Abstract
Cyr61 has been reported to participate in the development and progression of various cancers; however, its role in prostate cancer (PCa) still remains poorly understood. In this study, we explored the function of Cyr61 in a series of malignant PCa cell lines, including LnCap, Du145, and PC3. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and crystal violet assays demonstrated that Cyr61 was essential for the proliferation of PCa cells. Soft agar assay and xenograft analysis showed that downregulation of Cyr61 suppressed the tumorigenicity of Du145 cells both in vitro and in vivo. Either silencing the cellular Cyr61 by RNA interference or neutralising the endogenous Cyr61 by antibody inhibited the migration of Du145 cells. In contrast, purified protein of Cyr61 promoted the migration of LnCap cells in a dose-dependent manner. These results suggested that Cyr61 was involved in the migration of PCa cells. We also observed the accumulation of mature focal adhesion complexes associated with the impaired migration through Cyr61 downregulation. Also, further studies showed that Cyr61 regulated the level of activated Rac1 as well as its downstream targets, including phosphorylated JNK, E-cadherin, and p27kip1, which are key molecules involved in cell growth, migration, and invasion. The in vivo mouse tail vein injection experiment revealed that Cyr61 affected the metastatic capacity of Du145 cells, suggesting that Cyr61 was required for prostate tumour metastasis. Altogether, our results demonstrated that Cyr61 played an important role in the tumorigenicity and metastasis of PCa cells, which will benefit the development of therapeutic strategy for PCas.
Keywords: PCa, Du145, Cyr61, Rac1
Prostate cancer (PCa) is one of the main diseases influencing men today, ranked the third most common cancer worldwide (Ziober et al, 2001; Sim and Cheng, 2005). Though the molecular mechanisms underlying remain poorly understood, accumulating evidence indicates the involvement of various growth factors in the development and progression of PCa (Ware, 1998; Melton et al, 2007), such as insulin-like growth factor (Gennigens et al, 2006), vascular endothelial growth factor (Delongchamps et al, 2006), transforming growth factor-β (Zhu and Kyprianou, 2005), epidermal growth factor (Spiegel and Milstien, 2005), and hepatocyte growth factor (Hurle et al, 2005).
CCN growth factor family is named after the three first identified members – Cyr61 (cysteine-rich protein 61, CCN1), CTGF (connective tissue growth factor, CCN2), and Nov (nephroblastoma overexpressed, CCN3) (Bork, 1993). So far, this family has included six members: Cyr61, CTGF, Nov, WISP-1 (Wnt-1-induced secreted protein 1) (CCN4), WISP-2 (CCN5), and WISP-3 (CCN6) (Brigstock, 2003; Perbal, 2004). All CCN molecules share four conserved structural modules with sequence homologies similar to cysteine knot, thrombospondin, von Willebrand factor, and insulin-like growth factor-binding protein, respectively (Bork, 1993). Earlier studies have reported that CTGF and Nov promoted tumorigenesis of PCa (Maillard et al, 2001; Yang et al, 2005), whereas the expression of Cyr61 was downregulated in PCa (Pilarsky et al, 1998).
Cyr61 was the first cloned member of the CCN family and reported to mediate a variety of cellular processes, including cell adhesion, stimulation of chemostasis, enhancement of growth factor-induced DNA synthesis, cell survival, and angiogenesis (Grzeszkiewicz et al, 2002; Lin et al, 2004). Moreover, Cyr61 has been reported to be involved in the development of several kinds of tumours (Bleau et al, 2005). Overexpressed Cyr61 could stimulate the progression of breast cancers (Xie et al, 2001a, 2001b). A gastric adenocarcinoma cell line became more tumorigenic when the cells were genetically engineered to express high levels of Cyr61 (Babic et al, 1998). High expression level of Cyr61 was reported in rhabdomyosarcomas, malignant melanomas, colon adenocarcinomas, and bladder papillomas (Genini et al, 1996; Babic et al, 1998). Cyr61 also exhibited high levels in malignant gliomas and enhanced the tumorigenicity through the integrin-linked kinase signalling pathway (Xie et al, 2004). Upregulation of Cyr61 expression was recently identified in peritoneal metastases from human pancreatic cancer (Holloway et al, 2005). Paradoxically, Cyr61 was downregulated in lung cancers, and forced expression of Cyr61 inhibits tumorigenicity of lung cancer cells (Tong et al, 2001, 2004). Cyr61 was also reported to inhibit the growth of endometrial cancer (Chien et al, 2004) and leiomyomas (Sampath et al, 2001).
Although it has been reported that Cyr61 was upregulated and required for prostatic cell proliferation in benign prostatic hyperplasia (Sakamoto et al, 2004), there are rare reports on the role of Cyr61 in malignant PCa cells. In this study, therefore, we investigated the functions of Cyr61 in Du145, a high-grade metastatic PCa cell line (Stone et al, 1978), and found that Cyr61 played an essential role in the proliferation, migration, and metastasis of this PCa cells both in vitro and in vivo.
Materials and methods
Prostate cancer tissue samples and cell lines
Twenty pairs of primary PCa samples and their corresponding normal tissues were obtained from PCa patients treated at the First Affiliated Hospital of Zhengzhou University (Henan, China) from 2002 to 2005 after their written informed consent, and none of the patients received any neoadjuvant therapy. All specimens were frozen at once in liquid nitrogen after surgical excision and stored at −80°C until use. Our study was approved by the Institutional Review Board of the Institute for Nutritional Sciences, Chinese Academy of Sciences. PC3, Du145, LnCap, and 22RV1 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640, supplemented with 10% foetal bovine serum, 10 U ml−1 penicillin, and 10 U ml−1 streptomycin, at 37°C in a humidified atmosphere containing 5% CO2.
Reagents
Rabbit anti-human Cyr61 polyclonal antibody was purchased from Santa Cruz Biotechnology (SC-13100(H-78); Santa Cruz, CA, USA); anti-focal adhesion kinase (anti-FAK, clone 77), anti-Rac1 (clone 102), anti-paxillin (clone 68), FAK (pY397), Paxillin (pY118), anti-phosphorylated JNK (pT183/pY185, clone 41), and pan-p-JNK (clone 37) monoclonal antibodies were from BD Transduction Laboratories (San Diego, CA, USA); Rac1-specific inhibitor NSC23766 was purchased from EMD Biosciences (Darmstadt, Germany); Lipofectamine 2000 was from Invitrogen (Carlsbad, CA, USA); 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Roche Molecular Biochemicals (Basel, Switzerland); and D-luciferin was from Biosynth International Inc.(Naperville, IL, USA).
RNA interference of Cyr61 and rescue experiments
To knock down the endogenous Cyr61, we selected three siRNA hairpin sequences against different sites of Cyr61 mRNA that were designed using Ambin web software: (1) GGCCAGAAATGTATTGTTC; (2) GAAATGCAGCAAGACCAAG; and (3) GAACGTCATGATGATCCAG. These sequences were cloned to the FG12 vector and RNA interference (RNAi) cell lines were produced as described previously (Qin et al, 2003). A silent mutant Cyr61 expression construct was made resistant to an siRNA sequence. Between the nucleic acids 676 and 694, GGCCAGAAATGTATTGTTC were replaced with GGACAAAAGTGCATCGTAC. The silent mutant Cyr61 was delivered into the Cyr61-silenced Du145 cells with lentivirus (Figure 1H).
