Abstract
Background
BK virus (BKV), JC virus (JCV) and simian virus 40 (SV40) are nonenveloped DNA viruses, members of the family Polyomaviridae. BK and JC viruses establish persistent infections in humans, and evidence suggests that SV40 can infect humans, as well. Whether persistence occurs in the lymphoid system is unknown.
Methods
Paraffin-embedded tonsils from 220 immunocompetent children (mean age 9.3 years) were examined by polymerase chain reaction (PCR) to detect viral DNA of BKV, JCV, SV40, and Epstein-Barr virus (EBV).
Results
Polyomavirus-specific DNA sequences were detected in 8.3% (29/351) of specimens collected from 220 children. Twenty-one (9.5%) children had polyomavirus DNA present in at least one tonsil, with sequences identified as SV40 (n=20) and BKV (n=1). Polyomavirus JCV was not detected. Among patients positive for SV40, 8 of 14 (57%) contained viral DNA in both available tonsils. EBV DNA was detected in 99 (28.2%) samples from 67 (30.5%) patients. Eleven samples (3.1%) from 8 (3.6%) children were positive for both polyomavirus and EBV. SV40-positive children were significantly older than the SV40-negative subjects (P < 0.001). T-antigen expression was detected in an SV40 DNA-positive tonsil by immunohistochemistry.
Conclusions
These results suggest that SV40 can infect tonsils, that lymphoid tissue may represent a site for polyomavirus persistence, and that immunohistochemistry is not a useful detection assay when there are very few virus-infected cells in a tissue.
Keywords: Polyomavirus, BK virus, SV40, EB virus, Tonsils, Lymphoid tissue, Persistence
1. Introduction
Polyomaviruses BK virus (BKV) and JC virus (JCV) persist in humans following infection early in life. Approximately 60–80% of the human population produce antibodies to BKV and JCV by the second decade of life, with seroconversion occurring mostly during childhood and earlier for BKV than for JCV (Imperiale and Major, 2007; Knowles, 2006; Major et al., 1992; Walker and Frisque, 1986). Simian virus 40 (SV40), a polyomavirus of macaque origin, was introduced into humans presumably as a result of the widespread use of poliovaccines inadvertently contaminated with this virus (Butel and Lednicky, 1999; Cutrone et al., 2005; Stratton et al., 2003; Vilchez and Butel, 2004). The contamination occurred because vaccines were produced in cultures of kidney cells derived from rhesus macaques, which are frequently infected with SV40. Millions of people worldwide were exposed to live SV40 via these early vaccines (Butel and Lednicky, 1999; Cutrone et al., 2005; Proceedings of the Second International Conference on Live Poliovirus Vaccines, 1960; Stratton et al., 2003; Vilchez and Butel, 2004). The Institute of Medicine concluded that some SV40 infections in the human population today are related to SV40 exposure from early forms of the poliovaccine (Stratton et al., 2003). SV40 has been detected in specimens from children and adults not exposed to known contaminated poliovaccines, suggesting that SV40 is transmitted in humans today (Butel and Lednicky, 1999; Stratton et al., 2003; Vilchez and Butel, 2004).
BKV, JCV, and SV40 have been detected in a variety of organ systems including urinary, central nervous, and gastrointestinal (Khalili and Stoner, 2001; Major et al., 1992; Vanchiere et al., 2005a, 2005b). This suggests that the pathogenesis of these viruses involves dissemination within the host. While the route of viral spread is unknown, BKV, JCV, and SV40 DNA sequences have been identified in B-lymphocytes from healthy blood donors and immunosuppressed patients (Azzi et al., 1996; Chatterjee et al., 2000; David et al., 2001; Dolei et al., 2000; Martini et al., 1996; Monaco et al., 1996). Both BKV and JCV DNA have been found in tonsil tissue in children, and JCV DNA has been found in spleen and lymph nodes (Dorries et al., 1994; Goudsmit et al., 1982; Kato et al., 2004; Monaco et al., 1998). These findings suggest that the lymphoid system plays a role in polyomavirus infections and may be a site for polyomavirus persistence. Epstein-Barr virus (EBV) is a herpesvirus found in a majority of the human population. After primary infection the virus maintains a state of lifelong, asymptomatic latent persistence in lymphoid tissues with sporadic reactivation (Rickinson and Kieff, 2007).
SV40, like other polyomaviruses, is known to establish persistent infections. The level of virus present may be very low in immunocompetent hosts (Butel, 2000; Lednicky and Butel, 1999), although SV40 can cause widespread infections in immunocompromised monkeys (Lednicky et al., 1998). The nature of interaction of SV40 with lymphoid tissue is unclear. B-cell lymphomas occur in small-animal models of SV40 infection (Cicala et al., 1992; Diamandopoulos, 1973; Matthews et al., 1987), and SV40 is associated with some B-cell lymphomas in humans (Amara et al., 2007; David et al., 2001; Meneses et al., 2005; Shivapurkar et al., 2002; Vilchez et al., 2002b; Zekri et al., 2007). Therefore, B-cell-rich tissues such as tonsils may represent a site that harbors SV40.
