Abstract
The cAMP-PKA cascade is a recognized signaling pathway important in inhibition of inflammatory injury events such as endothelial permeability and leucocyte trafficking, and a critical target of regulation is believed to be inhibition of Rho proteins. Here, we hypothesize that PKA directly phosphorylates GTP dissociation inhibitor (GDI) to negatively regulate Rho activity. Amino acid analysis of GDIα showed two potential protein kinase A (PKA) phosphorylation motifs, Ser174 and Thr182. Using in vitro kinase assay and mass spectrometry, we found that the purified PKA catalytic subunit phosphorylated GDIα-GST fusion protein and PKA motif-containing GDIα peptide at Ser174, but not Thr182. Transfection of COS-7 cells with mutated full-length GDIα at Ser174 to Ala174 (GDIα-Ser174A) abrogated the ability of cAMP to phosphorylate GDIα. However, mutation of Thr182 to Ala182 (GDIα-Thr182A) did not abrogate, and cAMP increased phosphorylation of GDIα to a similar extent as wild-type GDIα transfectants. The mutant GDIα-Ser174A, but not GDIα-Thr182A, was unable to prevent cAMP-mediated inhibition of Rho-dependent serum-response element reporter activity. Furthermore, the mutant GDIα-Ser174A was unable to prevent the thrombin-induced RhoA activation. Coprecipitation studies indicated that neither mutation of the PKA consensus sites nor phosphorylation alter GDIα binding with RhoA, suggesting that phosphorylation of Ser174 regulated preformed GDIα-RhoA complexes. The findings provide strong support that the selective phosphorylation at Ser174 by PKA is a signaling pathway in the negative regulation of RhoA activity and therefore could be a potential protective mechanism for inflammatory injury.
Keywords: adenosine 3′,5′-cyclic monophosphate-dependent protein kinase; protein kinase A consensus phosphorylation sites; single-site mutated GDIα
rho is a member of the superfamily of Rho GTPases (Rho, Rac, and Cdc42) and is a critical signaling intermediate in regulation of vascular inflammatory activities, such as increases in endothelial permeability (7, 11, 21, 38, 39, 41) and leukocyte extravasation (1, 2). Therefore, there is much current interest in understanding mechanisms that could inhibit its activity. In the vascular endothelium, the cAMP signaling cascade is a recognized protective pathway against inflammatory activities through its direct targets protein kinase A (PKA) (8, 26, 36, 40) and EPAC1 (8, 13, 23, 37). We (36) and others (13, 18) have found that Rho proteins are potential direct targets of the cAMP-mediated protective pathway that could be responsible in prevention of inflammatory activities in endothelial cells.
Although the precise mechanisms by which cAMP inhibits Rho remain yet to be fully delineated, there is clear evidence that the cAMP-activated PKA can inhibit Rho by multiple pathways, including direct phosphorylation of RhoA (17, 24) or phosphorylation of upstream determinants controlling Rho activity such as regulator of G protein signaling (3) and Gα13 (28). In endothelial cells, Essler and coworkers (18) observed that RhoA is not phosphorylated by an elevation of intracellular cAMP, suggesting that the preferred target(s) of regulation are factors upstream of RhoA.
Therefore, we postulate that one such possible upstream regulator is GDP dissociation inhibitor (GDI), which functions to prevent GDP dissociation from Rho proteins.
It is well established that the activation of Rho proteins is determined by binding of GTP, and inactivation by GDP, with this cycling of GTP/GDP binding controlled by GDI, GDP exchange factor (GEF), which facilitates exchange of GDP for GTP, and GTPase-activating protein (GAP), which stimulates hydrolysis of GTP to GDP. Of these, GDI is a pivotal regulator that controls access of Rho proteins to GEF and GAP, as well as effector targets (20). Recent evidence has linked specific sites phosphorylated by protein kinas Cα (PKCα) (22), PAK1 (16), and Src (15) to selective functions of GDI.
We have already reported that PKA increased GDI phosphorylation in endothelial cells (36), but a direct phosphorylation by PKA and specific GDI residues phosphorylated were not determined. Here, we hypothesize that PKA directly phosphorylates GDI to negatively regulate Rho activity. The specific goals of the study were to identify the residues on GDI phosphorylated directly by PKA and the effects of their phosphorylation on Rho function. The findings indicate that of the two predicted PKA consensus phosphorylation sites (Ser174 and Thr182), only Ser174 was phosphorylated as determined by in vitro kinase activity assay and transfection studies of single-point mutations of human GDIα. The phosphorylation of GDIα-Ser174 resulted in inhibition of RhoA activity as evaluated by serum-response element (SRE)-reporter activity and affinity binding for rhotekin. The results indicate that phosphorylation of GDIα at Ser174 by PKA suppresses RhoA activity, providing a potential protective signaling mechanism for inflammatory injury.
