Abstract
Background and purpose:
Arachidonyl trifluoromethyl ketone (ATK) is widely used as an inhibitor of cytosolic group IV phospholipase A2 (cPLA2) and calcium-independent group VI phospholipase A2 (iPLA2). ATK thus reduces arachidonic acid (AA) substrate for cyclooxygenase (COX; also known as prostaglandin H synthase) and attenuates prostaglandin (PG) synthesis. It has been shown previously, that ATK blocks thromboxane B2 production induced by exogenous AA in human platelets. It remains, however, unknown whether ATK also directly modulates the activity of cyclooxygenase (COX).
Experimental approach:
Time courses for inhibition of COX by ATK was obtained using osteoblast-like MC3T3-E1 cells, with exogenous AA as substrate and the pure enzymes COX-1 and COX-2. PGE2 was measured by GC-MS.
Key results:
ATK was a potent inhibitor of COX-1 and COX-2 with IC50 values of 0.5 and 0.1 μM in MC3T3-E1 cells and of 1.7 and 2.6 μM using the pure enzymes. Inhibition was reversible, with slow- and tight-binding characteristics. The arachidonyl carbon chain was essential, as the saturated palmitoyl analogue had no effect.
Conclusions and implications:
Attenuation of PG synthesis by ATK is taken to be the consequence of PLA2 inhibition and the findings of many studies are interpreted on that basis. If there are, however, alternative routes for AA liberation (such as phospholipase C/diacyl glycerol lipase or phospholipase D), this interpretation can lead to false conclusions. As ATK is a widely used and important pharmacological tool in eicosanoid research, knowledge of its interactions with other major enzymes of the cascade is of considerable importance.
Keywords: arachidonyl trifluoromethyl ketone, COX, prostaglandin, phospholipase, cyclooxygenase, MC3T3-E1, osteoblast
Introduction
Phospholipase A2 (PLA2) enzymes hydrolyze the fatty acid ester bond at the sn-2 position in phospholipids (Dennis, 1997). PLA2 activity results in the formation of lysophospholipids and the eicosanoid precursor arachidonic acid (AA). Alternatively, PLC and PLD enzymes can indirectly provide free AA. PLCs metabolize phosphatidylinositol (PI) and phosphatidylinositol phosphates to inositol phosphates and diacylglycerol (DAG) (Rebecchi and Pentyala, 2000). DAG is further metabolized sequentially by DAG lipase to 2-arachidonylglycerol and monoacylglycerol lipase or fatty acid amidohydrolase to release free AA (Chau and Tai, 1981), as found in platelets (Bell et al., 1979) and bovine pulmonary artery endothelial cells (Whatley et al., 1993). PLD hydrolyzes phospholipids, such as phosphatidylcholine, phosphatidylethanolamine and phosphatidylinositol to form phosphatidic acid, which can be further metabolized to DAG and lysolphosphatidic acid (Exton, 1990). DAG can provide AA through the route described above.
Free AA is subsequently metabolized to prostaglandins (PGs) by COX enzymes. Two isoforms exist, the constitutively expressed COX-1 and the inducible COX-2. In MC3T3-E1 osteoblastic cells both enzymes lead to the formation of PGE2, which is considered to be a powerful modulator of bone cell function. As in osteoblasts, prostanoids are ubiquitously produced by many cell types and serve as autocrine and paracrine mediators of numerous cellular functions. Inhibition of COX by non-steroidal anti-inflammatory drugs is only one example of the extensive potential of pharmacological intervention at the site of prostanoid synthesis.