The objective of this study was to test the hypothesis that polyomaviruses establish infections in lymphoid tissue. We investigated the frequency of infection by polyomaviruses in tonsils of immunocompetent children. The results suggest that tonsillar tissue is a site for SV40 infection and might play a role in viral persistence.
2. Patients, Materials, and Methods
2.1. Patients and lymphoid tissue specimens
A total of 385 paraffin-embedded, nonmalignant tonsils from 237 immunocompetent children from November 2003 to June 2006 were obtained from Texas Children’s Hospital, Houston, Texas. Demographic data collected on patients included age, gender, and pathologic diagnosis. The study was approved by the Institutional Review Board at Baylor College of Medicine. The most common reason for removal of tonsils from children at this hospital is tonsillar hypertrophy. Currently, only gross examination is performed on all tonsils. Previously, when tonsils were examined microscopically, the majority were designated as having “benign lymphoid hyperplasia”. That was the case for both virus-positive and virus-negative tonsils in this study for which microscopic evaluations were available. A retrospective chart review of 19 of the SV40-positive patients revealed that all had a diagnosis of tonsillar hypertrophy.
2.2. DNA extraction from tonsil samples
Paraffin-embedded tonsillar tissues (two 20-μm sections) were deparaffinized with xylene and then washed with 100%, followed by 70%, ethanol. DNA was extracted using a proteinase K and phenol/chloroform extraction method as previously described (Lednicky and Butel, 1998). All sample processing was performed in a laminar flow hood within a BSL-2 facility free from viruses and plasmids.
2.3. PCR amplification and DNA sequence analysis
Polymerase chain reaction (PCR) assays were set-up in a “PCR Clean Room” using barrier pipette tips to avoid contamination. For each sample, a total of 250–500 ng of DNA was used per reaction. All DNA samples were tested for suitability for amplification using primers specific for the human β-hemoglobin gene (primers P0C3/KM38) (Vilchez et al., 2002b) and only specimens from which cellular sequences were amplified (n=351) were examined further. Primers PYVfor/PYVrev directed at a conserved sequence in the amino (N)-terminus of the large T-antigen (T-ag) gene of BKV, JCV, and SV40 were used for viral analysis (Bergsagel et al., 1992; Lednicky et al., 1995). Our laboratory has established the use of these primers to detect BKV and JCV in clinical samples (Thomas et al., 2007; Vanchiere et al., 2005a, 2005b) as well as SV40 (Meneses et al., 2005; Thomas et al., 2007; Vilchez et al., 2002b). Primers TP1Q3/TP1Q5 were used to detect EBV latent membrane protein 2a (LMP2a) gene sequences (Vilchez et al., 2002a). Positive control plasmids were added to the control PCR reactions outside of the clean rooms after tubes containing negative controls and test DNAs were closed. The positive controls were a plasmid containing cloned SV40 (pSV40-B2E) (McNees et al., 2005) for polyomavirus PCR reactions and a plasmid containing cloned LMP2a gene (O’Sullivan et al., 2002) or cellular DNA from the EBV-positive lymphoma cell line Namalwa (Meneses et al., 2005) for EBV PCR reactions. Negative controls for PCR assays were reactions without added DNA template. Two to 10 separate PCR reactions were run per sample for the polyomavirus assay and two PCR reactions for the EBV assay. PCR products were sequenced to confirm the identity of polyomavirus DNA recovered from lymphoid tissues and only sequence-proven cases are reported here. Samples for which sequence analysis of a PCR product was not successful were not designated as virus-positive tissues.
2.4. Immunohistochemistry
Immunohistochemical staining for detection of SV40 T-ag was as described (Meneses et al., 2005), except that citrate buffer (pH 6) was used for antigen retrieval and the monoclonal antibody against SV40 T-ag (PAb101) was used at a dilution of 1:5000. SV40-induced hamster tumors served as positive controls.
2.5. Statistical analysis
Differences among samples were calculated with the 2-tailed Student’s t test using statistical software (STATA 9.0 for Windows). Differences among groups were considered statistically significant at P < 0.05.
3. Results
3.1. Detection of SV40 and EBV DNA sequences in lymphoid tissue samples
DNAs were extracted from 385 tonsil specimens from 237 healthy children (Table 1). Some specimens represented separate left and right tonsils from the same patient. DNAs were screened for suitability for PCR analysis by amplifying a cellular β-globin gene sequence. β-globin gene sequences were successfully amplified from 351 (91%) of 385 lymphoid specimens from 220 (93%) of 237 subjects. These included both left and right tonsils from 131 patients. These specimens were analyzed for the presence of polyomavirus and EBV DNA sequences.
