Abstract
The ubiquitous α(E)-catenin is an essential actin cytoskeletal linker. The transcription factor, serum response factor (SRF), induces transcription via binding to the serum response element (SRE) in gene promoters, and in many cases responds to actin dynamics. Here, we report that α(E)-catenin expression in HEK293 cells activates the SRE.L transcriptional reporter, a reporter containing the isolated SRF-binding site, and a stably integrated SRE.L reporter in fibroblasts. α-Catenin-induced reporter activity appears only partly dependent on RhoA GTPase and Rho kinase function. α-Catenin expression has no effect on RhoA activation or localization, and α-catenin-induced SRE.L reporter activation is insensitive to the actin-modulating agent latrunculin B. Ectopic α-catenin expression was not sufficient to induce actin filament assembly as measured by stress fiber formation. SRE.L reporter is activated by the C-terminal ~300 residue region of α(E)-catenin. These results suggest induction of SRF-mediated transcription by α(E)-catenin either downstream of RhoA or via a parallel pathway.
Keywords: α-Catenin, Signaling, Transcription, Serum response factor, Serum response element, Rho, Actin, Rho kinase
The ubiquitous α(E)-catenin is a key component of the cadherin/catenin complex that mediates cell–cell adhesion in many vertebrate tissues, and is required during development and for adult tissue integrity [1]. In this complex, β- or γ-catenin directly binds to the cadherin cytoplasmic tail in a mutually exclusive fashion and α-catenin binds to β- or γ-catenin [1]. Whereas β- and γ-catenin share sequence similarity, α-catenin is a distinct protein [1]. The canonical function of α(E)-catenin is thought of as linking the adhesion complex to the actin cytoskeleton, and recent evidence shows that this is a more dynamic process than previously perceived, with α-catenin being present in distinct complexes [2]. The N-terminal region of α-catenin binds β-catenin, the central region binds α-actinin/vinculin, and the C-terminal region binds ZO-1, with both N- and C-terminal regions binding actin [3,4]. α-Catenin is part of a super-family which includes vinculin and α-catulin [5,6]. Vinculin links integrin adhesion complexes to the actin cytoskeleton [5]; whether α-catulin associates with adhesion complexes is currently unknown.
Increasing evidence indicates that α-catenin modulates signaling pathways. Ectopic α(E)-catenin expression inhibits Wnt-mediated Xenopus development [7] and potently blocks β-catenin signaling [8,9], a key component of the Wnt pathway [10]. Moreover, α(E)-catenin acts as a tumor growth suppressor in cancer cells [11]. Furthermore, α(E)-catenin down-regulates skin proliferation in mice by inhibiting MAP kinase- and NF-κB-dependent pathways [12,13], and regulates cerebral cortex size via Hedgehog pathway inhibition [14], although the mechanisms remain to be defined. Additionally, αN-catenin can interact and co-translocate to the nucleus with the transcriptional repressor ZASC1 [15].
The transcription factor serum response factor (SRF) binds to the serum response element (SRE) found in many gene promoters and induces transcription [16], such as skeletal and smooth muscle-specific genes [17]. SRF binds to a core sequence of the SRE distinct from the ternary complex factor (TCF) binding site found in some promoters [16]. SRF is essential during embryonic development and for differentiation-specific gene transcription such as neuronal- and muscle-specific genes references in [17]. Rho GTPase function is required for extracellular signal-induced SRF activation [18] and this has led to the use of SRE transcriptional reporter assays to assess Rho-dependent signaling. Rho controls actin filament assembly [19], and SRF activity is linked to actin treadmilling in that G-actin depletion and increased actin filament polymerization can activate SRE reporters and a subset of SRF target genes [20].
Reports suggest links between α-catenin-related proteins and Rho. For example, α-catenin associates with Drosophila Rho1 [21], while α-catulin associates with Lbc Rho GEF [22], a direct Rho activator [23]. The involvement of actin dynamics in the SRF pathway, and the association of α-catenin family members with signaling and the actin cytoskeleton led us to investigate the potential involvement of α(E)-catenin in SRF modulation. Our findings detailed here in cultured cells suggest for the first time that α-catenin can activate SRF/SRE-dependent transcription.
Materials and experimental methods
Cell culture
HEK293 cells (ATCC) are maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies) plus 10% FBS. Immortalized mouse embryonic fibroblasts (MEFs) containing stably integrated SRE.L reporter [24] were generated and maintained as described in [25]. Quiescent serum-starved Swiss 3T3 fibroblasts were maintained as described [19].