AA release is considered as the rate-limiting step in PG synthesis and many studies have been performed to clarify the function of various PL enzymes in this event. Classifying and assigning PL activity relies largely on the use of pharmacological modulators of these enzymes. Thus, Lucas and Dennis (2005) have reviewed how to distinguish PLA2 types in biological samples using group-specific assays and inhibitors. One of the most widely used inhibitors is arachidonyl trifluoromethyl ketone (ATK). It is considered to be a specific, slow- and tight-binding inhibitor of group IV cPLA2 (Street et al., 1993) and group VI iPLA2 (Ackermann et al., 1995; Ghomashchi et al., 1999). It has been used in numerous studies to link PG synthesis to the activity of certain PLA types (Kurusu et al., 1997; Kuwata et al., 1998; Saunders et al., 1999; D'Orazi et al., 2006). Attenuation of PG synthesis was thereby attributed to the inhibitory effect of ATK on the above-mentioned PLA2s. Inhibition of PLAs certainly blunts PG formation, and the conclusions of such inhibition experiments may be correct if there are no other effects of ATK on the PG synthesizing system. It has been previously shown that ATK blocks AA-induced thromboxane B2 production in human platelets, but direct effects of this compound on COX enzymes have not been investigated so far (Riendeau et al., 1994). This study was therefore conducted to investigate such effects. Our results showed that ATK potently inhibited both COX-1 and COX-2.
Materials and methods
Cell culture
MC3T3-E1 cells (passage number 10–20, kindly provided by) were cultured routinely in α-minimum essential medium (α-MEM) containing 5% fetal calf serum (FCS), gentamycin sulphate (83.4 mg L−1), 50 μg mL−1 ascorbate and L-glutamine (0.584 g L−1) in a humidified atmosphere of 5% CO2 in 80 cm2 flasks (initial plating density 2 × 104 cells cm−2) and transferred to 4 cm2 12-well culture dishes before experiments. Experiments were carried out at confluency (day 7 of culture). For serum induction of COX-2, cells were cultured under starving conditions (0.2% FCS in α-MEM) for 24 h. Serum induction was accomplished with 5% FCS in α-MEM (Pilbeam et al., 1993).
COX-1 and COX-2 enzyme assays
Enzyme inhibitor potencies were measured in Tris–HCl buffer (30 mM, pH 8.0) containing glutathione (0.49 mM), adrenaline (1 mM) and haematin (1 μM). One unit of COX and 1 μM sodium arachidonate were used. Pre-incubation with ATK was carried out for 30 min at room temperature. Incubation with sodium arachidonate was performed for 30 min at 37 °C. The mixture was then cooled in an ice bath and the reaction terminated by the addition of ice-cold formic acid (0.2%). Assay for PGE2 was then performed as described below.
PGE2 analysis
For assessment of short-term PGE2 production, medium (1 mL) was removed and the cell monolayer was incubated in 1 mL of HEPES-buffered (20 mM) Hank's balanced salt solution without calcium. Incubations with AA, test compounds or vehicle were carried out for 30 min. Pre-incubations with ATK were carried out for 30 min. For determination of long-term PGE2 synthesis, COX-2 was induced by serum in the presence of ATK for 3 h (Pilbeam et al., 1993). Subsequent stimulation with AA was carried out for 30 min. The incubation buffer was removed and PGE2 measured by GC-NICI-MS. Briefly, PGE2 was converted to its pentafluorobenzyl ester-trimethylsilyl ether-O-methyloxime derivative. Quantitation was carried out by the use of tetradeuterated PGE2 (Leis et al., 1987). A TACE GC-MS system (Thermo) was used. GC was performed on a 15 m DB-5MS fused silica capillary column (Thermo). The temperature of the splitless Grob injector was kept at 290 °C, initial column temperature was 160 °C for 1 min, followed by an increase of 40 ° min−1 to 310 °C. Negative ion chemical ionization was carried out in the single ion recording mode with methane as a moderating gas.