Table 1.
Presence of polyomavirus and Epstein-Barr virus sequences in tonsils from immunocompetent childrena
| No. of patients | No. DNA-positive patients (%)b |
No. of tissues | No. of DNA-positive tissues (%)b |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β-globin | PYV T-agc | EBV LMP2a | Both PYV and EBV | β-globin | PYV T-ag | EBV LMP2a | Both PYV and EBV | ||
| 237 | 220 (92.8) | 21 (9.5)d | 67 (30.5) | 8 (3.6) | 385 | 351 (91.2) | 29 (8.3) | 99 (28.2) | 11 (3.1) |
Polymerase chain reaction (PCR) assay was used to detect DNA sequences from paraffin-embedded tonsil tissue. Human β-globin gene, a cellular-control gene, was analyzed by PCR first to determine suitability of DNA. Only those samples that amplified the human β-globin gene were analyzed further. Polyomavirus PCR products were DNA sequenced to confirm the presence and identity of the polyomavirus type present.
The numbers of patients (n=220) and tissues (n=351) that yielded a positive reaction for β-globin DNA were used as baselines to calculate the percent viral DNA-positive values.
Abbreviations used: PYV, polyomavirus; T-ag, large tumor antigen; EBV, Epstein-Barr virus; LMP2a, latent membrane protein 2a.
Of polyomavirus-positive tonsils from 21 children, 20 contained simian virus 40 and 1 contained BK virus-specific DNA sequences.
Polyomavirus DNA was detected in 29 (8.3%) of 351 tonsil samples from 21 (9.5%) of 220 immunocompetent children (Table 1). Sequence analysis of the polyomavirus large T-ag PCR amplicons revealed that 28 contained sequences unique to SV40, whereas BKV DNA sequence was present in one sample. JCV DNA sequences were not detected in tissue samples. Among the 20 SV40-positive patients, samples of both left and right tonsils were available from 14 patients, 8 (57%) of whom contained detectable SV40 DNA in both tonsils. EBV LMP2a gene DNA was detected in 99 (28.2%) of 351 tonsil samples from 67 (30.5%) of 220 children (Table 1). Among the 67 EBV-positive patients, 28 of 48 (57%) contained EBV DNA in both left and right tonsils. Eleven (3.1%) samples from 8 (3.6%) children were positive for both polyomavirus and EBV DNAs.
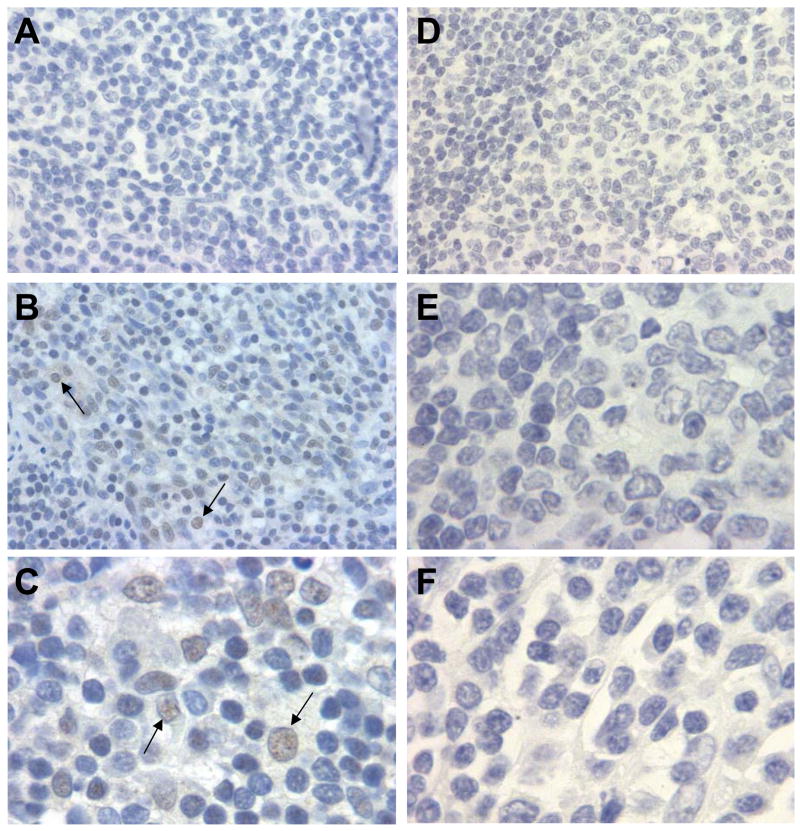
Specimens in which polyomavirus DNA was present were tested for T-ag expression by immunohistochemistry (IHC). T-ag gene expression was detected in 1 (4.8%) of 21 tissues tested, a sample that was SV40 DNA-positive (Fig. 1).
