Abstract
Background and purpose:
Fipronil is the active ingredient in a number of widely used insecticides. Human exposure to fipronil leads to symptoms (headache, nausea and seizures) typically associated with the antagonism of GABAA receptors in the brain. In this study, we have examined the modulation of the common brain GABAA receptor subtype by fipronil and its major metabolite, fipronil sulphone.
Experimental approach:
Whole-cell and single-channel recordings were made from HEK 293 cells transiently expressing rat α1β2γ2L GABAA receptors.
Key results:
The major effect of fipronil was to increase the rate of current decay in macroscopic recordings. In single-channel recordings, the presence of fipronil resulted in shorter cluster durations without affecting the intracluster open and closed time distributions or the single-channel conductance. The α1V256S mutation, previously shown alleviate channel inhibition by inhibitory steroids and several insecticides, had a relatively small effect on channel block by fipronil. The mode of action of fipronil sulphone was similar to that of its parent compound but the metabolite was less potent at inhibiting the α1β2γ2L receptor.
Conclusions and implications:
We conclude that exposure to fipronil induces accumulation of receptors in a novel, long-lived blocked state. This process proceeds in parallel with and independently of, channel desensitization. The lower potency of fipronil sulphone indicates that the conversion serves as a detoxifying process in mammalian brain.
Keywords: GABAA receptor, ion channel, insecticide
Introduction
Fipronil (5-amino-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[1(R,S)-(trifluoromethyl)sulphinyl]-1H-pyrazole-3-carbonitrile; Figure 1a) is the active ingredient in a number of widely used insecticides. It was introduced in Europe and in the United States in the mid 1990s, and has been successfully used for pest control in agriculture, public hygiene and veterinary medicine since then. Fipronil's mode of action is through the inhibition of glutamate- and GABA-activated chloride channels resulting in uncontrolled neural excitation and, eventually, the death of the insect (see Narahashi et al., 2007).
Figure 1.
Structures of fipronil and fipronil sulphone. Fipronil (a) is the active ingredient in a number of widely used insecticides. Fipronil sulphone (b) is the major metabolite of fipronil in mammals and insects.
Besides inhibiting insect channels, the compound also antagonizes mammalian GABAA receptors, although with lower potency. In electrophysiological and biochemical tests, fipronil fully blocks the insect chloride channels at concentrations below 100 nM (Zhao et al., 2003). In contrast, the IC50 for the mammalian GABAA receptor in dorsal root ganglia (DRG) lies in the micromolar range (Ikeda et al., 2001).
Notwithstanding the lower potency of fipronil on mammalian GABAA receptors, frequent contact with the compound for some groups of society (for example, field workers) means that accidental overexposure can, and has been reported to, occur. This results in symptoms and complications typically associated with the blockade of the GABAA receptor function, for example, headache, nausea and seizures (Mohamed et al., 2004). The presence of fipronil-containing products in many households suggests that the possibility of contamination and efficient ways to treat it should be considered (Jennings et al., 2002).
Despite such strong incentives, the molecular mechanism by which fipronil acts on GABAA receptors in the brain remains largely unknown. In DRG neurons, exposure to micromolar concentrations of fipronil results in the inhibition of GABA-elicited currents. Both unliganded closed and open channels can interact with fipronil, although channel activation appears to enhance the association and dissociation rates for the drug (Ikeda et al., 2001). Fipronil does not compete with picrotoxinin for a common binding site, and it was concluded that fipronil is a negative allosteric modulator of the GABAA receptor in DRG neurons (Ikeda et al., 2001).
But the GABAA receptor subtypes in the DRG are different from the ones found in the brain. Most notably, DRG neurons lack the α1- and γ2L-subunits (Maddox et al., 2004), whereas receptors containing these subunits (along with β2) are considered the most prevalent subtype in the brain (McKernan and Whiting, 1996). Subunit composition differences are known to affect both kinetics and pharmacology of the GABAA receptor (see Ebert et al., 1997; Picton and Fisher, 2007) suggesting that the findings from the work on fipronil actions on GABAA receptors in DRG cannot be simply translated to the brain setting. The symptoms seen following fipronil exposure suggest that most of the effects are associated with actions on the GABAA receptor function in the brain, not in the periphery, providing an additional motivation for these studies.
In both mammals and insects, fipronil is rapidly converted to fipronil sulphone (Figure 1b). Importantly, fipronil sulphone is found in brain samples of mice following intraperitoneal injections of fipronil (Hainzl et al., 1998). It is not clear how the conversion process affects toxicity of fipronil in mammals. Inhibition of cytochrome P450 monooxygenase with piperonyl butoxide does not affect, in mice, the toxicity of fipronil (Hainzl et al., 1998). This suggests that fipronil and its metabolite possess similar toxicity. In contrast, Zhao et al. (2005) found that fipronil sulphone is more potent (by almost an order of magnitude) than fipronil in blocking GABAA receptor currents in rat DRG neurons.
In this study, we have investigated the mechanism of action of fipronil and fipronil sulphone on the rat α1β2γ2L GABAA receptor. We found that fipronil and fipronil sulphone inhibit the receptor. In whole-cell recordings, the presence of fipronil resulted in faster current decay, whereas in single-channel recordings, the effect was manifested as briefer single-channel clusters. Fipronil was without effect on the intracluster kinetic properties suggesting that it does not influence the binding of GABA to the receptor or the channel gating properties. The kinetic effect of fipronil sulphone was similar to that of fipronil, but the metabolite was 10-fold less effective in producing channel inhibition.
Methods
Cells and culture conditions
Experiments were conducted on HEK 293 cells transiently expressing recombinant rat α1β2γ2L GABAA receptors. The cells (ATCC number CRL-1573) were grown in Dulbecco's Modified Eagle's Medium with 10% foetal bovine serum (Hyclone, Logan, UT, USA), penicillin (100 u mL−1) and streptomycin (100 μg mL−1) in a humidified atmosphere with 5% CO2 at 37 °C. The cells were passaged twice a week at 80–90% confluency and subcultured in 35 mm dishes for electrophysiological experiments.
Expression of GABAA receptors
The GABAA receptor subunit cDNAs were subcloned into the pcDNA3 expression vector (Invitrogen, Carlsbad, CA, USA) and transiently transfected into HEK cells using a calcium phosphate precipitation-based technique. A total of 3 μg of cDNA in the ratio of 1:1:1 (α:β:γ) was mixed with 12.5 μL of 2.5 M CaCl2, and distilled H2O to a final volume of 125 μL. The solution was added slowly, without mixing, to an equal volume of 2 × BES-buffered solution. The combined mixture was incubated at room temperature for 10 min followed by mixing the contents and an additional incubation for 15 min. The precipitate was then added to the cells in a 35 mm dish for overnight incubation at 37 °C, followed by the replacement of medium in the dish. The experiments were conducted during the next 2 days after changing the medium.