MATERIALS AND METHODS
Materials
The following reagents were purchased from commercial sources. Amersham Pharmacia Biotech (Piscataway, NJ): ECL Kit, protein A sepharose CL-4B, horseradish peroxidase-conjugated anti-rabbit IgG antibodies; Cytoskeleton (Denver, CO): human GDIα-GST protein; GIBCO (Gaithersburg, MD): Dulbecco's modified Eagle's medium (DMEM), penicillin-streptomycin, phosphate-buffered saline (PBS), Lipofectamine; HyClone Laboratories (Logan, UT): fetal bovine serum (FBS). Pierce (Rockford, IL): BCA kit, bovine serum albumin standard; Promega (Madison, WI): Luciferase kit; Qiagen (6): PhosphoProtein purification kit; Santa Cruz Biotechnology (San Diego, CA): polyclonal anti-GDI antibody, polyclonal anti-RhoA antibody; Sigma Chemical (St. Louis, MO): protein kinase A catalytic subunit (PKA), ATP, phenylmethylsulfonyl fluoride (PMSF); Stratagene (LaJolla, CA): QuikChange Site-directed Mutagenesis Kit; Upstate Biotechnology (Lake Placid, NY): Kemptide phosphate acceptor peptide; Molecular Probes (Eugene, OR): Pro-Q Diamond Phosphoprotein Gel Staining Kit. All other reagents are indicated in the text.
GDI Peptide Synthesis
PKA is a cAMP-dependent serine-threonine protein kinase and phosphorylates its substrates through recognition of consensus motifs (Arg-X-Ser/Thr or Arg-Arg/Lys-X-Ser/Thr). Our amino acid sequence analysis of GDIα showed that there are possibly two potential PKA consensus phosphorylation sites (Arg-Gly-Ser174 and Arg-Phe-Thr182) located near the COOH-terminus (Fig. 1). We designed GDIα peptides that contain the PKA phosphorylation consensus sites as follows. PeptideS174/T182 contained both consensus PKA phosphorylation sites: Met-Leu-Ala-Arg-Gly-Ser174-Tyr-Ser-Ile-Lys-Ser-Arg-Phe-Thr182-Asp-Asp. A negative control peptide was made containing the same sequence except Ser174 and Thr182 were changed to Ala174 and Ala182, respectively. Peptides containing a single PKA phosphorylation site were synthesized as Met-Leu-Ala-Arg-Gly-Ser174-Tyr-Ser (peptideS174) and Ile-Lys-Ser-Arg-Phe-Thr182-Asp-Asp (peptideT182).
Fig. 1.
Putative PKA phosphorylation sites on GTP dissociation inhibitor α (GDIα). Two potential PKA consensus phosphorylation sites (underlined) are located in the hydrophobic binding domain of GDIα: Arg-Gly-Ser (amino acids 172-174) and Arg-Phe-Thr (amino acids 180-182). Additionally, two GDI regions bind Rho proteins: NH2-terminal regulatory domain binds the switch region of Rho; COOH-terminal hydrophobic binding domain binds the isoprenylated membrane-anchoring region of Rho.
The peptides were synthesized by solid-phase peptide synthesis with 9-fluorenylmethyl-oxycarbonyl (Fmoc) chemistry (Research Resources Core Facility, University of Illinois, Chicago, IL). Synthesis took place from COOH-terminus to NH2-terminus; the COOH-terminal Fmoc-amino acid (Anaspec, CA) was attached to an insoluble support resin via an acid-labile linker. Fmoc group of this amino acid was deprotected by 20% piperidine. The second Fmoc-amino acid was coupled to the COOH-terminal residue using the activator 0.1 M 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate in dimethylformamide containing 0.4 M 4-methyl morpholine for 60 min and later washed to remove all unbound amino acid. Fmoc groups were again removed, and the cycle was continued to generate the peptide. The resin-bound peptide was deprotected of its side chain protection groups as well as cleaved from the resin using trifluoroacetic acid (TFA). Ethyl ether was added to precipitate the peptide from the TFA solution. The precipitated peptide was lyophilized and was then characterized by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (Voyager, DE PRO, Applied Biosystems, Foster City, CA). The peptide was verified by HPLC chromatogram and NH2-terminus sequencing.
In Vitro Phosphorylation
In vitro phosphorylation was made by reacting purified PKA catalytic subunit with purified recombinant GDIα-GST fusion protein or synthetic GDIα peptides. The reaction mixture contained either 5 μg GDIα-GST or 10 μg GDIα peptides, purified PKA from bovine heart (30–65 U/μg protein), and 20 μM ATP in a reaction buffer [50 mM Tris (pH 7.4), 10 mM MgCl]. For control, 7.7 μg kemptide and 25 units PKA were added into the reaction buffer. The reactions were incubated at 30°C for 10 min and immediately analyzed by mass spectrometry. For determination of phosphorylation, a mass spectrometric value of an increase of 80 Da would be indicative of incorporation of one phosphate into a protein. In brief, samples were spotted onto a matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) target and analyzed by a Voyager-DE PRO Mass Spectrometer (Applied Biosystems, Foster City, CA) equipped with a 337-nm pulsed nitrogen laser. Peptide mass was measured using a positive-ion linear mode over the 1,000- to 6,500-m/z range. External mass calibration was performed using peaks of a mixture of bradykinin fragments 1–7 at 757 Da, angiotensin II (human) at 1,046 Da, P14R (synthetic peptide) at 1,533 Da, and adrenocorticotropic hormone fragment 18–39 (human) at 2,465 Da.