Fig. 1.
(A–C) SV40 T-ag expression in an SV40 DNA-positive tonsil from a 14-year-old male. (A) No staining with negative mouse antibody control. (B,C) SV40 T-ag-positive staining with antibody PAb101. (D–F) Negative T-ag staining with antibody PAb101 on other tonsils (D and E are the same specimen). Arrows point to representative T-ag-positive cells in panels B and C. Original magnification for panels A, B and D, 40×; panels C, E and F, 100×.
3.2. Clinical Correlations
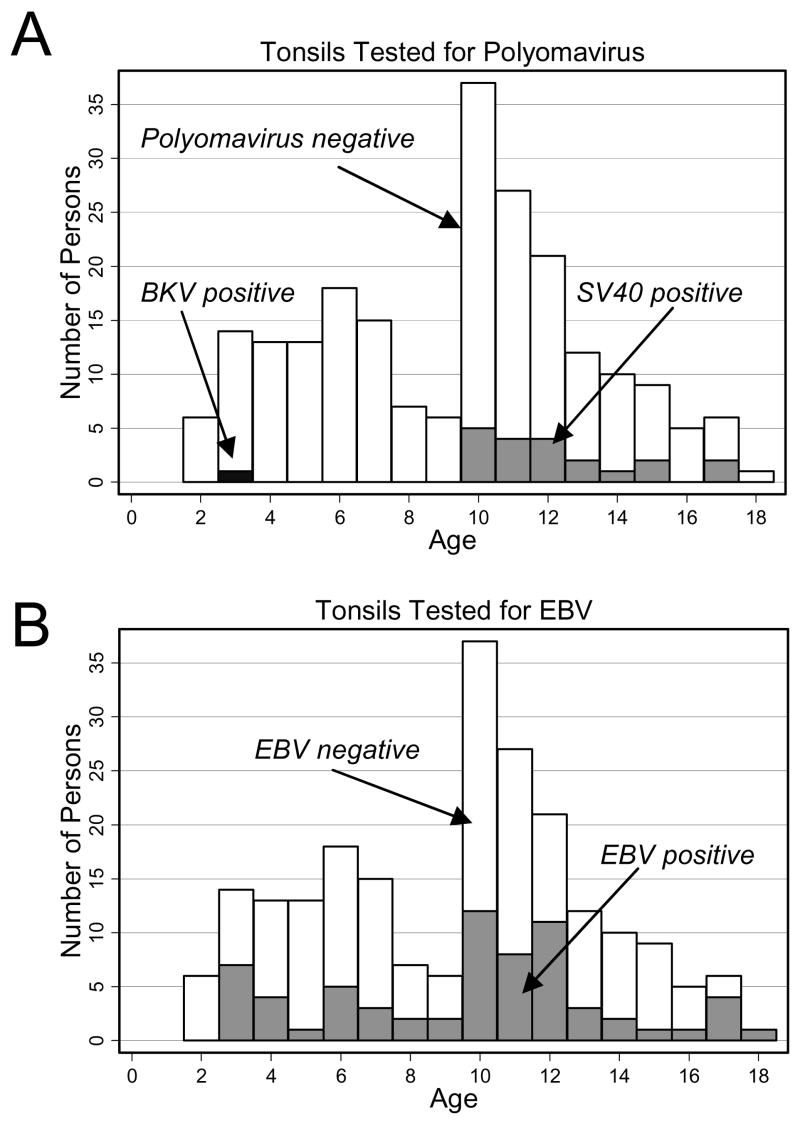
The demographics of the study population are shown in Table 2. The mean age of the study population was 9.3 years, ranging from 2 years to 18 years. The mean age of children whose tonsils were SV40-positive was 12.3 years (range 10–17 years) compared to 8.7 years (range 2–18 years, P < 0.001) for children whose tonsils were SV40-negative (Fig. 2). The BKV-positive patient was a 3-year-old male. The mean age of children who were EBV DNA-positive was 9.7 years (range 3–18 years), compared to 9.1 years (range 2–17 years, P = 0.29) for children whose tonsils contained no detectable EBV DNA (Fig. 2).
Table 2.