The α1-subunit is epitope (FLAG; Ueno et al., 1996) tagged in the aminoterminal end of the subunit. Cells expressing high levels of receptors (or, more precisely, the α-subunit) were identified using a bead-binding technique where the surface expression of the FLAG peptide was determined using a mouse monoclonal antibody to the FLAG epitope (M2, Sigma-Aldrich, St Louis, MO, USA), which had been adsorbed to beads with a covalently attached goat antimouse immunoglobulin G antibody (Dynal, Great Neck, NY, USA). The presence of the FLAG tag in the α-subunit is without effect on the kinetics of macroscopic currents or GABA dose–response properties (Ueno et al., 1996). In whole-cell recordings, the presence of the γ-subunit in the receptor complexes was verified from the position of the GABA dose–response curve, which is significantly left-shifted for the αβ-containing receptors compared to the αβγ receptors (Boileau et al., 2003; P Li and G Akk, unpublished data). Our previous tests have verified reliable incorporation of the γ-subunit in receptor complexes using our transfection techniques (Li et al., 2006).
Electrophysiological experimental conditions
The experiments were carried out using standard whole-cell voltage clamp and single-channel patch clamp methods. The bath solution contained (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose and 10 HEPES; pH 7.4. In whole-cell recordings, the pipette solution contained (in mM): 140 CsCl, 4 NaCl, 4 MgCl2, 0.5 CaCl2, 5 EGTA and 10 HEPES; pH 7.4. In single-channel recordings, the pipette solution contained (in mM): 120 NaCl, 5 KCl, 10 MgCl2, 0.1 CaCl2, 20 tetraethylammonium, 5 4-aminopyridine, 10 glucose and 10 HEPES; pH 7.4.
The agonist and modulator were applied through the bath using an SF-77B fast perfusion stepper system (Warner Instruments, Hamden, CT, USA) in whole-cell experiments, or added to the pipette solution in single-channel recordings. The drug delivery system in whole-cell recordings had a solution exchange time (measured by switching the bath solution from 100% Ringer's to 75% Ringer's) of 2–3 ms when measured with open tip, and 10–20 ms when measured with a typical HEK cell.
Stock solutions of fipronil and fipronil sulphone were made in dimethyl sulphoxide at 10–20 mM concentrations, and final dilutions were made immediately before the experiment. The maximal dimethyl sulphoxide concentration in diluted solutions was 0.3%. We have previously shown that the channel activation by GABA is not affected by the presence of 0.3% dimethyl sulphoxide (Li et al., 2007). All experiments were carried out at room temperature.
Recording and analysis in whole cells
The recording and analysis of whole-cell currents were carried out as described previously (Li et al., 2006). In most experiments, the cells were clamped at −60 mV. The drug application and washout lengths were specific to the particular experiment, and are further described and justified in the text. The currents were recorded using an Axopatch 200B amplifier (Molecular Devices, Union City, CA, USA), low-pass filtered at 2 kHz and digitized with a Digidata 1320 series interface (Molecular Devices) at 10 kHz. The analysis of whole-cell currents was carried out using the pClamp 9.0 software package, and was aimed at determining the amplitudes of the peak and steady-state responses, and the time constants for current decay or recovery. In agreement with previous data (Benkwitz et al., 2004), we found that the time course of current desensitization in the presence of GABA was dominated by a single exponential.
Recording and analysis of single channels
The recording and analysis of single-channel currents have been described in detail previously (Akk et al., 2001, 2004). The experiments were conducted in the cell-attached or inside-out configurations. The currents were obtained at 50 μM GABA, a concentration, which corresponds to approximately EC40 in the open probability dose–response curve (Steinbach and Akk, 2001), and in the presence of 1 mM GABA (a saturating concentration). In most experiments, the pipette potential was held at +60 to +80 mV, which translates to an approximately −120 to −100 mV potential difference across the patch membrane. Channel activity was recorded using an Axopatch 200B amplifier, low-pass filtered at 10 kHz, and acquired with a Digidata 1320 series interface at 50 kHz using pClamp software.
The analysis of single-channel currents was limited to clusters, that is, episodes of intense activity originating from the activation of a single ion channel. A cluster was defined as a series of openings separated by closed periods shorter than a critical duration (τcrit). The τcrit was chosen arbitrarily, but was at least 10 times longer (150–500 ms) than the longest-lived intracluster closed time component. In addition, we imposed a minimum duration (20 ms) and a minimum number of events (three) for a cluster to be accepted for further analysis. This was performed to eliminate channel activity of unknown origin. The currents were digitally filtered at 2–3 kHz and idealized using the QuB Suite (Qin et al., 1996; www.qub.buffalo.edu). The intracluster open and closed times were estimated from the idealized currents using a maximum likelihood method, which incorporates a correction for missed events (QuB Suite).
Statistical methods
The statistical analysis was performed using the Student's t-test with significance levels given in the text.
Chemicals
Fipronil sulphone was purchased from AccuStandard, Inc (New Haven, CT, USA). Fipronil and other chemicals were purchased from Sigma Chemical Co (St Louis, MO, USA).
Results
Exposure to fipronil inhibits GABAA receptor function through an increase in the apparent desensitization rate
Exposure to fipronil results in the modulation of α1β2γ2L GABAA receptor currents. Figure 2a shows sample recordings from a cell exposed to 1 mM GABA (a saturating concentration) in the absence and presence of 10 μM fipronil. The coapplication of the insecticide with GABA had minimal effect on the peak response, but the current decay during exposure to agonist, that is, apparent desensitization was significantly faster in the presence of fipronil than under control conditions. Current decay was adequately fitted to a single exponential, and in the presence of 1 mM GABA+10 μM fipronil, the decay time constant was 407±105 ms (n=10 cells). For comparison, the desensitization time constant was 5.6±2.3 s for 1 mM GABA alone (n=11 cells).
Figure 2.
Fipronil inhibits rat α1β2γ2L GABAA receptor function by increasing the apparent desensitization rate. (a) Sample whole-cell current traces obtained in response to 4-s application of 1 mM GABA or 1 mM GABA+10 μM fipronil. The traces shown had peak amplitudes of 2005 pA (GABA) or 2058 pA (GABA+fipronil). Fits of the current decay to a single exponential gave time constants of 6644 ms and 577 ms for GABA and GABA+fipronil, respectively. (b) A plot showing the relationship between the normalized decay time constant and fipronil concentration. The curve was fitted to the Hill equation with best-fit parameters IC50=1.1±0.2 μM and n=1.3±0.1. All data points were obtained from coapplication of fipronil with 1 mM GABA. Data (mean±s.e.mean) from four cells were used for the concentration–response relationship. The drug applications lasted for 4 s and were separated from successive applications with 30 s washout periods. Control responses were obtained between successive fipronil applications to verify full washout of the insecticide.