Plasmids
The following plasmids were gifts: pcGN:HA-α(E)-catenin (NM_001903) (1–906), α(E)-catenin 1–161, α(E)-catenin 84–907, and α(E)-catenin 129–907 (Bullions et al. [11]); pcGN:α(E)-catenin ΔN′MD (Ozawa [3]); pGFP:vinculin (Bershadsky); SRE.L luciferase reporter (Kaibuchi [24]); pGL2:human vinculin promoter luciferase reporter (Pilz [26]); pGEX-2T-Rhotekin RBD (Schwartz [27]) and pEFLINK C3 transferase (Treisman). pSRF:luciferase reporter plasmid was from Stratagene (CA). pcDNA:Myc-α-catenin 46–149 is described in [9], pSR:Lbc Rho GEF in [23], and pRK5myc:p115 Rho GEF in [28]. pcDNA:Myc-RhoAL63 was from Guthrie cDNA Resource (PA).
Antibodies and reagents
Anti-α(E)-catenin (1G5), RhoA (26C4), c-Myc (9E10), actin (C-2), and GFP (B2) antibodies were from Santa Cruz Biotechnology (CA). Anti-HA antibody (HA-7) was from Sigma (MO). Latrunculin B, swinholide A, and Y27632 were from Calbiochem (CA).
Cell transfection
Cells were transfected using Lipofectamine 2000 reagent (Invitrogen) as recommended. Backbone vector was included to ensure that equal plasmid amounts were transfected.
Transcriptional reporter assay
HEK293 cells were co-transfected with 500 ng SRE.L and 20 ng pTK-RL reporters (6-well plates) in triplicate. Induction of SRE.L luciferase reporter was assayed by Dual-Luciferase Reporter Assay System (Promega) 48 h later as described in [29].
Immunoblotting
Immunoblotting was carried out as in [22] and visualized using the Perkin-Elmer enhanced chemiluminescence (ECL) detection system. Band densities were quantified by Alpha Innotech IS2200 Digital Imaging System.
RBD assay
GTP-RhoA was affinity-purified using GST-Rhotekin Rho-binding domain (RBD) fusion protein as in [27].
Subcellular fractionation
Cell lysates were fractionated into S-100 soluble and P-100 particulate fractions as described in [22].
G:F-actin fractionation
Following transfection in 6-well dishes (6 wells per group) overnight, pelleted cells were resuspended in Triton X-100 lysis buffer [20], and the procedure described in [20] was followed to obtain equal total volumes of G and F fractions which were immunoblotted for actin.
Cell microinjection
Confluent quiescent, serum-starved Swiss 3T3 fibroblasts were microinjected with epitope tagged expression vectors (0.1 μg/μl), incubated for 2–3 h, and stained with phalloidin to detect actin, as previously described [19]. Fluorescence images were recorded on a CCD camera and processed using Openlab software.
Statistical analysis
Data are presented as means ± standard deviation (SD). Using Student’s t test; a p value <0.05 was considered to indicate statistical significance.
Results and discussion
The SRE.L transcriptional reporter contains multiple copies of a modified SRE encoding an intact SRF-binding site and a mutated TCF-binding site [24], driving luciferase expression. As expected, transient transfection of RhoAL63 with SRE.L reporter plasmid in HEK293 cells led to increased luciferase expression (Fig. 1A). Interestingly, expression of increasing amounts of α-catenin readily induced SRE.L reporter activation of up to ~15-fold at the highest dose tested. In contrast, vinculin and β-catenin expression had no inductive effect and neither did α-catulin as reported earlier [22], highlighting the distinct activity of α-catenin. Use of another reporter plasmid that encodes only the isolated SRF-binding site (pSRF:Luc) shows that α-catenin also induced pSRF:Luc reporter activation (Fig. 1B). Under serum-withdrawal, expression of α-catenin in MEFs which contain a stably integrated SRE.L reporter [25] also led to luciferase expression comparable in magnitude to that induced by RhoAL63 (Fig. 1C), confirming the results obtained from HEK293 transient transfection. The reduced magnitude of the effect in stably transfected MEFs is likely due to the higher expression levels obtained by transient expression and the higher transfectability of HEK293. α-Catenin also activates the pGL2:vinculin luciferase reporter encoding the human vinculin promoter which contains an SRF-binding site [26] in HEK293 cells at levels comparable to 10% serum (Fig. 1D), suggesting that the effect also applies to native SRF-containing promoters. These results demonstrate that α-catenin activity is not limited to a particular SRF-responsive reporter or cell type.