Demographic characteristics of study population of immunocompetent childrena
| Characteristics | Total | SV40 in tonsils
|
P value | EBV in tonsils | P value | ||
|---|---|---|---|---|---|---|---|
| SV40 positive | SV40 negative | EBV positive | EBV negative | ||||
| Age (years) | |||||||
| Mean age | 9.3 | 12.3 | 8.7 | <0.001 | 9.7 | 9.1 | 0.29 |
| Age range | 2–18 | 10–17 | 2–18 | 3–18 | 2–17 | ||
| Female/male | 116/104 | 7/13 | 109/91 | 32/35 | 82/71 | ||
| Total subjects | 220 | 20 | 200 | 67 | 153 | ||
Abbreviations used: SV40, simian virus 40; EBV, Epstein-Barr virus.
Fig. 2.
Age distributions among children with virus-positive tonsils. (A) Histogram plot of the age distribution of children with SV40-positive (grey bar) and SV40-negative (white bar) tonsils. SV40-positive children were significantly older (12.3 yr vs. 8.7 yr, respectively; P < 0.001). One tonsil from a 3-year-old male was positive for BKV DNA (black bar). (B) Histogram plot of the age distribution of children with EBV-positive (grey bar) and EBV-negative (white bar) tonsils (9.7 yr vs. 9.1 yr, respectively; P = 0.29).
Of the polyomavirus-positive patients, 33% were Caucasian, 33% were Hispanic, 19% were African-American, 5% were Asian-American, and 10% were other or not specified. This is similar to the ethnicities of the Texas Children’s Hospital patient population of 38,579 patients from the same time period (34% Caucasian, 31% Hispanic, 19% African-American, 2% Asian-American, and 14% other or unknown).
4. Discussion
This investigation demonstrates that polyomaviruses SV40 and BKV can be present in tonsils from immunocompetent children. Polyomavirus DNA was detected in tonsils from 21 (9.5%) children, with SV40 detected most frequently [20/21 (95.2%)]. To our knowledge, this is the first report of the presence of SV40 DNA in human tonsillar tissue. The validity of these findings is substantiated by the fact SV40 was detected in both left and right tonsils in over half of the SV40-positive children from whom both tonsils were available. The mean age of children with SV40-infected tonsils was significantly older than children in whom polyomavirus DNA was absent. EBV DNA was also found in tonsils from this patient population (30.5%), with no age difference between the EBV-positive and -negative children. Previous reports have estimated the prevalence of EBV in nonneoplastic tonsils to be between 29% and 51% (Endo et al., 2001; Pai et al., 2004) and our findings were similar (30%). Eight patients (3.6%) had tonsillar coinfections with SV40 and EBV.
These findings support the hypothesis that polyomaviruses infect the human lymphoid system. The frequency of detection of BKV and JCV in the present study is lower than reported for the few studies in the published literature (Goudsmit et al., 1982; Kato et al., 2004; Monaco et al., 1998). These results probably reflect differences in samples and detection methods used. Goudsmit et al. (1982) utilized hybridization of 32P-labeled BKV DNA to tonsil DNAs that had been cleaved with restriction enzyme BamHI. Half a frozen tonsil was used for each DNA extraction and a large amount (10 μg) of tonsil DNA was added per lane for gel electrophoresis. Five of 12 tonsils from young children yielded a DNA band of the correct size that hybridized to BKV DNA. Monaco et al. (1998) tested tonsils from children (3–15 yr) and adults (18–57 yr) for JCV using a nested PCR approach followed by Southern blot hybridization. One microgram of tonsil DNA was used per reaction. Using this sensitive approach, 19/54 (35%) tonsils were JCV-positive. Kato et al. (2004) tested tonsillar tissues from adults (21–61 yr) for JCV, also using a nested PCR assay. Ten reactions with 1–2 μg DNA/reaction were run per sample to increase sensitivity to detect very low copy numbers of viral DNA. JCV DNA was detected in 14 of 32 donors (44%). For 10/17 (59%) JCV-positive tonsils, only 1 of 10 replicate nested PCR reactions was positive. In contrast to these studies, we extracted DNA from only two 20-micron sections from each formalin-fixed, paraffin-embedded tonsil and used 250–500 ng of DNA per PCR reaction. We did not use a nested PCR assay and in those cases in which we were able to run replicate PCR reactions, similar to the observation of Kato et al. (2004), usually a single reaction was positive for polyomavirus DNA. This suggests a very low viral load in the tonsillar tissue, with SV40 perhaps tending to be higher than BKV in this patient population. Considering that T-ag expression was detected in a single tonsil, this shows that IHC is not a useful screening test when there are very few virus-infected cells in a tissue. The absence of JCV in this study probably reflects the young age of our study population (mean 9.3 years) as JCV infections tend to occur in older children and adolescents (Kato et al., 2004; Knowles, 2006; Monaco et al., 1998). The detection of SV40 DNA in tonsil tissue (9.1%) in this study was similar to the seroprevalence of SV40 in hospitalized children (5.9%) in the Houston area (Butel et al., 1999). In addition, a recent study of stool samples from 99 children at the same hospital found that 8% and 38% of children excreted polyomavirus SV40 and BKV, respectively (Vanchiere et al., 2005a).