Western blot analysis of COX-2 induction
MC3T3-E1 cells were treated with different concentrations of ATK and COX-2 expression was induced with serum. Cells were washed with chilled phosphate-buffered saline (pH 7.4) and lysed on ice for 15 min in 100 μL of lysis buffer (HEPES, 50 mM; NaCl, 150 mM; EDTA, 1 mM; Na4P2O7, 10 mM; Na3VO4, 2 mM; NaF, 10 mM; Triton X-100, 1%, v/v; glycerol, 10%, v/v; protease inhibitor cocktail tablets and pH 7.4). Cell lysates were scraped off and cell debris was removed by centrifugation at 11 000 × g at 4 °C for 10 min. Protein lysates (130 μg) were diluted in NuPAGE LDS sample buffer and NuPAGE sample reducing agent to a final volume of 30 μL. The samples were heated for 10 min at 70 °C and then subjected to electrophoresis on 4–12% NuPAGE Bis–Tris gels, 1.5 mm in NuPAGE MES SDS running buffer (80 min at 130 V). Proteins were transferred to nitrocellulose membranes (2 h, 0.3 A) and blocked with non-fat milk powder. The blots were incubated with goat polyclonal antibody COX-2 (dilution 1:200 in 3% bovine serum albumin) for 2 h at room temperature. The membranes were washed and then incubated with horseradish peroxidase-conjugated donkey anti-goat IgG (dilution 1:200 000) for 1 h at room temperature. After washing, immunoreactive signals were detected with SuperSignal West Pico chemiluminescent substrate and exposure to Hyperfilm MP. For loading controls, membranes were stripped and re-probed with a primary antibody recognizing β-actin and horseradish peroxidase-conjugated goat anti-mouse IgG was used as a secondary antibody.
Association and dissociation time course of ATK in MC3T3-E1 cells
Experiments were performed with 10 μM ATK and 6 μM AA. For association time course, cells were pre-incubated with ATK for different times. At time 0, ATK and AA were added simultaneously. Dissociation behaviour was elaborated as follows: cells were pre-incubated with ATK for 30 min, medium was removed, cells were washed twice with incubation buffer and left for indicated periods of time before stimulating with AA for 30 min. Assay for PGE2 was then performed as described above.
Statistical methods and nomenclature
Statistical analysis was performed with Student's t-test for paired samples, where appropriate. All data shown are representative of at least three independent experiments. Sigmoidal fits were calculated with ORIGIN software version 6.0 from Microcalc using pooled data from triplicate measurements.
Nomenclature used in this paper conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Reagents
ATK and palmitoyl methyltrifluoro ketone (PTK) were purchased from Biomol, Hamburg, FRG. AA and HEPES buffer was from Sigma Chemical Co., Vienna, Austria, α-MEM and FCS were obtained from Sera-lab, Haywarth, UK. COX-1, COX-2 and PGH2 were from Cayman Chemical, Ann Arbor, MI, USA. Goat polyclonal antibody COX-2 (C20) and β-actin antibody (C4) were from Santa Cruz Biotechnology via Szabo, Vienna, Austria. Horseradish peroxidase-conjugated donkey anti-goat IgG was from Jackson and horseradish peroxidase-conjugated goat anti-mouse IgG was obtained through Rockland via Biomol, Hamburg, FRG. The L-glutamine was from Serva, via AL-Labortechnik, Amstetten, Austria. Trypsin-EDTA was purchased from Böhringer, Vienna, Austria. Pentafluorobenzyl bromide, bis(N,O-trimethylsilyl)-trifluoroacetamide, silylation grade pyridine, acetonitrile and O-methoxyamine hydrochloride were from Pierce Chemical Co., Vienna, Austria. Culture dishes were from Falcon through Szabo, Vienna, Austria. MC3T3-E1 cells were kindly donated by Dr Klaushofer (Vienna). Deuterated PGE2 was obtained through MSD Isotopes through IC Chemikalien GmbH, Vienna, Austria. All other chemicals and reagents were from Merck, Vienna, Austria.
