Abstract
Earlier we reported defects in D1 receptor function in renal proximal tubules (RPTs) of aged Fischer 344 (F344) and obese Zucker rats. However, the defects in the receptor function in RPTs of obese Zucker rats do not pass onto primary cultures of RPTs from these animals. Here, we determined whether the defects in D1 receptor function in RPTs of aged F344 rats pass onto the primary cultures. RPTs from aged (24-mo) and adult (6-mo) F344 rats were grown into primary cultures. The microscopic studies showed that cells in cultures from adult and old rats were healthy as determined by the shape and size of the cells and nuclei. D1 receptor agonist SKF-38393 produced inhibition of 86Rb (rubidium) uptake, index of Na-K-ATPase activity, in cells from adult rats, but this was reduced in old rats. Also, SKF-38393 increased the [35S]GTPγS binding, index of receptor activation, in the membranes of cells from adult rats but to a lesser extent from old rats. Furthermore, there was a downward trend in the levels of D1 receptor numbers and in the receptor proteins in old rats. Interestingly, gp91phox subunit of NADPH oxidase and cellular protein carbonyl levels (oxidative stress marker) were higher in cultures from old rats. These results show that RPTs from adult and old F344 rats grow into epithelial cells in cultures. Furthermore, cells in cultures from old rats are at a higher level of oxidative stress, which may be contributing to the reduced D1 receptor function in the cells from old compared with adult rats.
Keywords: dopamine, NADPH oxidase, G protein-coupled receptor, aging
during increased sodium intake renal dopamine maintains sodium homeostasis by promoting sodium excretion via activation of D1 receptor and inhibition of sodium transporter Na-K-ATPase in renal proximal tubules (RPT) (1, 9, 19, 20). Diminished D1 receptor signaling in RPT has been linked to the reduced natriuretic response to dopamine and development of hypertension in animals and humans (16, 25, 34).
Aging initiates various structural and functional changes in several body organs including in the kidney (13). In the elderly, reduced ability of the kidney to respond to dopamine and increase sodium excretion during salt loading has been linked to the development of age-associated hypertension (33). In old Fischer 344 rats (F344), renal dopamine D1 receptor function is also impaired, partly due to reduced D1 receptor G protein coupling in RPT, resulting in diminished natriuretic response to dopamine in aging (6). Age-related increase in oxidative stress has been linked to uncoupling of D1 receptors from G proteins leading to D1 receptor dysfunction in RPTs of old rats (14).
Primary cell cultures from different organs such as the brain, heart, and kidney have been useful in studying the etiology of Alzheimer's disease, heart failure, and hypertension, respectively (7, 16, 18, 21, 25, 30). For example, studies in cell cultures from RPTs of spontaneously hypertensive rats (SHR) and essential hypertensive patients demonstrated that a variant in GRK4 genes is responsible for the dysfunction of dopamine D1 receptors (16). Whereas, in obesity-related hypertensive rats (obese Zucker rats), the cause of the receptor dysfunction has been attributed to systemic circulating factors, which also cause a defect in renal D1 receptor function (4).
Previously, we reported that D1 receptor dysfunction in RPTs of old Fischer 344 was mainly due to uncoupling of the D1 receptor from G proteins. However, it is not known whether D1 receptor dysfunction, in terms of inability of D1 receptor agonist to stimulate G protein and inhibit Na-K-ATPase, in RPTs of old F344 rats pass onto the primary proximal tubular epithelial cell cultures. Therefore, in the present study we wanted to determine the effects of D1 receptor agonist SKF-38393 on G protein coupling and Na-K-ATPase activities in primary cell cultures of RPTs from adult and old F344 rats. Since age-associated increase in oxidative stress contributes to D1 receptor dysfunction, we also determined markers of oxidative stress and subunits of NADPH oxidase, an enzyme that generates oxygen radicals.
METHODS AND MATERIALS
Animals.
Male Fischer (F344/NNiaHsd) rats of 6 mo (adult) and 24 mo (old) of age were purchased from National Institute on Aging (Bethesda, MD) raised by Harlan-Sprague-Dawley (Indianapolis, IN). Rats were allowed to acclimate for at least 3 days before any studies were conducted, fed commercial rat chow and water ad libitum, and housed in a temperature-, humidity-, and light-controlled (12-h light/dark cycle) environment in the University of Houston Animal Care Facility. The animals were used in the study with the approval of Institution's Animal Care and Use Committee and according to the National Institutes of Health guidelines.
Primary proximal tubular epithelial cell cultures.
Primary cell cultures from RPTs of adult and old F344 rats were prepared by a method routinely used in our laboratory (15). Briefly, under pentobarbital anesthesia (50 mg/kg), a midline abdominal incision was made to perfuse the kidney with buffer A (in mM: 142 NaCl, 7 KCl, 10 HEPES, 40 dextrose, 25 mannitol, 0.5 EDTA, and 2 Na citrate) followed by with buffer B (in mM: 142 NaCl, 7 KCl, 10 HEPES, 40 dextrose, 25 mannitol, 5 CaCl2, collagenase 0.1%, and hyaluronidase 0.1%). The kidneys were isolated and kept in ice-cold buffer C (buffer B without collagenase and hyaluronidase but with twice the concentration of antibiotic and antimycotic).