The faster rate of current decay in the presence of fipronil indicated that the insecticide was acting as an inhibitor of receptor function. Although the apparent kinetic mechanism is consistent with fipronil acting by enhancing the rate of desensitization, in the absence of detailed molecular data demonstrating that fipronil promotes channel desensitization, we have chosen to call the effect of fipronil on the GABAA receptor function inhibition or block.
In the simplest model, the current decay rate in the presence of 1 mM GABA+10 μM fipronil (1/407 ms) consists of the sum of the desensitization rate (k+D*) estimated in the absence of fipronil (1/5.6 s), and the rate of fipronil-induced inhibition (kon*).
We note that k+D*, as determined from the desensitization time constant in macroscopic recordings, is not the microscopic desensitization rate constant but rather represents the composite rate of entry into long-lived desensitized states under particular experimental conditions. Similarly, kon* and the recovery rate, koff, are not the microscopic association and dissociation rate constants for fipronil, instead reflecting the rates with which the inhibition develops or channels recover from the inhibition induced by fipronil. In the presence of 10 μM fipronil, kon* can be calculated as 2.5−0.2 s−1=2.3 s−1. Assuming a second order reaction, the rate constant for the development of fipronil-induced block was 0.23 μM−1 s−1 (∼2 × 105 M−1 s−1).
The concentration–effect relationship for the decay time constant was measured at 1 mM GABA in the absence and presence of 0.3–10 μM fipronil. At each fipronil concentration, the macroscopic current decay time constant was estimated and normalized to control value. In these experiments, GABA and fipronil were coapplied and successive test applications alternated with one or more control (GABA alone) applications to verify full washout of fipronil and the lack of slowly accumulating drug effect. The summary of data is shown in Figure 2b. The curve was fitted to the Hill equation yielding an IC50 of 1.1±0.2 μM and a Hill slope of 1.3±0.2. In most cells, coapplication of fipronil with GABA had a small (<10%) effect on the peak response.
In a simple second order reaction, the rate of development of inhibition increases linearly with blocker concentration. To test the linearity of block by fipronil, we plotted the rate of development of block (inverse of the decay time constant) as a function of fipronil concentration (Figure 3a). Linear regression analysis of data obtained in the presence of 0.3–10 μM fipronil gave a slope of 0.21±0.01 μM−1 s−1 and an intercept of 0.23±0.03 s−1 (R>0.99). Significant deviations from linearity were observed at 30–100 μM fipronil (data not shown). It is likely that poor aqueous solubility of fipronil (Stark and Vargas, 2005) is the cause for this, rather than a slow and rate-limiting conformational change following the binding of the insecticide. Accordingly, we have limited most experiments to the highest fipronil concentration of 10 μM.
Figure 3.
Kinetics of fipronil-induced inhibition. (a) The inverse of the decay time constant in the presence of GABA+fipronil is plotted as a function of fipronil concentration. The linear fit gave a slope of 0.21±0.01 and an intercept of 0.23±0.03. (b) Recovery from fipronil-induced inhibition. Two superimposed current traces are shown. The first (grey) trace shows a response to a 1-min application of 1 mM GABA. A single exponential fit of the desensitization phase gave a time constant of 10.5 s and a steady-state response of 371 pA. The second (black) trace shows a response to a 1-min application of 1 mM GABA+10 μM fipronil followed by a 1-min application of 1 mM GABA. The decay phase had a time constant of 458 ms. The recovery phase was fitted to a single exponential yielding a time constant of 8.4 s. Both traces are from the same cell.
The steady-state current level at the end of the drug application depends on the rates leaving and entering the active state of the channel. During a short pulse of GABA+10 μM fipronil, most of the current decay is due to fipronil-induced inhibition. Thus, the fractional steady-state current level can be expressed as koff/([fipronil] kon+koff). In 10 cells, the steady-state current level for 1 mM GABA+10 μM fipronil was 3.4±2.9% of the peak response (Figure 2a). From these data we calculate the koff as (fractional steady-state current [fipronil] kon)/(1−fractional steady-state current) yielding 0.081 s−1.
An additional estimate of the rate of recovery from the blocked state was obtained by first inducing the accumulation of receptors in the blocked state by a 60 s exposure to 1 mM GABA+10 μM fipronil, and then measuring the rate of current recovery upon switching the extracellular solution to one containing GABA alone (Figure 3b). In this experimental protocol, the recovery time constant was 8.2±4.2 s (n=10 cells), yielding a koff of 0.12±0.06 s−1.
We next studied the ability of fipronil to interact with unliganded receptors by examining the effect of preapplication of fipronil on the peak current. In this experiment, 10 μM fipronil was applied to the cell for 60 s, after which the solution was switched to one containing 1 mM GABA+10 μM fipronil. Sample current traces from this experiment are shown in Figure 4a. Preincubation with fipronil had a strong effect on the peak response during a subsequent exposure to GABA+fipronil. Following a 60 s exposure to 10 μM fipronil, the peak response was reduced to 50±14% (n=11 cells) of control. In the same cells, coapplication of 10 μM fipronil with GABA, in the absence of preincubation, had no discernible effect (103±10% of control) on the peak current. These findings suggest that fipronil is capable of blocking unliganded closed channels.
Figure 4.
Preapplication of fipronil reduces peak response. (a) Comparison of fipronil effect on peak current in response to a 4-s application of 1 mM GABA+10 μM fipronil without (left trace) or following a 60 s preapplication of 10 μM fipronil (right trace). The peak current was 2227 pA in control (GABA), 2310 pA for coapplied GABA+fipronil, and 1227 pA for GABA+fipronil preceded by a fipronil preapplication. All current traces are from the same cell. (b) Normalized peak current for 1 mM GABA+10 μM fipronil following a 1–90 s preapplication of 10 μM fipronil. A single exponential fit gave a time constant of 19±4 s and a constant (steady-state level) of 0.43±0.03. Data from two cells (90 s preincubation) or five cells (all other time points) were used for the graph.
To estimate the kon and koff values for unliganded receptors, we examined the reduction of peak current as a function of preincubation duration. The relationship between the fractional peak current and preincubation duration (Figure 4b) was fitted with a single exponential yielding a time constant of 19±4 s saturating at 43±3% of control. From these values, we postulate (10 μM kon+koff)=1/19 s and koff/(10 μM kon+koff)=0.43, yielding a koff of 0.023 s−1 and a kon of 0.003 μM−1 s−1 (3 × 103 M−1 s−1). These values are 5 times and 77 times, respectively, slower than the respective rates in the presence of 1 mM GABA.
The presence of fipronil affects single-channel cluster durations but not intracluster open and closed time distributions
We next examined the effect of fipronil on single-channel currents. Intracluster open and closed times can yield valuable information on the receptor binding and gating properties, whereas the mean cluster duration is inversely related to the desensitization rate. Additionally, the amplitudes of single-channel events can be used to determine the single-channel conductance. The findings from the whole-cell recordings, where faster current decay in the presence of fipronil was observed, strongly suggested that the insecticide acts by modifying single-channel cluster durations. Accordingly, we first measured the single-channel cluster durations in the presence of 1 mM GABA in the absence and presence of 10 μM fipronil. The goal of these experiments was to verify the mechanism of inhibition of fipronil and to get an independent estimate for the rate of development of block.
