Abstract
Background and purpose:
Antagonism of the gastric inhibitory polypeptide (GIP) receptor with daily injection of proline-3 gastric inhibitory polypeptide ((Pro3)GIP) can reverse or prevent many of the metabolic abnormalities associated with diet-induced obesity-diabetes (diabesity). This study has examined the ability of a novel and longer-acting form of (Pro3)GIP, (Pro3)GIP mini-polyethylene glycol ((Pro3)GIP[mPEG]), to counter diet-induced diabesity in mice, using a daily and intermittent dosing regime.
Experimental approach:
We studied the actions of (Pro3)GIP[mPEG] at the GIP receptor in vitro and in vivo in both dietary and genetic diabesity.
Key results:
(Pro3)GIP[mPEG] was completely resistant to degradation by dipeptidyl peptidase IV. (Pro3)GIP[mPEG] inhibited GIP-induced cAMP and insulin production in vitro. A greater and prolonged antagonism of GIP-induced glucose-lowering action was followed (Pro3)GIP[mPEG] administration, compared with (Pro3)GIP. In contrast with (Pro3)GIP, mice injected once every 3 days for 48 days with (Pro3)GIP[mPEG] displayed reduced body weight gain and hyperinsulinemia with improved glucose tolerance and insulin secretory responses, compared with high-fat-fed controls. Daily i.p. injection of (Pro3)GIP, (Pro3)GIP[mPEG] or (Pro3)GIP b.i.d. for 21 days also decreased body weight, circulating plasma insulin levels and improved glucose tolerance, compared with high-fat controls. Plasma triglycerides were decreased by (Pro3)GIP[mPEG] and (Pro3)GIP b.i.d. treatment groups. The observed changes were accompanied by enhancement of insulin sensitivity in all treatment regimes. (Pro3)GIP[mPEG] was also effective over 16 days treatment of genetically obese-diabetic ob/ob mice.
Conclusions and implications:
These data demonstrate the utility of GIP receptor antagonism for the treatment of diabesity and the potential offered by (Pro3)GIP[mPEG] as a long-acting stable GIP receptor antagonist.
Keywords: gastric inhibitory polypeptide, obesity, antagonist, high-fat feeding, PEGylation, obesity
Introduction
Gastric inhibitory polypeptide (GIP) is a gastrointestinal hormone secreted from enteroendocrine K-cells in response to nutrient absorption following feeding (Brown, 1994). Although for some time it was believed to be purely an incretin hormone, moderating pancreatic β-cell insulin release, several studies have indicated that GIP has wider effects on extra-pancreatic sites (Vella and Rizza, 2004). The GIP receptor is expressed on various extra-pancreatic tissues, including bone, intestine, heart, stomach, brain and adipose tissue (Usdin et al., 1993). Furthermore, the potent and protracted secretion of GIP following fat ingestion (Ross and Dupre, 1978) would indicate a crucial role of GIP in fat metabolism (Yip and Wolfe, 2000). In this regard, early and more recent studies have now identified overstimulation of the GIP receptor as a key link between consumption of energy-rich high-fat diets and obesity-diabetes (hereafter ‘diabesity') (Flatt et al., 1983; Bailey et al., 1986; Yamada et al., 2006; Hansotia et al., 2007).
On the basis of these observations, a clear rationale for the use of GIP receptor antagonists as a treatment of diabesity has emerged. Thus, both normal and obese-diabetic (ob/ob) mice with genetic knockout of the GIP receptor are protected from diet-induced obesity (Miyawaki et al., 2002). Our previous studies in ob/ob and diet-induced obese mice have shown that subchronic daily administration of the specific and stable GIP receptor antagonist, proline-3 gastric inhibitory polypeptide ((Pro3)GIP) (Gault et al., 2002), can prevent or reverse many of the established metabolic abnormalities associated with diabesity (Gault et al., 2005, 2007a; Irwin et al., 2007; McClean et al., 2007). Ultimately however, the circulating half-life of (Pro3)GIP is subject to renal clearance, which detracts from its therapeutic utility (Meier et al., 2004).
One possible approach to avoid renal filtration and clearance from the body involves the design and synthesis of fatty acid derivatives of (Pro3)GIP. We have previously characterized a fatty acid-derivatized (Pro3)GIP analogue and shown that acylation of Lys16 with palmitic acid in (Pro3)GIP does not readily improve its biological effectiveness as a GIP receptor antagonist during a once-daily dosing regime in ob/ob mice (Gault et al., 2007b). Consequently, a second approach to avoid renal filtration and clearance of (Pro3)GIP might be through addition of a mini-polyethylene glycol (mPEG) residue. PEGylation of peptides and proteins has been shown to prolong circulating half-lives through decreased renal clearance and increased proteolytic stability (Harris and Chess, 2003). The usefulness of this approach has been successfully exploited using glucagon-like peptide-1 (GLP-1) and positive effects on stability and bioactivity have been obtained with a truncated form of GIP (Salhanick et al., 2005; Lee et al., 2006).
We have recently reported that repeated daily administration of (Pro3)GIP is associated with elevated (Pro3)GIP levels at least 24 h after the previous injection (McClean et al., 2007), reminiscent of the delayed clearance of exendin-4 (Simonsen et al., 2006). This study was designed to investigate the metabolic stability, biological activity and duration of action of a novel long-acting (Pro3)GIP peptide, (Pro3)GIP mini-polyethylene glycol ((Pro3)GIP[mPEG]). (Pro3)GIP[mPEG] has a mPEG group conjugated to the C-terminal of (Pro3)GIP. This smaller mPEG molecule possesses similar properties to larger PEG conjugates, but should cause less interference with biological activity (Gault et al., 2008). The stability of (Pro3)GIP[mPEG] and biological activity were examined and compared with (Pro3)GIP. In addition, normal mice fed with a high-fat diet or mutant ob/ob mice were used to examine whether prolonged daily injections of (Pro3)GIP[mPEG] were able to reverse diet- or genetically induced diabesity in a similar fashion to (Pro3)GIP (McClean et al., 2007). Furthermore, high-fat-fed mice were also used to examine the subchronic metabolic effects of (Pro3)GIP[mPEG] administration once every 3 days.
Materials and methods
Peptide synthesis
Native GIP, (Pro3)GIP and (Pro3)GIP[mPEG] were purchased from Sigma Genosys (Cambridge, UK). (Pro3)GIP[mPEG] was created by addition of a 145 Da PEG residue to the C-terminus of (Pro3)GIP. All peptides were characterized using matrix-assisted laser desorption ionization-time of flight mass spectrometry as described previously (Gault et al., 2007b).
