Abstract
Background and purpose:
The purinergic system through the A2A adenosine receptor regulates addiction induced by different drugs of abuse. The aim of the present study was to investigate the specific role of A2A adenosine receptors (A2ARs) in the behavioural and neurochemical responses to morphine associated with its motivational properties.
Experimental approach:
Mice lacking A2ARs (A2A knockout (KO) mice) and wild-type littermates were used to evaluate behavioural responses induced by morphine. Antinociception was assessed using the tail-immersion and the hot-plate tests. Place-conditioning paradigms were used to evaluate the rewarding effects of morphine and the dysphoric responses of morphine withdrawal. Microdialysis studies were carried out to evaluate changes in the extracellular levels of dopamine in the nucleus accumbens of A2A KO mice after morphine administration.
Key results:
The acute administration of morphine induced a similar enhancement of locomotor activity and antinociceptive responses in both genotypes. However, the rewarding effects induced by morphine were completely blocked in A2A KO mice. Also, naloxone did not induce place aversion in animals lacking the A2ARs.
Conclusions and implications:
Our findings demonstrate that the rewarding and aversive effects associated with morphine abstinence were abolished in A2A KO mice, supporting a differential role of the A2A adenosine receptor in the somatic and motivational effects of morphine addiction. This study provides evidence for the role of A2ARs as general modulators of the motivational properties of drugs of abuse. Pharmacological manipulation of these receptors may represent a new target in the management of drug addiction.
Keywords: knockout mice, A2A adenosine receptors, place conditioning, microdialysis, reward, morphine
Introduction
Adenosine is an endogenous purine nucleoside, which acts as a neuromodulator in the CNS, and regulates a wide range of pathophysiological processes, such as epilepsies, sleep disorders, pain and drug addiction (Hack and Christie, 2003). The effects of adenosine are mediated through the activation of four receptor types: A1, A2A, A2B and A3 (Fredholm et al., 2005). A2A adenosine receptors (A2ARs) are found at high concentrations in the brain areas involved in the control of motivational responses including the olfactory tubercle, the caudate-putamen and the nucleus accumbens (NAc) (Moreau and Huber, 1999).
The generation of knockout (KO) mice with complete and specific inactivation of the A2AR (Ledent et al., 1997) provides a useful genetic model to investigate the role of these receptors in addictive processes. We have previously demonstrated that A2A gene deletion in mice modifies the behavioural effects induced by different drugs of abuse. Thus, nicotine-induced conditioned place preference was suppressed in A2A KO mice (Castañé et al., 2006). Additionally, both rewarding and aversive effects of Δ9-tetrahydrocannabinol were reduced, whereas the expression of rimonabant-precipitated Δ9-tetrahydrocannabinol withdrawal was found to be attenuated in A2A KO mice (Soria et al., 2004). Moreover, the reinforcing effects of cocaine decreased in these mutants in the self-administration paradigm (Soria et al., 2006).
Dopamine (DA) neurotransmission in the mesolimbic system plays a crucial role in the reward processes and other addictive-related behaviours (Koob, 1996; Di Chiara, 2002). Interestingly, A2A KO mice have been shown to have hypodopaminergic activity in the striatum (Dassesse et al., 2001), which could account for the attenuated reward effects induced by psychostimulants observed in KO mice. In the striatum, A2AR are expressed on GABAergic striatopallidal neurons and are colocalized with D2 DA receptors (Ferré et al., 1997). Adenosine appears to regulate DA neurotransmission through antagonistic interactions between adenosine A1/DA D1 receptors and adenosine A2A/DA D2 receptors (Franco et al., 2000).
Interactions between the purinergic and opioid systems have also been reported. Thus, A2ARs regulate proenkephalin gene expression in the striatum (Fink et al., 1992; Schiffmann and Vanderhaeghen, 1993). A reduction of [3H]-deltorphin-I binding to δ-opioid receptors and an increase in [3H]-Cl-977 binding to κ-opioid receptors were observed in the spinal cord of A2A KO mice (Bailey et al., 2002) associated with functional changes in opioid antinociception. Interestingly, adenosine has been suggested to regulate pharmacological responses induced by opioids. Thus, the spinal antinociceptive effects of morphine seem to be mediated, at least in part, by the release of endogenous adenosine and subsequent activation of A1 and A2 receptors (Sweeney et al., 1987, 1991). In addition, adenosine participates in opioid addictive responses and A2A blockade eliminates heroin-seeking behaviour in rats by preventing the synergy between μ-opioid and CB1 cannabinoid receptors (Yao et al., 2006). Furthermore, the blockade of adenosine metabolism by adenosine kinase inhibitors (Kaplan and Coyle, 1998) and the administration of adenosine agonists (Kaplan and Sears, 1996) decrease the severity of morphine abstinence, whereas adenosine antagonists increase the expression of morphine withdrawal symptoms in rats (Salem and Hope, 1997). In agreement, we have shown that the severity of morphine withdrawal was increased in mice lacking A2AR (Berrendero et al., 2003; Bailey et al., 2004). However, the involvement of A2AR in the motivational effects induced by opioids remains to be clarified.
The aim of this study was to investigate the specific role of A2AR in morphine-induced behavioural and neurochemical responses related to its motivational properties. For this purpose, we have evaluated the acute locomotor and antinociceptive effects induced by morphine in mice lacking A2AR. In addition, the rewarding properties of morphine and the aversive effects associated with morphine withdrawal were evaluated in these mutant mice by using the place-conditioning paradigm. Finally, in vivo microdialysis studies were performed to determine whether the acute effects of morphine on the extracellular levels of DA in the NAc are modified in these KO animals.
Methods
Drug and molecular target nomenclature conforms to Br J Pharmacol Guide to Receptors and Channels (Alexander et al., 2008).
Animals
Mice lacking A2AR were generated as previously described (Ledent et al., 1997). To homogenize the genetic background of the mice, the first-generation heterozygotes were bred for 30 generations on a CD1 background (Charles River, France) with selection for the mutant A2A gene at each generation. Beginning with the 30th generation of backcrossed mice, heterozygote–heterozygote matings of A2A KO mice produced wild-type (WT) and KO littermates for subsequent experiments. Breeding couples were periodically renovated by crossing heterozygote mice with WT CD1 females (Charles River, France) to maintain a genetically diverse outbred background.