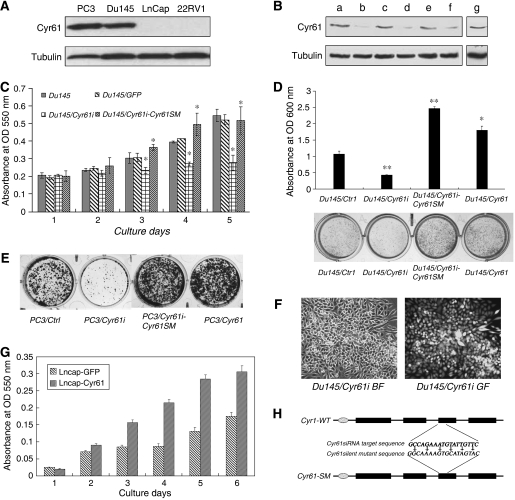
Figure 1.
Silencing of Cyr61 inhibited the proliferation of Du145 cells. (A) The protein level of Cyr61 in four PCa cell lines. (B) Du145 cells were infected with lentivirus either expressing Cyr61 siRNA (Du145/Cyr61i) or the control siRNA (Du145/Ctrli), which processed the same A/T composition but different sequences, and not matched with any genes. Three sequences of either Cyr61 siRNA or control siRNA were designed. Lane a: Du145/Ctrli #1 cells; lane b: Du145/Cyr61i #1 cells; lane c: Du145/Ctrli #2 cells; lane d: Du145/Cyr61i #2 cells; lane e: Du145/Ctrli #3 cells; lane f: Du145/Cyr61i #3 cells; and lane g: wild-type Du145 (Du145/WT) cells. Tubulin was the loading control of protein samples. (C) In vitro proliferation of Du145/WT, Du145/Ctrli #1, Du145/Cyr61i #1, and Du145/Cyr61i-Cyr61SM cells was examined by MTT assay. (D) Crystal violet assay of colony formation of Du145/Cyr61i #1 and Du145/Ctrli #1 cells. Cells (3 × 103) were seeded each well in 12-well plates and photographed 2 weeks later. Number of cells was measured by detecting under OD 600 nm. A typical experiment was shown. (E) Crystal violet assay of PC3/Ctrl, PC3/Cyr61i, PC3/Cyr61i-Cyr61SM and PC3/Cyr61 cells was taken under the same way. (F) Du145 cells were infected with lentivirus expressing both EGFP and control RNAi or Cyr61 RNAi as evidenced by representative light-field or fluorescence photos. (G) LnCap cells were infected with lentivirus either expressing Cyr61 (LnCap/Cyr61) or the control GFP (LnCap/GFP). In vitro proliferation of LnCap/Cyr61 and LnCap/GFP cells was examined by MTT assay. (H) Schematic diagram showing the domain structure of wild-type Cyr61, silent mutation of Cyr61. Six nucleic acid changed Cyr61SM without changing the amino acid of Cyr61 making it resistant to the RNA interference function of 1# siRNA sequence. *P<0.05, **P<0.01, Student's t-test.
Forced expression of Cyr61 in Du145 and LnCap cells
A modified lentivirus-based FG12 vector with a CMV promoter to introduce Cyr61 gene was expressed in Du145 and LnCap cells (Qin et al, 2003). After infection, the cells were sorted by FACS to collect GFP-positive cells.
Western blot analysis
Total cell lysates (40 μg of protein) were separated by 10% SDS–PAGE gels and electrotransferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). After blocking with 5% milk, the membranes were probed with primary antibodies at 1 : 1000 dilutions. The membrane was washed and then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Immunoreactive proteins were detected with enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA) and photographed with Kodak X-Omat blue autoradiography film.
Cell proliferation analysis
To analyse the effect of Cyr61 on PCa cell proliferation activity, MTT assay and crystal violet assay were taken. In MTT assay, cells were plated into 96-well plates at 2 × 103 cells per well, cultured in 0.5∼1% FBS RPMI-1640 for various durations, and cell numbers were measured by MTT assay according to the protocol provided by MTT manufacturer. In crystal violet assay, equal number of cells and their control cells were seeded in 12-well plates and cultured in media supplemented with 0.5∼1% FBS for 7 days; media were changed every other day. Cellular growth was stopped after 7 days in culture by removing the media and adding 0.5% crystal violet solution in 20% methanol. After staining for 5 min, the fixed cells were washed with phosphate-buffered saline (PBS) and photographed (Thomas et al, 2004).
Soft agar assay
Cells were plated in 24-well flat-bottomed plates using a two-layer soft agar system with 1 × 103 cells per well in a volume of 400 μl per well as described earlier (Munker et al, 1986). After 14 days of incubation, colonies were counted and measured. All the experiments were repeated at least three times using triplicate plates per experimental point.
Purification of recombinant hCyr61 protein
Cyr61 was cloned into PIZT/V5-His vector between EcoRI and XbaI, followed by transfecting into SF9 insect cells with Cellfectin (Invitrogen). Cells were selected for antibiotic resistance to Zeocin. A Zeocin-resistant pool of cells stably expressed secreted r-hCyr61 recombinant protein. Cyr61-contained medium was collected and the recombinant protein was purified with chromatography on Ni2+ metal-chelating resins.
Tumorigenesis assay in vivo
Stably infected and FACS-sorted cells (1 × 107 cells per flank) suspended in 200 μl of RPMI and 150 μl Matrigel were injected into 5-week-old female SCID mice (CB-17TM/IcrCrl-scidBR) purchased from Shanghai Laboratory Animal Center, CAS (Shanghai, China) and treated in accordance with the American Association for the Accreditation of Laboratory Animal Care guidelines. Each animal was subcutaneously injected at two sites in the flanks. The resulting tumours were measured once a week and tumour volume (mm3) was calculated using the standard formula: length × width × height × 0.5236. Tumours were harvested 8 weeks after injection and individually weighed.
Wound-healing assays
Cells were seeded onto six-well dishes at 1 × 105 per well in growth medium. Confluent monolayers were starved overnight in assay medium and a single scratch wound was created using a micropipette tip. Cells were washed with PBS to remove cell debris, supplemented with assay medium, and monitored. Images were captured by phase microscopy using a × 10 objective at 0 and 24 h post-wounding. The percentage of cells in the wound-healing area was averagely calculated from four experiments.
Boyden chamber assay
Boyden chamber (8-μm pore size polycarbonate membrane) was obtained from Neuroprobe Corp., Bethesda, MD, USA. Cells (2 × 105) in 0.05 ml medium with 1% FBS were placed in the upper chamber, and the lower chamber was loaded with 0.152 ml medium containing 10% FBS. Cells that migrated to the lower surface of filters were detected with traditional H&E staining, and five fields of each well were counted after 4–24 h of incubation at 37°C with 5% CO2. Three wells were examined for each condition and cell type, and the experiments were repeated thrice. For inhibitor experiment, the migrated cells were detected as described above only after overnight incubation with or without series concentrations of NSC23766.
Cloning and production of GST-PAK-CD fusion protein
The Rac1 activity assay was based on the Rap1 activity assay described by Franke et al (1997). We used a glutathione-S-transferase (GST)-PAK-CD (PAK-CRIB domain) fusion protein, containing the Rac1- and Cdc42-binding regions from human PAK1B (GenBank accession number AF071884). A fragment encoding amino acids 56–272 of PAK1B was generated by standard PCR using the oligos 5′-AGCTGGATCCATTTTACCTGGAGAT-3′ and 5′-AGCTCTCGAGATTTCTGGCTGTTGGATGTC-3′, digested with BamHI/XhoI, and then inserted between BamHI with XhoI sites of pGEX4T1 (Pharmacia Biotech, Piscataway, NJ, USA) to yield GST-PAK-CD. Escherichia coli BL21 cells transformed with the GST-PAK-CD construct were grown at 37°C to an absorbance of 0.3. Expression of recombinant protein was induced by the addition of 0.1 mM isopropylthiogalactoside for 2 h. Cells were harvested, resuspended in lysis buffer (50 mM Tris pH 8, 2 mM MgCl2, 0.2 mM Na2S2O, 10% glycerol, 20% sucrose, 2 mM dithiothreitol, 1 μg ml−1 leupeptin, 1 μg ml−1 pepstatin, and 1 μg ml−1 aprotinin), and then sonicated. Cell lysates were centrifuged at 4°C for 20 min at 45 000 g and the supernatant was incubated with glutathione-coupled Sepharose 4B beads (Pharmacia Biotech) for 30 min at 4°C. Protein bound to the beads was washed three times in lysis buffer and the amount of bound fusion protein was estimated using Coomassie-stained SDS gels.