Assessment of peptide degradation
Degradation of native GIP, (Pro3)GIP and (Pro3)GIP[mPEG] was performed using HPLC analysis as described previously (Gault et al., 2007b). Briefly, (Pro3)GIP[mPEG] was incubated in vitro at 37 °C in 50 mM triethanolamine–HCl (pH 7.8) with dipeptidyl peptidase IV (DPP-IV) (5 mU) for 0, 2, 4, 8 and 24 h. Reactions were subsequently terminated by addition of 10% (v/v) trifluoroacetic acid/water and intact GIP separated from the major degradation product GIP(3–42) by HPLC and peaks collected manually before electrospray ionization-MS. HPLC peak area data were used to calculate percentage intact peptide remaining at various time points throughout incubation.
In vitro biological activity
BRIN-BD11 cells were harvested (McClenaghan et al., 1996), seeded into 96-well plates (3 × 104 cells per well) and grown for 16 h. Cells were washed twice in Hank's buffered saline buffer and incubated (20 min; 37 °C) with varying concentrations of (Pro3)GIP or (Pro3)GIP[mPEG] in the presence of native GIP (10−7 M) in Hank's buffered saline∣ buffer containing 1 mM IBMX. Medium was subsequently removed, cells were lysed and cAMP levels in the lysate were measured using an high throughput screening (HTS) chemiluminescent immunoassay kit (Millipore, Watford, UK).
For assessment of insulin-release, BRIN-BD11 cells were seeded into 24-well plates (105 cells per well) and allowed to attach overnight at 37 °C. Before acute tests, cells were pre-incubated (40 min; 37 °C) in Krebs–Ringer bicarbonate buffer (pH 7.4) supplemented with 0.5% (w/v) BSA and 1.1 mM glucose. Test incubations were performed in the presence of 5.6 mM glucose with a range of concentrations of (Pro3)GIP or (Pro3)GIP[mPEG] in the presence of native GIP (10−7 M). After incubation (20 min; 37 °C), the buffer was removed from each well and aliquots (200 μL) stored at −20 °C before measurement of insulin.
Animals
All animals had free access to drinking water, and experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986. Young (8-week-old) male NIH Swiss mice (Harlan Ltd, Oxon, UK) were divided into groups and housed individually in an air-conditioned room at 22±2 °C with a 12 h light:12 h dark cycle (0800–2000 hours). Groups of mice had access to a high-fat diet (45% fat, 20% protein and 35% carbohydrate; percentage of total energy of 26.15 kJ g−1; Special Diets Service, Essex, UK). Age-matched control mice from the same source had free access to standard rodent maintenance diet (10% fat, 30% protein and 60% carbohydrate; percentage of total energy of 12.99 kJ g−1; Trouw Nutrition, Cheshire, UK) and were used for comparative purposes as appropriate. Before commencement of experimental studies, all mice with diet-induced obesity were maintained on a high-fat diet for 154 days. Obesity and diabetes were clearly manifest as judged by body weight and plasma glucose and insulin analyses.
For another set of experiments, obese-diabetic (ob/ob) mice (18 weeks old) obtained from the colony maintained at Aston University were divided into groups and housed individually in an air-conditioned room at 22±2 °C with a 12 h light:12 h dark cycle (0800–2000 hours). Ob/ob mice had free access to standard rodent maintenance diet (as detailed above).
Acute in vivo studies
An initial acute experiment was preformed in high-fat mice to compare the duration of pharmacological activity of (Pro3)GIP and (Pro3)GIP[mPEG] by examining metabolic responses to i.p. injection of glucose (18 mmol kg−1) in combination with native GIP (25 nmol kg−1) at 4, 24 and 48 h after (Pro3)GIP, (Pro3)GIP[mPEG] or saline vehicle administration (25 nmol kg−1).
Long-term in vivo studies
Mice fed previously with a high-fat diet received i.p. injections of (Pro3)GIP, (Pro3)GIP[mPEG] (both at 25 nmol kg−1) or saline vehicle (control) once every 3 days (1700 hours) for 48 days. Food intake and body weight were recorded daily, whereas plasma glucose and insulin concentrations were monitored at intervals of 3–5 days. Intraperitoneal glucose tolerance (18 mmol kg−1) and insulin sensitivity (25 U kg−1) tests were performed at the end of the study period. In a separate series, evaluation of pancreatic β-cell secretory response to glucose (18 mmol kg−1), arginine (0.25 g kg−1), GLP-1 and glucagon (both at 25 nmol kg−1) were assessed in (Pro3)GIP[mPEG]-treated mice at the end of the study period.
In a second series of experiments, additional groups of high-fat animals received once-daily i.p. injections (1700 hours) of either saline vehicle (0.9% (w/v), NaCl), (Pro3)GIP, (Pro3)GIP[mPEG] (both at 25 nmol kg−1) or (Pro3)GIP b.i.d. (0900 and 1700 hours; both injections at 25 nmol kg−1) for 21 days. Observations were continued following cessation of all treatment regimes for a further 21 days. Plasma for measurement of cholesterol and triglycerides was taken on day 21. Intraperitoneal glucose tolerance (18 mmol kg−1) and insulin sensitivity (25 U kg−1) tests were performed on days 21 and 42.
In a third series of experiments, ob/ob mice received once-daily i.p. injections (1700 hours) of either saline vehicle (0.9% (w/v), NaCl), (Pro3)GIP or (Pro3)GIP[mPEG] (each at 25 nmol kg−1) for 16 days. At the end of the treatment period, i.p. glucose tolerance (18 mmol kg−1) and insulin sensitivity (50 U kg−1) tests were performed.
Biochemical analysis
Blood samples (approximately 120 μL per bleed) were taken from the cut tip of the tail vein of conscious mice at times indicated in the figures and immediately centrifuged using a Beckman microcentrifuge (Beckman Instruments, Galway, Ireland) for 30 s at 13 000 g. The resulting plasma was then aliquoted into fresh Eppendorf tubes and stored at −20 °C before analysis. Where appropriate, liver was excised, weighed and stored for measurement of triglyceride content as described previously (Carr et al., 1993). Furthermore, locomotor activity tests were performed as assessed from total distance traversed in an open field (120 × 120 cm surface area with 35-cm-high walls), calculated from measurement of line breaks (15 × 15 cm grid) (McClean et al., 2007). Plasma glucose was assayed by an automated glucose oxidase procedure using a Beckman Glucose Analyzer II (Beckman Instruments, Galway, Ireland) (Stevens, 1971), with the exception of insulin sensitivity tests where plasma glucose was measured from whole blood using the ‘plasma calibrated' Ascensia Contour Blood Glucose Meter (Bayer AG, Leverkusen, Germany). Plasma insulin was assayed by a modified dextran-coated charcoal radioimmunoassay (Flatt and Bailey, 1981). Plasma and tissue triglyceride and cholesterol levels were measured using a Hitachi Automated Analyzer 912 (Boehringer, Mannheim, Germany).
