development of EIU. The rats were treated with VGX-1027 at 30 min after lipopolysaccharide (LPS) or with dexamethasone (Dex) 30 min before, simultaneously with and 30 min after LPS injection. The aqueous humor was collected from individual rats 16 h after LPS injection. Data are expressed as mean±s.d. (five animals for each group, n=10 eyes). *P<0.0001 vs vehicle.
Figure 4.
The effect of VGX-1027 on cellular infiltration in the iris ciliary body. The rats were treated with VGX-1027 at 30 min (a), 6 h and 12 h (b) and 16 h (c) after lipopolysaccharide (LPS) injection or with dexamethasone (Dex) 30 min before, simultaneously with and 30 min after LPS (a) 6 h and 12 h (b) and 16 h (c) after LPS injection. The eyes were collected from the rats of the different experimental groups 16 h (a and b) or 72 h (c) after LPS injection. Data are expressed as mean±s.d. (n=10 eyes). *P<0.0001; **P<0.01 vs vehicle.
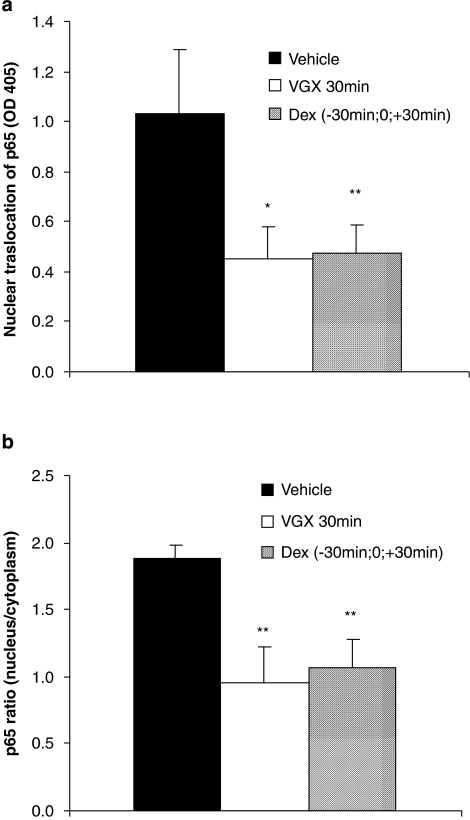
VGX-1027 reduces intraocular content of NF-κB p65 during EIU
As shown in Figure 5, NF-κB p65 was found at increased levels in ocular tissue homogenates from vehicle-treated control rats with concomitant increases in the nuclear/cytoplasmic ratios of the transcription factor subunit. In contrast, the nuclear levels and the nuclear/cytoplasmic ratios of NF-κB p65 were significantly reduced in the rats treated with VGX-1027 and in those treated with dexamethasone (Figure 5a and b).
Figure 5.
The effect of VGX-1027 on NF-κB nuclear translocation (a) and p65 nuclear/cytoplasm ratio (b) in ocular homogenates in rats with EIU. The rats were treated with VGX-1027 30 min after lipopolysaccharide (LPS) or with dexamethasone (Dex) 30 min before, simultaneously with and 30 min after LPS injection. Data are expressed as mean±s.d. (n=10 eyes). *P<0.05; **P<0.01 vs vehicle.
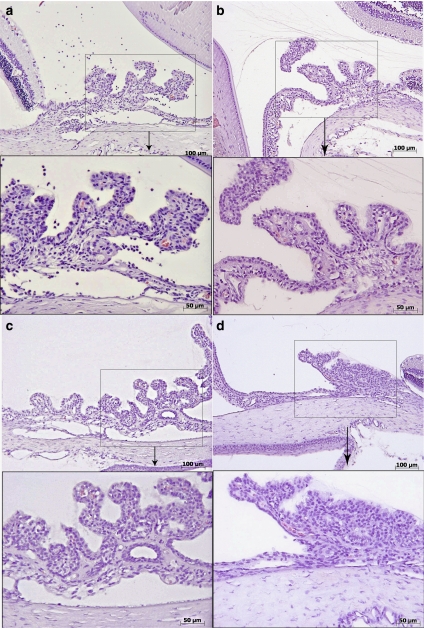
VGX-1027 suppresses histological signs of EIU
As reported previously (Bucolo et al., 2003), the histological evaluation of iris ciliary body tissues from the LPS-treated rats revealed signs of severe uveitis with massive neutrophil infiltration (Figure 6a). Epithelial cells from these rats showed markedly reduced cytoplasm and scarcely visible nuclei (Figure 6a). On the other hand, the rats treated with VGX-1027 or dexamethasone exhibited a significantly milder uveitis and maintenance of the epithelium compared with the vehicle-treated rats (Figure 6b and c). Confirming the clinical finding, VGX-1027 and dexamethasone were also equally capable of suppressing histological signs of uveitis when first administered 6 h, but not 12 h, after LPS (data not shown). The histological appearance of iris ciliary bodies in the rats treated with VGX-1027 was similar to that observed in the group of sham-treated rats that exhibited no signs of uveitis and intact epithelium (Figure 6d).