All the subsequent steps were carried out under sterile condition in a cell culture hood unless otherwise specified. After the capsule was removed, the kidneys were washed three times with ice-cold buffer C. The kidneys were cut open longitudinally with a sterile razor blade, and cortical tissues were obtained, minced into paste, and incubated with buffer B for 10 min at 37°C with occasional mixing with pipette. The tissue solution was filtered through a mesh (80 μm pore size), collected in culture tubes, and centrifuged at 100 g at 4°C. The pellet was washed twice with buffer D (buffer C without Ca2+ and antibiotic and antimycotic). Finally, the pellet was resuspended in 15 ml of buffer D, layered over 5 ml of 25% Ficoll solution (in mM: 142 NaCl, 7 KCl, and 10 HEPES), and centrifuged for 20 min at 4°C. The RPTs band between the interface was carefully obtained and washed twice by centrifugation in DMEM/F12 culture medium. The trypan blue exclusion test was performed to determine the viability of isolated renal proximal tubules, which were 95% viable. Finally, RPTs were resuspended in DMEM/F12 medium with supplements [0.573 ng/ml insulin, 5 μg/ml transferrin, 40 ng/ml hydrocortisone, 5 ng/ml selenium, 4 pg/ml 3,3,5-triiodo-l-thyronine (T3), 10 ng/ml epidermal growth factor, 1.2 mg/ml sodium bicarbonate, 0.29 mg/l l-glutamine, 25 IU/ml penicillin, 25 μg/ml streptomycin, and 10% fetal calf serum], plated, and grown in a humidified 5% CO2 chamber maintained at 37°C. The cell culture nutrient medium was replaced every 48 h. The cells in cultures were pure epithelial cells as seen under a microscope (Fig. 1) and used in the studies after 5–6 days of first seeding.
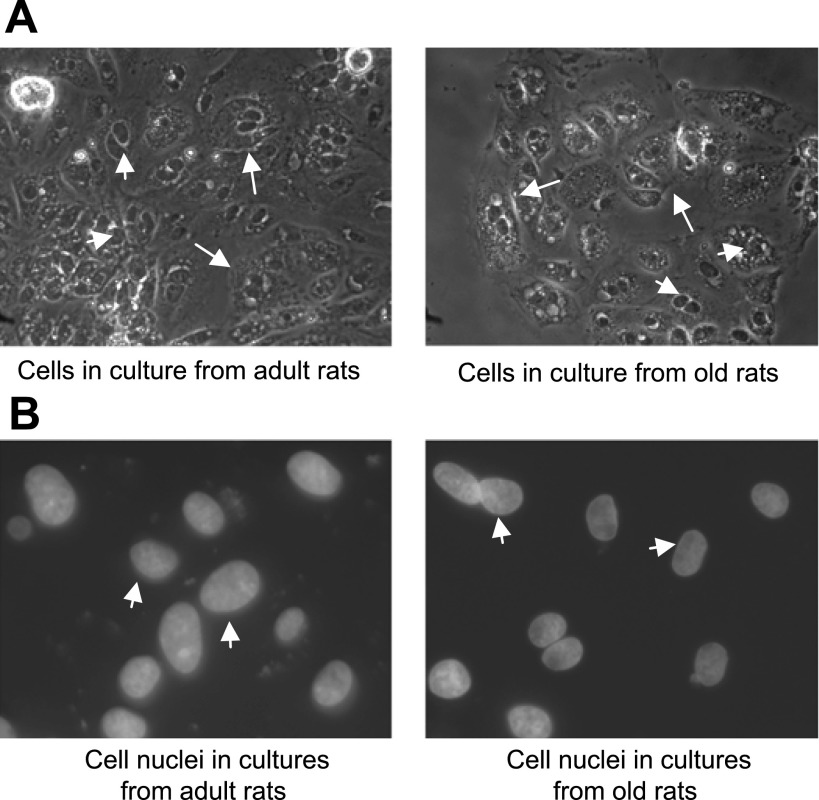
Fig. 1.
Renal proximal tubules (RPTs) from adult (6 mo) and old (24 mo) Fischer 344 (F344) rats grow into epithelial cells in primary cultures. Epithelial cells grown on glass cover-slips (details in methods and materials) were scanned under microscope using ×20 phase (A) and ×60 fluorescence (B) objectives. Arrows in A show cell boundaries. Arrowheads in A and B show cell nuclei.
86Rubidium (86Rb) uptake.
Primary cell cultures grown in 12-well plates were used for 86Rb uptake measurement according to our published method (5). The cells were incubated without or with SKF-38393 for 10 min at 37°C. 86Rb uptake was initiated by the addition of 1 ml DMEM containing 3 μCi/ml 86Rb. Cells were lysed with 3% sodium dodecyl sulfate, and radioactivity was measured directly in cell lysate using a gamma counter. Na-K-ATPase activity was determined as the difference between 86Rb uptake in the absence and presence of ouabain (1 mM).
Microscopy studies.
Proximal tubular epithelial cells were cultured on poly-d-lysine-coated glass coverslips. The cells were washed with PBS and fixed with ice-cold 2% paraformaldehyde in PBS for 20 min at 4°C. After being washed with PBS, the cells were scanned under a microscope using ×20 phase 1 objective (Olympus, Japan) for morphological evaluation. To visualize cellular nuclei, the glass coverslips containing cells were mounted onto glass slides using 4,6-diamidino-2-phenylindole (DAPI nuclear dye) containing mount media (Vector Laboratory, Burlingame, CA). The nuclei were observed under fluorescence microscope using a ×60 oil objective having 1.4 numerical aperature (Olympus, Japan) and specific DAPI filter (360–370 nm). The cell images were recorded by using digital camera (Hamamatsu, Japan) and Imaging Systems (Compix, Cranberry Township, PA). The blue-colored DAPI-stained cell nuclei have been shown as black and white images in results.
Preparation of membranes.
Cellular membranes were prepared according to our published method (14, 15). Briefly, cells grown on 35-mm plastic culture dishes were taken in homogenization buffer (in mM: 10 Tris, 250 sucrose, 1 PMSF, and protease inhibitor cocktail; pH 7.4), sonicated for 10 s, and subjected to low-speed centrifugation. The supernatant was subjected to high-speed centrifugation at 35,000 g for 30 min. The resulting pellet was resuspended in a small volume of homogenization buffer and considered membrane fraction. All the above steps were carried at 4°C using ice-cold buffer.