Statistics
Results are expressed as mean±s.e.mean. Data were compared using ANOVA, followed by a Student's–Newman–Keuls post hoc test. Area-under-the-curve (AUC) analyses were calculated using the trapezoidal rule with baseline subtraction (Burington, 1973). P<0.05 was considered to be statistically significant.
Results
Degradation and in vitro actions of GIP, (Pro3)GIP and (Pro3)GIP[mPEG] by DPP-IV
Gastric inhibitory polypeptide was rapidly degraded by DPP-IV with a half-life of 2.2 h (Figure 1a). After 8 h, GIP was completely degraded to GIP(3–42). In contrast, both (Pro3)GIP and (Pro3)GIP[mPEG] remained fully intact after prolonged incubations up to and including 24 h. When incubated in the presence of native GIP, both (Pro3)GIP and (Pro3)GIP[mPEG] significantly inhibited (2.9- and 2.3-fold, respectively; P<0.001) GIP-induced cAMP production with maximal inhibition observed at 10−6 M (Figure 1b). Figure 1c shows the effects of (Pro3)GIP and (Pro3)GIP[mPEG] on GIP-induced insulin secretion from clonal pancreatic BRIN-BD11 cells. Both (Pro3)GIP and (Pro3)GIP[mPEG] dose-dependently inhibited GIP-induced insulin-release (1.1- to 1.4-fold; P<0.01–P<0.001).
Figure 1.
Effects of (Pro3)GIP[mPEG] and (Pro3)GIP on (a) stability to DPP-IV, (b) cAMP production, (c) insulin secretion and (d–f) glucose homeostasis and insulin release before a GIP plus glucose peptide response test in mice with diet-induced obesity. (a) Resistance of (Pro3)GIP[mPEG] to degradation by DPP-IV (5 mU) was measured (n=3) following 0, 2, 4, 8 and 24 h incubations. Reaction products were subsequently analysed by HPLC and degradation expressed as a percentage of intact peptide relative to the major degradation fragment, GIP(3–42). (b) BRIN-BD11 cells were exposed to various concentrations of (Pro3)GIP[mPEG] in the presence of stimulatory GIP (10−7 M) for 20 min (n=8) and cAMP production was subsequently measured using ELISA. (c) BRIN-BD11 cells were incubated with a range of concentrations of (Pro3)GIP[mPEG] in the presence of stimulatory GIP (10−7 M) for 20 min and insulin release measured using RIA. (d–f) Tests were conducted 4, 24 and 48 h after a single injection of (Pro3)GIP (25 nmol kg−1), (Pro3)GIP[mPEG] (25 nmol kg−1) or saline vehicle (0.9% (w/v), NaCl) in mice previously fed with a high-fat-diet for 22 weeks. GIP (25 nmol kg−1) in combination with glucose (18 mmol kg−1) was administered by i.p. injection at time 0 min. Overall plasma glucose AUC values for 0–60 min post-injection are shown in Supplementary Table 1. Values represent means±s.e.mean. **P<0.01, ***P<0.001 compared with control. ΔΔP<0.01 compared with (Pro3)GIP.
Persistent antagonistic effects of (Pro3)GIP and (Pro3)GIP[mPEG] on GIP-induced glucose lowering in high-fat-fed mice
Administration of (Pro3)GIP or (Pro3)GIP[mPEG], 4 h previously, increased plasma glucose levels following combined i.p glucose (18 mmol kg−1) and GIP (25 nmol kg−1) injection, corresponding to significantly elevated (2.0-fold; P<0.01) overall glycaemic excursions, compared with control (Figure 1d). Similarly, administration of (Pro3)GIP and particularly (Pro3)GIP[mPEG] 24 h previously also significantly elevated (1.9- and 2.0-fold; P<0.05 and P<0.01, respectively) the overall glycaemic excursion (Supplementary Table 1). The protracted duration of action of (Pro3)GIP[mPEG] was still evident 48 h after administration, where the glycaemic excursion was still significantly raised (1.8-fold; P<0.05) compared with control (Supplementary Table 1).
Subchronic effects of once-daily injection of (Pro3)GIP[mPEG] and (Pro3)GIP versus (Pro3)GIP b.i.d. on food intake, body weight, plasma glucose and insulin concentrations in high-fat-fed mice
Administration of (Pro3)GIP, (Pro3)GIP b.i.d or (Pro3)GIP[mPEG] had no effect on energy intake (Figure 2a). However, from day 13 onwards, body weights of animals treated with (Pro3)GIP and (Pro3)GIP b.i.d. were significantly reduced (by 5.7–7.5 g; P<0.05–P<0.01) compared with high-fat controls (Figure 2b). This was accompanied by significantly reduced circulating glucose levels in mice treated with (Pro3)GIP b.i.d. and (Pro3)GIP once daily from day 7 and 17 onwards, respectively (Figure 2c). Similarly, (Pro3)GIP[mPEG] significantly lowered plasma glucose (1.3-fold; P<0.05) by day 17, and body weights were correspondingly reduced (by 3.3 g; P<0.01) compared with high-fat controls by day 16. Non-fasting plasma insulin concentrations followed a similar pattern with significant (2.2–4.0-fold; P<0.05–P<0.001) reductions in all groups from day 10 onwards (Figure 2d). Discontinuation of treatment regimes for 21 days revealed a persistent beneficial effect on body weight, plasma glucose and insulin in all treatment groups (Figure 2).
Figure 2.
Effects of once-daily injection of (Pro3)GIP[mPEG] and (Pro3)GIP versus twice-daily (Pro3)GIP administration on energy intake, body weight, plasma glucose and insulin in mice with diet-induced obesity. (a) Energy intake, (b) body weight, (c) plasma glucose and (d) plasma insulin were measured (at 3- to 4-day intervals) for 4 days before and 21 days during treatment with once-daily injection of (Pro3)GIP[mPEG] (25 nmol kg−1), (Pro3)GIP (25 nmol kg−1) or twice-daily (Pro3)GIP (2 × 25 nmol kg−1). Parameters continued to be monitored for 21 days following cessation of treatment. Mice had previously been fed with standard rodent maintenance diet (control) or high-fat-diet for 22 weeks. Values are mean±s.e.mean. for eight mice. *P<0.05, **P<0.01 and ***P<0.001 compared with control group. ΔP<0.05, ΔΔP<0.01, ΔΔΔP<0.001 compared with high-fat control group.