Mutagenesis of GDIα
Single mutations of full-length human GDIα were made with the use of the QuikChange Site-directed mutagenesis kit. The wild-type (wt) GDIα cDNA (wtGDIα) (GenBank no. AF498926) was obtained from Guthrie Research Institute (Sayre, PA) and had been cloned into pcDNA3.1+ (Invitrogen, Carlsbad, CA). One pair of mutagenic primers was designed to replace Ser174 with alanine (sense: 5′-CTG GCC CGG GGC gcC TAC AGC ATC AAG-3′; antisense: 5′-CTT GAT GCT GTA Ggc GCC CCG GGC CAG-3′). Another pair of mutagenic primers was designed to replace Thr182 with alanine (sense: 5′-C AAG TCC CGC TTC gCA GAC GAC GAC AAG ACC-3′; antisense: 5′-GGT CTT GTC GTC GTC TGc GAA GCG GGA CTT G-3′). The mismatched nucleotides are indicated by lowercase letters. The PCR reaction was set up using the primers and plasmid pcDNA 3.1 containing wtGDIα according to the KIT instruction. Extension of the oligonucleotide primers generated mutated plasmids containing either GDIα-Ser174A (Ser174 changed to Ala174) or GDIα-Thr182A (Thr182 changed to Ala182). After temperature cycling, the products were treated with Dpn I to digest the parental DNA template and to select for mutation-containing synthesized DNA. The plasmids, wtGDIα, GDIα-Ser174A, and GDIα-Thr182A were transformed into XL1-Blue supercompetent cells and grown on agar plates containing the antibiotic ampicillin. Positive clones were selected and verified by sequencing.
Transfection Studies
GDIα constructs.
COS 7 cells were cultured in DMEM containing 4.5 g/l glucose, 5% FBS, and 1% penicillin-streptomycin and grown in culture dishes until 60–70% confluent. The medium was replaced with serum-free DMEM, and the cells were transfected with 1∼2 μg of wtGDIα, GDIα-Ser174A, or GDIα-Thr182A using Lipofectamine according to standard transfection protocol. After incubation for 4 h at 37°C, the medium was replaced with DMEM containing 10% FBS, and cells were incubated overnight and used for studies as described.
Rho-dependent SRE-driven reporter.
The luciferase reporter plasmid containing SRE was kindly provided by Dr. Dolly Mehta (University of Illinois, Chicago, IL) for evaluation of the Rho-dependent transcription activity. The SRE-driven transcriptional activation requires SRF, which is an ubiquitous transcription factor shown to be regulated by mediators and other factors (i.e., lysophosphatidic acid, thrombin, serum, and stress) in a Rho-dependent manner (19, 29). For these assays, COS-7 cells were plated on 12-well dishes, grown to 70–80% confluence, and cotransfected with 1 μg of the SRE-luciferase plasmid plus either 1 μg of wtGDIα, GDIα-Ser174A, or GDIα-Thr182A as described. After transfection, the cells were treated according to experimental protocol and collected for assay of luciferase activity using Promega Luciferase kit according to the manufacturer's protocol. The luciferase activity was measured with a Femtomaster FB12 luminometer (Zylux; Maryville, TN). Assays were made in duplicates per group, and luciferase activity was normalized to microgram of protein from each of the duplicates.
Recombinant adenovirus.
For overexpression of the PKA inhibitor gene using the recombinant adenovirus, an E1−, E3− replication-deficient adenovirus containing full-length PKI cDNA (AdPKI) was constructed and characterized as described previously (26). The gene product of PKI binds with high-affinity and selectivity to PKA (9, 25, 26, 30). COS-7 cells were infected with AdPKI at 100 multiplicities of infection (MOI) = plaque-forming units/target cell for overnight and were transfected with 1 μg of wtGDIα, GDIα-Ser174A, or GDIα-Thr182A using Lipofectamine according to transfection protocol. The Adnull, which has no inserted gene, served as control virus.
In Situ Cellular Phosphorylation
The effects of cAMP on GDIα phosphorylation in cells were determined using the PhosphoProtein purification kit. COS-7 cells transfected with wtGDIα, GDIα-Ser174A, or GDIα-Thr182A were treated according to experimental protocol and lysed in PhosphoProtein lysis buffer, which contained the zwitterionic detergent CHAPS and a mixture of protein inhibitors. The supernatant from the cell lysate (2 mg for each group) was poured into the PhosphoProtein purification column preequilibrated with PhosphoProtein lysis buffer. The columns were washed with lysis buffer to remove unphosphorylated proteins, and the phosphorylated proteins were eluted with the PhosphoProtein elution buffer containing CHAPS. The phosphorylated proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane, and Western blot analysis was made with anti-GDI antibody. Bands corresponding to phosphorylated GDI (∼25 kD) were quantified by scanning densitometry (Scion Image, Beta, 4.0.2; Frederick, MD).
Affinity-binding assay for RhoA-GTP.
The GTP-bound form of RhoA was determined by affinity-binding assay to evaluate RhoA activation as previously described (21, 36). In brief, glutathione-S-transferase-C21 fusion protein (rhotekin, a Rho target molecule) was prepared from induction of cultures of transformed Escherichia coli with 0.1 mM isopropylthiogalactoside. COS-7 cells were grown to 60–70% confluence and transfected with either pcDNA3.1 empty vector or containing wtGDIα, GDIα-Ser174A, GDIα-Thr182A, respectively, using Lipofectamine. At 24 h posttransfection, the cells were treated according to experimental protocol and collected in GST-FISH buffer [50 mM Tris (pH 7.4), 10% glycerol, 100 mM NaCl, 1% NP-40, 2 mM MgCl2, 25 mM NaF, and 1 mM EDTA] plus protease inhibitor cocktail (10 μg/ml of pepstatin A, 10 μg/ml each of aprotinin and leupeptin, and 1 mM PMSF). Cell lysates were pelleted by centrifugation at 10,000 g at 4°C for 5 min, and equal volumes of supernatant were incubated with purified GST-rhotekin coupled to glutathione sepharose 4B beads at 4°C for 1 h. The GTP form of RhoA bound specifically to the rhotekin-sepharose beads was eluted by boiling in 2.5× Laemmli sample buffer and electrophoresed on 12.5% SDS-PAGE, and Western blot was made with affinity-purified antibody directed against RhoA.