Fig. 1.
α(E)-Catenin activates SRF-dependent transcriptional reporters. (A) HEK293 cells were transfected with either pcGN vector (500 ng), pcDNA:β-catenin Δ45 (500 ng) or pcGN:HA-α-catenin (100, 500, and 1000 ng), pcDNA:RhoAL63 (50 and 200 ng) or pcDNA:GFP-vinculin (250, 500, 750, and 1000 ng) with 500 ng SRE.L reporter. Panels show immunoblots (IB) of HA:α-catenin-transfected lysates probed with anti-HA antibody, and GFP:vinculin-transfected lysates probed with anti-GFP antibody to verify expression. (B) HEK293 cells were transfected with either pcGN (500 ng), RhoAL63 (200 and 500 ng), HA-α-catenin (500 and 1000 ng), or vinculin (500 and 1000 ng) with 500 ng pSRF-Luc reporter. (C) Serum-starved MEFs with stably integrated SRE.L reporter were transfected with pcGN (500 ng), RhoAL63 (200 ng) or α-catenin (400, 800 ng). *p < 0.05 for 1C groups vs. pcGN vector control. (D) HEK293 was transfected with 200 ng vinculin promoter luciferase reporter (pVinc promoter-Luc) and two doses of α-catenin, RhoAL63, or vinculin as in (B). RLU, relative luciferase units. Shown are representative experiments of three.
Given the links of Rho function with SRF activity, we co-expressed increasing amounts (5–50 ng) of pEF-C3 plasmid encoding the Rho-specific inhibitor, C3 transferase exoenzyme [30] along with RhoAL63 or α-catenin in HEK293. Even the lowest amount of C3 plasmid (5 ng) was sufficient to block RhoA-induced reporter induction by 95% or more (Fig. 2A). In contrast, α-catenin-induced activity was reduced by no more than 50% even with 10-fold greater amounts of C3 transferase plasmid (50 ng). This implies that α-catenin is unlikely to be upstream of Rho; and is either downstream of Rho, or on a parallel pathway converging on SRF. The partial inhibition by C3 on reporter activation may reflect a basal requirement for Rho for cell viability. The potential role of the Rho effector Rho kinase (ROCK) was next tested and 10 μM Y27632 ROCK inhibitor significantly, though not completely, attenuated both RhoA- and α-catenin-induced reporter activation (Fig. 2B). The partial inhibition may in part reflect a general requirement for ROCK for cell viability.
Fig. 2.
α-Catenin-induced SRE.L reporter activation is only partly Rho/ROCK-dependent, and α-catenin does not modulate Rho. (A) HEK293 cells were transfected with pcGN, RhoAL63, α-catenin, and increasing amounts of pEFpLINK C3 (5, 10, 25, and 50 ng) along with SRE.L. (B) HEK293 transfected with full-length or 129–906 α-catenin or RhoAL63 were treated overnight with vehicle or 7 μM Y27632 and % RLU calculated where the vehicle group value is set to 100%. RLU, relative luciferase units. Shown are representative experiments repeated at least twice. (C) HEK293 cells were transfected with 1.5 μg of pcDNA, Myc-RhoAL63, HA-α-catenin or HA-α-catenin 129–906 in 100 mm dishes. Equal volumes of S-100 soluble (S) and P-100 pellet (P) cell fractions were immunoblotted (IB) for endogenous RhoA or HA:RhoAL63. The ratios of soluble (S) to pellet RhoA (P) band density from three experiments were normalized to pcDNA control set to 1, and the relative ratios plotted as shown. (D) HEK293 cells were transfected with 2 μg of either pcGN vector, Lbc Rho GEF, or α-catenin. GTP-RhoA pull-down assays were carried out and the ratio of GTP-RhoA to total RhoA from two experiments was normalized to the pcGN vector group value which was set to 1.