Figure 6.
The effects of VGX-1027 on histological appearance of uveitis. Histological appearance of the iris ciliary body 16 h after lipopolysaccharide injection. Vehicle (a), VGX-1027 (b), dexamethasone (Dex) (c) and Sham (d). Original magnification (a–d) × 375; panel below (enlargement × 2 of the square) × 750.
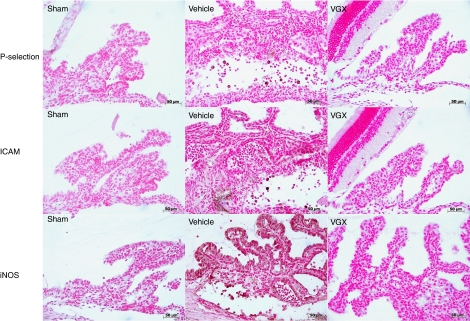
P-selectin, ICAM-1 and iNOS immunostaining
The results of immunohistochemical staining for P-selectin, ICAM-1 and inducible nitric oxide synthase (iNOS) correlated with the histological results and the intensity of the cell infiltration. Hence, whereas eye tissue sections from the sham-treated rats showed no staining for P-selectin, ICAM-1 and iNOS, the tissue sections obtained from rats injected with LPS and treated with the vehicle of VGX-1027 revealed intense positive staining for P-selectin, ICAM and iNOS (Figure 7). In contrast, a much weaker positive staining for these three proteins was observed in rats treated with VGX-1027 (Figure 7).
Figure 7.
The effects of VGX-1027 on P-selectin, ICAM-1 and iNOS expression in the iris ciliary body of rats with EIU. Immunohistochemical localization of P-selectin, ICAM and iNOS in the iris ciliary body 16 h after lipopolysaccharide injection. Transmission light micrograph. Original magnification, × 125.
Discussion
We have shown here that the novel immunomodulatory drug VGX-1027 inhibits clinical, seroimmunological and histological signs of ocular inflammation in rat EIU when administered within 6 h after LPS challenge, that is, during the relatively early onset of disease. Compared with vehicle-treated and LPS-challenged rats, animals treated with VGX-1027 exhibited reduced numbers of inflammatory cells and proteins and lower levels of TNF-α in the aqueous humor, and this was associated with reduced infiltration of inflammatory cells in the iris ciliary body and suppressed nuclear modification of vascular endothelial cells. The staining for P-selectin, ICAM-1 and iNOS was also markedly reduced in tissue ocular sections of rats treated with VGX-1027 as compared with those treated with vehicle only. Finally, VGX-1027 downregulated intraocular levels of activated NF-κB.
