Abstract
Glucagon receptor (GR) activity and expression are altered in several diseases, including Type 2 diabetes. Previously, we investigated the mechanism of GR desensitization and internalization. The present study focused on the fate of internalized GR. Using both hamster hepatocytes and human embryonic kidney (HEK)-293 cells, we showed that internalized GR recycled to the plasma membrane within 30–60 min following stimulation of the cells with 100 nM glucagon. In HEK-293 cells and during recycling, GR colocalized with Rab4, Rab11, β-arrestin1, β-arrestin2, and actin filaments, in the cytosolic and/or perinuclear domains. Glucagon treatment triggered redistribution of actin filaments from the plasma membrane to the cytosol. GR coimmunoprecipitated with β-actin in both hepatocytes and HEK-293 cells. Downregulation of β-arrestin1 and β-arrestin2 or disruption of the cytoskeleton inhibited recycling, but not internalization of GR. Deletion of the GR carboxyl-terminal 70 amino acids abolished internalization of GR in response to glucagon while deletion of the last 40 amino acids only did not affect GR internalization and recycling. After exposure of the cells to either high concentrations or prolonged duration of glucagon, GR colocalized with lysosomes. GR degradation was inhibited by lysosomal, but not proteosomal, inhibitors. In conclusion, GR recycles through Rab4- and Rab11- positive vesicles. The actin cytoskeleton, β-arrestin1, β-arrestin2, and the receptor's carboxyl terminus are involved in recycling. Prolonged stimulation with glucagon targets GR for degradation in lysosomes. Therefore, the present study provides a better understanding of the GR recycling mechanism, which could become useful in the treatment of certain diseases, including diabetes.
Keywords: actin, G protein-coupled receptor, lysosome, Rab4, Rab11
in type 2 diabetes, the basal level of glucagon is elevated and the insulin-to-glucagon relationship is altered (19, 22, 25), leading to hyperglycemia. Recently, there has been an increased interest in the glucagon receptor (GR) as a target in diabetes management. Understanding the mechanisms of GR desensitization and downregulation may facilitate identifying novel therapeutic targets in diabetes management.
The GR is a prototypical Family B G protein-coupled receptor (GPCR). After exposure to agonists, GPCRs are desensitized and sequestered in the cytosol. Internalized receptors are either recycled to the plasma membrane or targeted for degradation. Recycling of receptors is an important mechanism of resensitization. Rab4 mediates rapid recycling of the transferrin and β2-adrenergic receptors (20, 21), whereas Rab11 mediates slower recycling through perinuclear recycling endosomes (21). β-Arrestins modulate postendocytic sorting of some GPCRs, in particular recycling (16, 18, 29). The receptor's carboxyl (COOH) terminus interacts with the cytoskeleton, which plays an important role in trafficking, signaling, and allosteric regulation of many GPCRs (3). In particular, actin filaments play a role in receptor recycling (6, 9, 30) and its targeting to lysosomes (9, 13, 28) for degradation.
Previously, we and others have shown that glucagon triggers desensitization and internalization of the GR (2, 5, 14). The current study was undertaken to elucidate the pathways of postendocytic sorting of the GR, specifically, receptor recycling and degradation. Using isolated Golden Syrian hamster hepatocytes and/or GR stably transfected human embryonic kidney (HEK)-293 cells, we have investigated 1) the timeline and routes of GR recycling; 2) the roles of the cytoskeleton, β-arrestins, and the GR's COOH terminus in recycling; and 3) the subcellular site of GR degradation.
MATERIALS AND METHODS
Materials
Cell culture media, penicillin/streptomycin, l-glutamine, and amino acids were from Cellgro (Kansas City, MO). Geneticin/G418, cytochalasin D, cyclohexamide, tetracycline, sodium butyrate, DABCO, and di(N-succinimidyl) 3,3′-dithiodipropionate were from Sigma-Aldrich (St. Louis, MO). Glucagon was purchased from Bachem (King of Prussia, PA). 125I-labeled glucagon was purchased from Linco (St. Charles, MO). Phorbol myristate acetate, Mowiol, MG-132, nocodazole, and poly-l-lysine were purchased from Calbiochem (San Diego, CA). Lipofectamine 2000 and OptiMEM were from Invitrogen (Rockville, MD).
Plasmids.