[3H]SCH-23390 binding assay.
To determine the number of D1 receptors on the membranes, binding of a D1 receptor antagonist [3H]SCH-23390 to membranes was performed as described previously (14, 15). Briefly, for saturation binding, 50 μg of membrane proteins were incubated with 20 nM of [3H]SCH-23390 in a final volume of 250 μl binding buffer at 25°C for 90 min. Unlabeled SCH-23390 (10 μM) was used for determining nonspecific bindings. Specific binding was calculated as the difference between the total and nonspecific bindings.
[35S]GTPγS binding assay.
As previously described (14, 15), membrane proteins (5 μg) in the presence of [35S]GTPγS (0.6 nM corresponding to ∼100,000 cpm) and GDP (10 μM) were incubated with various concentrations of SKF-38393 (10−10 to 10−7 mol/l) in a final volume of 100 μl for 1 h at 30°C. Nonspecific binding was determined by adding 100 μM unlabeled GTP to the assay media. Specific binding was calculated as the difference between total and nonspecific bindings.
Western blotting of D1 receptors.
D1 receptor proteins were determined by our published method (14, 15). Membrane proteins (10 μg) were resolved by SDS-polyacrylamide gel electrophoresis and transblotted onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P, Millipore, Bedford, MA). The PVDF membrane was blocked with 5% nonfat dry milk in PBST overnight at 4°C followed by incubation with rabbit polyclonal D1 receptor antibody (1:1,000) (Chemicon, Temecula, CA) for 60 min. Horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1,000) was used to probe primary antibody. The protein bands were visualized using chemiluminescence reagents (Santa Cruz Biotech, Santa Cruz, CA) and X-ray films.
Western blotting of NADPH oxidase subunits.
Membrane proteins (15 μg) were resolved by SDS-PAGE followed by transblotting onto PVDF membranes. The PVDF membrane was blocked with 5% BSA in PBST for 1 h followed by incubation with mouse monoclonal NADPH gp91phox subunit antibody (1:500) (BD Biosciences, San Jose, CA) for 1 h. Goat-anti-mouse-horseradish peroxidase-conjugated antibody (1:2,000) was used to probe primary antibody. The protein bands were visualized on X-ray film using chemiluminescence reagents. The same blot was stripped off using a reagent kit from Alpha Diagnostics (San Antonio, TX), blocked with 5% BSA in PBST, and incubated with mouse monoclonal rac1 antibody (1:500) (BD Biosciences, San Jose, CA) for 1 h. Goat-anti-mouse-horseradish perxoidase-conjugated secondary antibody (1:2,000) was used to probe primary antibody. The protein bands were visualized on X-ray films using chemiluminescence reagents.
Western blot analysis of protein carbonyls.
Protein carbonyls (addition of aldehydes and ketones) were determined using a Western blotting detection assay kit (Chemicon, Temecula, CA) according to the manufacturer's protocol. Protein carbonyl is a measure of marker of oxidative stress (26) and has been used in our previous studies (2).
Proteins measurement.
Proteins were measured using BCA protein assay kit (Pierce, Rockford, IL) and BSA as standards.
Statistics.
Results are presented as means ± SE. Data were analyzed by ANOVA followed by Newman-Keuls multiple comparison test and by Student's t-test where applicable. A value of P < 0.05 was considered significant.
RESULTS
As shown in Fig. 1, RPTs when cultured grew into typical epithelial cells from both adult and old F344 rats. The shapes of the nuclei were of typical epithelial cells in both the cultures of adult and old rats indicating a normal development of the cells (Fig. 1).
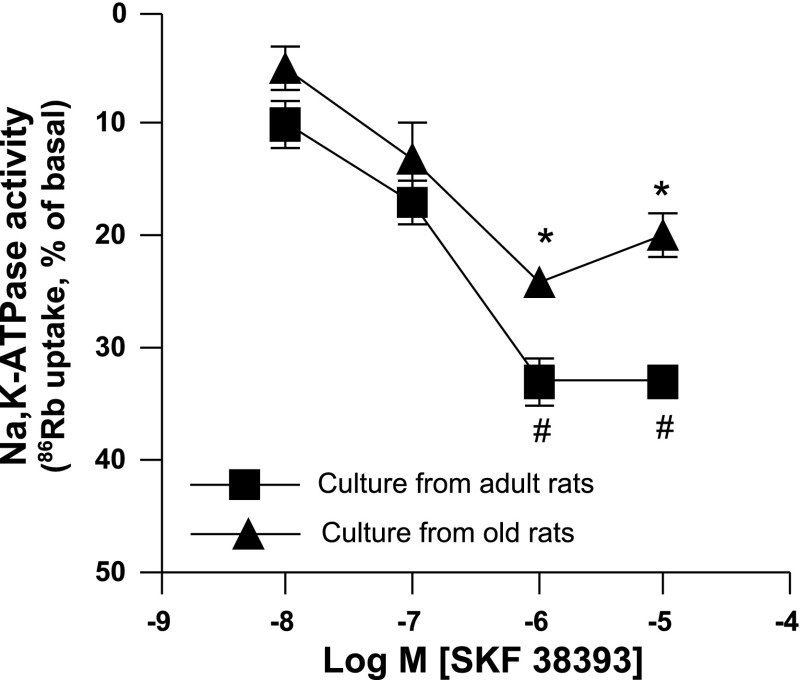
Dopamine D1 receptor agonist SKF-38393 produced a concentration-dependent inhibition of 86Rb uptakes in proximal tubular epithelial cell cultures from both adult and old rats (Fig. 2). However, SKF-38393-mediated inhibition of 86Rb uptake was reduced in the cell cultures of old compared with adult rats. The basal uptakes of 86Rb in cell cultures of adult and old rats were similar (adult vs. old: 858 ± 97 vs. 897 ± 92 cpm). The results are presented as percentage of basal (Fig. 2).