Subchronic effects of once-daily injection of (Pro3)GIP[mPEG] and (Pro3)GIP versus (Pro3)GIP b.i.d. on glucose tolerance and insulin sensitivity in high-fat-fed mice
High-fat-fed control animals exhibited impaired glucose tolerance (1.8-fold; P<0.001) compared with control animals on day 21 (Figure 3a, Supplementary Table 2). Groups treated with (Pro3)GIP b.i.d and (Pro3)GIP[mPEG] had significantly reduced glycaemic excursions compared with high-fat control (2.1- and 1.9-fold; P<0.001, respectively) and (Pro3)GIP animals (1.4- and 1.3-fold; P<0.001 and P<0.01, respectively). Glucose-induced insulin levels were significantly lowered in all treatment groups compared with high-fat controls (2.7- to 6.6-fold; P<0.001) (Figure 3b, Supplementary Table 2). Cessation of treatment regimes for 21 days resulted in similar glycaemic and insulin responses for all treatment groups (Supplementary Table 2).
Figure 3.
Effects of once-daily injection of (Pro3)GIP[mPEG] and (Pro3)GIP versus twice-daily (Pro3)GIP administration on glucose tolerance (a, b), plasma insulin response to glucose (c, d) and insulin sensitivity (e, f) in mice with diet-induced obesity. Glucose tolerance tests were conducted after 21 days of treatment with once-daily injection of (Pro3)GIP[mPEG] (25 nmol kg−1), (Pro3)GIP (25 nmol kg−1) or twice-daily (Pro3)GIP (2 × 25 nmol kg−1) (a, b). Responses were also measured at 21 days following cessation of treatment (c, d). Mice had previously been fed with standard rodent maintenance diet (control) or high-fat diet for 22 weeks. (a–d) Glucose (18 mmol kg−1) was administered at the time indicated by the arrow. Overall plasma glucose and insulin area-under-the-curve (AUC) values for 0–60 min post injection are shown in Supplementary Table 2. (e, f) Insulin sensitivity tests were conducted after 21 days of treatment with once-daily injection of (Pro3)GIP[mPEG] (25 nmol kg−1), (Pro3)GIP (25 nmol kg−1) or twice-daily (Pro3)GIP (2 × 25 nmol kg−1) (e). Responses were also measured at 21 days following cessation of treatment (f). Insulin (25 U kg−1) was administered at the time indicated by the arrow. Overall plasma glucose AUC values for 0–60 min post-injection are shown in Supplementary Table 2. Values are mean±s.e.m. for nine mice. *P<0.05, **P<0.01 and ***P<0.001 compared with control group. ΔP<0.05, ΔΔP<0.01, ΔΔΔP<0.001 compared with high-fat control group.
Following exogenous administration of insulin, plasma glucose concentrations were significantly lowered (1.9- to 2.4-fold; P<0.05–P<0.001) at 30 and 60 min post-injection in all treatment groups compared with high-fat controls (Figure 3e). Furthermore, there were no significant differences between treatment groups and normal controls in terms of post-injection and AUC glucose levels (Supplementary Table 2). Interestingly, following 21 days discontinuation of treatment regimes, plasma glucose responses were still significantly improved compared with high-fat-fed controls (Figure 3f, Supplementary Table 2).
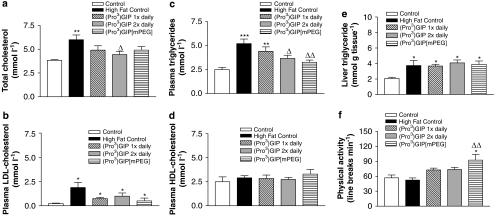
Subchronic effects of once-daily injection of (Pro3)GIP[mPEG] and (Pro3)GIP versus (Pro3)GIP b.i.d. administration on lipid profile, liver triglyceride content and physical activity in high-fat-fed mice
(Pro3)GIP b.i.d. treatment significantly reduced total plasma cholesterol and triglyceride levels (1.4-fold; P<0.05) compared with high-fat controls (Figures 4a and c). Furthermore, (Pro3)GIP[mPEG] treatment significantly (1.6-fold; P<0.01) lowered plasma triglyceride levels (Figure 4c). Neither high-fat feeding nor treatment regimes had any effect on plasma HDL-cholesterol concentrations (Figure 4d). Furthermore, although there was a trend for decreased LDL-cholesterol levels in all treatment groups compared with high-fat control mice, this failed to reach significance by day 21 (Figure 4b). High-fat feeding caused a significant elevation of liver triglyceride content (1.8-fold; P<0.05), which was unaffected by any of the treatment regimes (Figure 4e). High-fat feeding also had no significant effect on physical activity compared with control diet (Figure 4f). However, mice treated for 21 days with (Pro3)GIP[mPEG] exhibited significantly greater locomotor activity, compared with mice on a normal (1.6-fold; P<0.05) and high-fat diet (1.8; P<0.01).
Figure 4.
Effects of once-daily injection of (Pro3)GIP[mPEG] and (Pro3)GIP versus twice-daily (Pro3)GIP administration on lipid profile, liver triglyceride content and physical activity in mice with diet-induced obesity. (a) Total cholesterol, (b) plasma triglycerides, (c) HDL, (d) LDL, (e) liver triglyceride content and (f) physical activity were measured 21 days following treatment with once-daily injection of (Pro3)GIP[mPEG] (25 nmol kg−1), (Pro3)GIP (25 nmol kg−1) or twice-daily (Pro3)GIP (2 × 25 nmol kg−1). Mice had previously been fed with standard rodent maintenance diet (control) or high-fat diet for 22 weeks. Values are mean±s.e.mean for nine mice. *P<0.05, **P<0.01 and ***P<0.001 compared with control group. ΔP<0.05, ΔΔP<0.01 compared with high-fat control group.