Fourteen-week-old male A2A KO mice and WT littermates (30–35 g) were housed five per cage in temperature (21±1 °C) and humidity (55±10%) controlled rooms, with a 12 h light/12 h dark cycle (light between 0800 and 2000 hours). For the microdialysis experiments, animals were housed three per cage. Food and water were available ad libitum during all experiments except during the exposure to the different behavioural paradigms. Mice were handled for 1 week before the experiments were started. Animal procedures were conducted in accordance with the guidelines of the UK Animals Act 1986 (Scientific Procedures), the guidelines of the European Communities Directive 86/609/EEC regulating animal research, and the behavioural experiments performed in the laboratory of Barcelona were approved by the local ethical committee (CEEA-PRBB). All experiments were performed under blind conditions.
Drugs
Morphine used for behavioural studies was obtained from Ministerio de Sanidad y Consumo (Madrid, Spain). Morphine and cocaine for microdialysis experiments were purchased from Sigma Chemical Co. (Dorset, UK). Naloxone was purchased from Sigma Chemical Co. (Barcelona, Spain).
All the compounds were dissolved in sterile 0.9% physiological saline.
Acute effects induced by morphine
Locomotor activity responses induced by an acute injection of morphine (5 and 10 mg kg−1, s.c.) or vehicle were evaluated by using locomotor activity boxes (9 × 20 × 11 cm) (Imetronic, Bordeaux, France). The boxes were provided with two lines of photocells, one 2 cm above the floor to measure horizontal activity, and the other located 6 cm above the floor to measure vertical activity (rears), in a low luminosity environment (5 lux). Mice were habituated to the locomotor cages for 30 min over 3 consecutive days. On day 4, mice were placed in the locomotor activity boxes immediately after morphine (5 and 10 mg kg−1, s.c.) or vehicle injection, and locomotor activity was recorded for 30 min.
Antinociceptive effects induced by an acute administration of morphine (5 and 10 mg kg−1, s.c.) or vehicle were evaluated 30 min after the injection by using the tail-immersion test, as previously described (Simonin et al., 1998). The latency to a rapid tail-flick in the bath (50±0.5 °C) was registered with a cutoff latency of 15 s to prevent tissue damage. Subsequently, the hot-plate test (Eddy and Leimbach, 1953) was performed 31 min after morphine (5 and 10 mg kg−1, s.c.) or vehicle injection in the same experimental sequence. A glass cylinder was used to maintain the heated surface of the plate, which was kept at a temperature of 52±0.5 °C (Columbus Instruments, Columbus, OH, USA). The nociceptive threshold was evaluated by measuring the licking and the jumping responses, and a cutoff of 240 s was used to prevent tissue damage.
Morphine-induced conditioned place preference
The rewarding effects of morphine were evaluated using the conditioned place preference paradigm, as previously described (Maldonado et al., 1997). The apparatus consisted of two main square conditioning compartments (15 × 15 × 15 cm), with differences in texture and colours, separated by a triangular central area (Matthes et al., 1996). The light intensity within the conditioning chambers was 30 lux. During the preconditioning phase, drug-naive mice were placed in the middle of the central area and had free access to both compartments of the apparatus for 18 min. The time spent in each compartment was recorded by computerized monitoring software (Videotrack; View Point, Lyon, France). During the conditioning phase, mice received alternating injections of morphine (5 or 10 mg kg−1, s.c.) or vehicle and were immediately confined into one of the two conditioning compartments for 20 min. Three pairings were carried out with morphine and three pairings with vehicle on alternate days. Treatments were counterbalanced as closely as possible between compartments. Control animals received vehicle every day. The post-conditioning phase was conducted exactly as the preconditioning phase, that is, free access to each compartment for 18 min.
Conditioned place aversion to morphine withdrawal
Dysphoric effects of morphine withdrawal were investigated using the conditioned place aversion paradigm, as previously described (Valverde et al., 1996). Naloxone-induced place aversion in morphine-dependent mice was evaluated by use of the same apparatus described in the previous experiment. The preconditioning phase (day 1) was performed in the same way as in the place preference experiment and animals were free of drugs. The day after the preconditioning phase was conducted, opioid dependence was induced by administration of increasing doses of morphine (from 20 to 100 mg kg−1, i.p.) twice a day at 1000 and 1900 hours for 7 days; 20 and 20 mg kg−1 on day 2; 40 and 40 mg kg−1 on day 3; 60 and 60 mg kg−1 on day 4; 80 and 80 mg kg−1 on day 5; 100 and 100 mg kg−1 on days 6–8. A control group of animals received saline by using the same injection schedule. On day 7, animals received the morning morphine (100 mg kg−1, i.p.) or saline injection, and 2 h later naloxone (0.05 or 0.1 mg kg−1, s.c.) was administered and the animal was confined in the corresponding compartment for 15 min. On day 8, 2 h after morphine (100 mg kg−1, i.p.) or saline injection, animals received saline and were then confined in the other compartment. On day 9, animals did not receive any treatment. The post-conditioning phase (day 9) was performed in the same way as the preconditioning phase (free access to both compartments of the apparatus for 18 min). The doses of naloxone were selected to produce a conditioned place aversion without precipitating the presence of physical somatic signs of withdrawal, according to the previous studies (Valverde et al., 1996).