Fig. 2.
D1 receptor agonist SKF-38393 (10−8 to 10−5 M) inhibits Na-K-ATPase to a lesser extent in the proximal tubular epithelial cell cultures of old F344 rats. Na-K-ATPase activity was measured as rubidium (86Rb) uptake, an index of Na-K-ATPase activity. Results are means ± SE from proximal tubular epithelial cells in cultures from 4 rats in each group. *Significantly different from SKF-38393 (10−8 M) in culture from adult rats. #Significantly different from SKF-38393 (10−8 M) in culture from old rats. ANOVA and post hoc Newman-Keuls test was applied to determine the significance at P < 0.05.
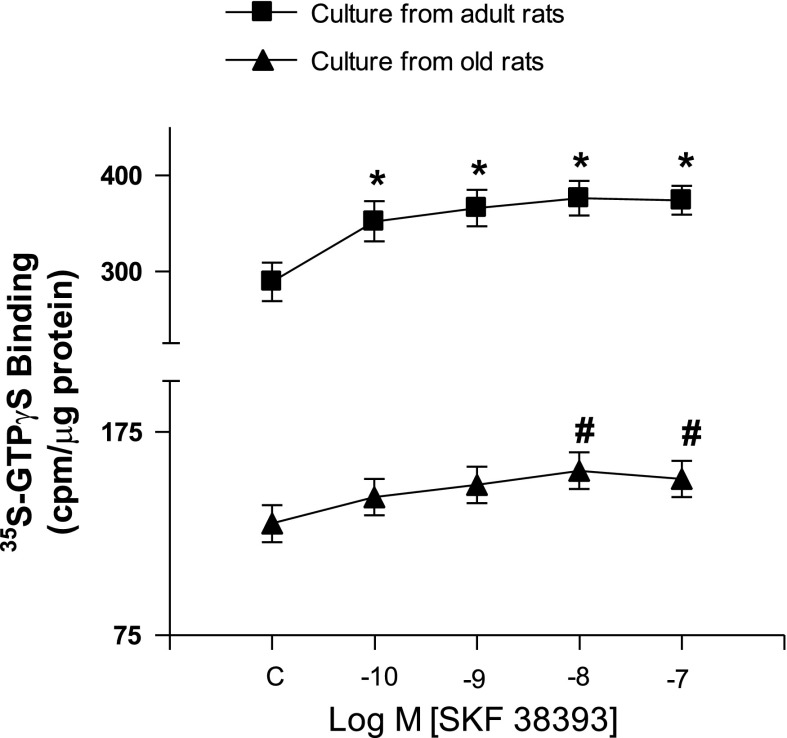
Similarly, D1 receptor activation with SKF-38393 produced a concentration-dependent stimulation of G proteins as measured by [35S]GTPγS binding in both the cell cultures of adult and old rats (Fig. 3). SKF-38393-mediated stimulation of G proteins was reduced in the cultures from old than in adult rats (Fig. 3).
Fig. 3.
D1 receptor agonist SKF-38393 (10−10 to 10−7 M) stimulates D1 receptor to a lesser extent in the proximal tubular epithelial cell cultures of old F344 rats. D1 receptor stimulation was measured as binding of [35S]GTPγS to G proteins, an index of receptor G protein coupling. Results are means ± SE from proximal tubular epithelial cells in cultures from 5 rats in each group. *Significantly different from SKF-38393 (10−10 M) in culture from adult rats. #Significantly different from SKF-38393 (10−10 M) in culture from old rats. ANOVA and post hoc Newman-Keuls test was applied to determine the significance at P < 0.05.