Subchronic effects of (Pro3)GIP and (Pro3)GIP[mPEG] injection once every 3 days on food intake, body weight and non-fasting plasma glucose and insulin levels in high-fat mice
Administration of (Pro3)GIP[mPEG] once every 3 days caused a reduction in body weight. However, this failed to reach significance over the 48-day study period (Figure 5a). In spite of this, examination of 48-day body weight changes revealed a significant (1.1- to 1.6-fold; P<0.05, respectively) reduction in body weight gain in (Pro3)GIP[mPEG]-treated mice compared with (Pro3)GIP-treated and control mice (Figure 5c). These effects were not associated with changes in food intake (Figure 5b). Plasma insulin was significantly decreased (2.8- to 3.3-fold; P<0.05–P<0.001) in mice treated with (Pro3)GIP[mPEG] once every 3 days from day 2 onwards (Figure 5e). (Pro3)GIP-treated mice also displayed significantly (2.1- to 2.8-fold; P>0.05 to P<0.01) lowered plasma insulin levels compared with controls, but this effect diminished after day 13. These changes in insulin levels were not accompanied by significantly altered non-fasting glucose concentrations (Figure 5d).
Figure 5.
Effects of (Pro3)GIP and (Pro3)GIP[mPEG] injections once every 3 days on body weight, energy intake, plasma glucose and insulin in mice with diet-induced obesity. (a) Body weight, (b) energy intake, (c) body weight change (d) plasma glucose and (e) plasma insulin were measured (at 3- to 4-day intervals) for 4 days before and 48 days during treatment with (Pro3)GIP[mPEG] or (Pro3)GIP once every 3 days (25 nmol kg−1). Mice had previously been fed with standard rodent maintenance diet (control) or high-fat diet for 22 weeks. Values are mean±s.e.mean for eight mice. *P<0.05, **P<0.01, ***P<0.001 compared with control and ΔP<0.05 compared with (Pro3)GIP-treated mice.
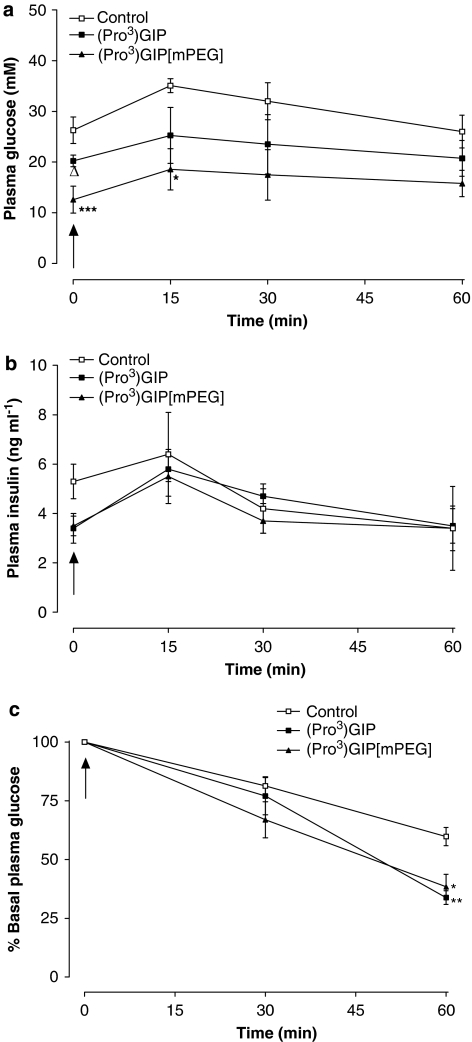
Subchronic effects of (Pro3)GIP and (Pro3)GIP[mPEG] injection once every 3 days on glucose tolerance and insulin sensitivity in high-fat-fed mice
Administration of (Pro3)GIP[mPEG] once every 3 days for 48 days significantly decreased (1.4-fold; P<0.05) plasma glucose levels compared with controls and (Pro3)GIP-treated mice at 60 min post-intraperitoneal glucose injection in high-fat-fed mice (Figure 6a). In harmony with this, the 0–60 min overall glycaemic excursion was significantly (1.3-fold; P<0.05) decreased in (Pro3)GIP[mPEG]-treated mice compared with high-fat saline control mice (Figure 6c). Overall plasma insulin concentrations were significantly (1.3-fold; P<0.05) enhanced following (Pro3)GIP[mPEG] treatment compared with controls (Figure 6d). The hypoglycaemic action of insulin was significantly (1.5-fold; P<0.01 to P<0.001) augmented 60 min post-insulin injection in high-fat-fed mice treated once every 3 days with (Pro3)GIP or (Pro3)GIP[mPEG] (Figure 6e). However, 0–60 min AUC measures revealed a significant (1.3-fold; P<0.05) enhancement of the actions of exogenous insulin in only (Pro3)GIP[mPEG]-treated animals (Figure 6f).
Figure 6.
Effects of (Pro3)GIP and (Pro3)GIP[mPEG] injections once every 3 days on glucose tolerance (a, c), plasma insulin response to glucose (b, d) and insulin sensitivity (e, f) in mice with diet-induced obesity. Glucose tolerance and insulin sensitivity tests were conducted after 48 days of treatment with (Pro3)GIP[mPEG] or (Pro3)GIP injections once every 3 days (25 nmol kg−1). Mice had previously been fed with standard rodent maintenance diet (control) or high-fat diet for 22 weeks. (a–d) Glucose (18 mmol kg−1) was administered at the time indicated by the arrow. Plasma glucose (a) and insulin (b) responses together with area-under-the-curve (AUC) values (c, d) for 0–60 min post-injection are shown. (e, f) Insulin (25 U kg−1) was administered at the time indicated by the arrow. Plasma glucose responses together with AUC values for 0–60 min post-injection are shown. Values are mean±s.e.mean for nine mice. P<0.05, **P<0.01 and ***P<0.001 compared with control and ΔP<0.05 compared with (Pro3)GIP-treated mice.
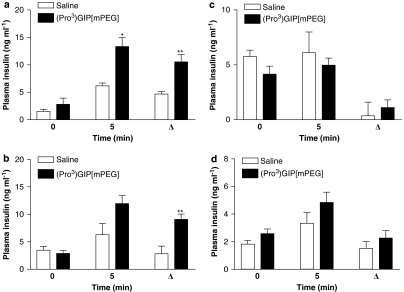
Subchronic effects of (Pro3)GIP[mPEG] injection once every 3 days on acute pancreatic β-cell response to glucose, arginine, GLP-1 and glucagon in high-fat-fed mice
Administration of (Pro3)GIP[mPEG] once every 3 days for 48 days evoked a pronounced rise in insulin release 5 min after glucose and arginine injection (Figures 7a and b). Furthermore, the 0–5 min difference in plasma insulin concentrations following glucose and arginine administration was significantly (2.2- and 3.2-fold; P<0.01, respectively) increased by (Pro3)GIP[mPEG] treatment compared with control (Figures 7a and b). This treatment had no effect on the acute pancreatic β-cell response to GLP-1 and glucagon (Figures 7c and d).