The single-channel clusters were identified and isolated in records from four cell-attached patches in the presence of 1 mM GABA. The average cluster duration was 3.4±3.4 s (n=90 clusters). This is similar to previous estimates of cluster durations in the presence of 1 mM GABA (Akk et al., 2001). The inclusion of fipronil in the pipette medium resulted in shorter clusters. In five patches exposed to 1 mM GABA+10 μM fipronil, the average cluster duration was 407±449 ms (n=532 clusters). Sample currents for both conditions are shown in Figure 5a.
Figure 5.
Fipronil modulates single-channel cluster durations but not the intracluster open and closed time distributions. (a) Two 16 s data segments showing single-channel activity elicited by 1 mM GABA (top trace) or 1 mM GABA+10 μM fipronil (bottom trace). The putative single-channel clusters are marked with lines above the traces. The presence of fipronil results in shorter cluster durations. (b) Segments of channel activity showing single-channel clusters in the presence of 50 μM GABA (top trace) or 50 μM GABA+10 μM fipronil (bottom trace). The putative clusters are marked with lines above the traces. The open and closed time histograms pertaining to the two patches are given next to the data traces. For 50 μM GABA, the open times were 0.25 ms (27%), 2.3 ms (65%) and 5.5 ms (8%), and the closed times were 0.18 ms (54%), 1.0 ms (15%) and 11.2 ms (31%). For 50 μM GABA+10 μM fipronil, the open times were 0.34 ms (31%), 3.0 ms (58%) and 7.9 ms (11%) and the closed times were 0.19 ms (66%), 1.6 ms (12%) and 13.9 ms (21%). All current traces were recorded in the cell-attached configuration.
In the presence of GABA, termination of a cluster reflects entry into long-lived desensitized state(s), allowing the estimation of the rate of entry into the long-lived desensitized state (k+D*) as an inverse of mean cluster duration. Thus, k+D* is calculated as 0.3±0.3 s−1 in the presence of 1 mM GABA. We note that k+D* is not the microscopic desensitization rate constant but rather a cumulative rate of channel desensitization being influenced by the fractional occupancy of states from which desensitization occurs and the rate constants connecting these states to the long-lived desensitized states.
For single-channel activity obtained in the presence of GABA and fipronil, the inverse of the cluster duration equals the sum of rates for baseline desensitization (k+D*) and fipronil-induced inhibition (kon). Thus, for GABA+10 μM fipronil, we calculate kon=(2.5−0.3 s−1)/10 μM=0.22 μM−1 s−1. This value is gratifyingly similar to the kon estimate obtained in macroscopic recordings (0.23 μM−1 s−1).
Although the rate of return from the blocked state can theoretically be established from the durations of inactive periods between single-channel clusters, these kinds of calculations are usually not practical because the intercluster closed times depend on the intrinsic rate of recovery and the number of receptors in the patch. The latter was unknown in this study. As a result, no attempt to determine the koff in single-channel recordings was made.
To get insight into fipronil effects on receptor affinity to GABA and the channel gating properties, we examined the effect of fipronil on the intracluster open and closed time distributions. The experiments were conducted in the presence of 1 mM or 50 μM GABA. Sample currents are shown in Figure 5, and the summary of the findings is presented in Tables 1 and 2. In agreement with previous single-channel recordings from HEK cells expressing rat α1β2γ2L receptors (Steinbach and Akk, 2001), we found that the intracluster open and closed time histograms were best fitted to sums of three exponentials. The three open time components reflect dwells in distinct open states and are differentially affected by various GABAA receptor modulators (Akk et al., 2004; Li et al., 2007). The present data demonstrate that 10 μM fipronil was without significant effect on the intracluster open time distributions (Table 1).
Table 1.
The summary of single-channel kinetic analysis of the OT distributions from the wild-type receptor under control conditions and in the presence of fipronil
| Agonist, modulator | OT1 (ms) | Fraction OT1 | OT2 (ms) | Fraction OT2 | OT3 (ms) | Fraction OT3 | N |
|---|---|---|---|---|---|---|---|
| 50 μM GABA | 0.32±0.17 | 0.25±0.04 | 2.5±0.9 | 0.58±0.08 | 7.8±2.3 | 0.17±0.11 | 3 |
| 50 μM GABA+10 μM fipronil | 0.26±0.12 | 0.35±0.09 | 2.3±0.8 | 0.42±0.11 | 6.5±1.3 | 0.23±0.09 | 4 |
| 1 mM GABA | 0.53±0.13 | 0.16±0.11 | 2.6±1.2 | 0.68±0.07 | 7.3±3.6 | 0.16±0.07 | 4 |
| 1 mM GABA+10 μM fipronil | 0.38±0.10 | 0.26±0.06 | 2.0±0.4 | 0.60±0.06 | 6.6±1.5 | 0.14±0.11 | 5 |
Abbreviation: OT, open time.
The intracluster OT histograms were fitted to a sum of three exponentials. The table gives the mean durations (OT1–3) and relative contributions (fraction OT1–3) for the three OT components. The presence of fipronil did not lead to statistically significant changes in the OT distributions (P>0.05; Student's t-test).
Table 2.
The summary of single-channel kinetic analysis of the CT distributions from the wild-type receptor under control conditions and in the presence of fipronil
| Agonist, modulator | CT1 (ms) | Fraction CT1 | CT2 (ms) | Fraction CT2 | CT3 (ms) | Fraction CT3 | N |
|---|---|---|---|---|---|---|---|
| 50 μM GABA | 0.17±0.03 | 0.57±0.10 | 1.4±0.2 | 0.14±0.01 | 16.4±6.5 | 0.28±0.10 | 3 |
| 50 μM GABA+10 μM fipronil | 0.21±0.05 | 0.54±0.13 | 1.6±0.5 | 0.16±0.05 | 11.9±1.9 | 0.30±0.10 | 4 |
| 1 mM GABA | 0.24±0.02 | 0.69±0.04 | 0.9±0.1 | 0.29±0.04 | 7.8±4.0 | 0.02±0.01 | 4 |
| 1 mM GABA+10 μM fipronil | 0.22±0.02 | 0.69±0.04 | 1.0±0.1 | 0.29±0.03 | 7.2±2.0 | 0.03±0.01 | 5 |
Abbreviation: CT, closed time.
The intracluster CT histograms were fitted to a sum of three exponentials. The table gives the mean durations (CT1–3) and relative contributions (fraction CT1–3) for the three CT components. The presence of fipronil did not lead to statistically significant changes in the CT distributions (P>0.05; Student's t-test). The CT2 component represents the activation-related CT (CTβ, see text) at 1 mM GABA. In the presence of 50 μM GABA, the CTβ coincides with the CT3 component.