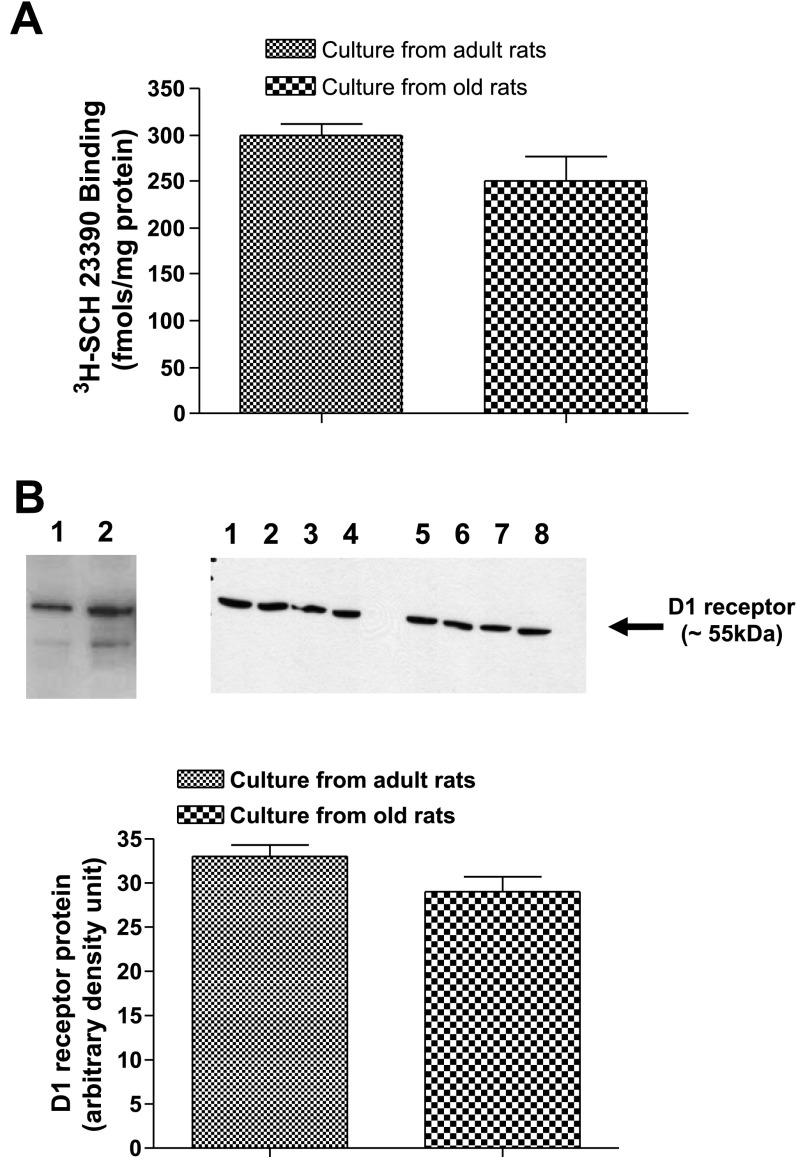
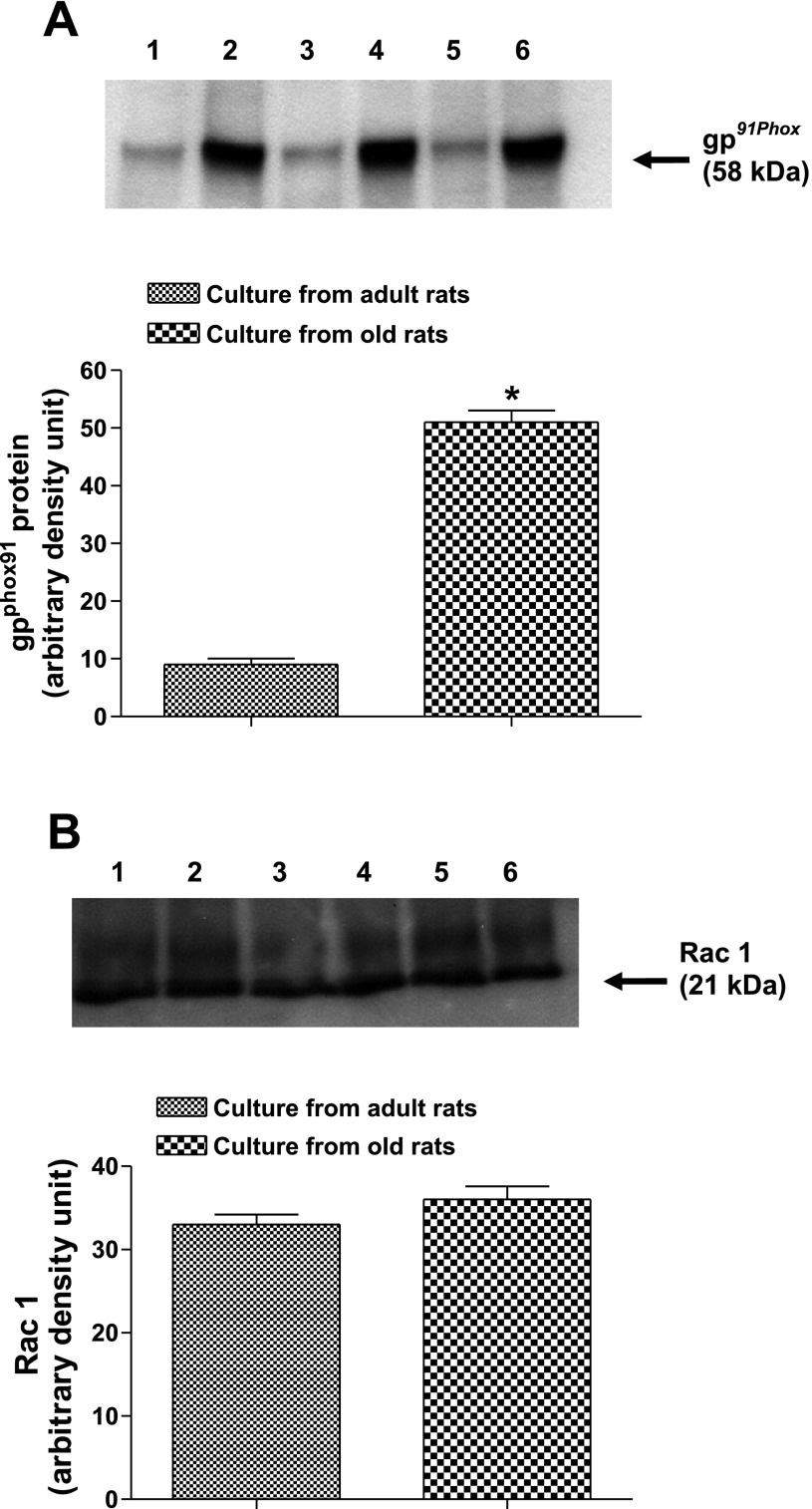
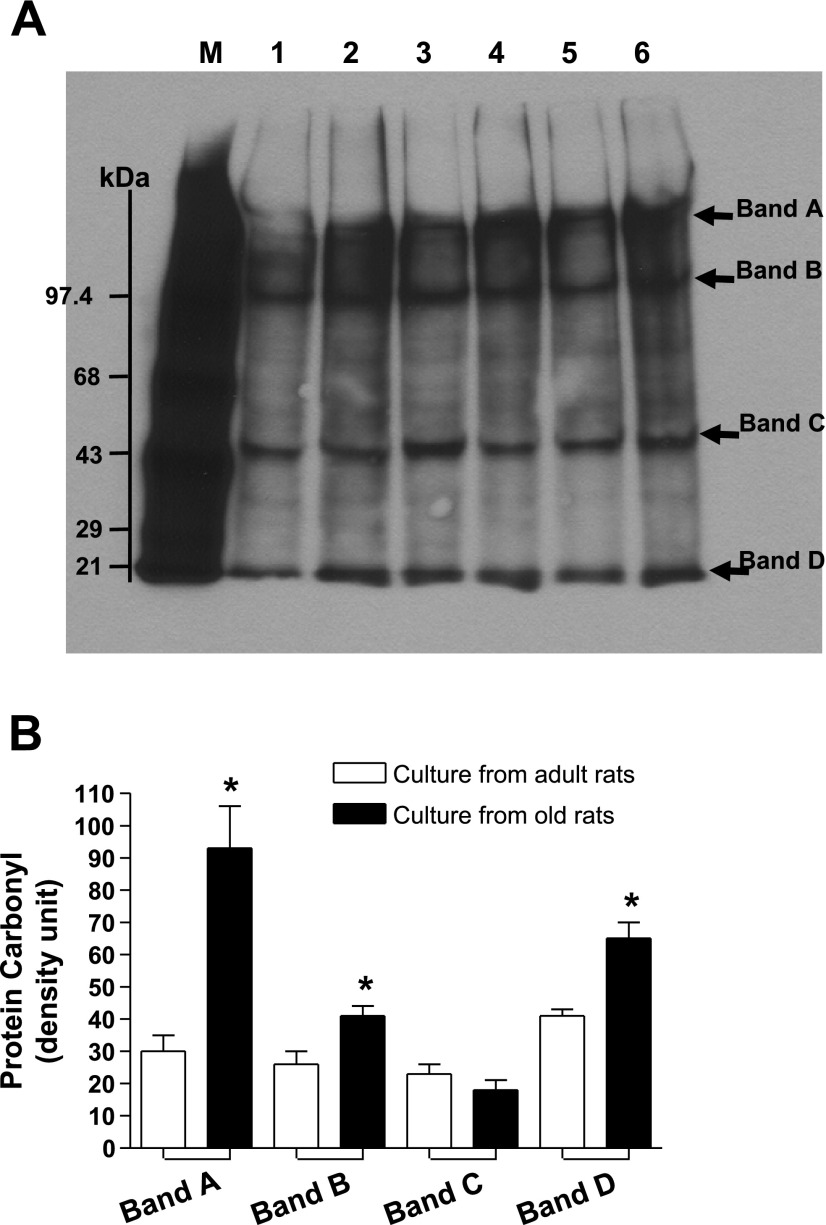
There was a downward trend in the levels of dopamine D1 receptor numbers (−16%) measured as [3H]SCH-23390 binding and D1 receptor proteins in cell cultures of old compared with adult rats (Fig. 4, A and B). Interestingly, gp91phox subunit of NADPH oxidase increased approximately sixfold in the membranes of cell cultures from old rats (Fig. 5A). Also, there was an upward trend (∼10%) in the levels of rac1 proteins in the membranes of cell cultures from old rats (Fig. 5B). Furthermore, there were increased levels of protein carbonyls in the cultures from old compared with adult rats (Fig. 6).
Fig. 4.
D1 receptor numbers and D1 receptor proteins in the membranes of proximal tubular epithelial cells of adult and old F344 rats. A: D1 receptor numbers were determined using 20 nM of radioactive D1 receptor-specific antagonist [3H]SCH-23390. B: D1 receptor proteins were measured by Western blot analysis using specific D1 receptor antibody. Bars are results as means ± SE (n = 4 cultures from 4 different rats). B: top right, immunoblot of D1 receptor proteins (lanes 1, 2, 5, 6: cultures from adult F344 rats; lanes 3, 4, 7, 8: cultures from old F344 rats); left, a positive control for D1 receptors in brain striatum of Sprague-Dawley rats (lane 1, 5 μg protein; lane 2, 15 μg protein).
Fig. 5.