Microdialysis studies
Surgery
One day before microdialysis, mice were anaesthetized with isofluorane (3.5–4.5%) and a microdialysis guide cannula (CMA 7, CMA microdialysis, Solna, Sweden) was stereotaxically implanted targeting the NAc. Each animal was placed on a heated mat, supported in a stereotaxic frame and a small bore hole drilled in the skull. A microdialysis guide cannula was implanted at coordinates relative to bregma and skull (AP: +1.5 mm, L: −0.9 mm, DV: −4.0 mm) targeting the left NAc core-shell border. The cannula was secured to the skull with a single anchor screw and dental acrylic cement. Animals were allowed to recover until they regained their righting reflex, and then housed individually for subsequent microdialysis. Microdialysis was carried out in freely moving mice. Animals were equilibrated in a locomotor cage (25.4 × 25.4 × 40.64 cm) 1 h before the start of microdialysis. A microdialysis probe (CMA/7/7/1) was connected to a microdialysis system (CMA/120) and perfused with artificial CSF (aCSF: NaCl 145 mM, KCL 2.8 mM, CaCl2 1.2 mM, MgCl2 1.2 mM). Under light and brief isofluorane anaesthesia the probe was gently inserted into the guide cannula. The final depth of the probe relative to the skull was −5.0 mm. The probe was perfused with aCSF at a flow rate of 1 μL min−1, dialysate collected for 2 h and discarded to ensure a stable basal DA level. Subsequently, five consecutive dialysate fractions (F3–7) were collected at intervals of 20 min to monitor basal DA release. Animals were then injected with 0.9% saline (5 mL kg−1, s.c.), morphine (20 mg kg−1, s.c.) or cocaine (20 mg kg−1, s.c). Sample fractions were collected at 20 min intervals for 120 min (F8–13). All sample fractions were collected directly into 35 μL of mobile phase (NaH2PO4 0.05 M, OSA 0.8 mM, EDTA 0.1 mM, methanol 10% (v/v), adjusted to pH 3.3 with orthophosphoric acid) and frozen immediately in dry ice. After the completion of the microdialysis procedure, mice were killed by cervical dislocation, the brains were removed and frozen in isopentane with dry ice (−20, −30 °C) for verification of probe placement by histological examination.
Histology
Coronal cryostat sections (20 μm) were cut from each brain using a cryostat (Zeiss Microm 505E). Probe placement was checked visually using haematoxilin and eosin histological staining. Only data obtained from animals with correct probe tracts were used for analysis.
DA analysis
Dopamine concentration in the dialysate was determined by HPLC with electrochemical detection. The HPLC system consisted of an ESA582 pump, ESA542 refrigerated automatic sampler, ESA5020 screening guard cell (E=+400 mV), ESA5014B dual potential coulometric microdialysis cell, CoulochemII electrochemical detector (ESA Analytical Ltd, Bucks, UK) and a Waters spherisorb ODS2 (100 × 4.6 mm × 5 μm) analytical column (Waters Ltd, Hertsfordshire, UK) protected by a guard column (Phenomenex, Cheshine, UK). The mobile phase (see surgery section), filtered through a 0.2 μm nylon membrane and degassed, was pumped at a flow rate of 1 mL min−1. All reagents used for the mobile phase were of analytical grade. DA was detected on a dual porous graphite electrode system (first electrode reduction potential E1=−70 mV, working electrode oxidation potential E2=+340 mV). Under these conditions, DA had a retention time of 9.2 min with a detection limit of 0.2 nM. Dialysate DA levels were quantified by external standard curve calibration, using peak area for quantification; data were not corrected for probe recovery.
Statistical analysis
Acute effects of morphine were compared by using two-way ANOVA (genotype and treatment as factors of variation) between subjects, followed by one-way ANOVA and Dunnet's post hoc comparisons when required. For the conditioned place preference and place aversion experiments, Student's paired two-tailed t-tests were used to compare the post-conditioning and preconditioning time spent in the drug-paired compartment. For microdialysis studies, data from the morphine-induced effects on extracellular levels of DA (as absolute values) were analysed using a three-way ANOVA with fraction (F), genotype (G) and treatment (T) as between factors of variation. Data from the cocaine-induced effects on extracellular levels of DA are presented as area under the curve (AUC) values, represented by the DA increase from basal levels. AUC was calculated by using a standard trapezoid method (Gibaldi and Perrier, 1975). The following equation was used:
where DAb is the basal fraction before the administration of drug, DAn is the increase in DA following the drug challenge, t is the time (min) between the consecutive measurements, B is the average basal measurements and n is the number of samples. AUC values were analysed by using Student's t-tests. The level of significance was P<0.05.
Results
Acute effects induced by morphine
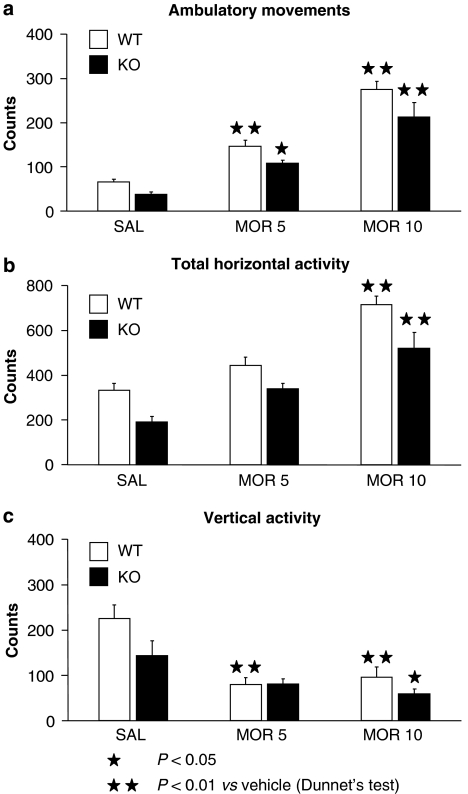
The acute administration of morphine (5 and 10 mg kg−1) induced a similar enhancement of locomotor activity and antinociceptive responses in the tail-immersion and hot-plate tests in both genotypes. Two-way ANOVA revealed treatment effects in all the acute responses, but no interaction between treatment and genotype (Table 1). Two-way ANOVA calculated for different locomotor responses revealed a main effect of the genotype, indicating a decrease in ambulatory movements, vertical activity and total activity in KO mice (Table 1). These data are in agreement with previous studies using A2A KO mice (Ledent et al., 1997). One-way ANOVA calculated for ambulatory movements (Figure 1a) showed a significant effect of treatment in WT (F [2.29]=59.414; P<0.01) and KO mice (F [2.29]=19.326; P<0.01). Post hoc analysis showed differences in morphine-treated WT and KO mice at the dose of 5 (P<0.01 for WT; P<0.05 for KO) and 10 mg kg−1 (P<0.01 in all the cases) when compared with saline-treated mice. One-way ANOVA calculated for total horizontal activity (Figure 1b) showed a significant effect of treatment in WT (F [2.29]=26.954; P<0.01) and KO mice (F [2.29]=12.541; P<0.01). Post hoc analysis showed differences in morphine-treated WT and KO mice at the dose of 10 mg kg−1 (P<0.01) when compared with saline-treated mice. One-way ANOVA calculated for vertical activity (Figure 1c) showed a significant effect of treatment in WT (F [2.28]=11.487; P<0.01) and KO mice (F [2.29]=4.188; P<0.05). Post hoc analysis showed differences in morphine-treated WT mice at the doses of 5 and 10 mg kg−1 (P<0.01), as well as in morphine-treated KO mice at the dose of 10 mg kg−1 (P<0.05) when compared to saline-treated mice. No difference was revealed between genotypes.