The powerful inhibitory action of VGX-1027 on the ocular expression of P-selectin and ICAM-1 may have an important function in its beneficial action in this model. Both P-selectin and ICAM-1 are expressed on endothelial cells in response to specific stimuli, and both proteins are upregulated by pro-inflammatory cytokines such as TNF-α (Van de Stolpe and Van der Saag, 1996; Ley, 2003). These molecules are essential for the migration of inflammatory cells into tissues of the eye during EIU development. Accordingly, an increased expression of ICAM-1 has been observed both in the iris of patients with uveitis (La Heij et al., 1998) and in vascular endothelium of the rat ciliary body during EIU (Withcup et al., 1993, 1995). P-selectin is also expressed in the vessels of the iris of rats with EIU (Suzuma et al., 1997). The involvement of both P-selectin- and ICAM-1-mediated pathways in the pathogenesis of EIU was demonstrated by the blockade of this process by monoclonal antibodies directed against these adhesion molecules (Suzuma et al., 1997). In addition, TNF-α may have an important function in the pathogenesis of EIU by inducing ICAM-1 and selectins, and by contributing to leukocyte adhesion, vascular leakage and apoptotic cell death in EIU (Koizumi et al., 2003). As VGX-1027 reduces the in vitro synthesis of TNF-α from macrophages in response to different stimuli, including LPS (Stojanovic et al., 2007), it is possible that the diminished expression of P-selectin and ICAM-1 in the iris ciliary bodies during development of EIU may be attributed to drug-induced downregulation of TNF-α production. Thus, by inhibiting TNF-α induction and intraocular expression of P-selectin and ICAM-1, VGX-1027 may have prevented infiltration of the eyes with inflammatory cells and, hence, local production of pro-inflammatory mediators. This pharmacological scenario is consistent with the lower levels of TNF-α and activated NF-κB found in the aqueous humor and ocular tissue homogenates as well as with the reduced expression of iNOS detected by immunohistochemical analysis in the ocular tissues from VGX-1027-treated rats.
A direct immunomodulatory action of VGX-1027 on leukocytes infiltrating the eyes, despite diminished expression of adhesion molecules, is also likely. In fact, an important immuno-inflammatory pathway in the development of LPS-induced EIU is activation of NF-κB that leads to local upregulated production of TNF-α and subsequent activation of the iNOS–NO pathways. TNF-α is implicated in the NO synthesis pathway in that the induction of iNOS is mediated by the release of endogenous TNF-α. It has been further demonstrated that epithelial cells of the iris ciliary body and cells infiltrating the anterior segment of the eye during EIU are major sources of NO (Goureau et al., 1995; Jacquemin et al., 1996; Mandai et al., 1996; Szabò et al., 1996). It has recently been shown that specific blockade of NF-κB, TNF-α and NO exerts beneficial effects on the course of EIU (Goureau et al., 1995; Avunduk et al., 2004; Kitamei et al., 2006; Suzuki et al., 2006).
This study adds the rat EIU model to the list of experimental immuno-inflammatory conditions that are favourably influenced by VGX-1027. Others include preclinical models of type 1 diabetes, inflammatory bowel diseases, endotoxemia, pleurisy and rheumatoid arthritis (Stojanovic et al., 2007; Stosic-Grujicic et al., 2007; Mangano et al., 2008).
Importantly, VGX-1027 was effective in counteracting the uveitis-inducing effects of LPS, even when administered, 30 min or 6 h after challenge with LPS. The later time is a point when inflammatory responses, including TNF-α and NO release in the aqueous humor, have already commenced (Medeiros et al., 2008). However, delaying initial treatment with VGX-1027 to 12 or 16 h after LPS challenge neither prevented development of EIU nor accelerated the spontaneous recovery, respectively. This may indicate that the drug lacks activity on the late efferent phase of the disease and that it is not capable of potentiating the naturally occurring anti-inflammatory pathways responsible for spontaneous resolution of EIU. Alternately, it is possible that a higher effective dose might be indicated in the rat model for established disease. Although these studies do not distinguish between the two scenarios, these latter data should not negate the possible translation of these findings to the clinical setting, or potentially exclude patients with fully established uveitis from treatment. In our studies, in spite of the well-known efficacy of steroidal therapy in uveitis patients, the same results were obtained for the positive controls—dexamethasone-treated rats. Dexamethasone was not capable of ameliorating the clinical or histological course of EIU when administered 12 or 16 h after LPS. It is possible that the short time frame of EIU development limits the possible window of therapeutic intervention in the rats, and perhaps also limits the additional upregulation of naturally occurring anti-inflammatory mechanisms responsible for the recovery of the disease that are activated by either VGX-1027 or dexamethasone. Nonetheless, the capacity of VGX-1027 to revert actively ongoing uveitis-inducing pathways when administered within 6 h after LPS challenge indicates that this compound is capable of suppressing actively ongoing immuno-inflammatory responses and supports its use for the clinical setting.