NADPH gp91phox over expresses in the membranes of proximal tubular epithelial cell cultures from old F344. A: top, gp91phox in the membranes of cell cultures of adult (lanes 1, 3, 5) and old (lanes 2, 4, 6) F344 rats. B: top, rac1 in the membranes of cell cultures of adult (lanes 1, 3, 5) and old (lanes 2, 4, 6) F344 rats. Bars are results as means ± SE (n = 3 cultures from 3 different rats). *Significantly different from cultures from adult rats in A (t-test, P < 0.01).
Fig. 6.
Proximal tubular epithelial cells in cultures from old F344 rats are endowed with higher protein carbonyls. Protein carbonyl, a marker of oxidative stress, was determined by Western blot analysis using a kit (details in methods and materials). A: lane M (markers of protein carbonyl); lanes 1, 3, 5 (primary cell cultures from adult F344 rats); lanes 2, 4, 6 (primary cell cultures from old F344 rats). Arrows point to protein bands with carbonyls. B: densities of protein carbonyls in bands A, B, C and D are means ± SE (n = 3 cultures from 3 different animals) and represented as bars. *Significantly different from respective cultures from adult rats (t-test, P < 0.05).
DISCUSSION
Our studies in primary cell cultures from RPTs of old F344 rats demonstrate the ability of D1 receptor agonist SKF-38393 to stimulate G proteins and inhibit Na-K-ATPase activity, however, to a lesser extent, compared with the primary cell cultures from adult F344 rats. In addition, the present study also provides the evidence that isolated RPTs from F344 rats, particularly from old F344 rats, can be cultured into epithelial cells. Furthermore, our data suggest that proximal tubular epithelial cells in cultures from old F344 rats are at higher levels of oxidative stress compared with the cultures from adult F344 rats.
