Abstract
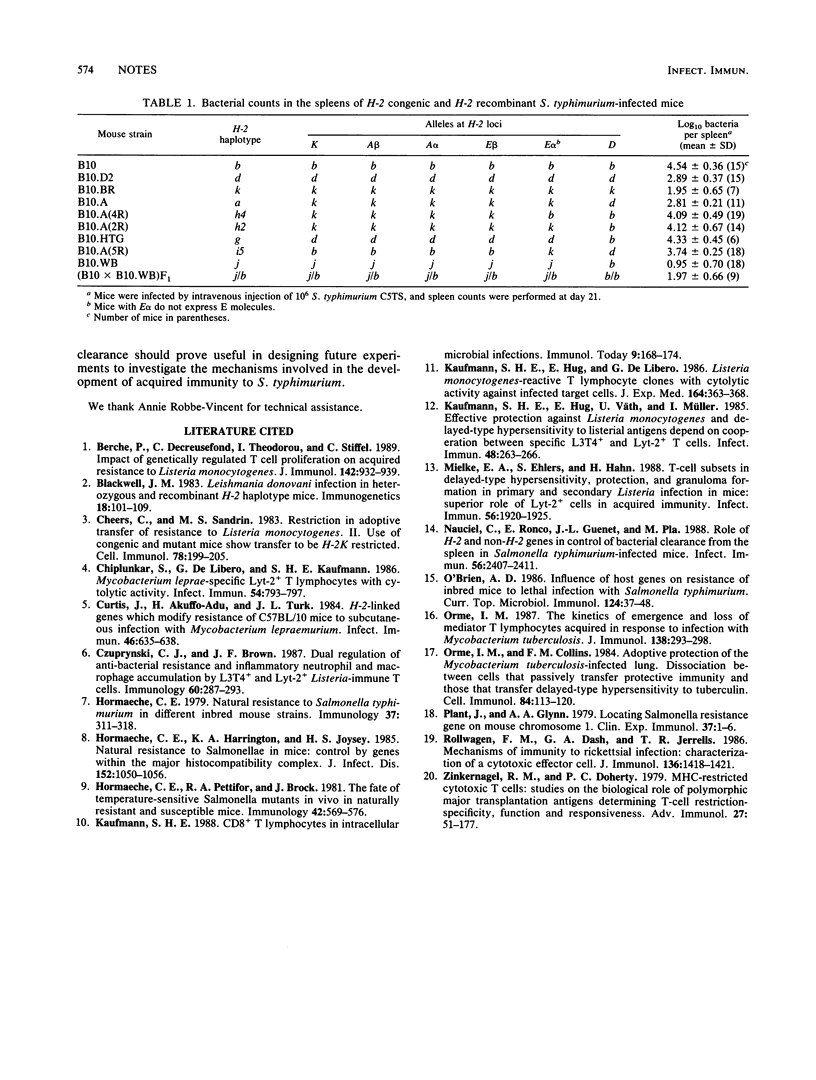
The rate of clearance of Salmonella typhimurium from the mouse spleen is under H-2 linked genetic control. The results of the present study, with H-2 recombinant mice on a C57BL/10 background, suggest the involvement of at least two loci, one in the D region and the other in the K-A alpha chromosomal segment.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berche P., Decreusefond C., Theodorou I., Stiffel C. Impact of genetically regulated T cell proliferation on acquired resistance to Listeria monocytogenes. J Immunol. 1989 Feb 1;142(3):932–939. [PubMed] [Google Scholar]
- Blackwell J. M. Leishmania donovani infection in heterozygous and recombinant H-2 haplotype mice. Immunogenetics. 1983;18(2):101–109. doi: 10.1007/BF00368537. [DOI] [PubMed] [Google Scholar]
- Cheers C., Sandrin M. S. Restriction in adoptive transfer of resistance to Listeria monocytogenes. II. Use of congenic and mutant mice show transfer to be H-2K restricted. Cell Immunol. 1983 Jun;78(2):199–205. doi: 10.1016/0008-8749(83)90274-5. [DOI] [PubMed] [Google Scholar]
- Chiplunkar S., De Libero G., Kaufmann S. H. Mycobacterium leprae-specific Lyt-2+ T lymphocytes with cytolytic activity. Infect Immun. 1986 Dec;54(3):793–797. doi: 10.1128/iai.54.3.793-797.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Akuffo-Adu H., Turk J. L. H-2-linked genes which modify resistance of C57BL/10 mice to subcutaneous infection with Mycobacterium lepraemurium. Infect Immun. 1984 Dec;46(3):635–638. doi: 10.1128/iai.46.3.635-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Dual regulation of anti-bacterial resistance and inflammatory neutrophil and macrophage accumulation by L3T4+ and Lyt 2+ Listeria-immune T cells. Immunology. 1987 Feb;60(2):287–293. [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E., Harrington K. A., Joysey H. S. Natural resistance to salmonellae in mice: control by genes within the major histocompatibility complex. J Infect Dis. 1985 Nov;152(5):1050–1056. doi: 10.1093/infdis/152.5.1050. [DOI] [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E., Pettifor R. A., Brock J. The fate of temperature-sensitive salmonella mutants in vivo in naturally resistant and susceptible mice. Immunology. 1981 Apr;42(4):569–576. [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Ronco E., Guenet J. L., Pla M. Role of H-2 and non-H-2 genes in control of bacterial clearance from the spleen in Salmonella typhimurium-infected mice. Infect Immun. 1988 Sep;56(9):2407–2411. doi: 10.1128/iai.56.9.2407-2411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D. Influence of host genes on resistance of inbred mice to lethal infection with Salmonella typhimurium. Curr Top Microbiol Immunol. 1986;124:37–48. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Rollwagen F. M., Dasch G. A., Jerrells T. R. Mechanisms of immunity to rickettsial infection: characterization of a cytotoxic effector cell. J Immunol. 1986 Feb 15;136(4):1418–1421. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]