Results
Inhibition of COX-1 and COX-2 by ATK in MC3T3-E1 cells
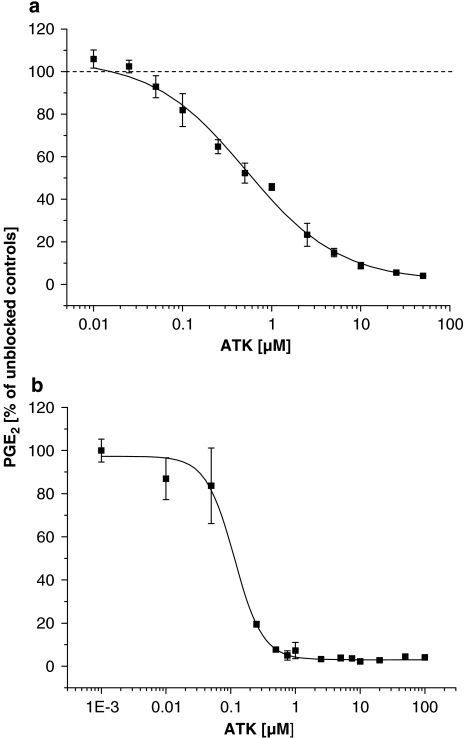
Inhibitor potencies of ATK on short-term (COX-1 related) PGE2 production in MC3T3-E1 cells are shown in Figure 1a. When stimulated with exogenous AA (6 μM), ATK blocked PGE2 synthesis in a dose-dependant manner by 96% with a calculated IC50 concentration of 0.5±0.08 μM (sigmoidal fit data: order=0.824±0.0981; χ2=1.67901; R2=0.99522). Calcium-free conditions and the use of exogenous AA substrate ensured uncoupling of the measured PGE2 synthesis from the effects of ATK on PL activities. Figure 1b shows the inhibitory effect of ATK on long-term (COX-2 related) PGE2 production in MC3T3-E1 cells. As induction of COX-2 was accomplished with serum for 3 h in the presence of ATK, the inhibitory effect seen in Figure 1b relates to the combined inhibition of both COX-1 and COX-2. Essentially, the same results were observed when medium was removed after induction of COX-2 (3 h) and stimulation with AA carried out in the buffer (results not shown). ATK blocked long-term PGE2 synthesis almost completely with an IC50 of 0.1±0.02 μM (sigmoidal fit data: order=1.9707±0.303; χ2=2.07969; R2=0.96661). Western blot analysis of COX-2 confirmed the induction of the enzyme by serum and revealed no influence of ATK on COX-2 protein expression. The results are shown in Figure 2.
Figure 1.
Inhibition of immediate (a) and delayed (b) PGE2 production by ATK in AA-stimulated MC3T3-E1 cells. Cells were cultured as described under methods. For short-term (COX-1 related) PGE2 synthesis, medium was exchanged with HEPES buffer. Cells were pre-incubated with ATK for 30 min, followed by stimulation with AA (6 μM) for 30 min. For long-term (COX-2 related) PGE2 synthesis, COX-2 was induced with serum in the presence of ATK for 3 h, followed by stimulation with AA (6 μM) for 30 min. PGE2 was measured in buffer or medium as described under methods.
Figure 2.
Western blot analysis of COX-2 expression in MC3T3-E1 cells. COX-2 induction was accomplished with serum in the presence of the indicated concentrations of ATK. β-Actin was used as a loading control. Western blotting conditions are described in detail under methods.