Immunoprecipitation
COS-7 cells were plated on 60-mm dishes, grown to 70–80% confluence, and transfected with 2 μg of wtGDIα, GDIα-Ser174A, or GDIα-Thr182A as described. After transfection, the cells were treated according to experimental protocol. The cells were then quickly washed with ice-cold PBS and lysed in radioimmune precipitation buffer [50 mM Tris (pH 8), 150 mM NaCl, 1% NP-40, 1 mM EGTA, 1 mM EDTA, 1 mM orthovanadate, and 50 mM NaF] plus protease inhibitor cocktail (10 μg/ml of pepstatin A, 10 μg/ml each of aprotinin and leupeptin, and 1 mM PMSF). The cell lysate was passed through a 21-gauge needle eight times and centrifuged at 4°C at 10,000 g for 10 min. The supernatant was collected and protein determination made. Seven hundred micrograms of protein from each experimental group were incubated with 2.0 μg rabbit anti-GDI antibody for 1 h at 4°C and 20 μl Protein A Sepharose CL-4B was added and incubated overnight at 4°C on a rocker platform. The immunoprecipitated protein complex was collected by centrifugation at 2,500 rpm at 4°C for 5 min, washed four times with PBS, boiled in 1× electrophoresis sample buffer, and separated by SDS-PAGE. Western blot analysis was made using anti-RhoA or anti-GDI antibodies to determine coimmunoprecipitation of RhoA with GDIα. As negative control, a separate group of cells was used for immunoprecipitation without the precipitating antibody.
Western Blot Analysis
Cells were collected and lysates prepared in the appropriate extraction buffer. Protein concentration was determined using BCA Protein Assay kit with bovine serum albumin as standard. The cell lysates were loaded at constant protein concentrations, separated by SDS-polyacrylamide gel electrophoresis in 12.5% acrylamide as needed, and electrotransferred to nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween-20 (TBST) and incubated with the appropriate primary antibodies diluted in TBST with 1% nonfat dry milk for overnight at 4°C in a rocker. The blot was washed 5× with TBST and incubated with the appropriate anti-IgG secondary antibody conjugated with horseradish peroxidase. The bands were detected using the ECL kit.
Statistics
Single sample data were analyzed by the two-tail t-test; a multiple range test (Scheffé's test) was used for comparison of experimental groups with a single control group.
RESULTS
PKA Directly Phosphoryates GDIα
Purified GDIα protein.
We initially determined the effects of the catalytic PKA subunit on phosphorylation of purified GDIα-GST fusion protein by mass spectrometry analysis. Results indicated that PKA (25 units) increased the molecular mass of GDIα-GST from 49,655 Da in control to 49,789 Da, a shift of 146 ± 6 Da (Fig. 2A; Table 1), suggesting incorporation of two phosphates since an increase in 80 Da corresponds to incorporation of one phosphate. With the use of kemptide as a positive control, a specific substrate of PKA, we observed that incubation with the purified PKA catalytic subunit increased its molecular mass from 773 Da (control) to 853 Da (with PKA), a shift of 80 Da (Fig. 2B; Table 1). When mass spectra (Fig. 2, A and B) are compared, those from the GDIα-GST reaction mixture did not resolve into the sharp peaks as with Kemptide. We suspect that this likely was attributed to varying amounts of unphosphorylated, mono- and di-phosphorylated GDIα-GST proteins in the reaction mix.
Fig. 2.
Purified PKA phosphorylates GDIα-GST. Representative mass spectrometric graphs of molecular mass of purified GDIα-GST (A) and Kemptide (positive PKA substrate control, B), n = 3; C: phosphorylation detected by Pro-Q Diamond Phosphoprotein Gel staining Kit (lane 1 = molecular mass standards; lane 2 = GDIα-GST uncleaved; lane 3 = GDIα-GST cleaved with human α-thrombin, 10−6 μM); purified PKA catalytic subunit = 25 units; control, absence of PKA.
Table 1.
Summary of in vitro kinase phosphorylation of GST-GDI and GDI peptides
| Substrate | n | Control | (+) PKA | Difference |
|---|---|---|---|---|
| GDI-GST | 3 | 49,758±45 | 49,904±98 | 146±6 |
| Kemptide | 6 | 773±0 | 853±0 | 80±0 |
| PeptideS174/T182 | 6 | 1,851±0 | 1931±0 | 80±0 |
| PeptideS174 | 3 | 886±0 | 966±0 | 80±0 |
| PeptideT182 | 3 | 983±0 | 983±0 | 0 |
| Control peptide | 3 | 1,818±0 | 1,818±0 | 0 |
Values are daltons ± SE as determined by mass spectrometry; n = number of separate determinations. GDI, GTP dissociation inhibitor.