Sources of transmission for BKV and JCV are thought to be urinary, gastrointestinal, and/or respiratory tracts (Knowles, 2006; Ling et al., 2003; Vanchiere et al., 2005a), but transmission of SV40 remains unknown. Results described here suggest that tonsillar tissue represents a site of infection by SV40, but whether the virus reached the tonsils via blood or an oral or respiratory route is not known.
The presence of SV40 in tonsils adds to indications that SV40 can infect B-cells. SV40 DNA sequences have been detected in B-lymphocytes from healthy blood donors and immunosuppressed patients (David et al., 2001; Dolei et al., 2000; Martini et al., 1996). B-cell lymphomas arise in some hamsters inoculated with SV40 (Cicala et al., 1992; Diamandopoulos, 1973; Matthews et al., 1987) and human B-cell lymphomas were found in recent investigations to contain SV40 markers (Amara et al., 2007; David et al., 2001; Meneses et al., 2005; Nakatsuka et al., 2003; Shivapurkar et al., 2002; Vilchez et al., 2002b; Zekri et al., 2007). Our findings here suggest that B-cell-rich tonsil tissue might play a role in viral persistence.
In summary, the detection of polyomavirus DNA in tonsils from pediatric patients supports the hypothesis that these viruses can infect lymphoid tissue. Further studies are needed to delineate the significance of polyomavirus infections in lymphoid tissue and the possibility of tonsillar tissue serving as a reservoir for polyomavirus persistence.
Acknowledgments
This work was supported by the National Institutes of Health (K12 RR17665 Mentored Clinical Investigator Award to N.C.P.; research grant CA104818 to J.S.B.). We thank Nikita Malani for technical assistance with sample analysis. We acknowledge Denise Treece and Carolyn Smith for help with data collection.
Abbreviations
- BKV
BK virus
- JCV
JC virus
- SV40
simian virus 40
- EBV
Epstein-Barr virus
- PCR
polymerase chain reaction
- T-ag
large tumor antigen
- IHC
immunohistochemistry
Footnotes
Conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amara K, Trimeche M, Ziadi S, Laatiri A, Hachana M, Sriha B, Mokni M, Korbi S. Presence of simian virus 40 DNA sequences in diffuse large B-cell lymphomas in Tunisia correlates with aberrant promoter hypermethylation of multiple tumor suppressor genes. Int J Cancer. 2007;121:2693–702. doi: 10.1002/ijc.23038. [DOI] [PubMed] [Google Scholar]
- Azzi A, De Santis R, Ciappi S, Leoncini F, Sterrantino G, Marino N, Mazzotta F, Laszlo D, Fanci R, Bosi A. Human polyomaviruses DNA detection in peripheral blood leukocytes from immunocompetent and immunocompromised individuals. J Neurovirol. 1996;2:411–6. doi: 10.3109/13550289609146907. [DOI] [PubMed] [Google Scholar]
- Bergsagel DJ, Finegold MJ, Butel JS, Kupsky WJ, Garcea RL. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992;326:988–93. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- Butel JS. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis. 2000;21:405–26. doi: 10.1093/carcin/21.3.405. [DOI] [PubMed] [Google Scholar]
- Butel JS, Jafar S, Wong C, Arrington AS, Opekun AR, Finegold MJ, Adam E. Evidence of SV40 infections in hospitalized children. Hum Pathol. 1999;30:1496–502. doi: 10.1016/s0046-8177(99)90173-9. [DOI] [PubMed] [Google Scholar]
- Butel JS, Lednicky JA. Cell and molecular biology of simian virus 40: implications for human infections and disease. J Natl Cancer Inst. 1999;91:119–34. doi: 10.1093/jnci/91.2.119. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Weyandt TB, Frisque RJ. Identification of archetype and rearranged forms of BK virus in leukocytes from healthy individuals. J Med Virol. 2000;60:353–62. [PubMed] [Google Scholar]
- Cicala C, Pompetti F, Nguyen P, Dixon K, Levine AS, Carbone M. SV40 small t deletion mutants preferentially transform mononuclear phagocytes and B lymphocytes in vivo. Virology. 1992;190:475–9. doi: 10.1016/0042-6822(92)91237-o. [DOI] [PubMed] [Google Scholar]
- Cutrone R, Lednicky J, Dunn G, Rizzo P, Bocchetta M, Chumakov K, Minor P, Carbone M. Some oral poliovirus vaccines were contaminated with infectious SV40 after 1961. Cancer Res. 2005;65:10273–9. doi: 10.1158/0008-5472.CAN-05-2028. [DOI] [PubMed] [Google Scholar]