Inhibition of pure COX-1 and COX-2 by ATK
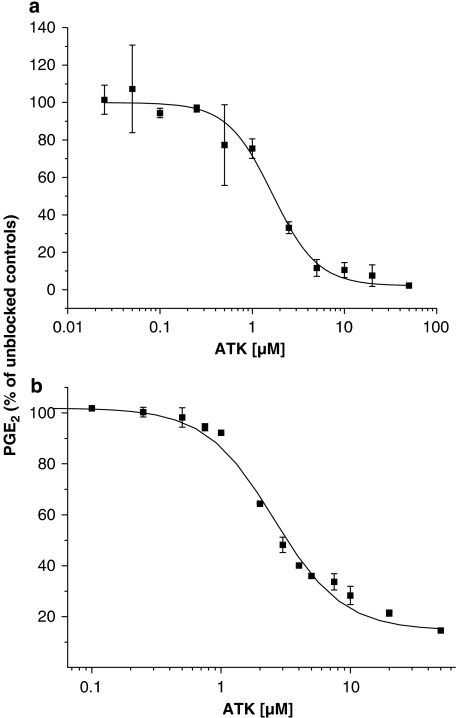
Inhibitor potencies of ATK on COX-1 and COX-2 are given in Figures 3a and b, respectively. Maximum inhibition caused by ATK was of 98% for COX-1 and 85% for COX-2. IC50 were 1.7±0.1 μM (sigmoidal fit data: order=1.7686±0.187; χ2=0.90592; R2=0.99971) for COX-1 and 2.6±0.2 μM (sigmoidal fit data: order=1.63448±0.15527; χ2=15.26087; R2=0.9951) for COX-2.
Figure 3.
Inhibition of PGE2 production of pure COX-1 (a) and COX-2 (b) by ATK in AA-stimulated MC3T3-E1 cells. Enzyme inhibitor potencies were measured in Tris–HCl buffer (30 mM, pH 8.0) containing glutathione (0.49 mM), adrenaline (1 mM) and haematin (1 μM). One unit of COX and 1 μM sodium arachidonate were used. Pre-incubation with ATK was carried out for 30 min at room temperature. Incubation with sodium arachidonate was performed for 30 min at 37 °C. PGE2 was measured as described under methods.
Effect of ATK on PGH2 conversion to PGE2
The effect of ATK was measured under the experimental conditions described above for pure COX-1 and COX-2. PGH2 at a concentration of 50 and 100 ng was used instead of COX enzymes. With 50 ng PGH2, the following amounts of PGE2 per experiment were measured: 8.8±0.6 ng (no ATK), 9.9±1.5 ng (10 μM ATK) and 8.6±0.1 ng (25 μM ATK). With 100 ng PGH2, the values for PGE2 per experiment were 24.0±1.9 ng (no ATK), 24.8±1.0 ng (10 μM ATK) and 23.8±0.3 ng (25 μM ATK).
Association and dissociation time course for ATK in MC3T3-E1 cells
Figures 4a and b show the association and dissociation time courses for ATK in MC3T3-E1 cells, respectively. As shown in Figure 4a, simultaneous addition of ATK and AA resulted in 43% inhibition of PGE2 synthesis. Ninty-three percent inhibition was achieved after 30 min pre-incubation time. Longer exposure to ATK did not significantly (P<0.05) enhance the inhibitory effect. However, up to 30 min there was a sustained attenuation of PGE2 production. Removing the inhibitor and equilibrating cells before AA stimulation for various periods of time yielded the dissociation time course displayed in Figure 4b. There was no significant recovery of PGE2 synthesis even after 90 min equilibration time (P<0.05).
Figure 4.
Association (a) and dissociation time course (b) of ATK in MC3T3-E1 cells. Experiments were performed with 10 μM ATK and 6 μM AA as described under methods. At time 0 in graph (a), ATK and AA were added simultaneously. Assay for PGE2 was then performed as described under methods.
Nature of ATK binding to COX
To check the binding behaviour of ATK to COX, varying concentrations of AA were used for stimulation after pre-incubation with ATK at different concentrations. The results are given in Figures 5a and b. In Figure 5a both, ATK (0.5, 1 and 2 μM) and AA (1, 2, 4 and 6 μM) were used at concentrations below the Km of COX (apparent 8.3 μM for COX-1 from ram seminal vesicle, Johnson et al., 1995). Inhibition kinetics is strictly dependant on the concentrations of substrate and inhibitor, suggesting a reversible mechanism. At ATK and AA concentrations above the Km of COX (ATK: 0, 10 and 25 μM; AA: 0.5, 10, 20 and 40 μM) the reversible nature is still apparent, as shown in Figure 5b.