Rac1-GTP pull-down assay
The Rac1 activity assay was performed by pull-down using GST fusion with the protein-binding domain of p21-activated kinase (GST-CIRB). Briefly, Du145 cells were plated onto the plate at a density of approximately 2 × 106 cells per 10 cm dish. When grown to 60–70% confluence, cells were washed twice with PBS, then lysed for 5 min in 250 μl ice-cold eukaryotic lysis buffer (25 mM Tris (pH 7.4) 1 mM EDTA, 5 mM MgCl2, 1 mM DTT, 0.1 mM EGTA, 100 mM NaCl, 1% NP-40, 5% glycerol, 1 mM PMSF, 1 μg ml−1 aprotinin, 1 μg ml−1 leupeptin and 1 μg ml−1 pestatin) and then incubated for 30 min with GST-PAK fusion protein that had been adsorbed to glutathione agarose beads. Beads were washed three times with 500 μl of cold eukaryotic lysis buffer and then resuspended in 10 μl reducing electrophoresis sample buffer (2% SDS, 10% glycerol, 80 mM (Tris pH 6.8), 2 mM EDTA, 100 mM DTT, and 0.1% bromophenol blue) and analysed by SDS–PAGE in a 12% gel. Following electrophoresis, samples were transferred to a nitrocellulose membrane and immunoblotted with mouse anti-human Rac1 antibody at a dilution of 1 : 1000. Normalised Rac1 activity values were determined by dividing the amount of Rac1 in the pull-down by the amount of Rac1 in the loading control.
In vivo bioluminescence imaging of tumour cells
Du145 cells were transfected with plasmids expressing firefly luciferase (PGL4-CMV-Luc2; Longmed Corporation, Beijing, China) using Lipofectamine 2000. Cells were selected for antibiotic resistance with G418. The surviving colonies were screened for bioluminescence in complete media supplemented with 150 μg ml−1 D-luciferin by in vitro imaging using the IVIS_ Imaging System (Xenogen, Alameda, CA, USA). Bioluminescent antibiotic-resistant clones were amplified in culture and characterised for stable luminescence in vitro and tumorigenic potential in vivo. One positive cell line, Du145-luc2-7, was selected for further studies. Du145-luc2-7 cells were infected with control siRNA virus (Du145-Luc2/Ctrli), Cyr61 siRNA virus (Du145-Luc2/Cyr61i), null virus (Du145-Luc2/GFP), and Cyr61 expression virus (Du145-Luc2/Cyr61), respectively. Four pools of Du145-Luc2/Ctrli, Du145-Luc2/Cyr61i, Du145-Luc2/GFP, and Du145-Luc2/Cyr61 were acquired after FACS sorting. They were injected, respectively, into the nude mice from the tail vein. Mice were anaesthetised by inhalation of 3% isoflurane/oxygen mixture delivered by the Xenogen XGI-85-port gas anaesthesia system. Imaging and image analysis were performed following the Xenogen protocol using a cooled CCD camera system (IVIS-100; Xenogen) and LivingImage software (Xenogen). The photographs were taken weekly.
Immunohistochemistry
For immunohistochemistry, primary PCa samples and their corresponding normal tissues were frozen in a cryostat chamber, and 10 μm sections were collected on glass slides. The sections were fixed in ice-cold acetone for 30 min, washed in 0.01 M PBS (8 mM Na2HPO4, 2 mM NaH2PO4, and 150 mM NaCl) for 3 × 5 min, blocked for 1 h in 0.01 M PBS supplemented with 0.3% Triton X-100 and 5% normal goat serum, and then incubated with Cyr61 antibody (1 : 500) at 4°C overnight. After brief washes in 0.01 M PBS, sections were incubated for 2 h in 0.01 M PBS with horseradish peroxidase-conjugated goat anti-rabbit IgG (1 : 1000), followed by development with 0.003% H2O2 and 0.03% DAB in 0.05 M Tris-HCl (pH 7.6). Immunohistochemistry for each sample was performed at least three times, and all sections were counterstained with haematoxylin.
Statistical analysis
The results were representative of at least three independent experiments performed in triplicate and were expressed as the mean±s.d. Statistical analysis of the data was performed using Student's t-test.
Results
Cyr61 stimulated the growth of PCa cells in vitro
We first examined the expression of Cyr61 in the four PCa cell lines 22RV1, LnCap, Du145, and PC3. In Figure 1A, the two highly malignant cell lines – PC3 and Du145 – exhibited significantly higher Cyr61 expression, whereas Cyr61 was nearly undetectable in the other two less malignant cell lines – LnCap and 22RV1 (Figure 1A). The level of Cyr61 in Du145 cells was fairly moderate among the four PCa cell lines; therefore, we explored the function of Cyr61 in Du145 cells by siRNA and antibody neutralisation, as well as forced expression and protein treatment, whereas overexpression and r-hCyr61 protein stimulation were employed in the study on LnCap cells.
After successful knockdown of endogeneous Cyr61 by RNAi (Figure 1B), we examined the cell growth using MTT assay, and the result showed that downregulation of Cyr61 in DU145 cells resulted in an obvious decrease in proliferation. Consistent with this, the introduction of the silent mutant Cyr61 rescued Cyr61 expression and the proliferation of Du145 cells (Figure 1C and H). Crystal violet experiment showed that the clones derived from Du145/Cyr61i cells were much smaller and fewer than those formed by Du145/Ctrli cells; in contrast, elevated Cyr61 level stimulated Du145 cells to form larger and more clones (Figure 1D). Similar results could also be observed in PC3 cells (Figure 1E). These results suggested that downregulation of Cyr61 Du145 cell proliferation and forced expression of Cyr61 in LnCap cells promoted cell proliferation (Figure 1G). Therefore, Cyr61 was required for the proliferation of PCa cells, which were consistent with their function reported in benign prostatic hyperplasia (Sakamoto et al, 2004).
Cyr61 promoted the tumorigenicity of Du145 cells both in vitro and in vivo
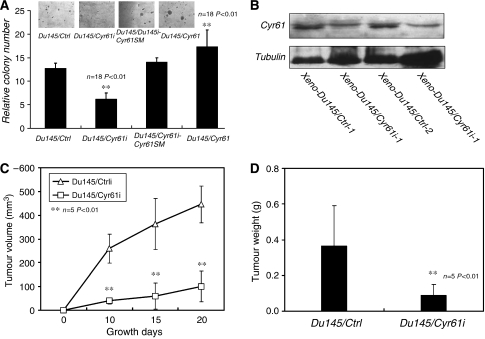
As the knockdown of Cyr61 inhibited the proliferation of PCa cells, we wondered whether it would affect the tumorigenicity either. Soft agar test was carried out to examine the anchorage-independent growth, which is a typical characteristic of the tumorigenicity of cancer cells in vitro (Dodson et al, 1981; Grisham et al, 1991). Du145/Ctrli, Du145/Cyr61i, Du145/Cyr61i-Cyr61SM, and Du145/Cyr61 cells were inoculated on the upper layer of soft agar. After 2 weeks, a number of obvious colonies of Du145/Ctrli cells were observed in each well, whereas much fewer colonies were formed by Du145/Cyr61i cells. Rescuing Cyr61 expression in Du145/Cyr61i cells by byCyr61SM resulted in almost as many colonies as those formed by Du145/Ctrli cells. Furthermore, the colonies derived from Du145/Cyr61i-Cyr61SM cells were larger than those from Du145/Cyr61i cells. Forced expression of Cyr61 in Du145 cells produced more and larger colonies when compared with the Du145/Ctrli cells (Figure 2A). These results indicated that Cyr61 promoted the tumorigenicity of Du145 cells in vitro.