Primary cell culture studies have been helpful in correlating the in vivo findings in organ tissues in understanding the mechanisms of toxicity induced by chemicals (8, 11, 22), and of diseases such as Alzheimer's disease, heart failure, and hypertension (7, 16, 18, 21, 25, 30). Age-related changes with regard to cell proliferation and migration also have been studied in primary cultures of anterior pituitary (29), aortic smooth muscle (23), and hepatocytes (27) in F344 rats. However, age-related studies in primary cell cultures from RPTs of F344 rats with regard to dopamine D1 receptor function are lacking. Although primary cell cultures from RPTs from F344 rats have been made using younger rats (11, 22). Herein, we provide evidence, to our knowledge for the first time that RPTs from old (24 mo) F344 rats can be grown into epithelial cells in cultures. These cells are healthy as determined by comparing the shapes of the cells and nuclei from the cultures of adult (6 mo) and old (24 mo) F344 rats. It should be noted that renal proximal tubular epithelial cells from adult and old F344 rats were cultured using standard cell culture conditions (normoxia, 21% O2). Satellite cells from skeletal muscle of 31-mo-old rats have successfully been grown using low oxygen concentration (hypoxia, 3% O2) (10). Since we did a comparative study between the cultures from adult and old rats and the fact that the cultures from adult rats grew well at standard culture conditions, we did not use low oxygen in our studies. Moreover, we were not sure how the cells from adult rats would grow at low oxygen, which will provide a hypoxic environment that may result in growth arrest in these cells.
Previously, we reported diminished natriuretic response to dopamine and D1 receptor agonists in old F344 rats, an animal model of aging, and in obese Zucker rats, an animal model of obesity-related hypertension and insulin resistance (6, 24). The cause for this was linked to the inability of dopamine and D1 receptor agonists to inhibit sodium transporter, such as Na-K-ATPase, due in part to reduced D1 receptor numbers and D1 receptor G protein uncoupling in RPTs. However, D1 receptor agonist stimulated G proteins and inhibited Na-K-ATPase activity to similar extent in primary cell cultures of RPTs from obese Zucker rats compared with their control counterpart, lean Zucker rats (4). Actually, the findings related to the normal D1 receptor function in primary cell cultures of RPTs from obese Zucker rats interested us to undertake the present studies in old F344 rats and their control counterpart, adult F344 rats. D1 receptor agonist SKF-38393 resulted in D1 receptor G protein coupling and in inhibition of Na-K-ATPase in primary cell cultures of adult and old F344 rats. However, unlike in obese Zucker rats, SKF-38393-mediated stimulatory and inhibitory responses on G proteins and Na-K-ATPase, respectively, were reduced in the cultures of old compared with adult F344 rats. This may be due to the effect of aging, per se, and/or perhaps to downward trend in the levels of D1 receptor density in the cultures of 24-mo-old F344 rats. The cultures from lean and obese Zucker rats were of similar age, 10–12 wk (4).
Reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase enzyme is the major source of reactive oxygen species in nonphagocytic cells (31, 32, 35). The primary redox component of all NADPH oxidases is known as Nox. In the kidney, Nox 2, also known as gp91phox, is highly expressed in the proximal convoluted tubule (17). Activation of gp91phox requires its assembly with a membrane subunit, p22phox; several cytosolic regulatory subunits p67phox, p47phox, and p40phox; and small GTPase Rac (12). Since we have demonstrated age-associated increase in oxidative stress in RPTs of old F344 rats (3, 14), we studied gp91phox in the primary cell cultures of RPTs from adult and old F344 rats. We found a marked increase (approximately sixfold) in the levels of gp91phox together with an upward trend in the levels of Rac1 in the cultures of RPTs from old F344 rats. The antibody from BD Biosciences (catalog no. 610354) used to detect the cytosolic regulatory subunit p47phox did not work in our studies. Nevertheless, increased expressions of gp91phox and Rac1 may be contributing to reactive oxygen species production resulting in higher levels of protein carbonyls, a marker of oxidative stress (26), seen in the cultures of RPTs from old F344 rats. It should be noted that cell cultures from RPTs of SHR are endowed with higher oxidative stress compared with the cultures from their normotensive counterpart (28). And, overexpression of gp91phox contributes to increased oxidative stress in the kidneys of SHRs compared with Wistar-Kyoto (WKY) rats (35). Also, agonist response to D1 receptor activation was reduced in the cultures from SHR than in the cultures from WKY rats, suggesting higher oxidative stress may be a contributing factor for the reduced D1 receptor function in SHR (25). Therefore, it is likely that a similar phenomenon exists in the cell cultures of RPTs from old F344 rats as reported in the present study.
In conclusion, we provide the evidence of the feasibility of preparing primary cell cultures from RPTs of 24-mo-old F344 rats, which may serve as an ex vivo cell model for cellular signaling studies. Moreover, D1 receptor agonist produced functional response in both the cultures of RPTs from adult and old F344 rats, however, to a lesser extent in old F344 rats. The increased oxidative stress in the cultures of RPTs from old F344 rats may be contributing to this phenomenon.