RhoA activation correlates with increased membrane localization [31], and we evaluated whether α-catenin expression modulates RhoA by altering RhoA subcellular localization. HEK293 cells were transfected with plasmids for either pcDNA vector, full-length α-catenin, RhoAL63 or an α-catenin mutant (α-catenin 129–906) which contains a deletion of the N-terminal 128 residues (see Fig. 4A). After 48 h, lysates were separated into cytosolic (soluble) and membrane-rich (pellet) fractions by high-speed fractionation and immunoblotted for endogenous RhoA or RhoAL63. As expected, a large proportion of the endogenous RhoA pool in unstimulated pcDNA transfected cells localizes to the cytosolic (soluble) fraction (Fig. 2C), whereas a substantial proportion of activated RhoAL63 localizes to the membrane- rich fraction. Expression of the α-catenin forms did not alter the relative ratio of soluble versus membrane- associated RhoA. Magie et al. [21] suggested that α-catenin may recruit Rho to its sites of action in Drosophila. However, we saw no effect of α-catenin on RhoA localization, at least in fractionated HEK293 cells. We next evaluated the effect of α-catenin on endogenous RhoA activation by GTP-Rho “pull-down” assay. Whereas Lbc Rho GEF predictably led to increased endogenous GTP-RhoA levels, α-catenin expression showed no effect in a representative experiment (Fig. 2D), consistent with the interpretation that α-catenin lies downstream of Rho or on a parallel pathway.
Fig. 4.
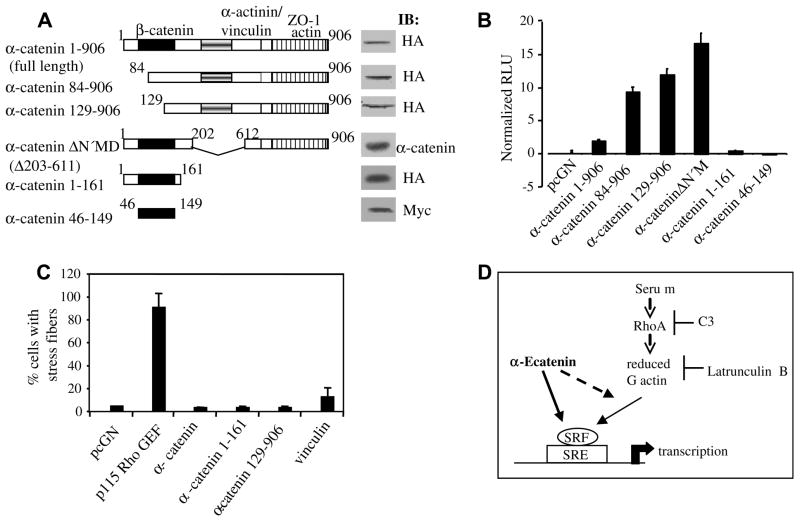
The α-catenin C-terminal region activates the SRE.L reporter, and α-catenin is not sufficient to induce actin stress fibers. (A) Schematic diagram of α-catenin and its mutants, and corresponding immunoblots (IB) of 50 μg of HEK293 lysates transfected with equimolar plasmid amounts. (B) HEK293 cells were transfected at equimolar amounts with full-length HA-α-catenin (1–906), 84–906, 129–906, α-catenin ΔN′M, 1–161, or Myc-α-catenin 46–149 as indicated, and SRE.L. RLU values for each group were normalized against their respective protein expression levels as determined by densitometric analysis of immunoblots using full-length α-catenin value as the reference set to 1. Shown is a representative experiment of three. (C) Quiescent Swiss 3T3 fibroblasts were microinjected with pcGN, p115 Rho GEF, α-catenin, α-catenin 1–161 or 129–906, or vinculin plasmids. % Microinjected cells containing actin stress fibers were determined from two separate experiments. (D) Proposed model in which α-catenin may activate SRF/SRE-mediated transcription downstream of Rho or via a parallel pathway.