β-Arrestin1 DN (V53D) was kindly provided by Dr. Marc Caron (Duke University, Durham, NC). β-Arrestin2 DN (arr2 319-418) was a generous gift from Dr. Jeffrey L. Benovic (Thomas Jefferson University, Philadelphia, PA). To obtain a fusion protein with a green fluorescent protein (GFP) tag at the COOH terminus, the rat GR gene was inserted into a TOPO-GFP plasmid (Stratagene, La Jolla, CA) (Krilov L, Nguyen A, David M, Miyazaki T, Meng J, Unson CG, and Bouscarel B, unpublished observations).
Antibodies and dyes.
The mouse anti-β-actin antibody, mouse anti-β-tubulin antibody, and anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody were from Sigma-Aldrich. The mouse anti-FLAG M2 antibody was purchased from Stratagene. The rabbit anti-Rab4 was from Upstate (Lake Placid, New York), and the rabbit anti-Rab11 was from Zymed (South San Francisco, CA). The fluorescently labeled secondary antibodies, goat anti-rabbit Alexa Fluor 568, and goat anti-mouse Alexa Fluor 488, as well as Lysotracker Red and Texas Red-phalloidin dyes, were from Molecular Probes (Carlsbad, CA). The HRP-conjugated anti-rabbit antibody was from Amersham Biosciences (Piscataway, NJ).
Cell Culture and Transfection
Hepatocytes were isolated from male Golden Syrian hamsters (100–130 g body wt) using the previously described collagenase perfusion technique (4). All procedures involving animals, including liver cell isolation, have been approved by the Institutional Animal Care and Use Committee at George Washington University. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 200 μM l-glutamine, MEM nonessential and essential amino acids, penicillin (5,000 IU/ml) and streptomycin (5,000 μg/ml). HEK-293 cells stably expressing the rat GR [HEK-GR (12)] were maintained in DMEM supplemented with 10% serum, 200 μM l-glutamine, MEM nonessential and essential amino acids, penicillin (5,000 IU/ml), and streptomycin (5,000 μg/ml). HEK-293 cells stably expressing a tetracycline-inducible FLAG-tagged GR [HEK-Flag-GR (26)] were maintained in DMEM/F-12 (50:50) supplemented with 200 μg/ml geneticin/G418, 10% fetal calf serum, and penicillin-streptomycin. The GR expression was induced in DMEM/F-12 supplemented with 5 mM sodium butyrate, 2 μg/ml tetracycline, 10% serum, penicillin (5,000 IU/ml), and streptomycin (5,000 μg/ml) for 24 h. The cells were transfected with 4–8 μg plasmid DNA using Lipofectamine 2000, following the manufacturer's instructions.
Fluorescence Microscopy
Cells grown on poly-l-lysine-coated coverslips were fixed in 3.7% paraformaldehyde for 10 min and permeabilized by a 15-min incubation with Triton X-100 at 37°C. The cells were then incubated in blocking buffer (8% BSA-PBS) for 1 h. Primary and secondary antibodies were diluted in 2% BSA-PBS buffer and incubated with the cells for 1 h each, respectively. The coverslips were mounted onto glass slides using Mowiol with DABCO. Images were taken with an Olympus IX81 fluorescence microscope. For each frame, four to eight planes were acquired and the projection images were deconvolved using Slidebook 4.1 software (Intelligent Imaging Innovations, Denver, CO).
Recycling Assay
Hepatocytes, HEK-GR, or HEK-Flag-GR cells were serum starved for 1 h and then incubated with 100 nM glucagon for 30 min at 37°C. Glucagon was removed by incubation with cold glycine buffer (pH 3), followed by several washes in binding buffer. To allow for recycling of receptors, the cells were returned to 37°C for the indicated period of time.
Radioligand Binding Assay
Method A.
Hepatocytes in suspension were incubated with 125I-labeled glucagon (∼10 nM) for 2 h at 4°C in binding buffer (DMEM containing 20 mM HEPES, pH 7.4, supplemented with 1 mM PMSF and 2% BSA). Cells were transferred to glass microfiber (GF/B) filters (Whatman) presoaked in 4% BSA and were washed five times with Tris-buffered saline (pH 7.4) to remove unbound 125I-labeled glucagon. Measurements were conducted in triplicate.
Method B.