Figure 7.
Effects of (Pro3)GIP and (Pro3)GIP[mPEG] injections once every 3 days on insulin response to glucose (a), arginine (b), GLP-1 (c) and glucagon (d) in mice with diet-induced obesity. (Pro3)GIP[mPEG] (25 nmol kg−1) or saline vehicle (control) were administered once every 3 days for 48 days before tests. Plasma insulin was measured immediately before and 5 min after i.p. injection of glucose (18 mmol kg−1), arginine (0.25 g kg−1), GLP-1 or glucagon (both at 25 nmol kg−1). Values are mean±s.e.mean for five mice. *P<0.05 and **P<0.01 compared with control.
Subchronic effects of daily injection of (Pro3)GIP and (Pro3)GIP[mPEG] on body weight, food intake, plasma glucose and insulin, glucose tolerance and insulin sensitivity in ob/ob mice
Administration of (Pro3)GIP or (Pro3)GIP[mPEG] for 16 days in ob/ob mice had no effect on food intake or body weight. In contrast, (Pro3)GIP or (Pro3)GIP[mPEG] significantly reduced plasma glucose concentrations (1.4-fold; P<0.01 and 2.1-fold; P<0.001, respectively) compared with controls (Table 1). Furthermore, Pro3)GIP[mPEG] treatment resulted in significantly reduced (1.4-fold; P<0.05) plasma glucose concentrations compared with animals receiving (Pro3)GIP. Both (Pro3)GIP or (Pro3)GIP[mPEG] significantly reduced plasma insulin (2.0-fold; P<0.05) (Table 1).
Table 1.
Effects of once-daily administration of (Pro3)GIP[mPEG] and (Pro3)GIP on food intake, body weight, plasma glucose and insulin in ob/ob mice
| Parameter |
Treatment |
|||||
|---|---|---|---|---|---|---|
|
Saline control |
(Pro3)GIP |
(Pro3)GIP[mPEG] |
||||
| Day 0 | Day 16 | Day 0 | Day 16 | Day 0 | Day 16 | |
| Food intake (g per day per mouse) | 8.6±0.5 | 7.4±0.7 | 9.7±0.3 | 8.9±0.3 | 8.7±0.5 | 7.7±0.9 |
| Body weight (g) | 89.5±2.7 | 89.3±3.0 | 90.2±2.6 | 87.8±2.9 | 90.3±3.1 | 88.6±2.8 |
| Glucose (mM) | 24.2±2.9 | 26.2±1.8 | 24.3±4.3 | 17.7±1.8** | 23.8±4.5 | 11.3±2.3***, Δ |
| Insulin (ng mL−1) | 9.7±1.3 | 8.4±1.4 | 8.0±1.4 | 3.5±1.2* | 8.0±1.5 | 4.1±0.7* |
Values are mean±s.e.mean for eight mice.
*P<0.05, **P<0.01 and ***P<0.001 compared with saline controls.
P<0.05 compared with (Pro3)GIP.
Daily administration of (Pro3)GIP or (Pro3)GIP[mPEG] for 16 days resulted in significantly reduced (1.3-fold; P<0.05 and 1.8-fold; P<0.001) plasma glucose concentrations following i.p. glucose load (Figure 8a, Supplementary Table 3). However, glucose-mediated plasma insulin concentrations were not significantly different (Figure 8b, Supplementary Table 3). As shown in Figure 8c, the hypoglycaemic action of insulin was significantly augmented in ob/ob mice treated with (Pro3)GIP (1.9-fold; P<0.05) or (Pro3)GIP[mPEG] (1.6-fold; P<0.01).
Figure 8.
Effects of once-daily injection of (Pro3)GIP[mPEG] or (Pro3)GIP on glucose tolerance and insulin sensitivity in ob/ob mice. Glucose tolerance tests (18 mmol kg−1) were conducted after 16 days of treatment with once-daily injection of (Pro3)GIP[mPEG] (25 nmol kg−1), (Pro3)GIP (25 nmol kg−1) or twice-daily injection of (Pro3)GIP (2 × 25 nmol kg−1). Plasma glucose (a) and insulin (b) responses together with area under the curve (AUC) values for 0–60 min post-injection are shown. In addition, insulin sensitivity (c) tests (25 U kg−1) were also conducted. Overall plasma glucose and insulin AUC values for 0–60 min post-injection are shown in Supplementary Table 3. Values are mean±s.e.mean for eight mice. *P<0.05, **P<0.01 and ***P<0.001 compared with saline controls.
Discussion
Recent observations have shown that antagonism of GIP signalling using (Pro3)GIP can both protect against and reverse aspects of genetically and diet-induced diabesity (Gault et al., 2005, 2007a; McClean et al., 2007). These studies have used (Pro3)GIP as an enzymatically stable and specific antagonist of the GIP receptor (Gault et al., 2002). However, despite resistance to enzymic degradation, (Pro3)GIP, similar to native GIP, is still subject to renal clearance (Meier et al., 2004). We have now taken a further step in optimizing the molecular design of the peptide-based GIP antagonist by specifically evaluating the effects of introducing a mPEG residue at the C-terminus of (Pro3)GIP.
As would be expected from previous research (Gault et al., 2002), (Pro3)GIP and (Pro3)GIP[mPEG] were completely resistant to enzymatic degradation by DPP IV. (Pro3)GIP[mPEG] exhibited similar dose-dependent inhibitory effects on GIP-induced cAMP production and insulin secretion as (Pro3)GIP (Gault et al., 2002). This indicates that (Pro3)GIP[mPEG] retained affinity and effectiveness at the GIP receptor, confirming expectations that (Pro3)GIP[mPEG] functions as a GIP receptor antagonist in vitro. Consistent with this view, (Pro3)GIP[mPEG] also displayed significant inhibitory effects on GIP-enhanced anti-hyperglycaemic actions when administered acutely to diet-induced obese mice. Moreover, the biological actions of (Pro3)GIP[mPEG] were greater and more protracted than (Pro3)GIP, indicative of increased bioactivity imparted by C-terminal mini-PEGylation.