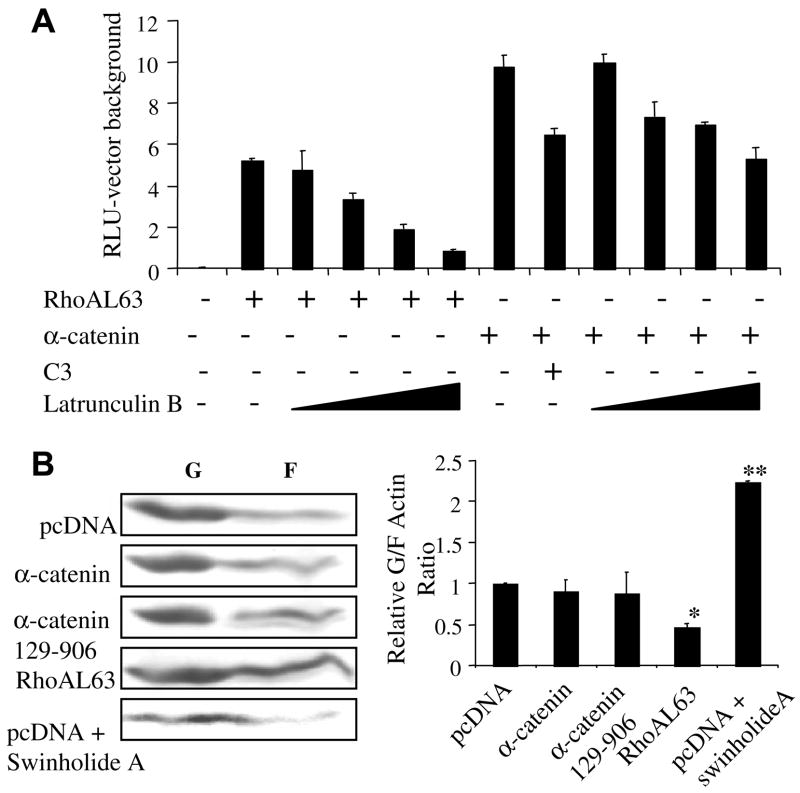
Given that a subset of SRF target genes is induced by Rho-induced actin treadmilling [20], we examined the role of actin dynamics. When HEK293 cells expressing either RhoAL63 or α-catenin along with SRE.L reporter were treated with latrunculin B (0.25–1.0 μM), a toxin which inhibits actin polymerization by sequestering G-actin monomers [32], RhoAL63-induced reporter activation was predictably progressively inhibited (Fig. 3A) to near-background levels. In contrast, α-catenin-induced reporter activation was largely refractory to latrunculin B treatment, with a maximum reduction of only ~40% at the highest dose (Fig. 3A). This finding further suggests that α-catenin does not function upstream of Rho. We next evaluated whether α-catenin influences the G:F-actin pool by fractionating G- and F-actin from HEK293 lysates expressing full-length α-catenin, α-catenin 129–906, or RhoA. As a control, vector transfected lysates were pretreated with swinholide A, an actin-binding agent that reduces filamentous actin levels by sequestering G-actin as dimers [33]. After fractionation, the G:F-actin fractions were immunoblotted for actin. As predicted, RhoAL63 expression led to an increase in the relative F-actin level and swinholide A treatment reduced the F-actin level (Fig. 3B graph). In contrast, no difference in the G:F-actin ratio was observed between α-catenin-expressing groups and vector control. Whereas actin dynamics are necessary for serum induction of certain SRF target genes such as srf and cytoskeletal actin other SRF target genes such as c-fos and egr-1 are unresponsive [20]. Our results suggest that α-catenin induces SRF-dependent transcription via a mechanism that is largely refractory to changes in the G-actin pool.
Fig. 3.
Effect of latrunculin B and swinholide A on α-catenin-induced SRE.L reporter activation. HEK293 cells were transfected with 300 ng RhoAL63 or 750 ng α-catenin as indicated and SRE.L. Some groups were also co-transfected with 50 ng pEFpLINK C3. For (A), 32 h post-transfection, some groups were treated with latrunculin B (0.25, 0.5, 0.75, and 1 μM) prior to lysis and luciferase activity measurement. (B) Cells transfected with designated plasmids were treated with swinholide A (0.03, 0.06, 0.09, and 0.12 μM) overnight, fractionated into Triton X-100 soluble (G-actin) and insoluble (F-actin) fractions, and immunoblotted for actin. The ratio of the G- to F-actin band density from three experiments was calculated and normalized against the pcDNA group value set to 1 to yield the relative G:F-actin ratios plotted in the graph. *p < 0.05 for RhoAL63-induced levels vs. pcDNA values; **p < 0.05 for the swinholide A-treated vs. pcDNA values.