The intracluster closed time distributions contain information on multiple kinetic parameters. The closed time component whose duration inversely scales with agonist concentration (CTβ) is associated with agonist binding and channel opening. At low agonist concentrations (for example, 50 μM GABA), the duration of CTβ is determined by receptor affinity to the agonist and the channel-opening rate constant. At high agonist concentrations (for example, 1 mM GABA), the channel-opening rate constant is the major determinant. Thus, changes in the duration of this component in the presence of fipronil would be indicative of the drug modifying receptor affinity to GABA or the channel-opening rate constant. The other intracluster closed time components, whose parameters are independent of agonist concentration, correspond to various blocked and/or short-lived desensitized states. Changes in the durations or prevalence of these closed time components may affect the deactivation kinetics of the receptor (Jones and Westbrook, 1995; Akk et al., 2007).
Our findings demonstrate that the presence of 10 μM fipronil was without effect on the intracluster closed time distributions. In particular, fipronil did not affect the properties of the activation-related closed time component, CTβ. Accordingly, we conclude that fipronil does not affect the receptor affinity to GABA or the channel-opening rate constant.
Finally, we examined the effect of fipronil on single-channel conductance. In the cell-attached configuration, the currents were recorded at applied pipette potentials of +25, +50 and +75 mV, and the channel conductance of the dominant amplitude class was determined from the slope of the I–V relationship. The data indicate that the presence of fipronil had minimal effect on the single-channel conductance that was estimated as 15.5±5.5 pS (n=4 patches) in the presence of 1 mM GABA and 13.9±1.0 pS (n=4 patches) in the presence of 1 mM GABA+20 μM fipronil. We also estimated the chord conductance at −100 mV membrane potential in inside-out patches, where the receptor is exposed to symmetrical chloride concentrations. The single-channel conductance was 24.7±1.1 pS (n=4 patches) in the presence of 1 mM GABA and 26.1±0.9 pS (n=4 patches) in the presence of 1 mM GABA+20 μM fipronil. The differences in the conductance estimates were statistically not significant, and we conclude that fipronil does not influence the single-channel conductance of the α1β2γ2L GABAA receptor.
Does fipronil promote channel desensitization?
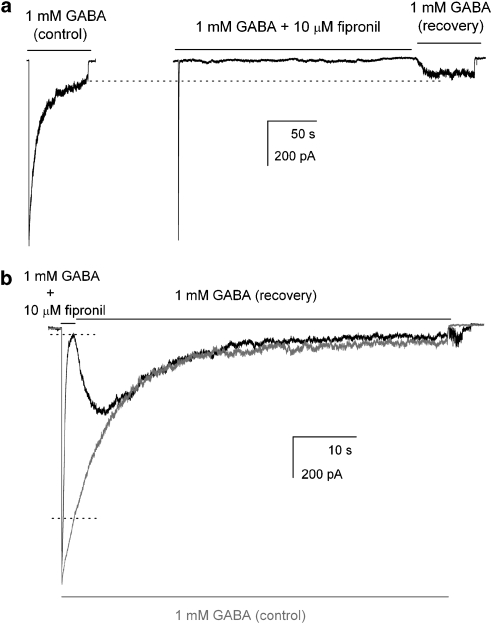
The experiments described above demonstrate that fipronil acts by speeding the decay rate in macroscopic recordings and reducing the mean cluster duration in single-channel recordings. Both actions are consistent with a mechanism in which fipronil acts by enhancing the intrinsic desensitization rate of the channel. However, the findings could also be accounted for by a mechanism in which fipronil inhibits the receptor by promoting entry into a distinct, long-lived non-conducting, that is, blocked state. Although the difference between the two mechanisms may, at first glance, appear trivial, the broader underlying question is whether fipronil promotes a naturally occurring mechanism, that is, desensitization, or modifies receptor behaviour by introducing a novel blocked receptor state. A variation of the two mechanisms is that the inhibition in the presence of fipronil and desensitization are distinct, but interacting processes, so that receptors driven into the inhibited state are incapable of undergoing desensitization.
To distinguish between the possible mechanisms, we first explored the hypothesis that fipronil-induced inhibition is distinct from desensitization and that inhibited receptors are unable to undergo normal desensitization. To test this, we exposed the receptors to a long (4 min) pulse of 1 mM GABA+10 μM fipronil and then examined the time course and magnitude of recovery of receptor activity upon switching the bath solution to one containing solely GABA. We reasoned that if fipronil-induced inhibition prevents channel desensitization, then the recovery phase should exhibit a prominent rebound current with a peak amplitude that may approach the level of the peak response to 1 mM GABA. And conversely, if inhibition and desensitization proceed independently, then recovery, upon the removal of fipronil from the bath solution, should proceed to the level identical to the steady-state current level observed during an application of 1 mM GABA. Figure 6a shows results from such an experiment. Exposure to 1 mM GABA+10 μM fipronil rapidly inhibited current response. Upon the removal of fipronil from the bath solution, recovery proceeded with a time constant of 5.3 s to a maximal current level of 77 pA. This is similar to the steady-state level in the control GABA response from the same cell (92 pA). Similar results were obtained in four additional cells. The mean recovery time constant following a 4-min exposure to 1 mM GABA+fipronil was 7.3±1.1 s (mean±s.e.mean; n=5 cells). The steady-state current level following the recovery from inhibition was 94±18% of the steady-state response in the end of a 1-min application of 1 mM GABA to the same cell (P>0.33; paired t-test). We interpret the results to indicate that the receptors inhibited by fipronil are capable of desensitizing.
Figure 6.
Fipronil-induced inhibition and receptor desensitization proceed independently. (a) The cell was exposed to 1 mM GABA+10 μM fipronil for 4 min, followed by a 1-min application of 1 mM GABA (right trace). The initial exposure to GABA+fipronil caused channel block that was followed by recovery when fipronil was removed from the bath. The peak response during recovery (77 pA) was similar to the steady-state current level in the control 1 mM GABA application (92 pA; left trace). We conclude that channels blocked by fipronil are capable of desensitizing (see text for more details). (b) The cell was exposed to 1 mM GABA+10 μM fipronil for 2 s, followed by a 1-min application of 1 mM GABA (black trace). The 2 s exposure to GABA+fipronil was sufficient to cause steady-state block, whereas in a control recording from the same cell (grey trace), 1 mM GABA caused desensitization to 75% of peak response at the 2 s mark. The current levels for both traces at the 2 s mark are shown with dotted lines. Upon the removal of fipronil from the bath (shown as 1 mM GABA (recovery)), the current recovered to the level predicted by the time course of desensitization in the control trace exhibiting a prominent peak before settling to steady-state level. We conclude that upon exposure to GABA+fipronil, inhibition and desensitization follow their individual time courses and are thus distinct processes.