GRANTS
National Institute on Aging Grants AG-25056 (to M. F. Lokhandwala) and AG-029904 (to M. Asghar) funded the study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aperia A Regulation of sodium/potassium ATPase activity: impact on salt balance and vascular contractility. Curr Hypertens Rep 3: 165–171, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Asghar M, Monjok E, Kouamou G, Ohia S, Bagchi D, Lokhandwala M. Super CitriMax (HCA-SX) attenuates increases in oxidative stress, inflammation, insulin resistance and body weight in developing obese Zucker rats. Molecular Cellular Biol 304: 93–99, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Asghar M, Lokhandwala MF. Antioxidant supplementation normalizes elevated protein kinase C activity in the proximal tubules of old rats. Exp Biol Med 229: 270–275, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Banday AA, Hussain T, Lokhandwala MF. Renal dopamine D(1) receptor dysfunction is acquired and not inherited in obese Zucker rats. Am J Physiol Renal Physiol 287: F109–F116, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopamine-mediated inhibition of renal Na,KATPase is reduced by insulin. Hypertension 41: 1353–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Beheray S, Kansra V, Hussain T, Lokhandwala MF. Diminished natriuretic response to dopamine in old rats is due to an impaired D1-like receptor-signaling pathway. Kidney Int 58: 712–720, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Bodyak N, Rigor DL, Chen YS, Han Y, Bisping E, Pu WT, Kang PM. Uncoupling protein 2 modulates cell viability in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H829–H835, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Brewer GJ, LeRoux PD. Human primary brain tumor cell growth inhibition in serum-free medium optimized for neuron survival. Brain Res 1157: 156–166, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Carey RM Renal dopamine system. Paracrine regulator of sodium homeostasis and blood pressure. Hypertension 38: 297–302, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarthy MV, Spangenburg EE, Booth FW. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cell Mol Life Sci 58: 1150–1158, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings BR, Zanger RC, Novak RF, Lash LH. Cytotoxicity of trichloroethylene and S-(1,2-dichlorovinyl)-l-cysteine in primary cultures of rat renal proximal tubular and distal tubular cells. Toxicology 83–98, 2000. [DOI] [PubMed]
- 12.Dang PM, Cross AR, Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558. Proc Natl Acad Sci USA 98: 3001–3005, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein M Aging and the kidney. J Am Soc Nephrol 7: 1106–1122, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Fardoun RZ, Asghar M, Lokhandwala MF. Role of oxidative stress in renal dopamine D1 receptor G-protein uncoupling and function in old Fischer 344 rats. Am J Physiol Renal Physiol 291: F945–F951, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Fardoun RZ, Asghar M, Lokhandwala M. Role of nuclear factor kappa B (NF-kappaB) in oxidative stress-induced defective dopamine D1 receptor signaling in the renal proximal tubules of Sprague-Dawley rats. Free Radic Biol Med 42: 756–764, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA 99: 3872–3877, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiszt M, Kopp JB, Várnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gellermann GP, Ullrich K, Tannert A, Unger C, Habicht G, Sauter SR, Hortschansky P, Horn U, Möllmann U, Decker M, Lehmann J, Fändrich M. Alzheimer-like plaque formation by human macrophages is reduced by fibrillation inhibitors and lovastatin. J Mol Biol 360: 251–257, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med 228: 134–42, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Jose PA, Eisner GM, Felder RA. Renal dopamine and sodium homeostasis. Curr Hypertens Rep 2: 174–183, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Kruger M, Sachse C, Zimmermann WH, Eschenhagen T, Klede S, Linke WA. Thyroid hormone regulates developmental titin isoform transitions via the phosphatidylinositol-3-kinase/AKT pathway. Circ Res Dec 20 [Epub ahead print], 2007. [DOI] [PubMed]
- 22.Lash LH, Putt DA, Hueni SE, Payton SG, Zwickl J. Interactive toxicity of inorganic mercury and trichloroethylene in rat and human proximal tubules: effects on apoptosis, necrosis, and glutathione status. Toxicol Appl Pharmacol 221: 349–362, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Cheng H, Lederer WJ, Froehlich J, Lakatta EG. Enhanced proliferation and migration and altered cytoskeletal proteins in early passage smooth muscle cells from young and old rat aortic explants. Exp Mol Pathol 64: 1–11, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Marwaha A, Lokhandwala MF. Diminished natriuretic response to dopamine D1 receptor agonist, SKF-38393 in obese Zucker rats. Clin Exp Hypertens 25: 509–515, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA. Dopamine-1 receptor coupling defect in renal proximal tubule cells in essential hypertension. Hypertension 33: 1036–1042, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Schacter E, Williams JA, Lim M, Levine RL. Differential susceptibility of plasma proteins to oxidative modification. Examination by Western blot immunoassay. Free Radic Biol Med 17: 429–437, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Shaddock JG, Chou MW, Casciano DA. Effects of age and caloric restriction on cell proliferation in hepatocyte cultures from control and hepatectomized Fischer 344 rats. Mutagenesis 11: 281–284, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Simão S, Fraga S, Jose PA, Soares-da-Silva P. Oxidative stress and alpha(1)-adrenoceptor-mediated stimulation of the Cl(-)/HCO(3)(-) exchanger in immortalized SHR proximal tubular epithelial cells. Br J Pharmacol 2008. Feb 25 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 29.Spik KW, Boyd RL, Sonntag WE. Effect of aging on GHRF-induced growth hormone release from anterior pituitary cells in primary culture. J Gerontol A Biol Sci Med Sci 46: B72–B77, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Tweedie D, Brossi A, Chen D, Ge YW, Bailey J, Yu QS, Kamal MA, Sambamurti K, Lahiri DK, Greig NH. Neurine, an acetylcholine autolysis product, elevates secreted amyloid-beta protein precursor and amyloid-beta peptide levels, and lowers neuronal cell viability in culture: a role in Alzheimer's disease? J Alzheimers Dis 1: 9–16, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res 97: 829–836, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox CS Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Zemel MB, Sowers JR. Salt sensitivity and systemic hypertension in the elderly. Am J Cardiol 61: 7H–12H, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder AR, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics 19: 233–246, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Zhan CD, Sindhu RK, Vaziri ND. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: reversal by lifelong antioxidant supplementation. Kidney Int 65: 219–227, 2004. [DOI] [PubMed] [Google Scholar]