α-Catenin partners with β-catenin, actin, α-actinin, vinculin, and ZO-1 through fairly well-defined regions (see Introduction). On this basis, mutants representing different regions of α-catenin shown in Fig. 4A were utilized. These mutants have been previously described [3,4,10,11], and the immunoblot panels of equal amounts of transfected HEK293 lysates confirm their expression. Complete or mutant α-catenin was transfected at equimolar amounts with SRE.L reporter and luciferase values normalized against their protein expression levels. Fig. 4B shows that α-catenin 84–906 and α-catenin 129–906 still induce SRE.L reporter activity, suggesting that α-catenin residues 129 onwards contain the functional domains required for SRE reporter activation. These mutants also appear to be more active than full-length α-catenin and possibly represent “activated” forms in this context. In contrast, α-catenin 1–161 and 46–149 induced negligible reporter activity, despite being expressed at comparable levels, and potently inhibiting TCF/LEF reporter activation, as reported [9]. The robust activity of the α-catenin ΔN′M mutant implies that the central ~400 residue region which contains α-actinin and vinculin binding regions is not required for SRE reporter induction. Overall, these results suggest that the α-catenin regions required for SRF induction lie within the C-terminal residues 612–906. Finally we assessed whether α-catenin expression affects actin stress fiber formation, a process of acto-myosin filament assembly requiring Rho [19]. Quiescent Swiss 3T3 fibroblasts containing few stress fibers were microinjected with plasmids for vector, activated p115 Rho GEF, full-length α-catenin, α-catenin 1–161, 129–906, or vinculin. After incubation, cells were stained for phalloidin to visualize actin. Whereas p115 Rho GEF microinjection predictably led to stress fiber formation in ~90% injected cells, none of the α-catenin microinjected groups showed a detectable inductive effect (Fig. 4C) and vinculin had a minor inductive effect (~15% of injected cells with stress fibers).
The lack of stress fiber is concordant with our finding that α-catenin does not induce G-actin depletion. Our results suggest a model shown in Fig. 4D where α-catenin may modulate SRF by either acting downstream of Rho, or via a parallel pathway, in keeping with recent reports that α-catenin is present in distinct intracellular complexes [2]. The possibility that α-catenin-induced SRE reporter activity may involve forms of actin dynamics other than the ones studied here cannot be currently ruled out. Alternatively, over-expressed α-catenin may modulate SRF activity in some other way. For example, α-catenin translocates to the nucleus upon β-catenin stimulation [8] and in conjunction with ZASC1 [15], a process that may be relevant in the context studied here. Overall, these data present a novel link between α-catenin and signaling pathways, and it is the first direct positive inductive signaling effect of α-catenin to our knowledge. SRF mediates the transcription of differentiation- associated genes [16,17], thus our finding is concordant with the documented anti-proliferative effects of α-catenin [8,9,11–13]. Loss of α-catenin expression is reported in cancers [34]; hence, α-catenin loss leading to dysfunctional SRF transcription could conceivably contribute in part to the de-differentiated phenotype of malignancy. Further elucidation of α-catenin-induced SRE/SRF activation may contribute to our understanding of the role of this multifunctional protein in signaling pathways.
Acknowledgments
We thank L. Bullions, A. Bershadsky, B. Geiger, K. Kaibuchi, M. Ozawa, R. Pilz, M.A. Schwartz, R. Treisman, B. Vogelstein for plasmids; and Brent Cochran for suggestions. Funding was by NIH T32DK07542 (supporting K.D.M. and P.D.), IACR (A.B.J.), Cancer Research UK (M.F.O. and A.H.), NIH HL32723 (B.L.F.), NIH HL79320 (UK), AHA-GIA, and HHMI Biomedical Resources grant (D.T.).