Figure 2.
Silencing of Cyr61 suppressed the tumorigenicity of Du145 cells. (A) Soft agar assay showed that Du145/Cyr61i #1 cells formed much fewer colonies in comparison with Du145/Ctrli #1 cells. Rescued expression of Cyr61 with cyr61 silent mutation and forced expression of cyr61 with lentivirus form much more and larger compared with the Cyr61/Cyr61i. (B) Xenografts were taken out after the mice were killed. Cyr61 expression level was detected by western blot. (C) Du145/Cyr61i #1 or Du145/Ctrli #1 cells (1 × 107) were subcutaneously injected into the dorsal skin of nude mice. Tumour volumes were measured every 7 days, and each point represented the mean volume±s.d. from five independent experiments. (D) Four weeks later, the mice were killed and the tumours were picked up for weighing. Each histogram represented the mean weight±s.d. from five independent experiments. **P<0.01, Student's t-test.
To further examine the effect of Cyr61 on the tumorigenicity of Du145 cells in vivo, either Du145/Cyr61i or Du145/Ctrli cells were subcutaneously injected into nude mice. Tumour volumes were measured every 7 days, and the result showed that Du145/Cyr61i cells formed much smaller tumours when compared with Du145/Ctrli cells (Figure 2C). Four weeks later, the mice were killed and all the tumours were weighed. In accordance with their volumes, the weight of tumours from Du145/Cyr61i cells was lighter than those derived from Du145/Ctrli cells (Figure 2D). Proteins from the xenografts were extracted and the expression of Cyr61 was examined. As shown in Figure 2B, the level of Cyr61 in the Du145/Cyr61i xenografts was lower than that in the Du145/Ctrli xenografts. These data thus suggested that Cyr61 downregulation also impaired the tumorigenicity of Du145 cells in vivo.
Cyr61 was essential for the migration of PCa cells in vitro
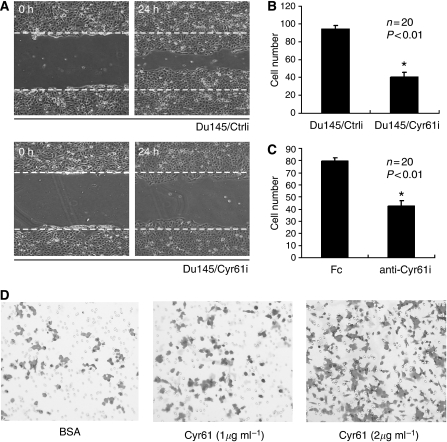
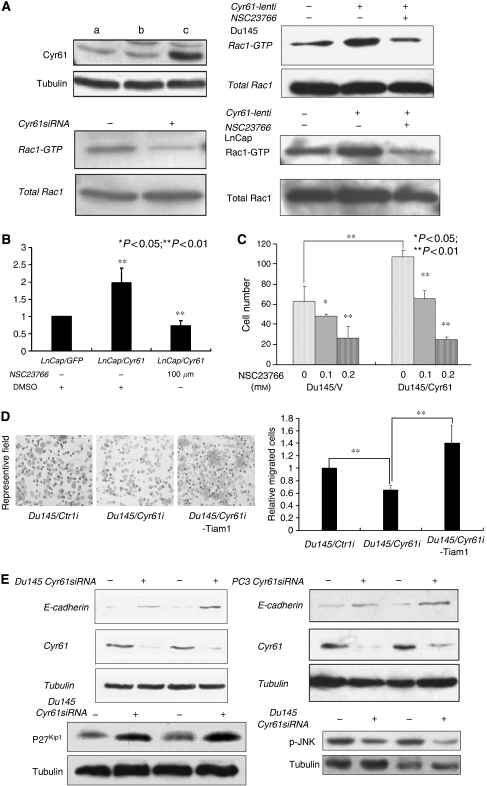
A number of studies have identified that Cyr61 functioned as a ligand of integrins (Kireeva et al, 1998) that play pivotal roles in cell migration (Hood and Cheresh, 2002; Carragher and Frame, 2004; Juliano et al, 2004; Moschos et al, 2007). Thus, we investigated the effects of Cyr61 on the mobility of Du145 and LnCap cells, which bore different levels of Cyr61 expression, respectively. Wound-healing results demonstrated that much fewer Du145/Cyr61i cells moved into the wound area than the Du145/Ctrli cells in the same interval (Figure 3A). Similarly, Boyden chamber assay revealed that the migration ability was impaired in Du145/Cyr61i cells compared with Du145/Ctrli cells (Figure 3B). To further confirm the effect of Cyr61 on cell migration, we used antibodies against Cyr61 (SC-13100) to block the endogenous Cyr61 in Du145 cells. Consistent with the results of an earlier study, the Boyden chamber assay showed that the migration was markedly suppressed upon antibody blocking (Figure 3C). As Cyr61 is a secreted protein, we hypothesised that Cyr61 exerted its effect on migration in a paracrine manner. To examine this hypothesis, we purified r-hCyr61 protein from insect cells and treated the LnCap cells, which express very low levels of Cyr61, with the r-hCyr61 protein. Expectedly, LinCap cells showed elevated migration ability in a dosage-dependent manner in r-hCyr61 protein treatment (Figure 3D). Thus, these results suggested that Cyr61 was closely involved in the migration of PCa cells.
Figure 3.
Silencing of Cyr61 inhibited the migration of Du145 cells. (A) Wound-healing assay for either Du145/Ctrli #1 or Du145/Cyr61i #1 cells. (B) Boyden chamber assay for Du145/Ctrli #1 or Du145/Cyr61i #1 cells, and the cells migrated through the polycarbonate membrane were counted following H&E staining. Student's t-test (*P<0.01). (C) Boyden chamber assay for Du145 cells treated with either rabbit IgG (Fc) or the antibody against Cyr61 (anti-Cyr61) and the cells migrated through the polycarbonate membrane were counted following H&E staining. Student's t-test (*P<0.01). (D) Boyden chamber assay for LnCap cells treated with either BSA (1 μg ml−1) or the purified Cyr61-his protein. Cyr61 stimulates LnCap cell migration in a dosage-dependent manner.