Table 1.
Two-way ANOVA for acute locomotor and antinociceptive responses induced by morphine in mice lacking the A2A adenosine receptor
|
Locomotor activity |
Antinociception |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ambulatory |
Total horizontal |
Vertical |
Tail-immersion |
Hot-plate: licking |
Hot-plate: jumping |
|||||||
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Treatment | F(2.54)=63.672 | 0.01 | F(2.54)=35.977 | 0.01 | F(2.53)=14.704 | 0.01 | F(2.53)=50.431 | 0.01 | F(2.54)=9.785 | 0.01 | F(2.54)=502.15 | 0.01 |
| Genotype | F(1.54)=9.526 | 0.01 | F(1.54)=17.606 | 0.05 | F(1.53)=4.523 | 0.05 | F(1.53)=1.884 | NS | F(1.54)=0.083 | NS | F(1.54)=1.164 | NS |
| TxG | F(2.54)=0.523 | NS | F(2.54)=0.587 | NS | F(2.53)=1.749 | NS | F(2.53)=0.930 | NS | F(2.54)=0.519 | NS | F(2.54)=0.291 | NS |
Abbreviation: NS, not significant.
Two-way ANOVA with treatment (T) and genotype (G) as factors of variation. See Methods for details.
Figure 1.
Acute locomotor effects induced by morphine in A2A knockout (KO) and wild-type (WT) mice. Locomotor activity ((a) ambulatory, (b) horizontal and (c) vertical) was evaluated for 30 min after acute morphine or saline injection. Data are expressed as mean±s.e.mean of locomotor activity counts (n=10 in all groups).
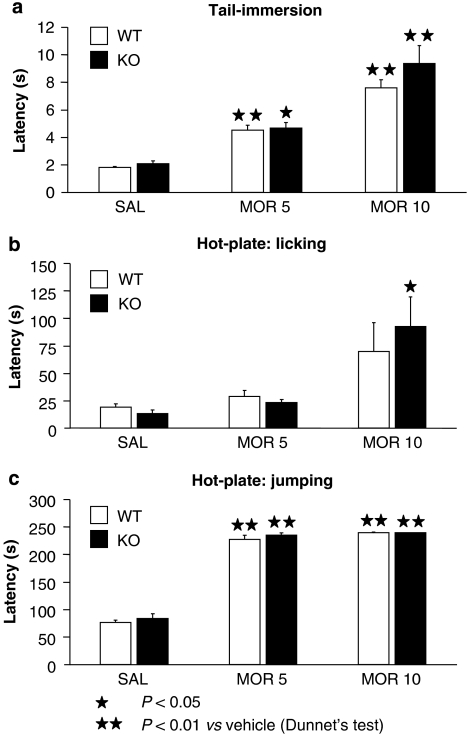
The antinociceptive effects of morphine (5 and 10 mg kg−1) were evaluated in A2A KO mice and WT littermates by using the tail-immersion (tail withdrawal latency) and the hot-plate test (licking and jumping responses). Two-way ANOVA for the responses in the tail-immersion test revealed a treatment effect, without genotype effect and no interaction between treatment and genotype (Table 1). One-way ANOVA calculated for tail withdrawal latencies showed a significant effect of treatment in WT (F [2.28]=42.652; P<0.01) and KO mice (F [2.29]=21.192; P<0.05). Post hoc analysis showed differences in morphine-treated WT and KO mice at the doses of 5 (P<0.05 for KO mice; P<0.01 for WT) and 10 mg kg−1 (P<0.01 in both genotypes), when compared with saline-treated mice (Figure 2a). Two-way ANOVA calculated for the licking latency in the hot-plate test showed that the treatment had an effect, but no interaction between treatment and genotype (Table 1). One-way ANOVA showed a significant effect of treatment in KO mice (F [2.29]=5.963; P<0.01), but not in WT animals (F [2.29]=3.019). Post hoc analysis showed differences in morphine-treated KO mice at the dose of 10 mg kg−1 (P<0.05) when compared with saline-treated mice (Figure 2b). Two-way ANOVA calculated for the jumping responses in the hot-plate test showed that the treatment had an effect, but no genotype effect and no interaction between treatment and genotype (Table 1). Subsequent one-way ANOVA showed that the treatment had a significant effect in WT (F [2.29]=287.530; P<0.01) and KO mice (F [2.29]=221.705; P<0.01). Post hoc analysis showed differences in morphine-treated WT and KO mice at the doses of 5 and 10 mg kg−1 (P<0.01 in all the cases), when compared with saline-treated mice (Figure 2c).
Figure 2.
Acute antinociceptive effects induced by morphine in A2A knockout (KO) and wild-type (WT) mice. Antinociceptive responses in the tail-immersion (a) and hot-plate (b, c) tests were measured 30 min after morphine or saline injection. Results are expressed as mean±s.e.mean of latency time in seconds (n=10 in all groups).
Morphine-induced conditioned place preference
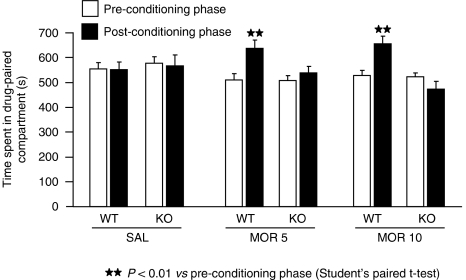
Rewarding responses induced by morphine (5 and 10 mg kg−1) were evaluated in WT and A2A KO mice by using the place-conditioning paradigm. One-way ANOVA revealed that a similar time was spent in the drug-associated compartment during the preconditioning phase by the different groups (F [5.86]=1.160), ensuring the use of an unbiased procedure (Figure 3). A significant rewarding effect of morphine was observed in WT at both doses of morphine used (5 and 10 mg kg−1), but not in mice lacking the A2AR. Accordingly, WT mice conditioned with 5 (t [1.9]=−6.903, P<0.01) and 10 mg kg−1 (t [1.11]=−3.186; P<0.01) of morphine spent significantly more time in the drug-associated compartment during the post-conditioning phase than during the preconditioning phase. In contrast, A2A KO mice receiving 5 and 10 mg kg−1 of morphine spent the same time in the drug-associated compartment during both phases (Figure 3).