In addition, the ability of VGX-1027 to counteract actively ongoing immuno-inflammatory events in this model is consistent with other in vivo studies, such as those conducted in the type II collagen-induced arthritis mouse model where the initial administration of VGX-1027 under a fully therapeutic regime led to rapid resolution of arthritis symptoms (Stojanovic et al., 2007). An effect of VGX-1027 was also observed in mice with immuno-inflammatory diabetes induced by five consecutive low doses of streptozotocin. In this study, commencing treatment with VGX-1027 10 days after the last streptozotocin injection and a few days before the development of hyperglycaemia significantly ameliorated the course of the disease (Stosic-Grujicic et al., 2007). Finally, VGX-1027 was still capable of exerting beneficial effects on the course of pleurisy, colitis or lethal endotoxaemia when administered after i.p. injection of carrageenan, challenge with LPS and intracolonic administration of dinitrobenzensulphonic acid (Stojanovic et al., 2007; Mangano et al., 2008).
In conclusion, this study demonstrates that VGX-1027 attenuates uveitis induced by LPS in rats at different mechanistic levels and suggests that the anti-uveitis effect of VGX-1027 may be dependent on a combination of pharmacological properties that could result in less efficient migration of inflammatory cells to the eyes and suppression of local proinflammatory activities of such cells. These results further suggest that VGX-1027, perhaps as a topical formulation, could have an important function in human uveitis as well.
Acknowledgments
We thank VGX Pharmaceuticals for the generous gift of VCX 1027. This work was partly supported from 60% research grant of the University of Catania to Professor FN.
Abbreviations
- EIU
endotoxin-induced uveitis
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κ-beta
- NO
nitric oxide
- PBS
phosphate-buffered saline
- TNF-α
tumour necrosis factor-α
Conflict of interest
Professor FN and Dr KM are stockholders of VGX Pharmaceuticals. Dr NYS and Dr JJK are employees of VGX Pharmaceuticals.
References
- Avunduk MC, Avunduk AM, Oztekin E, Baltaci AK, Ozyazgan Y, Mogolkoc R. Etanercept treatment in the endotoxin-induced uveitis of rats. Exp Eye Res. 2004;79:357–365. doi: 10.1016/j.exer.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Behar-Cohen FF, Savoldelli M, Parel JM, Goureau O, Thillaye-Goldenberg B, Courtois Y, et al. Reduction of corneal edema in endotoxin-induced uveitis after application of L-NAME as nitric oxide synthase inhibitor in rats by iontophoresis. Invest Ophthalmol Vis Sci. 1998;39:897–904. [PubMed] [Google Scholar]
- Bora NS, Kaplan HJ. Intraocular diseases—anterior uveitis. Chem Immunol Allergy. 2007;92:213–220. doi: 10.1159/000099272. [DOI] [PubMed] [Google Scholar]
- Bucolo C, Cuzzocrea S, Mazzon E, Caputi AP. Effects of cloricromene, a coumarin derivative, on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2003;44:1178–1184. doi: 10.1167/iovs.02-0559. [DOI] [PubMed] [Google Scholar]
- Goureau O, Bellot J, Thillaye B, Courtois Y, de Kozak Y. Increased nitric oxide production in endotoxin-induced uveitis reduction of uveitis by an inhibitor of nitric oxide synthase. J Immunol. 1995;154:6518–6523. [PubMed] [Google Scholar]
- Jacquemin E, de Kozak Y, Thillaye B, Courtois Y, Goureau O. Expression of inducible nitric oxide synthase in the eye from endotoxin-induced uveitis rats. Invest Ophthalmol Vis Sci. 1996;37:1187–1196. [PubMed] [Google Scholar]
- Jin XH, Ohgami K, Shiratori K, Suzuki Y, Hirano T, Koyama Y, et al. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2006;47:2562–2568. doi: 10.1167/iovs.05-1429. [DOI] [PubMed] [Google Scholar]
- Kitamei H, Iwabuchi K, Namba K, Yoshida K, Yanagawa Y, Kitaichi N, et al. Amelioration of experimental autoimmune uveoretinitis (EAU) with an inhibitor of nuclear factor-kappaB (NF-kappaB), pyrrolidine dithiocarbamate. J Leukoc Biol Jun. 2006;79:1193–1201. doi: 10.1189/jlb.0805453. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Poulaki V, Doehmen S, Welsandt G, Radetzky S, Lappas A, et al. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Invest Ophthalmol Vis Sci. 2003;44:2184–2191. doi: 10.1167/iovs.02-0589. [DOI] [PubMed] [Google Scholar]