Downregulation of Cyr61 increased focal adhesion assembly in Du145 cells
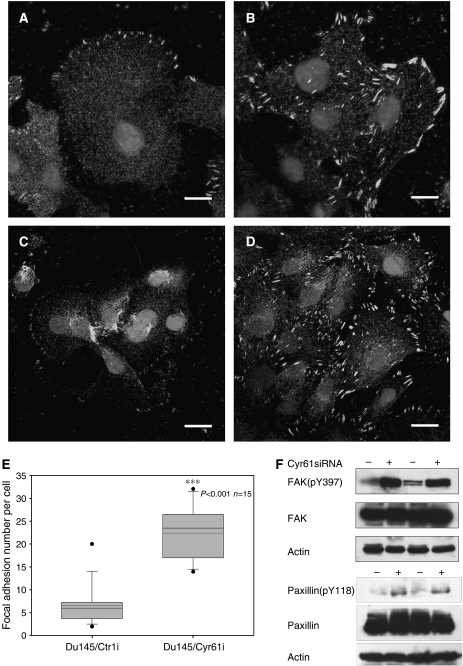
As the effects of integrins on migration often involve the regulation of focal adhesion (FA) (Miyamoto et al, 1995; Burridge and Chrzanowska-Wodnicka, 1996), we examined the status of FA after downregulation of Cyr61 by immunofluorescence of FAK (Figure 4A and B) and paxillin (Figure 4C and D), both of which serve as markers of FA (Miyamoto et al, 1995; Burridge and Chrzanowska-Wodnicka, 1996). As shown in Figure 4E, Du145/Cyr61i cells formed more FAs than Du145/Ctrli cells. Box-and-whisker plots of paxillin-positive-stained points within Du145/Ctrli or Du145/Cyr61i plated on FN for 8 h are shown. In Du145/Ctrli cells, the staining of FAK (Figure 4A) and paxillin (Figure 4C) was weak and mainly appeared on the polarised edge. In contrast, in Du145/Cyr61i cells, the staining signals of (Figure 4B) both FAK and paxillin (Figure 4D) were much more intense and exhibited uniform membrane distribution. This difference indicated altered FA assembly (Miyamoto et al, 1995; Burridge and Chrzanowska-Wodnicka, 1996). Phosphorylated FAK or paxillin, which are activated forms of the molecule, was probed with anti-FAK (pY397) or anti-paxillin (pY118) monoclonal antibodies, respectively. The phosphorylation level of both FAK and paxillin was elevated upon silencing of Cyr61 (Figure 3F), which indicated the increased activation of the two molecules. As the increase of FA often promotes the adhesion of host cells with the extracellular matrix and the loss of polar distribution of FA inhibits cell mobility (Hood and Cheresh, 2002; Carragher and Frame, 2004), the alteration of FA assembly in Du145/Cyr61i cells was consistent with their impaired mobility.
Figure 4.
Silencing of Cyr61 changed the intensity and distribution of focal adhesion. Immunofluorescence of FAK and paxillin, two markers of focal adhesion, on either Du145/Ctrli #1 ((A) for FAK staining and (C) for paxillin staining) or Du145/Cyr61i #1 cells ((B) for FAK staining and (D) for paxillin staining). Scale Bar=10 μm. (E) Increased focal adhesion formation upon reduced Cyr61 expression. Box-and-whisker plots of paxillin-positive-stained points within Du145/Ctrli or Du145/Cyr61i plated on FN for 8 h (***P<0.001; n=15 cells per point). (F) Increased FAK (pY397) and paxillin (pY118) levels upon reduced Cyr61 expression. Western blot detected protein level of total FAK and paxillin, together with FAK (pY397) and paxillin (pY118). β-Actin was used as a loading control.
Effect of Cyr61 on PCa cell migration was mediated through the activity of Rac1
To address the molecular mechanisms by which Cyr61 regulated the migration of Du145 cells, we further examined some key molecules involved in the integrin signalling pathways, such as ERK, AKT, and JNK (Juliano et al, 2004). In Du145/Cyr61i and the Du145/Ctrli cells, the activating forms of both ERK and AKT were almost the same (data not shown), whereas the activated JNK markedly decreased in the Du145/Cyr61i cells (Figure 5E). Previous studies have proved that the small GTP-binding protein Rac1 played a key role in the phosphorylation of JNK as well as cell migration (Juliano et al, 2004). Therefore, we assumed that the loss of activated JNK might be induced by a decreased level of activated Rac1. To test our hypothesis, the activated Rac1 was pulled down with GST-PKA (see Materials and Methods), and the result showed that there was much less activated Rac1 in Du145/Cyr61i cells than in Du145/Ctrli cells (Figure 5A). For further studying the molecular mechanism, we compared the activation of Rac1 in LnCap and Du145 cells overexpressing Cyr61 (LnCap/Cyr61 and Du145/Cyr61) with that in their parental cells. As expected, Cyr61 overexpression indeed enhanced the activation of Rac1 (Figure 5A) as well as the migration in both LnCap and Du145 cells (Figure 5B and C). To confirm that the influence of Cyr61 on the migration of PCa cell was Rac1 dependent, we employed a specific inhibitor NSC23766 for Rac1 to manipulate the activity of endogeneous Rac1 in LnCap/Cyr61 and Du145/Cyr61 cells. As shown in Figure 5B and C, the migration could be inhibited by NSC23766 in both LnCap and Du145 cells (Figure 5B and C). To further confirm the effect of Rac1 on Cyr61-induced cell migration, we transfected the Du145/Cyr61i cells with T-cell lymphoma invasion and metastasis 1 (Tiam1), which was reported as a specific activator of Rac1 (Hamelers et al, 2005; Cruz-Monserrate and O’Connor, 2008). Tiam1-transfected Du145/Cyr61i cells effectively reversed the migration-suppression effects of Cyr61 silencing (Figure 5D).
Figure 5.
Rac1 was implicated in the regulation of Du145 cell migration by Cyr61. (A) Protein levels of Cyr61 following forced expression of Cyr61 in Du145 cells. Lane a: Du145/WT cells; lane b: Du145 cells infected with lentivirus expressing GFP (Du145/GPF); lane c: Du145 cells infected with Cyr61 expression lentivirus (Du145/Cyr61). Activity of Rac1 was detected following silencing (down left) or forced expression of Cyr61 in Du145 cells (up right) and LnCap cells (down right) or treated with Rac1-specific inhibitor NSC23766. (B) Boyden chamber assay for either LnCap/GPF or LnCap/Cyr61 cells with or without priorly treated with NSC23766. **P<0.01. (C) Boyden chamber assay for either Du145/V or Du145/Cyr61 cells priorly treated with a series of concentrations of Rac1-specific inhibitor NSC23766. *P<0.05; **P<0.01. (D) Boyden chamber assay for either Du146/Ctrli or Du145/Cyr61i or Du145/Cyr61 transfected with Rac1 stimulator Tiam1. *P<0.01. (E) Rac1 downstream molecules that can affect cell migration and proliferation were detected both in Du145 cells and PC3 cells after silencing of Cyr61 expression. E-cadherin and p27Kip1 protein level are elevated after silencing of Cyr61. However, protein levels of phosphorylated JNK (p-JNK) were decreased following silencing of Cyr61 expression in Du145 cells with two different siRNA sequences.
Besides JNK, we also looked into other molecules downstream of Rac1, which are involved in both cell proliferation and migration. Protein level of E-cadherin was elevated in Cyr61-silenced Du145 and PC3 cells. p27kips, a famous CDK suppressor that was recently reported to have an important suppression function in cell migration (Bremnes et al, 2002; Supriatno et al, 2003), was also upregulated during silencing of Cyr61 in Du145 cells (Figure 5E). Therefore, the effects of Cyr61 on migration of Du145 cells might be mainly mediated through Rac1 signalling.