Figure 3.
Morphine-induced conditioned place preference in A2A knockout (KO) and wild-type (WT) mice. Results are expressed as mean±s.e.mean of time spent in the drug-paired compartment during the preconditioning and post-conditioning phases in WT and A2A KO mice after morphine or saline administration (saline groups, n=22; morphine groups, n=9–12).
Naloxone-induced place aversion in morphine-dependent mice
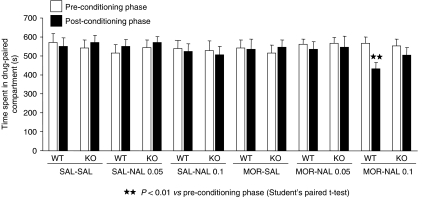
Naloxone-induced aversive effects in morphine-dependent mice were measured by using the place-conditioning paradigm. One-way ANOVA revealed a similar time spent in the drug-associated compartment during the preconditioning phase in the different groups (F [11,153]=0.233), ensuring the use of an unbiased procedure (Figure 4). Naloxone (0.1 mg kg−1, s.c.) induced a conditioned place aversion in morphine-dependent WT mice, as revealed by a significant decrease in the time spent in the drug-associated compartment during the post-conditioning vs the preconditioning phase (t [1.13]=4.410, P<0.01). In contrast, morphine-dependent A2A KO receiving such a dose of naloxone spent the same time in the drug-associated compartment during both phases. The administration of a lower dose of naloxone (0.05 mg kg−1) did not induce aversive responses in either WT or KO animals dependent on morphine (Figure 4).
Figure 4.
Naloxone-induced conditioned place aversion in morphine-dependent. A2A knockout (KO) and wild-type (WT) mice. Results are expressed as mean±s.e.mean of time spent in the drug-paired compartment during the preconditioning and post-conditioning phases in saline and morphine-treated WT and A2AKO mice after saline or naloxone administration (saline-treated groups, n=10–13; morphine-treated groups, n=11–15).
Morphine had no effect on extracellular levels of DA in the NAc
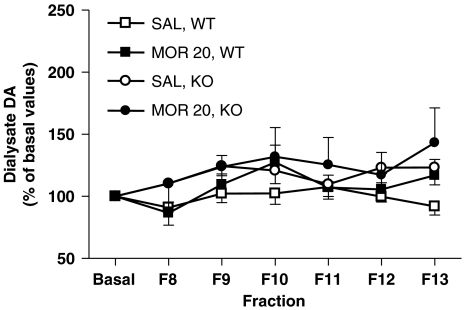
The extracellular levels of DA in the NAc were assessed by microdialysis in freely moving WT and adenosine A2A KO mice (n=5 for saline-treated WT mice, n=6 for saline-treated KO mice, n=6 for morphine-treated WT mice, n=7 for morphine-treated KO mice). Basal extracellular DA levels in the NAc measured over five dialysate fractions (F3–7) were comparable in WT (1.29±0.32 nM, n=11) and KO mice (1.08±0.16 nM, n=13) (Figure 5; genotype, P=0.450). Administration of morphine (20 mg kg−1, s.c.) had no effect on extracellular DA levels in the NAc of either treated WT or KO mice compared with saline-treated groups (Figure 5) despite inducing the marked behavioural responses of hyperlocomotion and straub tail (data not shown). Further studies using different probe target coordinates (left lateral shell of NAc AP: +0.98 mm, L: −1.7 mm, DV: −5.0 mm) or a longer time between microdialysis surgery and the day of the experiment (5 day protocol; day 1, implant cannula; day 3, implant probe; day 5, commence experiment) at two doses of morphine (10 and 20 mg kg−1) also failed to show any effect of morphine administration on extracellular DA in the NAc of WT or KO mice (data not shown). High-potassium (50 mM)-evoked extracellular accumbal DA was measured after both saline and morphine (20 mg kg−1) treatment and was not significantly different, nor was it altered in the A2A KO mice (AUC: WT saline 176.3±38.8; WT morphine 175.9±26.3; A2A KO saline 141.8±12.5; A2A KO morphine 118.1±23.4).
Figure 5.
Effects of the systemic morphine administration on dialysate dopamine (DA) concentrations in the nucleus accumbens (NAc) of A2A knockout (KO) and wild-type (WT) mice. Morphine did not increase DA output in the NAc of either WT (n=5–6) or A2A KO mice (n=6–7) in any dialysis fraction. Data are percentages of pretreatment (basal) values and are given as mean±s.e.mean values (see Methods section for details).
Increase in the extracellular levels of DA in the NAc after cocaine administration
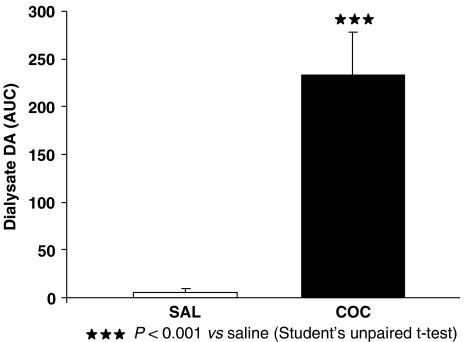
Acute cocaine (20 mg kg−1, s.c.) enhanced the extracellular levels of DA in the NAc in the WT mice; Student's t-test showed that cocaine had a significant effect on the AUC values (from 140 to 240 min) (t [1.8]=5.025, P<0.001, n=5) (Figure 6).
Figure 6.
Cocaine-evoked extracellular dopamine (DA) in the NAc in WT mice. Results are expressed as mean±s.e.mean of the area under the curve (AUC) calculated from F8–13 in WT mice (n=5) after saline (SAL) or cocaine (COC) administration.