Figure 5.
Inhibitor potency of ATK in MC3T3-E1 cells.
Effect of ATK analogues
A saturated analogue of ATK and PTK was used in this experiment. As shown in Figure 6, PTK has no effect on COX activity in MC3T3-E1 cells.
Figure 6.
Effects of PTK and ATK on PGE2 production in AA-stimulated MC3T3-E1 cells. Pre-incubation with ATK or PTK was carried out for 30 min at room temperature. Incubation with AA was performed for 30 min. PGE2 was measured as described under methods.
Discussion and conclusions
In this study, we found that ATK is a specific and potent blocker of cPLA2 and iPLA2, inhibited COX-1 and COX-2 in osteoblast-like MC3T3-E1 cells and in an enzyme assay using purified COX. At least in the experiments with MC3T3-E1 cells, PGE2 synthase must be considered as a potential target for ATK. However, as shown previously by another group, ATK-related inhibition of PGE2 synthase occurs only to a minimal extent, reaching 20% inhibition with 10 μM ATK (Quraishi et al., 2002).
For ATK-dependant inhibition of macrophage iPLA2, the IC50 value was found to be 15 μM (Ackermann et al., 1995). Thus, ATK must be considered as a strikingly more potent inhibitor of COX-1 and COX-2 on a molecular basis. As shown in our experiments, these effects are not related to some molecular interaction between ATK and the enzymatic product of COX, PGH2 and can thus be reasonably attributed to the inhibition of COX. Higher IC50 values obtained in the enzyme assay are not unusual, as the cellular environment assures more adequate conditions for optimal activity. The results from association time measurements indicate a relatively slow onset of ATK inhibition of COX, reaching its maximum effect only after 30 min. Surprisingly, apparent competition with AA substrate is rapid, which could be because of different binding affinities at the catalytic site of COX. Although we cannot infer what particular process is slow from our experiments, a slow binding velocity of ATK might reasonably explain the slow onset of inhibition.
Even more unique are the features of dissociation time courses. There was no noticeable reduction in blocking activity even after 90 min. At first glance this could be interpreted as an irreversible mode of action, but this can be ruled out for several reasons. First, AA competes dose-dependently with the inhibitor over a wide concentration range. Additionally, ATK shows slow- and tight-binding kinetics for human 85-kDa group IV cPLA2, showing only 14% dissociation of the Ca2+–cPLA2–ATK complex after 5 h (Street et al., 1993). In contrast, no such effects were observed with group VI iPLA2 (Ackermann et al., 1995). Nevertheless, such slow- and tight-binding could reasonably explain the findings of our dissociation experiments. Hypothetically, ATK could also act as a co-substrate of AA for COX-1 and COX-2. In this case, the inhibitor should be subjected to peroxidation and cyclooxygenation by COX, which would lead to the well-described suicide inhibition of the enzyme (Hemler and Lands, 1980; Wu et al., 1999). This would result in an irreversible loss of COX activity that could not be restored by AA. It is, however, clear that our dissociation and association data do not solely reflect substrate–enzyme binding rates. Membrane transport dynamics may significantly contribute to the time lag observed. In contrast, on the basis of similar polarity and structure to AA, ATK membrane transport should not be markedly different. In addition, our data suggest that inward membrane transport is effective enough to produce maximal inhibition after 30 min. Assuming a similar rate for the outward transport, some recovery of PG production should be observed after that time.
PTK, a saturated analogue of ATK, is a more potent inhibitor of group VI iPLA2 than ATK, whereas it is inactive towards group IV cPLA2 (Ackermann et al., 1995). In our study, PTK did not inhibit COX. This suggests that the unsaturated carbon backbone of ATK is essential for the binding and positioning of the inhibitor at the catalytic site of COX-1. In fact, there are more than 50 mostly hydrophobic interactions with 19 amino acid residues at this site, as described for AA (Thuresson et al., 2001).