Cyr61 stimulates Du145 metastasis in vivo and Cyr61 expression profile in clinical samples
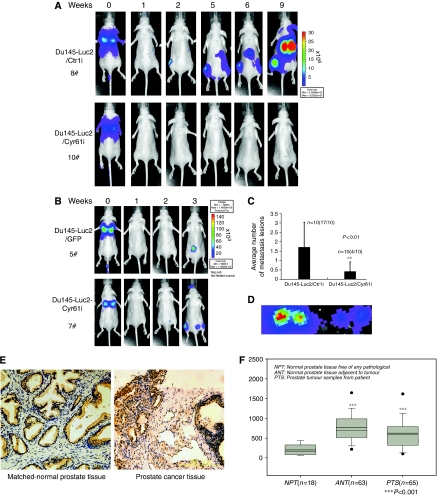
Considering the effect of Cyr61 on cell migration, we suspect it might also influence the metastasis of PCa cells in vivo. First, we constructed Du145-Luc2 cells, which were stably transfected with a firefly luciferase gene (see Materials and Methods), and subsequently infected with lentivirus delivering Cyr61 siRNA (Du145-Luc2/Cyr61i), control siRNA (Du145-Luc2/Ctrli), null (GFP only), or Cyr61, respectively. Four pools enriched with Du145-Luc2/Cyr61i, Du145-Luc2/Ctrli, Du145-Luc2/GFP, and Du145-Luc2/Cyr61 cells were selected by FACS. Also, cells from each pool were injected into the mouse through tail vein. It is well known that an important procedure of tumour metastasis in vivo is the formation of new colonies of tumour cells after movement in blood vascular system (Steeg, 2006), and the experimental model we used mimicked this process. As shown in Figure 6A, Du145-Luc2/Ctrli cells formed more tumour foci, which could be detected by bioluminescence imaging after a relatively short latency; whereas Du145-Luc/Cyr61i cells produced no tumour foci in the same time period. The average metastasis lesions were quantitated (Figure 6C). To exclude the possibility that the signal examined might be artificial, after the mouse were killed, we found out the metastasis lesions according to the previously obtained data (Figure 6D). Student's t-test revealed statistically significant difference between the control and Cyr61-silenced groups (P<0.01). Similarly, the Du145-Luc2/Cyr61 cells led to much more metastasis lesions than Du145-Luc2/GFP cells (Figure 6B). Conclusively, the results indicated the crucial role of Cyr61 in the metastasis of Du145 cells in vivo.
Figure 6.
Silencing of Cyr61 eliminated the systemic tumour growth of Du145 cells in vivo. (A, B, C, and D) Either Du145-Luc2/Ctrli #1 or Du145-Luc2/Cyr61i #1 cells (1 × 107) and 5 × 106 of either Du145-Luc2/GFP or Du145-Luc2/Cyr61 cells were injected into each 10 mice tail vein separately. The bioluminescence images were acquired using the IVIS imaging box at indicated time points. Representative mice (8# and 10#) of Du145-Luc2/Ctrli #1, Du145-Luc2/Cyr61i #1 (A) and Du145-Luc2/GFP, Du145-Luc2/Cyr61 (B) were presented for each time point. (C) Average number of metastasis lesions was qualified. (D) The metastasis lesion was picked out to confirm that bioluminescence signalling is not artificial. (E) Immunohistochemistry of Cyr61 in clinical prostate cancer samples and matched normal prostate tissue. Scale bar=100 μm. (F) Elevation of Cyr61 level upon tissue adjacent to tumour and the tumour samples compared with normal prostate tissues free of any pathological alteration; box-and-whisker plots of Cyr61 expression level at different stages of prostate cancer tissues. ***P<0.001; **P<0.01.
Cyr61 expression was changed with PCa progression
After analysing the Cyr61 expression profile in the gene expression array-based database of human metastatic prostate tumours and primary prostate tumours (GSE6919), we found that the normal prostate tissues showed very low level of Cyr61 expression, whereas the tumour-adjacent tissues and the tumour tissues demonstrated a significantly higher expression level of Cyr61 (P<0.001, Figure 6F). However, the expression level of Cyr61 in metastatic prostate tumour samples decreased when compared with that in the early stage of PCa (Figure 6F).
Metastasis largely results from the interaction between tumour cells and the surrounding environment. Yang et al (2005) found that mesenchymal stem cells within tumour stroma promoted breast cancer metastasis through the secretion of the chemokine CCL5. It was reported that the stromal expression of IGFBP3 was important for PCa progression (Massoner et al, 2008). Recently, Karnoub et al (2007) reported that stromal expression of CTGF promoted angiogenesis and tumorigenesis of PCa. These findings indicated the potential function of stromal Cyr61 in metastasis and tumour progression. Consistent with these studies, we found that Cyr61 indeed existed in the fibroblast cells in the region of cancerous tissues (Figure 6E). We further analysed the expression of Cyr61 in 20 pairs of clinical malignant PCa samples and their matched normal prostate tissues by immunostaining. A representative result showed that the expression of Cyr61 was mild in both epithelial cells and stromal fibroblast cells in normal prostate tissues, whereas higher level of Cyr61 expression was observed in epithelial PCa foci and the stromal fibroblast cells in tumour samples (Figure 6E), which indicated that Cyr61 played a promotive role in the tumorigenesis of PCa and served as an important paracrine growth factor to prompt the metastasis of malignant PCa cells.
Discussion
In this study, we explored the functions of Cyr61 in several representative PCa cell lines, including LnCap, Du145, and PC3 cells, and found that Cyr61 facilitated the proliferation and migration of the tumour cells, suggesting that Cyr61 might act as an oncoprotein in PCas.
Cyr61 is a well-established ligand for several integrins (Kireeva et al, 1998), including αvβ3 and α6β1, which were expressed in Du145 cells (Witkowski et al, 1993). Responding to the alteration of extracellular environment, for example, in ligand engagement, integrins can elicit intracellular signal pathways to regulate cell survival, proliferation, gene transcription, adhesion to ECM, and migration (Hood and Cheresh, 2002; Carragher and Frame, 2004; Juliano et al, 2004; Moschos et al, 2007). Earlier studies have demonstrated that Cyr61 induced cell proliferation, adhesion, and angiogenesis through activation of integrin (ávâ3) in endothelial cells (Babic et al, 1998). It was possible that the effect of Cyr61 exerted in Du145 cells might also be mediated through integrins.
In fact, we observed that the loss of Cyr61 facilitated the assembly of FAs, which is the intermediate for the interaction between cytoskeleton and ECM, and is largely regulated by integrin signalling pathways (Miyamoto et al, 1995; Burridge and Chrzanowska-Wodnicka, 1996; Juliano et al, 2004). Integrin-induced assembly or disassembly of FAs often resulted in the enhancement or attenuation of cell adhesion to the ECM, respectively, and the dynamic regulation of FA was a crucial determinant in cell migration (Hood and Cheresh, 2002; Carragher and Frame, 2004). The recruitment of many factors including FAK and paxillin promoted the assembly of FA, thus stabilising the interaction between cytoskeleton and ECM, which enhanced the cell adhesion while inhibited cell migration (Hood and Cheresh, 2002; Carragher and Frame, 2004). In contrast, the degradation or separation of FA components led to the disassembly of FA and elevated cell motility (Hood and Cheresh, 2002; Carragher and Frame, 2004). In our study, we found that knockdown of Cyr61 resulted in an excessive assembly of FAs in Du145 cells, which was consistent with the inhibition of migration. These results also reflected that loss of Cyr61 in Du145 cells might interfere with the normal regulation of FAs by integrins. It has been reported that CTGF promoted mesangial cell migration by disassembling FAs (Crean et al, 2004). As a high similarity existed in the structure and function between Cyr61 and CTGF, and both of them could serve as ligands for integrins (Brigstock, 2003), it was not strange that Cyr61 regulated FA in a similar way as CTGF does.