To address the possibility that GST was also phosphorylated, we used in vitro phosphorylation of GDIα-GST after cleavage of GST from the protein. Analysis of the amino acid sequence of GST in pGEX-2T (accession number AAA57089; cloning vector used for producing GDIα-GST) for PKA phosphorylation consensus sites indicated that GST contains one putative PKA phosphorylation site that overlapped with the thrombin cleavage site. This suggests that thrombin cleavage will likely prevent GST phosphorylation by PKA. After incubation of GDIα-GST with human α-thrombin (10−6 μM, 10 min), which effectively cleaved GST off from the fusion protein, subsequent detection for phosphorylation yielded phosphorylated GDIα, autophosphorylated PKA, and nonphosphorylated GST (Fig. 2C), which were subsequently confirmed by mass spectrometry. The results indicated that GST contained very likely only one PKA phosphorylation site, which is at the thrombin cleavage site.
Motif-containing GDIα peptides.
Subsequently, we synthesized peptides that contained either Ser174 (peptideS174), Thr182 (peptideT182), or both residues (peptideS174/T182) (Fig. 1) to determine the specific sites phosphorylated by PKA. Preliminary experiments indicated that the in vitro PKA-catalyzed phosphorylation of the synthetic peptides was optimal at 100 units of PKA (data not shown), and we subsequently used that PKA concentration for phosphorylation studies of the synthetic GDIα peptides. Incubation of peptideS174, but not peptideT182, with the PKA catalytic subunit increased the molecular mass by 80 Da (Fig. 3, A and B, respectively; Table 1), indicating that peptideS174 incorporated one phosphate, whereas peptideT182 did not. To confirm the phosphorylation site, we fragmented the precursor ions of peptideS174 by using high-energy collision (20 KeV) with an Axima TOF2 mass spectrometer (Shimadzu, Kyoto, Japan). Analysis of the resulting fragment ions using ProteinProspector software program (MS-Product, http://prospector.ucsf.edu) confirmed phosphosphorylation at Ser174 (see results in Supplemental Fig. A).
Fig. 3.

Purified PKA phosphorylates motif-containing GDIα peptides. Representative mass spectrometric graphs of molecular mass of purified GDIα peptides containing PKA phosphorylation consensus sites: GDIα peptideS174 (n = 3) (A), GDIα peptideT182 (n = 3) (B), GDIα peptideS174/T182 (n = 5) (C), and control peptideA174/A182 (n = 5) (D); PKA = 100 units PKA catalytic subunit; control = absence of PKA.
Mass spectrometry analysis of peptideS174/T182, which contained both phosphorylation sites showed that PKA catalyzed the incorporation of only one phosphate (Fig. 3C; Table 1). The negative control peptideA174/A182 was not phosphorylated by PKA (Fig. 3D; Table 1). The overall in vitro kinase results support that PKA directly phosphorylated GDIα, and the preferred residue was Ser174.
Ser174 of GDIα is Phosphorylated by Intracellular cAMP
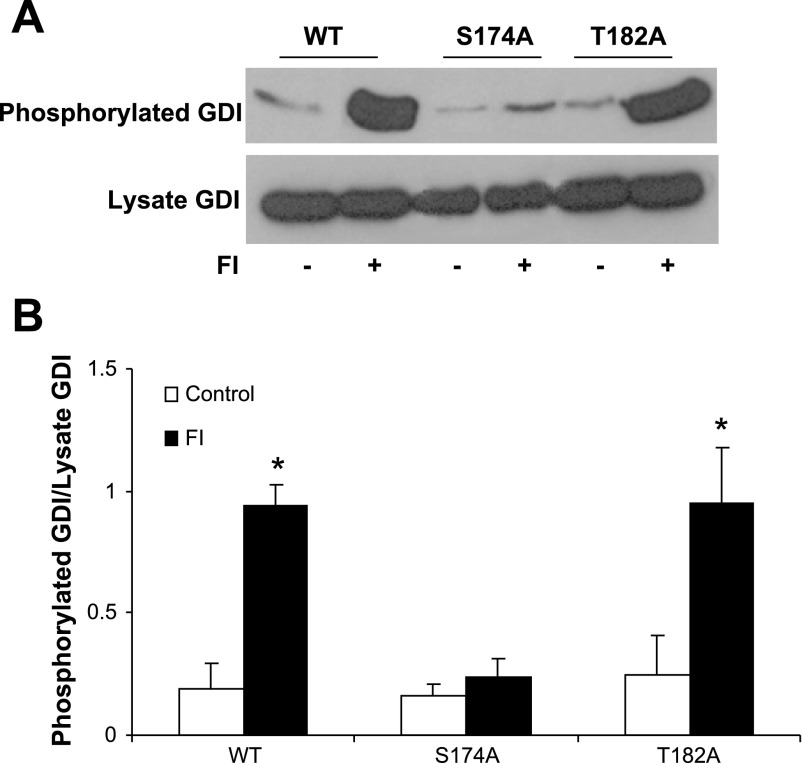
To test whether PKA also phosphorylates Ser174 in the in situ condition, we constructed single mutations of full-length GDIα at the PKA phosphorylation consensus sites, producing GDIα mutants with nonphosphorylatable residues at Ser174 (GDIα-Ser174A) or Thr182 (GDIα-Thr182A) (see materials and methods). COS-7 cells transfected with wtGDIα, GDIα-Ser174A, or GDIα-Thr182A were treated with 20 μM forskolin plus 2 μM IBMX (FI) for 30 min to increase intracellular cAMP levels, and the cells were analyzed for GDIα phosphoryation. The results showed that basal phosphorylation of GDIα was low and similar in the three transfectant groups. Treatment of wtGDIα transfectants with FI resulted in approximately fourfold increased GDIα phosphorylation over nonstimulated control (Fig. 4). However, cells transfected with the mutant GDIα-Ser174A were not responsive to FI treatment, showing GDI phosphorylation at similar levels as nontreated control. The nonsignificant slight increase was likely attributed to endogenous phosphorylated GDIα. With the mutant GDIα-Thr182A transfectants, FI caused a robust increase in phosphorylation similar in extent to the wtGDIα transfectants (Fig. 4). The results, together with the in vitro phosphorylation studies, provide strong evidence that Ser174 on GDIα is the more important residue targeted by PKA.