- La Heij E, Kuijpers RW, Baarsma SG, Kijlstra A, van der Weiden CM. Adhesion molecules in iris biopsy specimens from patients uveitis. Br J Ophthalmol. 1998;82:432–437. doi: 10.1136/bjo.82.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Mandai M, Mittag TW, Kogishi J, Masayoshi I, Masanori H, Yoshimura N. Role of nitric oxide synthase isozymes in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1996;37:826–832. [PubMed] [Google Scholar]
- Mangano K, Niranjan S, D'Alcamo M, Libra M, Malaguarnera L, Donia M, et al. In vitro inhibition of enterobacteria-reactive CD4+CD25- T cells and suppression of immunoinflammatory colitis in mice by the novel immunomodulatory agent VGX-102. Eur J Pharmacol. 2008;586:313–321. doi: 10.1016/j.ejphar.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Rodrigues GB, Figueiredo CP, Rodrigues EB, Grumman A, Jr, Menezes-de-Lima O, Jr, et al. Molecular mechanisms of topical antiinflammatory effects of lipoxin: in endotoxin-induced uveitis Mol Pharmacol 200874154–161.Epub 2008 Apr 15 [DOI] [PubMed] [Google Scholar]
- Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthalmol Vis Sci. 1994;35:924–930. [PubMed] [Google Scholar]
- Smith JR, Hart PH, Williams KA. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol Cell Biol. 1998;76:497–512. doi: 10.1046/j.1440-1711.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- Stojanovic I, Cuzzocrea S, Mangano K, Mazzon E, Miljkovic D, Wang M, et al. In vitroex vivo and in vivo immunopharmacological activities of the isoxazoline compound VGX-1027: modulation of cytokine synthesis and prevention of both organ-specific and systemic autoimmune diseases in murine models. Clin Immunol. 2007;123:311–323. doi: 10.1016/j.clim.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Stosic-Grujicic S, Cvetkovic I, Mangano K, Fresta M, Maksimovic-Ivanic D, Harhaji L, et al. A potent immunomodulatory compound, (S,R)-3-phenyl-4,5-dihydro-5-isoxazole acetic acid, prevents spontaneous and accelerated forms of autoimmune diabetes in NOD mice and inhibits the immunoinflammatory diabetes induced by multiple low doses of streptozotocin in CBA/H mice. J Pharmacol Exp Ther. 2007;320:1038–1049. doi: 10.1124/jpet.106.109272. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ohgami K, Shiratori K, Jin XH, Ilieva I, Koyama Y, et al. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-kappaB signaling pathway. Exp Eye Res. 2006;82:275–281. doi: 10.1016/j.exer.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Suzuma K, Mandai M Kogishi J, Shinichiro JT, Honda Y, Yoshimura N. Role of P-selectin in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1997;38:1610–1618. [PubMed] [Google Scholar]
- Szabò C, Zingarelli B, O'Connor M, Salzman A. DNA strand breakage, activation of poly-ADP-ribose synthetase, and cellular energy depletion are involved in the cytotoxicity in macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci USA. 1996;93:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier Z. Human immunoglobulins in intraocular inflammation. Ann NY Acad Sci. 2007;1110:337–347. doi: 10.1196/annals.1423.036. [DOI] [PubMed] [Google Scholar]
- Van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- Whitcup SM, Chan CC, Kozhich AT, Magone MT. Blocking both E-selectin and P-selectin inhibits endotoxin-induced leukocyte infiltration into the eye. Clin Immunol. 1999;93:107–113. doi: 10.1006/clin.1996.4324. [DOI] [PubMed] [Google Scholar]
- Withcup SM, DeBarge LR, Rosen H, Nussenblatt RB, Chan CC. Monoclonal antibody against CD11b/CD18 inhibits endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1993;34:673–681. [PubMed] [Google Scholar]
- Withcup SM, Hikita N, Shirao M, Miyasaka M, Tamatani T, Mochizuki M, et al. Monoclonal antibodies against CD54 (ICAM-1) and CD11a (LFA-1) prevent and inhibit endotoxin-induced uveitis. Exp Eye Res. 1995;60:597–601. doi: 10.1016/s0014-4835(05)80001-6. [DOI] [PubMed] [Google Scholar]