Our results also showed that the activation of Rac1, one important effector downstream of integrins (Hood and Cheresh, 2002; Carragher and Frame, 2004), was dramatically inhibited by the knockdown of Cyr61. The suppressive effect on cell migration by Cyr61 knockdown was also largely due to the decreased level of activated Rac1, which has also been shown important in cell migration in some other kinds of cancer cells (Almeida et al, 2000; Juliano et al, 2004). Rac1 is a member of Rho family and regulates cell migration by stimulating actin polymerisation to form lamellipodia (Carragher and Frame, 2004). As the extension of lamellipodia is an important step for cell migration, the decrease of mobile lamellipodia, which resulted from the inactivation of Rac1, inevitably lowers the cell motility. Therefore, Cyr61 might regulate the activity of Rac1 through the integrin pathway and then affect the migration of PCa cells. So far, several pathways have been found to participate in the regulation of migration by integrins, including AKT, ERK, and JNK signalling (Almeida et al, 2000; Juliano et al, 2004). However, we only detected the change in the activated status of JNK, suggesting that Cyr61 could activate JNK through integrin. Moreover, JNK is a downstream target of integrin-related Rac1 (Almeida et al, 2000; Juliano et al, 2004). Therefore, our results revealed that a novel Cyr61-integrin-Rac1-JNK signalling regulated the migration of malignant PCa cells.
Our in vivo study further showed that the metastasis of Du145 was inhibited by the silencing of Cyr61. A microarray analysis was conducted (Bacac et al, 2006) (data accessible at NCBI GEO database, accession GSE5945) to evaluate the response of stromal cells to tumour invasion. Cyr61 expression level was found higher in the stromal cells from invasive prostate tumour tissue than that in the stromal cells from intraepithelial neoplasias, suggesting the important role of Cyr61 in the metastasis of malignant PCa cells. Metastasis is the major barrier to eradicate cancers as well as the primary cause of death in patients (Steeg, 2006). So far, a lot of research have explored the complicated processes of cancer metastases and uncovered the general process of metastases as follows: tumour cells that have already acquired weakened adhesion and enhanced migration ability first separate from neighbouring cells; then the separated tumour cells enter into the circulation system (lymph or blood); during the process of transporting in the circulation system, the tumour cells translocated in the new proper tissues or organs by invasion and form new tumour foci, from which metastatic cancers eventually develop (Steeg, 2006). During the process, the abilities to propagate, migrate, and invade the metastatic tumour cells were all dramatically increased compared with homologous normal cells and non-metastatic tumour cells (Steeg, 2006). In our experimental model of metastasis, the tumour cells were directly injected into the blood system, which mimicked the movement of tumour cells in the circulation system. We found that Cyr61-downregulated Du145 cells could not develop metastatic lesions in vivo, whereas Du145 cells with normal Cy61 expression easily formed new tumour foci. The difference might be due to the decrease in cell proliferation and/or the attenuation of cell migration ability, which was proved in our study. In addition, Cyr61 was found to induce matrix metalloproteinase-1 production, which promoted the invasion of breast cancers (Nguyen et al, 2006). Moreover, Cyr61 was also a tumour-promoting protein in breast cancers (Liotta and Kohn, 2001; Xie et al, 2001a), which was similar to our findings in PCas; thus the loss of Cyr61 in PCa cells might also inhibit cell invasion by blocking the expression of matrix metalloproteinase-1. Recently, Cyr61 expression was also found to promote metastasis of human pancreatic cancer (Holloway et al, 2005). Therefore, Cyr61 might be a useful marker for metastasis in several types of malignant cancers including PCas.
Taken together, our results herein displayed that Cyr61 plays an important role in the proliferation and migration of malignant PCa cells, and the latter was largely due to the regulation of Cyr61 on Rac1 activity, as well as related FA assembly. The in vivo study further demonstrated that Cyr61 was also involved in the metastasis of PCa. Our results seemed contrary to the earlier report that Cyr61 might act as a tumour suppressor in PCa (Pilarsky et al, 1998). In that study, the researchers mainly used low-stage or well-differentiated PCa samples (Pilarsky et al, 1998). Therefore, we speculated that Cyr61 might play distinct roles in PCas with different degrees of malignance; however, this hypothesis required further investigations. After all, we successfully inhibited tumorigenicity of PCa cells both in vitro and in vivo by downregulating Cyr61 expression, and this suggested a potential therapeutic strategy for malignant PCas.
Acknowledgments
This study was sponsored by 973 Program 2007CB914704, 863 Program 2007AA02Z474, National Natural Science Funds for Distinguished Young Scholar 30725010, National Natural Science Foundation of China (NSFC) 30470847 and 30528003, Chinese Academy of Sciences Grant KSCX-YW-R-73, Shanghai Pujiang Program 06PJ14108, and Science and Technology Commission of Shanghai Municipality 04DZ14007 and 05DJ14009 (DX), China Postdoctoral Science Foundation, Shanghai Postdoctoral Scientific Program, and Chinese Academy of Sciences KC Wong Post-doctoral Fellowships (YW).
Footnotes
Conflict of intrest The authors have no conflicting financial interests.
References
- Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH (2000) Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol 149: 741–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF (1998) CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA 95: 6355–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacac M, Provero P, Mayran N, Stehle JC, Fusco C, Stamenkovic I (2006) A mouse stromal response to tumor invasion predicts prostate and breast cancer patient survival. PLoS ONE 1: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau AM, Planque N, Perbal B (2005) CCN proteins and cancer: two to tango. Front Biosci 10: 998–1009 [DOI] [PubMed] [Google Scholar]
- Bork P (1993) The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett 327: 125–130 [DOI] [PubMed] [Google Scholar]
- Bremnes RM, Veve R, Hirsch FR, Franklin WA (2002) The E-cadherin cell–cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer 36: 115–124 [DOI] [PubMed] [Google Scholar]
- Brigstock DR (2003) The CCN family: a new stimulus package. J Endocrinol 178: 169–175 [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M (1996) Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 12: 463–518 [DOI] [PubMed] [Google Scholar]
- Carragher NO, Frame MC (2004) Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol 14: 241–249 [DOI] [PubMed] [Google Scholar]
- Chien W, Kumagai T, Miller CW, Desmond JC, Frank JM, Said JW, Koeffler HP (2004) Cyr61 suppresses growth of human endometrial cancer cells. J Biol Chem 279: 53087–53096 [DOI] [PubMed] [Google Scholar]
- Crean JK, Furlong F, Finlay D, Mitchell D, Murphy M, Conway B, Brady HR, Godson C, Martin F (2004) Connective tissue growth factor [CTGF]/CCN2 stimulates mesangial cell migration through integrated dissolution of focal adhesion complexes and activation of cell polarization. FASEB J 18: 1541–1543 [DOI] [PubMed] [Google Scholar]
- Cruz-Monserrate Z, O’Connor KL (2008) Integrin alpha 6 beta 4 promotes migration, invasion through Tiam1 upregulation, and subsequent Rac activation. Neoplasia 10: 408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delongchamps NB, Peyromaure M, Dinh-Xuan AT (2006) Role of vascular endothelial growth factor in prostate cancer. Urology 68: 244–248 [DOI] [PubMed] [Google Scholar]
- Dodson MG, Slota J, Lange C, Major E (1981) Distinction of the phenotypes of in vitro anchorage-independent soft-agar growth and in vivo tumorigenicity in the nude mouse. Cancer Res 41: 1441–1446 [PubMed] [Google Scholar]