In this study, daily treatment of high-fat-fed mice for 21 days with (Pro3)GIP reproduced the previously noted beneficial effects on obesity and associated metabolic disturbances (McClean et al., 2007). Thus, attenuation of GIP signalling by (Pro3)GIP significantly decreased body weight and improved glucose tolerance, insulin sensitivity and lipid profile. Twice-daily (Pro3)GIP and daily (Pro3)GIP[mPEG] treatment modestly increased the effectiveness of the GIP antagonist compared with control high-fat-fed mice. In addition, administration of (Pro3)GIP[mPEG] to high-fat-fed mice once every 3 days resulted in a similar progressive lowering of body weights, a significant decrease in body weight change and accompanying metabolic benefits over 48 days of treatment. Furthermore, daily (Pro3)GIP[mPEG] treatment in ob/ob mice resulted in a significantly improved biochemical profiles similar to that of the parent (Pro3)GIP molecule. As expected, food intake was unchanged in all of these studies ruling out the possibility that reduction of body weight was merely the consequence of decreased food intake (Gault et al., 2007a). These findings clearly emphasize the potential therapeutic value of this approach in breaking the detrimental link between over-nutrition and adiposity (Flatt et al., 1983; Bailey et al., 1986; Miyawaki et al., 2002; Hansotia et al., 2007).
The main metabolic benefits observed with daily mPEGylation administration included improvements of glucose tolerance and plasma triglycerides in high-fat-fed mice and decreased hyperglycaemia in ob/ob mice. However, of greater significance is the finding that (Pro3)GIP[mPEG] administration once every 3 days evoked similar beneficial metabolic effects as daily (Pro3)GIP treatment in high-fat-fed mice, highlighting the protracted action of (Pro3)GIP[mPEG], which was originally observed in acute studies. The development of a specific assay to directly measure (Pro3)GIP[mPEG] in plasma would provide more precise details of circulating half-life. However, this is particularly encouraging given that previous modification of (Pro3)GIP to carry a C-16 palmitate fatty acid at the ɛ-amino group of the naturally occurring Lys at position 37 did not significantly augment bioactivity during a once-daily treatment regime in obese-diabetic (ob/ob) mice (Gault et al., 2007b).
As expected from previous studies, a key component of the beneficial action of GIP receptor antagonism by (Pro3)GIP[mPEG] was the significant improvement of insulin sensitivity. This was observed during dosing regimes of once daily and once every 3 days and was coupled with a substantial improvement in glucose tolerance. However, as the sampling times during glucose tolerance tests do not allow assessment of early-phase insulin responses, we conducted a separate series of experiments to evaluate the acute (5 min) response to glucose and several other secretagogues in high-fat-fed mice treated with (Pro3)GIP[mPEG] once every 3 days. We found that (Pro3)GIP[mPEG] treatment potentiated glucose- and arginine-stimulated insulin secretion and increased β-cell sensitivity to glucose. Pancreatic insulin secretory capacity, as measured by arginine stimulation, has been shown previously to be a sensitive indicator of functional β-cell mass (Ward et al., 1983). The effects of GLP-1 and glucagon on β-cell response were less obvious, but it is likely that the relatively low prevailing glucose levels did not allow the triggering of β-cell signalling by these glucose-dependent potentiators of insulin secretion. As detrimental changes in β-cell function following high-fat feeding is a direct result of the proportional change in obesity and islet lipid content (Hull et al., 2005; Tushuizen et al., 2007), it seems likely that facilitated uptake and oxidation of circulating fatty acids by muscle and liver in (Pro3)GIP[mPEG]-treated mice might also extend to β-cells.
The finding that high-fat-fed mice treated daily with (Pro3)GIP[mPEG] displayed increased locomotor activity agrees with previous studies of GIP receptor antagonism or ablation (Hansotia et al., 2007; McClean et al., 2007). However, neither daily (Pro3)GIP nor twice daily (Pro3)GIP had similar effects, which contrasts with previous findings (McClean et al., 2007). This may reflect the relatively short duration of the 21-day study and the enhanced pharmacodynamic profile of (Pro3)GIP[mPEG], evidenced during the interrupted dosing regime. Thus, increase of energy expenditure might partly contribute to the beneficial metabolic effects of (Pro3)GIP[mPEG] treatment. However, transgenic mice with overexpression of GIP have been reported to display increased locomotor activity (Ding et al., 2006), which contrasts with these other findings. Furthermore, triglyceride stores in liver of high-fat-fed mice were not significantly decreased by any of the treatments, as has been observed in more protracted studies using (Pro3)GIP or GIP receptor knockout mice (Hansotia et al., 2007; McClean et al., 2007). This may again reflect the relatively short duration of the present study period. An interesting observation in this study was the persistence of beneficial effects following discontinuation of the daily (Pro3)GIP and (Pro3)GIP[mPEG] treatment regimes. Thus, body weights, circulating glucose and insulin, glucose-induced insulin release and insulin sensitivity of high-fat-fed mice were still significantly improved in the (Pro3)GIP and (Pro3)GIP[mPEG] groups 21 days after cessation of treatment. Persistence of the metabolic advantages might largely reflect maintenance of decreased body weight, which is known to be beneficial for metabolic control. The absence of compensatory hyperphagia in these animals is important and suggests a possible long-term effect of GIP receptor blockade on the mechanisms regulating body energy balance.
In conclusion, antagonism of GIP action using (Pro3)GIP or (Pro3)GIP[mPEG] in mice with established diabesity resulted in significant and sustained improvement of obesity and associated metabolic abnormalities. The functional characterization and more protracted action of (Pro3)GIP[mPEG] suggests that PEGylation represents a potentially attractive advance in the design of GIP receptor antagonists for future diabesity therapy. These studies indicate that injection of (Pro3)GIP[mPEG] to high-fat-fed mice once every 3 days for 48 days resulted in a significant amelioration of diabetes and associated metabolic disturbances. Overall, (Pro3)GIP[mPEG] represents a new drug candidate for potential once- or twice-weekly treatment regimes in diabesity.
Supplementary Material
Acknowledgments
These studies were supported by the SAAD Trading and Contracting Company and project grants from Diabetes UK and Diabetes Research and Wellness Foundation OF07.