- David H, Mendoza S, Konishi T, Miller CW. Simian virus 40 is present in human lymphomas and normal blood. Cancer Lett. 2001;162:57–64. doi: 10.1016/s0304-3835(00)00628-5. [DOI] [PubMed] [Google Scholar]
- Diamandopoulos GT. Induction of lymphocytic leukemia, lymphosarcoma, reticulum cell sarcoma, and osteogenic sarcoma in the Syrian golden hamster by oncogenic DNA simian virus 40. J Natl Cancer Inst. 1973;50:1347–65. doi: 10.1093/jnci/50.5.1347. [DOI] [PubMed] [Google Scholar]
- Dolei A, Pietropaolo V, Gomes E, Di Taranto C, Ziccheddu M, Spanu MA, Lavorino C, Manca M, Degener AM. Polyomavirus persistence in lymphocytes: prevalence in lymphocytes from blood donors and healthy personnel of a blood transfusion centre. J Gen Virol. 2000;81:1967–73. doi: 10.1099/0022-1317-81-8-1967. [DOI] [PubMed] [Google Scholar]
- Dorries K, Vogel E, Gunther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198:59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- Endo LH, Ferreira D, Montenegro MC, Pinto GA, Altemani A, Bortoleto AE, Jr, Vassallo J. Detection of Epstein-Barr virus in tonsillar tissue of children and the relationship with recurrent tonsillitis. Int J Pediatr Otorhinolaryngol. 2001;58:9–15. doi: 10.1016/s0165-5876(00)00446-8. [DOI] [PubMed] [Google Scholar]
- Goudsmit J, Wertheim-van Dillen P, van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10:91–9. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- Imperiale MJ, Major EO. Polyomaviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2263–98. [Google Scholar]
- Kato A, Kitamura T, Takasaka T, Tominaga T, Ishikawa A, Zheng HY, Yogo Y. Detection of the archetypal regulatory region of JC virus from the tonsil tissue of patients with tonsillitis and tonsilar hypertrophy. J Neurovirol. 2004;10:244–9. doi: 10.1080/13550280490468663. [DOI] [PubMed] [Google Scholar]
- Khalili K, Stoner GL. Human Polyomaviruses: Molecular and Clinical Perspectives. Wiley-Liss; New York: 2001. [Google Scholar]
- Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV) In: Ahsan N, editor. Polyomaviruses and Human Disease. Vol. 577. Landes Bioscience; Georgetown, TX: 2006. pp. 19–45. [DOI] [PubMed] [Google Scholar]
- Lednicky JA, Arrington AS, Stewart AR, Dai XM, Wong C, Jafar S, Murphey-Corb M, Butel JS. Natural isolates of simian virus 40 from immunocompromised monkeys display extensive genetic heterogeneity: New implications for polyomavirus disease. J Virol. 1998;72:3980–90. doi: 10.1128/jvi.72.5.3980-3990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky JA, Butel JS. Consideration of PCR methods for the detection of SV40 in tissue and DNA specimens. Dev Biol Stand. 1998;94:155–64. [PubMed] [Google Scholar]
- Lednicky JA, Butel JS. Polyomaviruses and human tumors: a brief review of current concepts and interpretations. Frontiers Biosci. 1999;4:153–64. doi: 10.2741/lednicky. [DOI] [PubMed] [Google Scholar]
- Lednicky JA, Garcea RL, Bergsagel DJ, Butel JS. Natural simian virus 40 strains are present in human choroid plexus and ependymoma tumors. Virology. 1995;212:710–7. doi: 10.1006/viro.1995.1529. [DOI] [PubMed] [Google Scholar]
- Ling PD, Lednicky JA, Keitel WA, Poston DG, White ZS, Peng RS, Liu Z, Mehta SK, Pierson DL, Rooney CM, Vilchez RA, Smith EO, Butel JS. The dynamics of herpesvirus and polyomavirus reactivation and shedding in healthy adults: A 14-month longitudinal study. J Infect Dis. 2003;187:1571–80. doi: 10.1086/374739. [DOI] [PubMed] [Google Scholar]
- Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini F, Iaccheri L, Lazzarin L, Carinci P, Corallini A, Gerosa M, Iuzzolino P, Barbanti-Brodano G, Tognon M. SV40 early region and large T antigen in human brain tumors, peripheral blood cells, and sperm fluids from healthy individuals. Cancer Res. 1996;56:4820–5. [PubMed] [Google Scholar]