We note a caveat, that recovery from the inhibition must proceed rapidly compared with the development of desensitization. Slow recovery would tend to mask the peak response even if receptors blocked by fipronil were unable to desensitize, because the rapidly developing desensitization would limit the response from receptors recovering from block.
We next made an attempt to distinguish between a model where fipronil acts to enhance the intrinsic rate of desensitization (k+D*), and one where fipronil promotes channel entry into a distinct, blocked/inhibited state. For that, we briefly applied 1 mM GABA+10 μM fipronil to a cell, followed by washout in the presence of GABA alone. The duration of the initial application of GABA+fipronil (2 s) was chosen to elicit steady-state block but only relatively minor desensitization. If fipronil acts by enhancing k+D*, then the fractional steady-state current level in the presence of fipronil can be expressed as (k−D*/(k−D*+k+DF)), where k−D* corresponds to the baseline rate of current recovery from desensitization and k+DF corresponds to the rate of desensitization in the presence of fipronil. Upon removal of fipronil from the extracellular medium, the system equilibrates to a new steady-state current level that is determined by the baseline desensitization properties and equal to (k−D*/(k−D*+k+D*)). In contrast, if fipronil-induced inhibition and desensitization are independent processes, then, after a short pulse of GABA+fipronil, the receptors recover to a current level that is determined by the time course of desensitization. If the drug application was sufficiently brief, desensitization will not have reached a steady-state level and the recovery current should exhibit a prominent peak, arising from the receptors recovered from block but not yet desensitized, that then decays to the steady-state level.
Sample currents from this experiment are shown in Figure 6b. In a control recording (exposure to 1 mM GABA), the current amplitude was reduced to 75% of peak response at the 2 s mark, whereas a 2-s application of GABA+10 μM fipronil fully inhibited the response leading to the steady-state current level that equalled 2.6% of the peak response. Upon switching the extracellular solution to 1 mM GABA, the currents recovered and the recovery phase demonstrated a pronounced peak (426 pA) before decaying to a steady-state current level (43 pA). The presence of the peak cannot be explained by the model where fipronil acts to enhance the intrinsic k+D*. Instead, the peak is consistent with a model where fipronil elicits rapid accumulation of receptors in a novel blocked state, whereas channel desensitization proceeds more slowly and independently. Similar results were obtained in five additional cells. The rebound current had a peak amplitude that was 536±113% (mean±s.e.mean) of the steady-state current (P<0.05; paired t-test). We conclude that fipronil acts on the rat α1β2γ2L GABAA receptor by promoting entry into a novel, long-lived blocked state.
The α1V256S mutation slows the development and recovery from fipronil-induced inhibition
The whole-cell and single-channel data demonstrate that the kinetic mechanism of action of fipronil is to increase the rate of current decay. Previous work has shown a similar mechanism of action for inhibitory steroids and a tricyclic benz[e]indene neurosteroid analogue (Shen et al., 2000; Akk et al., 2001; Li et al., 2006). In the work with inhibitory steroids and steroid analogues, it was also shown that a valine-to-serine mutation of the 2′ residue of the α1-subunit M2 transmembrane domain (α1V256S) strongly diminished the ability of these compounds to block the GABAA receptor (Akk et al., 2001; Li et al., 2006). The role of the α1V256 residue in the actions of inhibitory steroids is unclear but the mutation may interfere with conformational changes underlying block, or alter the structure of the channel to reduce the on- rate of the blocker. To examine the possibility that, besides having the same kinetic mechanism, fipronil utilizes the same binding structures and/or transduction elements as inhibitory steroids, we tested the effect of the α1V256S mutation on channel block by fipronil.
Currents elicited from the mutant receptor by 1 mM GABA alone showed little desensitization (Figure 7a). We were unable to obtain a precise and reproducible value for the desensitization time constant, but, qualitatively, we estimate that desensitization proceeded more slowly in the mutant than in the wild-type receptor. We next probed the ability of 10 μM fipronil to inhibit whole-cell currents elicited by 1 mM GABA. Data from six cells show that the presence of fipronil accelerated the current decay resulting in a decay time constant of 1176±738 ms. From these data we calculate that kon=0.09±0.05 μM−1 s−1 for the α1V256S mutant receptor. This is almost three times slower than the kon for the wild-type receptor (P<0.01). The presence of 10 μM fipronil did not affect the peak current (103±3% of control GABA response; P>0.35).
Figure 7.
The α1V256S mutation slows the development and recovery from block. (a) Sample whole-cell current traces obtained in response to 1 mM GABA or 1 mM GABA+10 μM fipronil. The traces shown had amplitudes of 2313 pA (GABA) or 2614 pA (GABA+fipronil). The decay phase of the trace obtained in the presence of GABA+fipronil had a time constant of 2.1 s. (b) Recovery from desensitization. Two superimposed current traces are shown. The first trace shows a response to a 2-min application of 1 mM GABA. The peak amplitude was 927 pA, and a single exponential fit of the decay phase gave a time constant of 70 s and a steady-state response of 267 pA. The second trace shows a response to a 60-s application of 1 mM GABA+10 μM fipronil followed by a 2-min application of 1 mM GABA. The peak amplitude during the initial phase was 850 pA and the decay phase had a time constant of 2.0 s. The recovery phase was fitted to a single exponential yielding a time constant of 56 s. Both traces are from the same cell.
The rate of recovery from the blocked state was estimated by inducing the accumulation of receptors in the inhibited state by exposing the cell to a solution containing 1 mM GABA+10 μM fipronil, and then measuring the rate of recovery upon switching the extracellular solution to one containing GABA alone (Figure 7b). In this experimental protocol, the recovery time constant was 60.1±39.2 s (n=10 cells), yielding a koff of 0.017±0.011 s−1. This is 7-fold slower than the rate of recovery in the wild-type receptor.
Fipronil sulphone is a weak inhibitor of the α1β2γ2L GABAA receptor
In both mammals and insects, fipronil is metabolized to fipronil sulphone. Fipronil sulphone is a potent inhibitor of insect chloride channels, but its role in mammalian toxicity is unclear. Accordingly, we next examined rat α1β2γ2L receptor modulation by fipronil sulphone.
Coapplication of fipronil sulphone with GABA resulted in faster current decay (Figure 8a), similar to that observed in the presence of fipronil. However, the effect was much weaker than in the presence of fipronil. The decay time constant in the presence of 1 mM GABA+10 μM fipronil sulphone was 2.3±0.7 s (n=5 cells). From these data we calculate the kon for fipronil sulphone as (0.43−0.2 s−1)/10 μM=0.023 μM−1 s−1 (∼2 × 104 M−1 s−1). This is 10-fold lower than the rate of development of block in the presence of fipronil.
Figure 8.