Discussion
In this study, the participation of A2AR in the behavioural and neurochemical responses associated with morphine's motivational properties were evaluated; these included the reward and the dysphoric effects associated with the naloxone-precipitated morphine withdrawal. We also investigated the acute effects of morphine on nociception and locomotion to ensure that the motivational and neurochemical responses observed were not influenced by changes in these effects. Mice lacking A2AR exhibited similar acute responses after morphine administration to those in WT littermates. However, both the rewarding and aversive effects associated with morphine abstinence were completely abolished in mice lacking the A2AR.
In our study, deletion of the A2AR did not modify the acute effects induced by morphine. Previously, hypoalgesia was found to occur in A2A KO mice, but the thermal stimulus was stronger than that used in this study (Ledent et al., 1997). Thus, changes in basal nociceptive responses in A2A KO mice are not robust when using the tail-immersion test at 50 °C, and several studies now suggest that the hypoalgesia is dependent on the intensity of the stimulus used (for review see Ferré et al., 2007). Hence, our data demonstrate that A2AR do not participate in the acute effects induced by morphine.
Morphine-induced rewarding responses were abolished in A2A KO mice. Two different doses of morphine were used in this paradigm (5 and 10 mg kg−1), but no difference was observed in the effect of morphine in WT mice due to the ‘all or nothing' responses often observed in this behavioural model. However, the lack of the conditioned place preference in KO mice when morphine was given at the highest dose indicates that the morphine reward response is absent in mice lacking A2AR. A2AR are mainly located in striatal neurons where they interact with multiple neurotransmitter systems, being coexpressed with postsynaptic receptors in GABAergic neurons (Fink et al., 1992). Adenosine regulates DA transmission through antagonistic interactions of A2A/DA D2 receptors (Franco et al., 2000), and this interaction also modulates glutamate release and thereby affects the striatal neuronal output (Tozzi et al., 2007). In addition, developmental changes have been shown to occur in A2A KO mice that lead to a functional hypodopaminergic state (Dassesse et al., 2001); this is pertinent to the behavioural findings of this study. Hence, the neurotransmitter DA has been widely implicated as a key regulator of the pharmacological actions and rewarding properties of drugs of abuse (Berridge and Robinson, 1998; Spanagel and Weiss, 1999; Wise, 2004). Interestingly, DA D2 and A2AR interactions may also involve molecular mechanisms, and a functional and cooperative interaction between these two receptors has been described for the adenylate cyclase activity in neuronal cells (Vortherms and Watts, 2004). Additionally, recent findings have revealed the existence of a synergy between D2 and adenosine A2AR mediated by βγ dimmers of GTP-binding proteins. This interaction confers ethanol hypersensitivity and βγ dimmers are required for voluntary drinking (Yao et al., 2002). Moreover, DARPP-32, which plays an obligatory role in dopaminergic transmission, is altered in mice lacking A2AR (Svenningsson et al., 2000), and a large body of evidence supports a key role for DARPP-32-dependent signalling in mediating the action of multiple drugs of abuse, including morphine (Svenningsson et al., 2005). μ-Opioid receptor agonists, such as morphine, have been shown to increase DA release preferentially in the NAc by the disinhibition of GABAergic neurons (Di Chiara and Imperato, 1988; Johnson and North, 1992). In this study, microdialysis experiments in freely moving WT and adenosine A2A KO mice revealed no differences between the genotypes in the basal or saline-evoked extracellular DA levels in the Nac. In contrast, Dassesse et al. (2001) demonstrated hypodopaminergic activity in the striatum of KO mice. In this last study, the microdyalisis probe was placed in the caudate-putamen, whereas our microdialysis studies targeted the NAc, and the experimental conditions (length of the probe, number of animals, number of samples and so on) were also different in the two studies. In another study (Castañé et al., 2006), where the conditions were similar to those used in our experiments, the basal DA levels in the NAc were found to be the same in both genotypes. Also, Rethy et al. (1971) and Gupta et al. (1988) found that morphine, up to 20 mg kg−1, despite producing recognized behavioural responses of hyperlocomotion and straub tail, failed to increase extracellular DA release in either the core or shell of the NAc. However, others have shown morphine-induced DA release in a C57/B6 mouse strain (Chefer et al., 2003). Hence, the lack of a DA response in this study might be attributable to the outbred background strain of these mice (CD1); previous studies have suggested that differences in the background strain of experimental models can result in a variable sensitivity to drugs of abuse (Barbaccia et al., 1981; Shoaib et al., 1995; He and Shippenberg, 2000; Fadda et al., 2005). Interestingly, the CD1 strain has been previously characterized as ethanol-avoiding (Short et al., 2006). This suggests that the CD1 strain may have a differential sensitivity to drugs of abuse and may explain its weak response to the neurochemical effects of morphine in the NAc. Furthermore, the temporal resolution of the microdialysis technique could also be a limiting factor and any small increases in DA detected are unlikely to be significant. Notably, acute cocaine (20 mg kg−1) produced a significant enhancement of the DA levels in the NAc (Figure 6), confirming that DA release in response to addictive substance is present in these animals. Taken together, our results on the rewarding effects of morphine are in agreement with a recent finding revealing that adenosine A2A blockade prevents synergy between μ-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behaviour in rats. Indeed, an A2A antagonist administered directly into the NAc or indirectly through the i.p. route completely eliminates reinstatement in heroin-addicted rats (Yao et al., 2006), pointing out the essential role of A2AR in the addictive effects of opiates.
It is known that μ-opioid receptors are responsible for morphine-induced motivational responses (Matthes et al., 1996). Until now, no study has investigated the role of A2AR in the aversive responses that occur during morphine withdrawal. Interestingly, our findings demonstrate that the aversive responses exhibited during morphine abstinence are impaired in mice lacking A2AR. As we have shown that no significant changes in μ-opioid receptor binding occur in brains from naloxone-precipitated withdrawn A2A KO mice (Bailey et al., 2004), and also a lack of change in μ-opioid receptor binding in naive A2A KO mice (Bailey et al., 2002), compensatory changes in the expression of opioid receptors in A2A KO mice are unlikely to account for the absence of the dysphoric effects normally associated with morphine abstinence in these mice. However, a significant increase in the level of μ-opioid receptor-stimulated [35S]-GTPγS binding has been observed in the NAc but not in other brain structures of mutant mice during morphine abstinence (Bailey et al., 2004). It is, therefore, possible that this increased μ-opioid receptor-mediated G-protein activity in the NAc might be a compensatory mechanism to increase levels of extracellular DA that are extremely low in A2A KO mice during the morphine withdrawal syndrome. This increase in DA neurotransmission, through the enhancement of intracellular transduction mechanisms associated with μ-opioid receptor activation, could be responsible for the lack of dysphoric effects observed during morphine withdrawal in A2A KO mice and could progressively appear as an adaptive process while physical dependence is developing.