Fig. 4.
Mutation of GDIα-Ser174 abrogates cAMP-mediated phosphorylation. COS-7 cells were transfected with wild-type GDIα (wt) or the mutant constructs GDIα-Ser174A (S174A) or GDIα-Thr182A (T182A) overnight and treated with 20 μM forskolin and 2 μM IBMX (FI) to increase intracellular level of cAMP. GDIα phosphorylation was determined using the PhosphoProtein purification kit from Qiagen (materials and methods). A: top, representative Western blot showing detection of GDIα phosphorylation from the transfectants; bottom, total GDIα in cell lysates. B: bar graph summarizes results from 6 separate determinations; *P < 0.01 compared with control.
Phosphorylation of GDIα-Ser174 Inhibits RhoA
Effects of cAMP on SRE-luciferase reporter activity.
The functional significance of the PKA-mediated phosphorylation of GDIα-Ser174 was tested using a Rho-dependent, SRE-driven reporter plasmid (19). PKA specificity was determined by overexpression of COS-7 cells with a recombinant AdPKI gene as described by us (26) (see materials and methods). After infection with AdPKI or Adnull overnight, the cells were cotransfected with the SRE-luciferase reporter along with either wtGDIα or the GDI mutant constructs (GDIα-Ser174A or GDIα-Thr182A). In control Adnull cells, results show that FI treatment of wtGDIα transfectants significantly decreased reporter activity compared with control cells but not in the GDIα-Ser174A transfectants (Fig. 5). However, FI treatment of cells transfected with GDIα-Thr182A mutant resulted in significant inhibition of SRE reporter activity (Fig. 5). After PKI overexpression, the FI-mediated inhibition in both wtGDIα and GDIα-Thr182A transfectants was abrogated, indicating that the inhibited reporter activity was PKA specific (Fig. 5). As expected, PKI overexpression had no effects on reporter activity in the GDIα-Ser174A transfectants (Fig. 5). The results indicate that PKA phosphorylation of Ser174 enhanced the ability of GDIα to negatively regulate RhoA.
Fig. 5.
Mutation of GDIα-Ser174 prevents cAMP-induced inhibition of Rho-dependent activity. COS-7 cells were cotransfected overnight with the Rho-dependent SRE-luciferase reporter plasmid and with either wtGDIα (wt), GDIα-Ser174A (S174A), or GDIα-Thr182A (T182A). For PKA specificity, cells were overexpressed with the recombinant adenovirus containing PKI inhibitor gene; AdNull served as control (see materials and methods). One group was treated with FI for 8 h to increase intracellular cAMP levels, whereas another remained as untreated control. Luciferase activity was measured in all cell groups, and results are reported as %FI/control; n = 10–14 separate determinations. *P < 0.01 compare with nontreated control in wtGDIα and GDIα-Thr182A-transfected groups.
Effects of cAMP on thrombin-stimulated RhoA.
We next tested whether phosphorylation of GDIα-Ser174 can prevent agonist-stimulated activation of RhoA. COS-7 cells were transfected with wtGDIα or GDIα-Ser174A overnight, pretreated with FI, and then challenged with thrombin (100 nM) for 10 min. The cells were collected for affinity binding with rhotekin to determine the GTP-bound RhoA. Results showed that FI treatment significantly inhibited the thrombin-stimulated increase in RhoA-GTP (∼40%) in wtGDIα transfectants, whereas in GDIα-Ser174A transfectants, FI treatment was unable to prevent the thrombin-induced RhoA activation (Fig. 6).
Fig. 6.
Mutation of GDIα-Ser174 prevented the ability of cAMP to inhibit mediator-induced RhoA activation. COS-7 cells were transfected with wtGDIα (wt) or GDIαS174A (S174A) overnight. Controls and those treated with FI for 30 min were stimulated with human α-thrombin (Thr; 100 nM for 10 min). RhoA activation was determined by affinity-binding assay (see materials and methods), and densitometric scans of bands are summarized in the bar graph. A representative Western blot of the pull-downed RhoA-GTP is shown. Values are reported as means ± SE of normalized RhoA-GTP; n = 5. *P < 0.01.
Effects of phosphorylated GDIα-Ser174 on interaction with RhoA.
We investigated whether phosphorylation of GDIαS174 alters GDIα interactions with RhoA. COS-7 cell transfectants were treated with FI as previously described for phosphorylation determination, and cell lysates were immunoprecipitated with anti-GDI Ab, followed by Western blot detection for RhoA. Results showed that FI treatment of wtGDIα, GDIα-Ser174A, and GDIα-Thr182A transfectants did not alter GDI-RhoA complex formation (Fig. 7). Furthermore, the mutation of these residues per se did not alter interaction between the two proteins either since cells in the absence of FI showed similar levels of coprecipitated RhoA as those in the presence of FI (Fig. 7). Negative controls were immunoprecipitated in the absence of anti-GDI Ab or with the isotype-matched IgG and showed absence of precipitated bands (data not shown).
Fig. 7.