Fipronil sulphone is a weak inhibitor of the α1β2γ2L GABAA receptor. (a) Sample whole-cell current traces obtained in response to a 10-s application of 1 mM GABA or 1 mM GABA+10 μM fipronil sulphone. The traces shown had peak amplitudes of 3061 pA (GABA) or 2883 pA (GABA+fipronil sulphone). Fits of the current decay to a single exponential gave time constants of 7.6 and 1.7 s for GABA and GABA+fipronil sulphone, respectively. (b) Recovery from desensitization. The trace shows a response to a 60-s application of 1 mM GABA+10 μM fipronil sulphone followed by a 1-min application of 1 mM GABA. The recovery phase (shown with arrows) was fitted to a single exponential yielding a time constant of 22.9 s.
The rate for recovery from block induced by fipronil sulphone was estimated by exposing the receptors to 1 mM GABA+30 μM fipronil sulphone and then measuring the rate of recovery upon switching the extracellular solution to one containing GABA alone (Figure 8b). In this experimental protocol, the recovery time constant was 16.7±6.0 s (n=4 cells), yielding a koff of 0.06±0.02 s−1. This value is twofold less than the rate of recovery from block induced by 10 μM fipronil.
Discussion and conclusions
In this study, we report a characterization of the inhibitory effect of the insecticide fipronil and its major metabolite, fipronil sulphone, on the mammalian α1β2γ2L GABAA receptor. The effect was manifested as a rapid current decay during a prolonged pulse of agonist, whereas in single-channel recordings, the presence of fipronil resulted in shorter cluster durations. Fipronil did not affect the affinity of the receptor to GABA, the channel gating properties, or the conductance of the ion channel. The mode of action of fipronil sulphone was similar, but fipronil sulphone was a weaker antagonist of the receptor than fipronil.
Many aspects of our findings are in agreement with previous macroscopic data on fipronil modulation of GABAA receptors in rat DRG neurons (Ikeda et al., 2001). Similar to that study, we found that fipronil slowly and reversibly inhibits GABAA receptors. In both native (Ikeda et al., 2001) and recombinant receptors (the present study), the onset and recovery rates for the effect were faster for activated than unliganded receptors. But, whereas the rate for recovery from fipronil-induced inhibition was similar for native and recombinant receptors, the rate of onset proved to be highly dependent on the receptor origin. The kon was 0.23 μM−1 s−1 in α1β2γ2L receptors and more than 30-fold less (0.007 μM−1 s−1) in native receptors in DRG. Differences in GABAA receptor subunit combination are likely to underlie the differences in the kon estimates.
In an earlier study of single-channel currents from native spinal cord receptors, fipronil failed to alter single-channel conductance and had only a minor effect on open time distributions (Ikeda et al. 2004). The predominant effect of fipronil was an increase in the duration of the longest-lived closed time component. The lack of significant effects on channel conductance and open time distributions is similar to the present findings from recombinant receptors. But the interpretation of the closed time effect seen by Ikeda et al. (2004) is not trivial and is hindered by difficulties in assigning the specific closed time classes to specific activation processes (for example, agonist binding, channel opening and desensitization). Our data, on the basis of single-channel cluster analysis, indicate that fipronil does not modulate agonist binding or channel opening, instead, its presence leads to early termination of single-channel clusters.
The kinetic mechanism of action of fipronil is similar to that of an inhibitory neurosteroid, pregnenolone sulphate and some tricyclic neurosteroid analogues (Akk et al., 2001; Li et al., 2006). But in contrast to these drugs whose effect is drastically diminished in the α1V256S mutant receptor, the inhibitory actions of fipronil were little affected by the α1V256S mutation. In the mutant receptor, the kon for fipronil was less than threefold lower than in the wild-type receptor. We note that the serine mutation to the homologous site in the Drosophila receptor (alanine302) has been shown to markedly reduce receptor inhibition by fipronil (Hosie et al., 1995). We infer that the insecticide acts differently on insect and mammalian receptors.
Both in mammals and insects, fipronil is metabolized to fipronil sulphone. A previous study (Zhao et al., 2005) had found that fipronil sulphone is a potent antagonist of GABA receptors in insect and rat neurons. In rat DRG neurons, the off-rate for fipronil and fipronil sulphone were comparable, whereas the on-rate for fipronil sulphone was sevenfold greater than that for fipronil (Zhao et al., 2005). In contrast, we find that block develops 10-fold more slowly in the presence of fipronil sulphone compared with fipronil. Recovery from block was twofold faster in the presence of fipronil. Accordingly, our findings suggest that metabolism of fipronil in rat brain serves as detoxifying process.
Desensitization of the GABAA receptor and other ligand-gated ion channels is a process involving specific molecular rearrangements in the channel pore while leaving the structure of the agonist binding site largely unaffected (Wilson and Karlin, 2001; Muroi et al., 2006). Although, phenomenologically, the effect of fipronil could be accounted for by a more rapid desensitization in the presence of fipronil, we have no direct evidence that the mechanistic basis for channel inhibition by fipronil involves the elements participating in channel desensitization. Moreover, our data on recovery from block are consistent with a model where fipronil-induced inhibition and channel desensitization proceed in parallel and independently, indicating that fipronil induces the accumulation of receptors in a novel, long-lived blocked state.
What do we know about the structures involved in mediating the inhibitory effect of fipronil? Modelling studies predict that fipronil, as well as functionally related non-competitive antagonists of the GABAA receptor picrotoxinin and t-butylbicyclo-phosphorothionate, interact with the 2′, 6′ and 9′ residues of the M2 membrane-spanning domain (Chen et al., 2006a). When bound to its site, fipronil is predicted to inhibit the current flow by simply blocking the pore. Our data indicating that, besides blocking active receptors, fipronil is capable of acting on unliganded closed channels are somewhat unexpected as the activation gate is likely to be located extracellular from the 2′ residue (Bali and Akabas, 2007), thereby potentially making the fipronil-binding site inaccessible in closed channels. However, we note that the access route of non-competitive antagonists to their binding site may not totally be dependent on the channel pore and may involve movement through the water-filled cavities between neighbouring subunits (Chen et al., 2006b).
To the best of our knowledge, this is the first electrophysiological study of the modulation of α1β2γ2L GABAA receptors by fipronil and fipronil sulphone. Previous electrophysiological studies of fipronil effects have been conducted, besides native GABAA receptors from DRG, on insect GABA receptors (Hosie et al., 1995) and ρ1 (GABAC) receptors (Ratra et al., 2002). The insect receptors were found to be highly sensitive to fipronil but human homomeric ρ1 receptors were not affected by up to 30 μM fipronil. We also note that binding studies have demonstrated high, insect receptor-like affinity of β3 homomeric receptors to fipronil (Ratra and Casida, 2001). But the β3 homomeric receptors are unlikely to occur in great quantities in the brain and, in any case, they do not contribute to responses to the endogenous transmitter GABA (Wooltorton et al., 1997), making such receptors an unlikely target for fipronil in the CNS.
In summary, we have shown that the insecticide fipronil acts on mammalian α1β2γ2L GABAA receptors by promoting entry into a novel non-conducting state. Receptor affinity, channel gating properties and single-channel conductance are not affected in the presence of the drug. The major metabolite of fipronil, fipronil sulphone was less potent at inhibiting the GABAA receptor function suggesting that the conversion of fipronil to fipronil sulphone presents a detoxifying process in the mammalian brain.