Our results clearly demonstrate inhibitory effects of ATK on both COX-1 and COX-2. Under the conditions employed for measuring cellular COX-2 activity, however, there might be a concerted effect of ATK on enzyme inhibition and expression as well. Western blot analysis, however, clearly demonstrated that COX-2 protein expression was not influenced by ATK. Thus, the inhibitory effect of ATK is solely related to enzyme inhibition.
Inhibitors of COX enzymes have gained much attention and pharmacological applications are many (such as non-steroidal anti-inflammatory drugs). The main purpose of this study was not to define a potential function of ATK as another modulator of COX activity, but to prevent misinterpretation of experiments, where ATK-related attenuation of prostaglandin synthesis is accepted to be the direct consequence of PLA2 inhibition (Kurusu et al., 1997; Kuwata et al., 1998; Saunders et al., 1999; D'Orazi et al., 2006). If, however, alternative routes for AA liberation are present, such as DAG metabolism preceded by the action of PLC or PLD, then conclusions drawn on the AA liberation pathway involved could be untenable. As there is widespread use of ATK, which relies on the acknowledged specificity of ATK, it is of particular interest to elucidate effects of this compound on other key enzymes of the AA cascade.
In conclusion, ATK is a potent inhibitor of COX-1 and COX-2. On a molar basis, it is more effective in blocking COX than PLA2 enzymes. Inhibition by ATK is reversible and displays slow- and tight-binding characteristics.
Abbreviations
- α-MEM
α-minimum essential medium
- AA
arachidonic acid
- ATK
arachidonyl trifluoromethyl ketone
- COX
cyclooxygenase
- DAG
diacyl glycerol
- FCS
fetal calf serum
- GC-NICI-MS
gas chromatography-negative ion chemical ionization mass spectrometry
- PG
prostaglandin
- PL
phospholipase
- PLA2
phospholipase A2
- PLC
phospholipase C
- PLD
phospholipase D
- PTK
palmitoyl methyltrifluoro ketone
Conflict of interest
The authors state no conflict of interest.
References
- Ackermann EJ, Conde-Frieboess K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 Suppl. 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Kennerly DA, Stanford N, Majerus PW. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci USA. 1979;76:3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau LY, Tai HH. Release of arachidonate from diglyceride in human platelets requires the sequential action of a diglyceride lipase and a monoglyceride lipase. Biochem Biophys Res Commun. 1981;100:1688–1695. doi: 10.1016/0006-291x(81)90713-0. [DOI] [PubMed] [Google Scholar]
- D'Orazi G, Sciulli MG, Di Stefano V, Riccioni S, Frattini M, Falcioni R, et al. Homeodomain-interacting protein kinase-2 restrains cytosolic phospholipase A2-dependent prostaglandin E2 generation in human colorectal cancer cells. Clin Cancer Res. 2006;12:735–741. doi: 10.1158/1078-0432.CCR-05-1557. [DOI] [PubMed] [Google Scholar]
- Dennis EA. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- Exton JH. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990;265:1–4. [PubMed] [Google Scholar]
- Ghomashchi F, Loo R, Balsinde J, Bartoli F, Apitz-Castro R, Clark JD, et al. Trifluoromethyl ketones and methyl fluorophosphonates as inhibitors of group IV and VI phospholipases A2: structure-function studies with vesicle, micelle, and membrane assays. Biochim Biophys Acta. 1999;1420:45–46. doi: 10.1016/s0005-2736(99)00056-5. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Lands WE. Evidence for a peroxide-initiated free radical mechanism of prostaglandin biosynthesis. J Biol Chem. 1980;255:6253–6261. [PubMed] [Google Scholar]