References
- 1.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 2.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozawa M. Identification of the region of alpha-catenin that plays an essential role in cadherin-mediated cell adhesion. J Biol Chem. 1998;273:29524–29529. doi: 10.1074/jbc.273.45.29524. [DOI] [PubMed] [Google Scholar]
- 4.Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge AK, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JS, Nelson M, Wang L, Liu W, Qian CP, Shridhar V, Urrutia R, Smith DI. Identfication and chromosomal localization of CTNNAL1, a novel protein homologous to alpha-catenin. Genomics. 1998;54:149–154. doi: 10.1006/geno.1998.5458. [DOI] [PubMed] [Google Scholar]
- 7.Sehgal RN, Gumbiner BM, Reichardt LF. Antagonism of cell adhesion by an alpha-catenin mutant, and of the Wnt-signaling pathway by alpha-catenin in Xenopus embryos. J Cell Biol. 1997;139:1033–1046. doi: 10.1083/jcb.139.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannini AL, Vivanco M, Kypta RM. Alpha-catenin inhibits beta-catenin signaling by preventing formation of a beta-catenin*T-cell factor*DNA complex. J Biol Chem. 2000;275:21883–21888. doi: 10.1074/jbc.M001929200. [DOI] [PubMed] [Google Scholar]
- 9.Merdek KD, Nguyen NT, Toksoz D. Distinct activities of the alpha-catenin family, alpha-catulin and alpha-catenin, on beta-catenin- mediated signaling. Mol Cell Biol. 2004;24:2410–2422. doi: 10.1128/MCB.24.6.2410-2422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi A. Regulation of beta-catenin signaling in the Wnt pathway. Biochem Biophys Res Commun. 2000;268:243–248. doi: 10.1006/bbrc.1999.1860. [DOI] [PubMed] [Google Scholar]
- 11.Bullions LC, Notterman DA, Chung LS, Levine AJ. Expression of wild-type alpha-catenin protein in cells with a mutant alpha-catenin gene restores both growth regulation and tumor suppressor activities. Mol Cell Biol. 1997;17:4501–4508. doi: 10.1128/mcb.17.8.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 13.Kobielak A, Fuchs E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci USA. 2006;103:2322–2327. doi: 10.1073/pnas.0510422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. AlphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–1612. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogaerts S, Vanlandschoot A, van Hengel J, van Roy F. Nuclear translocation of alphaN-catenin by the novel zinc finger transcriptional repressor ZASC1. Exp Cell Res. 2005;311:1–13. doi: 10.1016/j.yexcr.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Price MA, Hill C, Treisman R. Integration of growth factor signals at the c-fos serum response element. Philos Trans R Soc Lond B Biol Sci. 1996;351:551–559. doi: 10.1098/rstb.1996.0054. [DOI] [PubMed] [Google Scholar]
- 17.Arsenian S, Weinhold B, Oelgeschlager M, Ruther U, Nordheim A, et al. Serum response factor is essential for mesoderm formation during mouse embryogenesis. EMBO J. 1998;17:6289–6299. doi: 10.1093/emboj/17.21.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 19.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 20.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;23:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 21.Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- 22.Park B, Nguyen NT, Dutt P, Merdek KD, Bashar M, Sterpetti P, Tosolini A, Testa JR, Toksoz D. Association of Lbc Rho guanine nucleotide exchange factor with alpha-catenin-related protein, alpha-catulin/CTNNAL1, supports serum response factor activation. J Biol Chem. 2002;277:45361–45370. doi: 10.1074/jbc.M202447200. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Olson MF, Hall A, Cerione RA, Toksoz D. Direct involvement of the small GTP-binding protein Rho in lbc oncogene function. J Biol Chem. 1995;270:9031–9034. doi: 10.1074/jbc.270.16.9031. [DOI] [PubMed] [Google Scholar]
- 24.Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Ikebe M, Kaibuchi K. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by Rho-kinase. J Biol Chem. 1997;272:25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- 25.Sousa AM, Liu T, Guevara O, Stevens J, Fanburg BL, Gaestel M, Toksoz D, Kayyali US. Smooth muscle alpha-actin expression and myofibroblast differentiation by TGFbeta are dependent upon MK2. J Cell Biochem. 2007;100:1581–1589. doi: 10.1002/jcb.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gudi T, Chen JC, Casteel DE, Seasholtz TM, Boss GR, Pilz RB. cGMP-dependent protein kinase inhibits serum-response element- dependent transcription by inhibiting rho activation and functions. J Biol Chem. 2002;277:37382–37393. doi: 10.1074/jbc.M204491200. [DOI] [PubMed] [Google Scholar]
- 27.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutt P, Nguyen N, Toksoz D. Role of Lbc RhoGEF in Galpha12/13-induced signals to Rho GTPase. Cell Signal. 2004;16:201–220. doi: 10.1016/s0898-6568(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 30.Chardin P, Boquet P, Madaule P, Popo MR, Rubin EJ, Gill DM. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamson P, Paterson HF, Hall A. Intracellular localization of the P21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Letts. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 33.Bubb MR, Spector I, Bershadsky AD, Korn ED. Swinholide A is a microfilament disrupting marine toxin that stabilizes actin dimers and severs actin filaments. J Biol Chem. 1995;270:3463– 3466. doi: 10.1074/jbc.270.8.3463. [DOI] [PubMed] [Google Scholar]
- 34.Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun. 1999;2:77–85. doi: 10.1006/mcbr.1999.0155. [DOI] [PubMed] [Google Scholar]