Phosphorylation of GDIα-Ser174 did not alter GDIα-RhoA complex formation. Coprecipitation analysis were determined from COS-7 cells transfected with wtαGDI (wt), GDIα-Ser174A (S174A), or GDIα-Thr182A (T182A) overnight, followed by treatment with FI to increase intracellular levels of cAMP. Top: affinity-purified anti-GDI Ab was used for immunoprecipitation, and separated proteins were detected by Western blot analysis with anti-RhoA or anti-GDI antibody; a representative Western blot from five separate determinations. Bottom: bar graph summarization of densitometric scans of bands.
DISCUSSION
The current study provides strong evidence that PKA directly phosphorylated GDIα at Ser174, which was sufficient to inhibit, at least in part, RhoA function. Mutation of this residue to Ala174 effectively abrogated the cAMP-stimulated phosphorylation response in COS-7 cell transfectants. This nearly 100% inhibition of phosphorylation in the GDIα-Ser174A mutant indicated that cAMP phosphorylated mostly Ser174 and not other residues. The finding is further underscored by the observation that mutation of Thr182, the other PKA consensus phosphorylation site, did not prevent cAMP-mediated phosphorylation of GDI. The Thr182A mutant construct was phosphorylated to the same extent as the wtGDIα. Results from in vitro phosphorylation of GDI peptides provide further evidence of the selectivity of Ser174 phosphorylation by PKA. The synthetic GDIα peptide containing both PKA phosphorylation consensus sites (Ser174 and Thr182) was phosphorylated at only one of these residues by PKA. This finding was substantiated with GDIα peptides containing single PKA phosphorylation sites, which showed that Ser174, but not Thr182, was phosphorylated.
Surprisingly, when whole GDIα-GST protein was used as substrate, PKA increased the molecular mass by 146 Da, indicating potential phosphorylation of two residues. This finding appears to be in contrast to the phosphorylation studies made with GDIα peptides and the single-site mutation studies. Analysis of the amino acid sequence of the GST component of the fusion protein revealed that it contains one putative PKA phosphorylation consensus site, located at the carboxy terminus and overlapping with the thrombin cleavage site, and suggests that this could account for the other phosphorylation site. The subsequent in vitro phosphorylation assay following thrombin cleavage detected phosphorylation of GDIα, but not GST, confirming that GST contained only that one phosphorylation site, which contributed to the overall phosphorylation of GDI-GST by PKA.
A key finding from the current work is that phosphoryation of GDIα-Ser174 by PKA enhances GDIα's negative regulation of RhoA. We found that the mutation of Ser174 to Ser174A, rendering GDIα not phosphorylatable by PKA, abrogated the ability of cAMP to inhibit basal RhoA and thrombin-stimulated RhoA activities. This observation is consistent with our previous report that PKA inhibited thrombin-induced RhoA activation and prevented the increase in endothelial permeability (36). Ser174 appears to be a critical residue for regulation of GDIα function, serving as a convergence point for multiple kinases (and phosphatases?). DerMardirossian and coworkers (16) found that PAK1 also phosphorylates GDI at Ser174, and the combined phosphorylation with Ser101 causes dissociation of Rac1 from GDI, leading to a selective activation of Rac1. Although no data were shown in the publication nor in the supplementary results, the authors (16) additionally noted that PKA phosphorylated Ser174, which corroborates this current important finding. In contrast to the consequences of PAK1-mediated phosphorylation (on both Ser101 and Ser174), PKA phosphorylated only Ser174. Together, these findings clearly suggest high selectivity of GDI function under control of specific residues as phosphorylated by different kinases.
GDI is believed to negatively regulate Rho proteins through its association with them, affecting both the cellular location and GDP/GTP cycling (14, 20, 31). However, in our model, the phosphorylation of GDIα-Ser174 by PKA did not increase GDIα association with RhoA, a finding consistent with our previous report in endothelial cells in which elevation of intracellular cAMP does not increase coprecipitated endogenous GDIα and RhoA (36). The results suggest that phosphorylated GDI inhibited RhoA not by formation of new complexes, but rather by exerting effects on preexisting GDI-RhoA complexes. The hydrophobic geranylgeranyl-binding domain of GDI comprises the carboxy-terminal two-thirds of the molecule (residues 74–204) (Fig. 1), folding into an immunoglobulin-like β sandwich for binding Rho proteins. Ser174 lies adjacent to Tyr175, a residue lining the hydrophobic binding pocket and forming hydrophobic contacts with Rho proteins (20). We speculate that phosphorylation of Ser174 may exert local steric changes on Tyr175, which could potentiate retention of Rho within GDI's hydrophobic binding pocket and thereby preventing Rho activation.
The cAMP-PKA signaling pathway is important in preventing a wide range of inflammatory activities (4, 5, 10, 26, 32–36, 40). The current results show that a direct target of the cAMP-PKA pathway is Ser174 of GDI, and this phosphorylation of GDI resulted in the inhibition of RhoA function. The findings are significant in that activation of RhoA is implicated in a variety of inflammation-linked diseases, including pulmonary hypertension, acute lung injury, atherosclerosis (27, 42), tumor progression, and metastasis (12).
Supplementary Material
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grant HL-71081 (to H. Lum) and American Heart Association Postdoctoral Fellowships, Greater Midwest Affiliate (to J. Qiao).