Abbreviations
- DPP IV
dipeptidyl peptidase IV
- GIP
gastric inhibitory polypeptide
- GLP-1
glucagon-like-peptide-1
- mPEG
mini-polyethylene glycol
- (Pro3)GIP
proline-3 gastric inhibitory polypeptide
Conflict of interest
N Irwin, VA Gault and PR Flatt are shareholders in Diabetica Ltd.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Bailey CJ, Flatt PR, Kwasowski P, Powell CJ, Marks V. Immunoreactive gastric inhibitory polypeptide and K cell hyperplasia in obese hyperglycaemic (ob/ob) mice fed high fat and high carbohydrate cafeteria diets. Acta Endocrinol (Copenh) 1986;112:224–229. doi: 10.1530/acta.0.1120224. [DOI] [PubMed] [Google Scholar]
- Brown JC.Enteroinsular axis Gut Peptides: Biochemistry and Physiology 1994Raven Press: New York; 765–784.In: Dockray GJ, Walsh JH (eds). [Google Scholar]
- Burington RS. Handbook of Mathematical Tables and Formulae. McGraw-Hill: New York; 1973. [Google Scholar]
- Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- Ding KH, Zhong Q, Xie D, Chen HX, Della-Fera MA, Bollag RJ, et al. Effects of glucose-dependent insulinotropic polypeptide on behaviour. Peptides. 2006;27:2750–2755. doi: 10.1016/j.peptides.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Flatt PR, Bailey CJ. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia. 1981;20:573–577. doi: 10.1007/BF00252768. [DOI] [PubMed] [Google Scholar]
- Flatt PR, Bailey CJ, Kwasowski P, Swanston-Flatt SK, Marks V. Abnormalities of GIP in spontaneous syndromes of obesity and diabetes in mice. Diabetes. 1983;32:433–435. doi: 10.2337/diab.32.5.433. [DOI] [PubMed] [Google Scholar]
- Gault VA, Hunter K, Irwin N, Greer B, Green BD, Harriott P, et al. Characterisation and glucoregulatory actions of a novel acylated form of the (Pro3)GIP receptor antagonist in type 2 diabetes. Biol Chem. 2007b;388:173–179. doi: 10.1515/BC.2007.019. [DOI] [PubMed] [Google Scholar]
- Gault VA, Irwin N, Green BD, McCluskey JT, Greer B, Bailey CJ, et al. Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes. 2005;54:2436–2446. doi: 10.2337/diabetes.54.8.2436. [DOI] [PubMed] [Google Scholar]
- Gault VA, Kerr BD, Irwin N, Flatt PR. C-terminal mini-PEGylation of glucose-dependent insulinotropic polypeptide exhibits metabolic stability and improved glucose homeostasis in dietary-induced diabetes. Biochem Pharmacol. 2008;75:2325–2333. doi: 10.1016/j.bcp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Gault VA, McClean PL, Cassidy RS, Irwin N, Flatt PR. Chemical GIP receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high fat and cafeteria diets. Diabetologia. 2007a;50:1532–1540. doi: 10.1007/s00125-007-0710-4. [DOI] [PubMed] [Google Scholar]
- Gault VA, O'Harte FPM, Harriott P, Flatt PR. Characterization of the cellular and metabolic effects of a novel enzyme-resistant antagonist of glucose-dependent insulinotropic polypeptide. Biochem Biophys Res Commun. 2002;290:1420–1426. doi: 10.1006/bbrc.2002.6364. [DOI] [PubMed] [Google Scholar]
- Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007;117:143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Chess RB. Effects of PEGylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–224. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005;48:1350–1358. doi: 10.1007/s00125-005-1772-9. [DOI] [PubMed] [Google Scholar]
- Irwin N, McClean PL, O'Harte FPM, Gault VA, Harriott P, Flatt PR. Early administration of the glucose-dependent insulinotropic polypeptide receptor antagonist, (Pro3)GIP, prevents the development of diabetes and related metabolic abnormalities associated with genetically-inherited obesity in ob/ob mice. Diabetologia. 2007;50:1532–1540. doi: 10.1007/s00125-007-0692-2. [DOI] [PubMed] [Google Scholar]
- Lee YS, Lee SH, Byun Y, Lee KC. PEGylated glucagon-like peptide-1 displays preserved effects on insulin release in isolated pancreatic islets and improved biological activity in db/db mice. Diabetologia. 2006;49:1608–1611. doi: 10.1007/s00125-006-0234-3. [DOI] [PubMed] [Google Scholar]
- McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP receptor antagonism reverses obesity, insulin resistance and associated metabolic disturbances induced in mice by prolonged consumption of high fat diet. Am J Physiol Endocrinol Metab. 2007;293:E1746–E1755. doi: 10.1152/ajpendo.00460.2007. [DOI] [PubMed] [Google Scholar]
- McClenaghan NH, Barnett CR, Ah-Sing E, Abdel-Wahab YHA, O'Harte FPM, Yoon T-W, et al. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes. 1996;45:1132–1140. doi: 10.2337/diab.45.8.1132. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Nauck MA, Kranz D, Holst JJ, Deacon CF, Gaeckler D, et al. Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes. 2004;53:654–662. doi: 10.2337/diabetes.53.3.654. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, et al. Inhibition of gastric inhibitory polypeptide signalling prevents obesity. Nat Med. 2002;8:738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- Ross SA, Dupré J. Effects of ingestion of triglyceride or galactose on secretion of gastric inhibitory polypeptide and on responses to intravenous glucose in normal and diabetic subjects. Diabetes. 1978;27:327–333. doi: 10.2337/diab.27.3.327. [DOI] [PubMed] [Google Scholar]
- Salhanick AL, Clairemont KB, Buckholz TM, Pellegrino CM, Ha S, Lumb KJ. Contribution of site-specific PEGylation to the dipeptidyl peptidase IV stability of glucose-dependent insulinotropic polypeptide. Bioorg Med Chem Lett. 2005;15:4114–4117. doi: 10.1016/j.bmcl.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Holst JJ, Deacon CF. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia. 2006;49:706–712. doi: 10.1007/s00125-005-0128-9. [DOI] [PubMed] [Google Scholar]
- Stevens JF. Determination of glucose by an automatic analyser. Clin Chem Acta. 1971;32:199–201. doi: 10.1016/0009-8981(71)90332-9. [DOI] [PubMed] [Google Scholar]
- Tushuizen ME, Bunck MC, Pouwels PJ, Diamant M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care. 2007;30:2916–2921. doi: 10.2337/dc07-0326. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133:2861–2870. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- Vella A, Rizza RA. Extrapancreatic effects of GIP and GLP-1. Horm Metab Res. 2004;36:830–836. doi: 10.1055/s-2004-82617. [DOI] [PubMed] [Google Scholar]
- Ward WK, Halter JB, Best JD, Beard JC, Porte D., Jr Hyperglycaemia and beta-cell adaptation during prolonged somatostatin infusion with glucagon replacement in man. Diabetes. 1983;32:943–947. doi: 10.2337/diab.32.10.943. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Miyawaki K, Tsukiyama K, Harada N, Yamada C, Seino S. Pancreatic and extrapancreatic effects of gastric inhibitory polypeptide. Diabetes. 2006;55:S86–S91. [Google Scholar]
- Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci. 2000;66:91–103. doi: 10.1016/s0024-3205(99)00314-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.