- Franke B, Akkerman JW, Bos JL (1997) Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J 16: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genini M, Schwalbe P, Scholl FA, Schafer BW (1996) Isolation of genes differentially expressed in human primary myoblasts and embryonal rhabdomyosarcoma. Int J Cancer 66: 571–577 [DOI] [PubMed] [Google Scholar]
- Gennigens C, Menetrier-Caux C, Droz JP (2006) Insulin-Like Growth Factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol 58: 124–145 [DOI] [PubMed] [Google Scholar]
- Grisham JW, Tsao MS, Lee LW, Smith GJ (1991) Clonal analysis of neoplastic transformation in cultured diploid rat liver epithelial cells. Basic Life Sci 57: 279–298; discussion 299–300 [DOI] [PubMed] [Google Scholar]
- Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF (2002) The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology 143: 1441–1450 [DOI] [PubMed] [Google Scholar]
- Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, Collard JG (2005) The Rac activator Tiam1 is required for (alpha)3(beta)1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol 171: 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway SE, Beck AW, Girard L, Jaber MR, Barnett Jr CC, Brekken RA, Fleming JB (2005) Increased expression of Cyr61 (CCN1) identified in peritoneal metastases from human pancreatic cancer. J Am Coll Surg 200: 371–377 [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA (2002) Role of integrins in cell invasion and migration. Nat Rev Cancer 2: 91–100 [DOI] [PubMed] [Google Scholar]
- Hurle RA, Davies G, Parr C, Mason MD, Jenkins SA, Kynaston HG, Jiang WG (2005) Hepatocyte growth factor/scatter factor and prostate cancer: a review. Histol Histopathol 20: 1339–1349 [DOI] [PubMed] [Google Scholar]
- Juliano RL, Reddig P, Alahari S, Edin M, Howe A, Aplin A (2004) Integrin regulation of cell signalling and motility. Biochem Soc Trans 32: 443–446 [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449: 557–563 [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Lam SC, Lau LF (1998) Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem 273: 3090–3096 [DOI] [PubMed] [Google Scholar]
- Lin MT, Chang CC, Chen ST, Chang HL, Su JL, Chau YP, Kuo ML (2004) Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-kappaB-dependent XIAP up-regulation. J Biol Chem 279: 24015–24023 [DOI] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC (2001) The microenvironment of the tumour–host interface. Nature 411: 375–379 [DOI] [PubMed] [Google Scholar]
- Maillard M, Cadot B, Ball RY, Sethia K, Edwards DR, Perbal B, Tatoud R (2001) Differential expression of the ccn3 (nov) proto-oncogene in human prostate cell lines and tissues. Mol Pathol 54: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoner P, Haag P, Seifarth C, Jurgeit A, Rogatsch H, Doppler W, Bartsch G, Klocker H (2008) Insulin-like growth factor binding protein-3 (IGFBP-3) in the prostate and in prostate cancer: local production, distribution and secretion pattern indicate a role in stromal–epithelial interaction. Prostate 68: 1165–1178 [DOI] [PubMed] [Google Scholar]
- Melton AC, Soon Jr RK, Park JG, Martinez L, deHart GW, Yee Jr HF (2007) Focal adhesion disassembly is an essential early event in hepatic stellate cell chemotaxis. Am J Physiol Gastrointest Liver Physiol 293: G1272–G1280 [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM (1995) Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol 131: 791–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos SJ, Drogowski LM, Reppert SL, Kirkwood JM (2007) Integrins and cancer. Oncology (Williston Park) 21: 13–20 [PubMed] [Google Scholar]
- Munker R, Norman A, Koeffler HP (1986) Vitamin D compounds. Effect on clonal proliferation and differentiation of human myeloid cells. J Clin Invest 78: 424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Kuliopulos A, Graham RA, Covic L (2006) Tumor-derived Cyr61(CCN1) promotes stromal matrix metalloproteinase-1 production and protease-activated receptor 1-dependent migration of breast cancer cells. Cancer Res 66: 2658–2665 [DOI] [PubMed] [Google Scholar]
- Perbal B (2004) CCN proteins: multifunctional signalling regulators. Lancet 363: 62–64 [DOI] [PubMed] [Google Scholar]
- Pilarsky CP, Schmidt U, Eissrich C, Stade J, Froschermaier SE, Haase M, Faller G, Kirchner TW, Wirth MP (1998) Expression of the extracellular matrix signaling molecule Cyr61 is downregulated in prostate cancer. Prostate 36: 85–91 [DOI] [PubMed] [Google Scholar]
- Qin XF, An DS, Chen IS, Baltimore D (2003) Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA 100: 183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S, Yokoyama M, Aoki M, Suzuki K, Kakehi Y, Saito Y (2004) Induction and function of CYR61 (CCN1) in prostatic stromal and epithelial cells: CYR61 is required for prostatic cell proliferation. Prostate 61: 305–317 [DOI] [PubMed] [Google Scholar]
- Sampath D, Zhu Y, Winneker RC, Zhang Z (2001) Aberrant expression of Cyr61, a member of the CCN (CTGF/Cyr61/Cef10/NOVH) family, and dysregulation by 17 beta-estradiol and basic fibroblast growth factor in human uterine leiomyomas. J Clin Endocrinol Metab 86: 1707–1715 [DOI] [PubMed] [Google Scholar]
- Sim HG, Cheng CW (2005) Changing demography of prostate cancer in Asia. Eur J Cancer 41: 834–845 [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S (2005) Critical role of acylglycerol kinase in epidermal growth factor-induced mitogenesis of prostate cancer cells. Biochem Soc Trans 33: 1362–1365 [DOI] [PubMed] [Google Scholar]
- Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12: 895–904 [DOI] [PubMed] [Google Scholar]
- Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF (1978) Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 21: 274–281 [DOI] [PubMed] [Google Scholar]
- Supriatno, Harada K, Kawaguchi S, Yoshida H, Sato M (2003) Effect of p27Kip1 on the ability of invasion and metastasis of an oral cancer cell line. Oncol Rep 10: 527–532 [PubMed] [Google Scholar]
- Thomas M, Finnegan CE, Rogers KM, Purcell JW, Trimble A, Johnston PG, Boland MP (2004) STAT1: a modulator of chemotherapy-induced apoptosis. Cancer Res 64: 8357–8364 [DOI] [PubMed] [Google Scholar]
- Tong X, O’Kelly J, Xie D, Mori A, Lemp N, McKenna R, Miller CW, Koeffler HP (2004) Cyr61 suppresses the growth of non-small-cell lung cancer cells via the beta-catenin-c-myc-p53 pathway. Oncogene 23: 4847–4855 [DOI] [PubMed] [Google Scholar]
- Tong X, Xie D, O’Kelly J, Miller CW, Muller-Tidow C, Koeffler HP (2001) Cyr61, a member of CCN family, is a tumor suppressor in non-small cell lung cancer. J Biol Chem 276: 47709–47714 [DOI] [PubMed] [Google Scholar]
- Ware JL (1998) Growth factor network disruption in prostate cancer progression. Cancer Metastasis Rev 17: 443–447 [DOI] [PubMed] [Google Scholar]
- Witkowski CM, Rabinovitz I, Nagle RB, Affinito KS, Cress AE (1993) Characterization of integrin subunits, cellular adhesion and tumorgenicity of four human prostate cell lines. J Cancer Res Clin Oncol 119: 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D, Miller CW, O’Kelly J, Nakachi K, Sakashita A, Said JW, Gornbein J, Koeffler HP (2001a) Breast cancer. Cyr61 is overexpressed, estrogen-inducible, and associated with more advanced disease. J Biol Chem 276: 14187–14194 [DOI] [PubMed] [Google Scholar]
- Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP (2001b) Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res 61: 8917–8923 [PubMed] [Google Scholar]
- Xie D, Yin D, Tong X, O’Kelly J, Mori A, Miller C, Black K, Gui D, Said JW, Koeffler HP (2004) Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways. Cancer Res 64: 1987–1996 [DOI] [PubMed] [Google Scholar]
- Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR (2005) Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res 65: 8887–8895 [DOI] [PubMed] [Google Scholar]
- Zhu B, Kyprianou N (2005) Transforming growth factor beta and prostate cancer. Cancer Treat Res 126: 157–173 [DOI] [PubMed] [Google Scholar]
- Ziober BL, Silverman Jr SS, Kramer RH (2001) Adhesive mechanisms regulating invasion and metastasis in oral cancer. Crit Rev Oral Biol Med 12: 499–510 [DOI] [PubMed] [Google Scholar]