Current address of J. Zhang: Center for Cardiovascular Sciences, Albany Medical Center, Albany, NY 12208; Current address of J. Qiao: St. Joseph's Regional Medical Center, Paterson, NJ 07503.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol 162: 2964–2973, 1999. [PubMed] [Google Scholar]
- 2.Anwar KN, Fazal F, Malik AB, Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol 173: 6965–6972, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanian N, Levay K, Keren-Raifman T, Faurobert E, Slepak VZ. Phosphorylation of the regulator of G protein signaling RGS9-1 by protein kinase A is a potential mechanism of light- and Ca2+-mediated regulation of G protein function in photoreceptors. Biochemistry 40: 12619–12627, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Balyasnikova IV, Pelligrino DA, Greenwood J, Adamson P, Dragon S, Raza H, Galea E. Cyclic adenosine monophosphate regulates the expression of the intercellular adhesion molecule and the inducible nitric oxide synthase in brain endothelial cells. J Cereb Blood Flow Metab 20: 688–699, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Barnard JW, Seibert AF, Prasad VR, Smart DA, Strada SJ, Taylor AE, Thompson WJ. Reversal of pulmonary capillary ischemia-reperfusion injury by rolipram, a cAMP phosphodiesterase inhibitor. J Appl Physiol 77: 774–781, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Bauer B, Mirey G, Vetter IR, Garcia-Ranea JA, Valencia A, Wittinghofer A, Camonis JH, Cool RH. Effector recognition by the small GTP-binding proteins Ras and Ral. J Biol Chem 274: 17763–17770, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313: 2504–2520, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 283: H1819–H1828, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Bruynzeel I, van der Raaij LM, Willemze R, Stoof TJ. Pentoxifylline inhibits human T-cell adhesion to dermal endothelial cells. Arch Dermatol Res 289: 189–193, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Chiba Y, Ishii Y, Kitamura S, Sugiyama Y. Activation of rho is involved in the mechanism of hydrogen-peroxide-induced lung edema in isolated perfused rabbit lung. Microvasc Res 62: 164–171, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Collard JG Signaling pathways regulated by Rho-like proteins: A possible role in tumor formation and metastasis (Review). Int J Oncol 8: 131–138, 1996. [PubMed] [Google Scholar]
- 13.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005. [DOI] [PubMed] [Google Scholar]
- 14.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 15: 356–363, 2005. [DOI] [PubMed] [Google Scholar]
- 15.DerMardirossian C, Rocklin G, Seo JY, Bokoch GM. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol Biol Cell 17: 4760–4768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell 15: 117–127, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278: 19023–19031, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol 164: 6543–6549, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Hill CS, Wynne J, Treisman R. The Rho Family GTPases RhoA, Rac1, and Cdc42Hs regulate transcriptional activity by SRF. Cell 81: 1159–1170, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman GR, Nassar N, Cerione RA. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell 100: 345–356, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCα and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol 289: L176–L185, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Knezevic N, Roy A, Timblin B, Konstantoulaki M, Sharma T, Malik AB, Mehta D. GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability. Mol Cell Biol 27: 6323–6333, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 579: 4966–4972, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 15: 510–519, 1996. [PMC free article] [PubMed] [Google Scholar]
- 25.Lum H, Hao Z, Gayle D, Kumar P, Patterson CE, Uhler MD. Vascular endothelial cells express isoforms of protein kinase A inhibitor. Am J Physiol Cell Physiol 282: C59–C66, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol Cell Physiol 277: C580–C588, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res 93: 884–888, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Manganello JM, Huang JS, Kozasa T, Voyno-Yasenetskaya TA,. Le Breton GC. Protein kinase A-mediated phosphorylation of the Galpha13 switch I region alters the Galphabetagamma13-G protein-coupled receptor complex and inhibits Rho activation. J Biol Chem 278: 124–130, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem 276: 22614–22620, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Meja KK, Catley MC, Cambridge LM, Barnes PJ, Lum H, Newton R, Giembycz MA. Adenovirus-mediated delivery and expression of a cAMP-dependent protein kinase inhibitor gene to BEAS-2B epithelial cells abolishes the anti-inflammatory effects of rolipram, salbutamol, and prostaglandin E2: a comparison with H-89. J Pharmacol Exp Ther 309: 833–844, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol 152: 111–126, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minnear FL, DeMichele MA, Leonhardt S, Andersen TT, Teitler M. Isoproterenol antagonizes endothelial permeability induced by thrombin and thrombin receptor peptide. J Appl Physiol 75: 1171–1179, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Ottonello L, Morone MP, Dapino P, Dallegri F. Tumour necrosis factor alpha-induced oxidative burst in neutrophils adherent to fibronectin: effects of cyclic AMP-elevating agents. Br J Haematol 91: 566–570, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Patterson CE, Lum H. Update on pulmonary edema: the role and regulation of endothelial barrier function. Endothelium 8: 75–105, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Pober JS, Slowik MR, De Luca LG, Ritchie AJ. Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J Immunol 150: 5114–5123, 1993. [PubMed] [Google Scholar]
- 36.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 284: L972–L980, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol 26: 6333–6346, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci 116: 4615–4628, 2003. [DOI] [PubMed] [Google Scholar]
- 39.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol 23: 211–217, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Waschke J, Drenckhahn D, Adamson RH, Barth H, Curry FE. cAMP protects endothelial barrier functions by preventing Rac-1 inhibition. Am J Physiol Heart Circ Physiol 287: H2427–H2433, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol 176: 150–165, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol 24: 1842–1847, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.