Motor and motivational responses to opioids have been found to be closely related (Salamone, 1996). In our case, the absence of place preference and place aversion were not dependent on the locomotor impairment of A2A KO mice as the motor response to morphine was preserved in these animals (Figure 1). In a previous study, using a similar procedure to induce morphine dependence, we observed an enhancement in the physical expression of the morphine withdrawal syndrome in mice lacking A2AR, as revealed by a higher global withdrawal score in KO morphine-dependent mice (Berrendero et al., 2003; Bailey et al., 2004). In agreement with these findings, in pharmacological studies A2A-selective adenosine receptor agonists have been shown to decrease the incidence of some morphine withdrawal signs, whereas the administration of adenosine antagonists produced opposite responses (Salem and Hope, 1997; Zarrindast et al., 1999). In addition, in electrophysiological studies the nonselective adenosine antagonist caffeine was found to enhance the electrical activity recorded in the nucleus paragigantocellularis (Khalili et al., 2001), a brain structure that participates in the somatic expression of the opioid withdrawal syndrome (Rasmussen and Aghajanian, 1989).
Our results demonstrate a clear dissociation between the mechanisms involved in the motivational properties of opiates, which are related to their addictive capacities, and the somatic signs of naloxone-precipitated withdrawal syndrome, which reveal the physical component of opiate dependence. Thus, adenosine A2AR appear to have a crucial role in the rewarding and aversive effects of opiates and the expression of the physical symptoms of opiate dependence, modulating the motivational effects and somatic abstinence in opposite directions. Our findings also support the hypothesis that receptors involved in the addictive behaviours induced by prototypic drugs require the activation of A2AR; this has been shown for ethanol, cocaine and heroin operant self-administration (Arolfo et al., 2004; Soria et al., 2006; Yao et al., 2006). Furthermore, we have also demonstrated that A2A KO mice elicit a decreased motivation for nicotine and Δ9-tetrahydrocannabinol (Soria et al., 2004; Castañé et al., 2006). The present findings now provide evidence that A2ARs are also required for the expression of morphine rewarding responses and the negative motivational effects associated with the morphine withdrawal syndrome. We conclude that adenosine acting through the A2AR represents a general system for modulating the motivational responses associated with drugs of abuse and the pharmacological manipulation of these receptors may represent a new target in the management of drug addiction.
Acknowledgments
This study was supported by grants from Spanish MEC (SAF2004/0568 and SAF2007/60249) and MSC (PNSD-2006), European Communities (GENADDICT LSHM-CT-2004-005166) and Joint Innovation Fund, University of Surrey and Roehampton University, UK. The Interuniversity Attraction Poles Programme (P6-14)—Belgian State—Belgian Science Policy, the Fondation Médicale Reine Elisabeth and the Fonds National Belge de la Recherche Scientifique. We acknowledge Dr Don Fisher (Roehampton University) for his assistance with HPLC-EC analysis.
Abbreviations
- A2AR
A2A adenosine receptors
- DA
dopamine
- KO
knockout
- Nac
nucleus accumbens
- WT
wild type
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- Bailey A, Davis L, Lesscher HM, Kelly MD, Ledent C, Hourani SM, et al. Enhanced morphine withdrawal and micro-opioid receptor G-protein coupling in A2A adenosine receptor KO mice. J Neurochem. 2004;88:827–834. doi: 10.1046/j.1471-4159.2003.02214.x. [DOI] [PubMed] [Google Scholar]
- Bailey A, Ledent C, Kelly M, Hourani SM, Kitchen I. Changes in spinal delta and kappa opioid systems in mice deficient in the A2A receptor gene. J Neurosci. 2002;22:9210–9220. doi: 10.1523/JNEUROSCI.22-21-09210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Reggiani A, Spano PF, Trabucchi M. Ethanol-induced changes of dopaminergic function in three strains of mice characterized by a different population of opiate receptors. Psychopharmacology. 1981;74:260–262. doi: 10.1007/BF00427106. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Castañé A, Ledent C, Parmentier M, Maldonado R, Valverde O. Increase of morphine withdrawal in mice lacking A2a receptors and no changes in CB1/A2a double KO mice. Eur J Neurosci. 2003;17:315–324. doi: 10.1046/j.1460-9568.2003.02439.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience. Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Castañé A, Soria G, Ledent C, Maldonado R, Valverde O. Attenuation of nicotine-induced rewarding effects in A2A knockout mice. Neuropharmacology. 2006;51:631–640. doi: 10.1016/j.neuropharm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Kieffer BL, Shippenberg TS. Basal and morphine-evoked dopaminergic neurotransmission in the nucleus accumbens of MOR- and DOR-KO mice. Eur J Neurosci. 2003;18:1915–1922. doi: 10.1046/j.1460-9568.2003.02912.x. [DOI] [PubMed] [Google Scholar]
- Dassesse D, Massie A, Ferrari R, Ledent C, Parmentier M, Arckens L, et al. Functional striatal hypodopaminergic activity in mice lacking adenosine A(2A) receptors. J Neurochem. 2001;78:183–198. doi: 10.1046/j.1471-4159.2001.00389.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- Fadda P, Scherma M, Collu M, Fratta W. Dopamine and serotonin release in dorsal striatum and nucleus accumbens is differentially modulated by morphine in DBA/2J and C57BL/6J mice. Synapse. 2005;56:29–38. doi: 10.1002/syn.20122. [DOI] [PubMed] [Google Scholar]
- Ferré S, Diamond I, Goldberg SR, Yao L, Hourani SMO, Huang ZL, et al. Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry. Implications for drug addiction, sleep and pain. Prog Neurobiol. 2007;83:332–347. doi: 10.1016/j.pneurobio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Franco R, Ferré S, Agnati L, Torvinen M, Gines S, Hillion J, et al. Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacology. 2000;23:S50–S59. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Gibaldi M, Perrier D.The trapezoid rule Pharmacokinetics 1975Dekker, New York; In: Gibaldi M, Perier D (eds).2nd edn.pp 494 [Google Scholar]