- Johnson JL, Wimsatt J, Buckel SD, Dyer RD, Maddipati KR. Purification and characterization of prostaglandin H synthase from sheep placental cotyledons. Arch Biochem Biophys. 1995;324:26–34. doi: 10.1006/abbi.1995.9934. [DOI] [PubMed] [Google Scholar]
- Kurusu S, Noguchi T, Kawaminami M, Hashimoto I. Role of cytosolic phospholipase A2 in eicosanoid generation by corpora lutea of pseudopregnant rats: effects of its specific inhibitor. Prostag Leukotr Essent Fatty Acids. 1997;57:119–124. doi: 10.1016/s0952-3278(97)90001-6. [DOI] [PubMed] [Google Scholar]
- Kuwata H, Nakatani Y, Murakami M, Kudo I. Cytosolic phospholipase A2 is required for cytokine-induced expression of type IIA secretory phospholipase A2 that mediates optimal cyclooxygenase-2-dependent delayed prostaglandin E2 generation in rat 3Y1 fibroblasts. J Biol Chem. 1998;273:1733–1740. doi: 10.1074/jbc.273.3.1733. [DOI] [PubMed] [Google Scholar]
- Leis HJ, Hohenester E, Gleispach H, Mayer B, Malle E. Measurement of prostaglandins, thromboxanes and hydroxy fatty acids by stable isotope dilution gas chromatography/mass spectrometry. Biomed Environ Mass Spectrom. 1987;14:617–621. doi: 10.1002/bms.1200141108. [DOI] [PubMed] [Google Scholar]
- Lucas KK, Dennis EA. Distinguishing phospholipase A2 types in biological samples by employing group-specific assays in the presence of inhibitors. Prostaglandins Other Lipid Mediat. 2005;77:235–248. doi: 10.1016/j.prostaglandins.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Pilbeam CC, Kawaguchi H, Hakeda Y, Voznesensky O, Alander CB, Raisz LG. Differential regulation of inducible and constitutive prostaglandin endoperoxide synthase in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993;268:25643–25649. [PubMed] [Google Scholar]
- Quraishi O, Mancini JA, Riendeau R. Inhibition of inducible prostaglandin E2 synthase by 15-deoxy-Δ12,14-prostaglandin J2 and polyunsaturated fatty acids. Biochem Pharmacol. 2002;63:1183–1189. doi: 10.1016/s0006-2952(02)00844-4. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, function and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Riendeau D, Guay J, Weech PK, Laliberte F, Yergey J, Li C, et al. Arachidonyl trifluoromethyl ketone, a potent inhibitor of 85-kDa phospholipase A2, blocks production of arachidonate and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets. J Biol Chem. 1994;269:15619–15624. [PubMed] [Google Scholar]
- Saunders MA, Belvisi MG, Cirino G, Barnes PJ, Warner TD, Mitchell JA. Mechanisms of prostaglandin E2 release by intact cells expressing cyclooxygenase-2: evidence for a ‘two-component' model. J Pharmacol Exp Ther. 1999;288:1101–1106. [PubMed] [Google Scholar]
- Street IP, Lin HK, Laliberté F, Ghomashchi F, Wang Z, Perrier H, et al. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- Thuresson ED, Lakkides KM, Rieke CJ, Sun Y, Wingerd BA, Micielli R, et al. Prostaglandin endoperoxide H synthase-1: the function of cyclooxygenase active site residues in the binding, positioning and oxygenation of arachidonic acid. J Biol Chem. 2001;276:10347–10357. doi: 10.1074/jbc.M009377200. [DOI] [PubMed] [Google Scholar]
- Whatley RE, Stroud ED, Bunting M, Zinnerman GA, Mclntyre TM, Prescott SM. Growth-dependent changes in arachidonic acid release from endothelial cells are mediated by protein kinase C and changes in diacylglycerol. J Biol Chem. 1993;268:16130–16138. [PubMed] [Google Scholar]
- Wu G, Wei C, Kulmacz RJ, Osawa Y, Tsai A. A mechanistic study of self-inactivation of the peroxidase activity in prostaglandin H synthase-1. J Biol Chem. 1999;274:9231–9237. doi: 10.1074/jbc.274.14.9231. [DOI] [PubMed] [Google Scholar]