- Matthews BJ, Levine AS, Dixon K. Deletion mutations in the small t antigen gene alter the tissue specificity of tumors induced by simian virus 40. J Virol. 1987;61:1282–5. doi: 10.1128/jvi.61.4.1282-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNees AL, White ZS, Zanwar P, Vilchez RA, Butel JS. Specific and quantitative detection of human polyomaviruses BKV, JCV, and SV40 by real time PCR. J Clin Virol. 2005;34:52–62. doi: 10.1016/j.jcv.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Meneses A, Lopez-Terrada D, Zanwar P, Killen DE, Monterroso V, Butel JS, Vilchez RA. Lymphoproliferative disorders in Costa Rica and simian virus 40. Haematologica. 2005;90:1635–42. [PubMed] [Google Scholar]
- Monaco MCG, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70:7004–12. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco MCG, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: Evidence for site of initial viral infection. J Virol. 1998;72:9918–23. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka S-I, Liu A, Dong Z, Nomura S, Takakuwa T, Miyazato H, Aozasa K the Osaka Lymphoma Study Group. Simian virus 40 sequences in malignant lymphomas in Japan. Cancer Res. 2003;63:7606–8. [PubMed] [Google Scholar]
- O’Sullivan CE, Peng RS, Cole KS, Montelaro RC, Sturgeon T, Jenson HB, Ling PD. Epstein-Barr virus and human immunodeficiency virus serological responses and viral burdens in HIV-infected patients treated with HAART. J Med Virol. 2002;67:320–6. doi: 10.1002/jmv.10080. [DOI] [PubMed] [Google Scholar]
- Pai P-C, Tsang N-M, Tseng C-K, Hao S-P, Kuo T-T, Wei K-C, Hsueh C, Chuang C-C. Prevalence of LMP-1 gene in tonsils and non-neoplastic nasopharynxes by nest-polymerase chain reaction in Taiwan. Head & Neck. 2004;26:619–24. doi: 10.1002/hed.20057. [DOI] [PubMed] [Google Scholar]
- Papers and discussions held. Proceedings of the Second International Conference on Live Poliovirus Vaccines; Washington, DC, Pan American Health Organization, Scientific Publication No. 50. 1960. [Google Scholar]
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Straus SE, Martin MA, Roizman B, editors. Fields Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2655–700. [Google Scholar]
- Shivapurkar N, Harada K, Reddy J, Scheuermann RH, Xu Y, McKenna RW, Milchgrub S, Kroft SH, Feng Z, Gazdar AF. Presence of simian virus 40 DNA sequences in human lymphomas. Lancet. 2002;359:851–2. doi: 10.1016/S0140-6736(02)07921-7. [DOI] [PubMed] [Google Scholar]
- Stratton K, Alamario DA, McCormick MC. Immunization Safety Review: SV40 Contamination of Polio Vaccine and Cancer. The National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Thomas LD, Vilchez RA, White ZS, Zanwar P, Milstone AP, Butel JS, Dummer S. A prospective longitudinal study of polyomavirus shedding in lung-transplant recipients. J Infect Dis. 2007;195:442–9. doi: 10.1086/510625. [DOI] [PubMed] [Google Scholar]
- Vanchiere JA, Nicome RK, Greer JM, Demmler GJ, Butel JS. Frequent detection of polyomaviruses in stool samples from hospitalized children. J Infect Dis. 2005a;192:658–64. doi: 10.1086/432076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanchiere JA, White ZS, Butel JS. Detection of BK virus and simian virus 40 in the urine of healthy children. J Med Virol. 2005b;75:447–54. doi: 10.1002/jmv.20287. [DOI] [PubMed] [Google Scholar]
- Vilchez RA, Butel JS. Emergent human pathogen simian virus 40 and its role in cancer. Clin Microbiol Rev. 2004;17:495–508. doi: 10.1128/CMR.17.3.495-508.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez RA, Kozinetz CA, Jorgensen JL, Kroll MH, Butel JS. AIDS-related systemic non-Hodgkin’s lymphoma at a large community program. AIDS Res Hum Retroviruses. 2002a;18:237–42. doi: 10.1089/088922202753472793. [DOI] [PubMed] [Google Scholar]
- Vilchez RA, Madden CR, Kozinetz CA, Halvorson SJ, White ZS, Jorgensen JL, Finch CJ, Butel JS. Association between simian virus 40 and non-Hodgkin lymphoma. Lancet. 2002b;359:817–23. doi: 10.1016/S0140-6736(02)07950-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Frisque RJ. The biology and molecular biology of JC virus. In: Salzman NP, editor. The Papovaviridae. Vol. 1. Plenum Press; New York: 1986. pp. 327–77. [Google Scholar]
- Zekri AR, Mohamed W, Bahnassy A, Refat L, Khaled M, Shalaby S, Hafez M. Detection of simian virus 40 DNA sequences in Egyptian patients with different hematological malignancies. Leuk Lymphoma. 2007;48:1828–34. doi: 10.1080/10428190701534408. [DOI] [PubMed] [Google Scholar]