- Gupta ML, Nath R, Gupta TK, Gupta GP. A study of central neurotransmitter mechanisms in morphine-induced ‘Straub reaction' in mice: role of central dopamine receptors. Clin Exp Pharmacol Physiol. 1988;15:727–732. doi: 10.1111/j.1440-1681.1988.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hack SP, Christie MJ. Adaptations in adenosine signaling in drug dependence: therapeutic implications. Crit Rev Neurobiol. 2003;15:235–274. doi: 10.1615/critrevneurobiol.v15.i34.30. [DOI] [PubMed] [Google Scholar]
- He M, Shippenberg TS. Strain differences in basal and cocaine-evoked dopamine dynamics in mouse striatum. J Pharmacol Exp Ther. 2000;293:121–127. [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiology. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Coyle TS. Adenosine kinase inhibitors attenuate opiate withdrawal via adenosine receptor activation. Eur J Pharmacol. 1998;362:1–8. doi: 10.1016/s0014-2999(98)00724-9. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Sears MT. Adenosine receptor agonists attenuate and adenosine receptor antagonists exacerbate opiate withdrawal signs. Psychopharmacology. 1996;123:64–70. doi: 10.1007/BF02246282. [DOI] [PubMed] [Google Scholar]
- Khalili M, Semnanian S, Fathollahi Y. Caffeine increases paragigantocellularis neuronal firing rate and induces withdrawal signs in morphine-dependent rats. Eur J Pharmacol. 2001;412:239–245. doi: 10.1016/s0014-2999(01)00718-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1:186–189. [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Huber G. Central adenosine A(2A) receptors: an overview. Brain Res Brain Res Rev. 1999;31:65–82. doi: 10.1016/s0165-0173(99)00059-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesions of the nucleus paragigantocellularis. Brain Res. 1989;505:346–350. doi: 10.1016/0006-8993(89)91466-2. [DOI] [PubMed] [Google Scholar]
- Rethy CR, Smith CB, Villarreal JE. Effects of narcotic analgesics upon the locomotor activity and brain catecholamine content of the mouse. J Pharmacol Exp Ther. 1971;176:472–479. [PubMed] [Google Scholar]
- Salamone JD. The behavioral neurochemistry of motivation: methodological and conceptual issues in studies of the dynamic activity of nucleus accumbens dopamine. J Neurosci Methods. 1996;64:137–149. doi: 10.1016/0165-0270(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Salem A, Hope W. Effect of adenosine receptor agonists and antagonists on the expression of opiate withdrawal in rats. Pharmacol Biochem Behav. 1997;57:671–679. doi: 10.1016/s0091-3057(96)00393-0. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ. Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci. 1993;13:1080–1087. doi: 10.1523/JNEUROSCI.13-03-01080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, et al. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Spanagel R, Stohr T, Shippenberg TS. Strain differences in the rewarding and dopamine-releasing effects of morphine in rats. Psychopharmacology. 1995;117:240–247. doi: 10.1007/BF02245193. [DOI] [PubMed] [Google Scholar]
- Short JL, Drago J, Lawrence AJ. Comparison of ethanol preference and neurochemical measures of mesolimbic dopamine and adenosine systems across different strains of mice. Alcohol Clin Exp Res. 2006;30:606–620. doi: 10.1111/j.1530-0277.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Soria G, Castañé A, Berrendero F, Ledent C, Parmentier M, Maldonado R, et al. Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. Eur J Neurosci. 2004;20:2203–2213. doi: 10.1111/j.1460-9568.2004.03682.x. [DOI] [PubMed] [Google Scholar]
- Soria G, Castañé A, Ledent C, Parmentier M, Maldonado R, Valverde O. The reinforcing efficacy of cocaine is diminished in mice lacking A2A adenosine receptors. Neuropsychopharmacology. 2006;31:978–987. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, et al. Regulation of the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci USA. 2000;97:1856–1860. doi: 10.1073/pnas.97.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7:E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MI, White TD, Jhamandas KH, Sawynok J. Morphine releases endogenous adenosine from the spinal cord in vivo. Eur J Pharmacol. 1987;141:169–170. doi: 10.1016/0014-2999(87)90428-6. [DOI] [PubMed] [Google Scholar]
- Sweeney MI, White TD, Sawynok J. Intracerebroventricular morphine releases adenosine and adenosine 3′,5′-cyclic monophosphate from the spinal cord via a serotonergic mechanism. J Pharmacol Exp Ther. 1991;259:1013–1018. [PubMed] [Google Scholar]
- Tozzi A, Tscherter A, Belcastro V, Tantucci M, Costa C, Picconi B, et al. Interaction of A2A adenosine and D2 dopamine receptors modulates corticostriatal glutamatergic transmission. Neuropharmacology. 2007;53:783–789. doi: 10.1016/j.neuropharm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Valverde O, Tzavara E, Hanoune J, Roques BP, Maldonado R. Protein kinases in the rat nucleus accumbens are involved in the aversive component of opiate withdrawal. Eur J Neurosci. 1996;8:2671–2678. doi: 10.1111/j.1460-9568.1996.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Vortherms TA, Watts VJ. Sensitization of neuronal A2A adenosine receptors after persistent D2 dopamine receptor activation. J Pharmacol Exp Ther. 2004;308:221–227. doi: 10.1124/jpet.103.057083. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, Fuchs S, et al. bg dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell. 2002;109:733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Ueda T, Diamond I. Adenosine A2a blockade prevents synergy between m-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc Natl Acad Sci USA. 2006;103:7877–7882. doi: 10.1073/pnas.0602661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Naghipour B, Roushan-zamir F, Shafaghi B. Effects of adenosine receptor agents on the expression of morphine withdrawal in mice. Eur J Pharmacol. 1999;369:17–22. doi: 10.1016/s0014-2999(99)00021-7. [DOI] [PubMed] [Google Scholar]