Acknowledgments
We thank Joe Henry Steinbach for stimulating discussions during the course of the work and John Bracamontes for help with molecular biology. This study was supported by the National Institutes of Health Grant ES16350.
Abbreviations
- GABAA receptor
γ-aminobutyric acid type A receptor
Conflict of interest
The authors state no conflict of interest.
References
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. J Physiol. 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol. 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Manion BD, Evers AS, Steinbach JH. Ethanol modulates the interaction of the endogenous neurosteroid allopregnanolone with the α1β2γ2L GABAA receptor. Mol Pharmacol. 2007;71:461–472. doi: 10.1124/mol.106.029942. [DOI] [PubMed] [Google Scholar]
- Bali M, Akabas MH. The location of a closed channel gate in the GABAA receptor channel. J Gen Physiol. 2007;129:145–159. doi: 10.1085/jgp.200609639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkwitz C, Banks MI, Pearce RA. Influence of GABAA receptor γ2 splice variants on receptor kinetics and isoflurane modulation. Anesthesiology. 2004;101:924–936. doi: 10.1097/00000542-200410000-00018. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA. Effects of the γ2S subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacol. 2003;44:1003–1012. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Chen L, Durkin KA, Casida JE. Structural model for a γ-aminobutyric acid receptor noncompetitive antagonist binding: widely diverse structures fit the same site. Proc Natl Acad Sci USA. 2006a;103:5185–5190. doi: 10.1073/pnas.0600370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Durkin KA, Casida JE. Spontaneous mobility of GABAA receptor M2 extracellular half relative to noncomeptitive antagonist action. J Biol Chem. 2006b;281:38871–38878. doi: 10.1074/jbc.M608301200. [DOI] [PubMed] [Google Scholar]
- Ebert B, Thompson SA, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human γ-aminobutyric acid type A receptors. Mol Pharmacol. 1997;52:1150–1156. [PubMed] [Google Scholar]
- Hainzl D, Cole LM, Casida JE. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem Res Toxicol. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Baylis HA, Buckingham SD, Sattelle DB. Actions of the insecticide fipronil, on dieldrin-sensitive and -resistant GABA receptors of Drosophila melanogaster. Br J Pharmacol. 1995;115:909–912. doi: 10.1111/j.1476-5381.1995.tb15896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Nagata K, Kono Y, Yeh JZ, Narahashi T. Fipronil modulation of GABAA receptor single-channel currents. Pest Manag Sci. 2004;60:487–492. doi: 10.1002/ps.830. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Zhao X, Nagata K, Kono Y, Shono T, Yeh JZ, et al. Fipronil modulation of γ-aminobutyric acidA receptors in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2001;296:914–921. [PubMed] [Google Scholar]
- Jennings KA, Canerdy TD, Keller RJ, Atieh BH, Doss RB, Gupta RC. Human exposure to fipronil from dogs treated with frontline. Vet Hum Toxicol. 2002;44:301–303. [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Mol Pharmacol. 2007;71:1582–1590. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- Li P, Covey DF, Steinbach JH, Akk G. Dual potentiating and inhibitory actions of a benz[e]indene neurosteroid analog on recombinant α1β2γ2 GABAA receptors. Mol Pharmacol. 2006;69:2015–2026. doi: 10.1124/mol.106.022590. [DOI] [PubMed] [Google Scholar]
- Maddox FN, Valeyev AY, Poth K, Holohean AM, Wood PM, Davidoff RA, et al. GABAA receptor subunit mRNA expression in cultured embryonic and adult human dorsal root ganglion neurons. Brain Res Dev Brain Res. 2004;149:143–151. doi: 10.1016/j.devbrainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mohamed F, Senarathna L, Percy A, Abeyewardene M, Eaglesham G, Cheng R, et al. Acute human self-poisoning with the N-phenylpyrazole insecticide fipronil—a GABAA-gated chloride channel blocker. J Toxicol Clin Toxicol. 2004;42:955–963. doi: 10.1081/clt-200041784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi Y, Czajkowski C, Jackson MB. Local and global ligand-induced changes in the structure of the GABAA receptor. Biochemistry. 2006;45:7013–7022. doi: 10.1021/bi060222v. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Zhao X, Ikeda T, Nagata K, Yeh JZ. Differential actions of insecticides on target sites: basis for selective toxicity. Hum Exper Toxicol. 2007;26:361–366. doi: 10.1177/0960327106078408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton AJ, Fisher JL. Effect of the α subunit subtype on the macroscopic kinetic properties of recombinant GABAA receptors. Brain Res. 2007;1165:40–49. doi: 10.1016/j.brainres.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratra GS, Casida JE. GABA receptor subunit composition relative to insecticide potency and selectivity. Toxicol Lett. 2001;122:215–222. doi: 10.1016/s0378-4274(01)00366-6. [DOI] [PubMed] [Google Scholar]
- Ratra GS, Erkkila BE, Weiss DS, Casida JE. Unique insecticide specificity of human homomeric ρ1 GABAC receptor. Toxicol Lett. 2002;129:47–53. doi: 10.1016/s0378-4274(01)00471-4. [DOI] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Covey DF, Zorumski CF. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. J Neurosci. 2000;20:3571–3579. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JD, Vargas RI. Toxicity and hazard assessment of fipronil to Daphnia pulex. Exotoxicol Environ Saf. 2005;62:11–16. doi: 10.1016/j.ecoenv.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Steinbach JH, Akk G. Modulation of GABAA receptor gating by pentobarbital. J Physiol. 2001;537:715–733. doi: 10.1111/j.1469-7793.2001.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Zorumski C, Bracamontes J, Steinbach JH. Endogenous subunits can cause ambiguities in the pharmacology of exogenous γ-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol Pharmacol. 1996;50:931–938. [PubMed] [Google Scholar]
- Wilson GG, Karlin A. Acetylcholine receptor channel structure in the resting, open, and desensitized states probed with the substituted-cysteine-accessibility method. Proc Natl Acad Sci USA. 2001;98:1241–1248. doi: 10.1073/pnas.031567798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Moss SJ, Smart TG. Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur J Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Zhao X, Salgado VL, Yeh JZ, Narahashi T. Differential actions of fipronil and dieldrin insecticides on GABA-gated chloride channels in cockroach neurons. J Pharmacol Exp Ther. 2003;306:914–924. doi: 10.1124/jpet.103.051839. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yeh JZ, Salgado VL, Narahashi T. Sulfone metabolite of fipronil blocks γ-aminobutyric acid- and glutamate-activated chloride channels in mammalian and insect neurons. J Pharmacol Exp Ther. 2005;314:363–373. doi: 10.1124/jpet.104.077891. [DOI] [PubMed] [Google Scholar]