Internalization and recycling conditions for the HEK-293 cells were similar to those used for the hepatocytes. For GR binding determination, the cells were labeled with 125I-labeled glucagon for 2 h at 4°C in binding buffer (DMEM containing 0.1% BSA and 20 mM HEPES, pH 7.4). The unbound glucagon was washed out with binding buffer, and the cells were lysed in 0.8 M NaOH. Measurements were conducted in quadruplicate. In both methods, high concentrations of unlabeled glucagon (>800 nM) were used to estimate nonspecific binding. All values were corrected for nonspecific binding. Where indicated, the calculated values for internalization were normalized to the ones obtained for control samples (nontreated) and are expressed as percentage different from control, set at 100%.
Role of Cytoskeleton in GR Trafficking
To assess the importance of cytoskeleton in GR internalization, HEK-GR cells were serum starved for 30 min at 37°C, then incubated for another 30 min at 37°C in the presence of either 10 μM nocodazole or 10 μM cytochalasin D to disrupt microtubules or actin filaments, respectively, followed by 30-min treatment with 100 nM glucagon at 37°C. The cells were then washed to remove unbound glucagon, and the number of surface receptors was assessed by binding as described above (method B). To explore the effect of cytoskeletal disruption on GR recycling, HEK-GR cells were serum starved for 60 min at 37°C, then incubated with 100 nM glucagon at 37°C for 30 min. The cells were then washed extensively, nocodazole (10 μM) or cytochalasin D (10 μM) was added on ice, and the cells were then returned to 37°C for 60 min to allow for recycling. Surface GR was measured by binding (method B).
Immunoprecipitation and Western Blot Analysis
HEK-GR cells or hepatocytes were incubated with the cross-linker 1.25 mM di(N-succinimidyl) 3,3′-dithiodipropionate for 30 min at room temperature, centrifuged, and the pellets resuspended in lysis buffer containing 100 mM Tris·HCl, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, and protease inhibitor cocktail (Roche, Palo Alto, CA). For hepatocytes, membrane fractions were separated from cytosolic fractions with ultracentrifugation at 100,000 g for 50 min at 4°C and were then resuspended in membrane isolation buffer (50 mM Tris·HCl, pH 7.4, 2.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 5 mM NaF, 1 mM Na3VO4 and protease inhibitor cocktail). Protein concentration in the lysates was determined by the BCA assay (Pierce, Rockford, IL). Lysates containing 400 μg protein were precleared with 50 μl anti-mouse agarose beads (E-Biosciences, San Diego, CA) for 30 min, centrifuged, and the supernatants incubated with the anti-FLAG (5 μg) or anti-GR (ST-18) antibody overnight at 4°C. The precipitates were incubated with 50 μl agarose beads for 1.5 h. The beads were washed and the pellets were resuspended in 20 μl lysis buffer containing glycoprotein denaturation buffer (New England Biolabs, Ipswich, MA) and were incubated for 30 min at 37°C to inactivate the cross-linking reagent. The proteins were separated on an 8–16% Tris-glycine gradient gel (Invitrogen) and transferred to nitrocellulose Hybond membranes (Amersham Biosciences). The membranes were blocked in 5% milk dissolved in wash buffer containing 25 mM Tris, 150 mM NaCl, and 0.1% Tween 20 for 1 h. The primary and HRP-conjugated secondary antibodies were incubated with the membrane for 1 h, and the bands visualized using Western Lightning ECL detection reagent (PerkinElmer, Boston, MA). The blots were scanned, and densitometric analysis was conducted using ImageQuant software (CGRB Core Laboratories, Amersham Biosciences).
GR Degradation
HEK-GR cells were serum starved for 1 h and were incubated with 25 μM cycloheximide and 100 nM glucagon in the presence or absence of lysosomal inhibitor chloroquine (200 μM, Sigma-Aldrich) and proteosomal inhibitor MG-132 (20 μM) for 2–8 h. At the end of the incubation period, total cell lysates were prepared and immunoblotting was conducted as described above. For live-cell microscopy, HEK-293 cells transfected with GR-GFP were seeded onto glass-bottom dishes (MatTek, Ashland, MA). The cells were incubated with 50 nM Lysotracker Red in serum-free medium for 30 min to label lysosomes, were washed, and were treated with 100 or 1,000 nM glucagon for 1–4 h. Images were taken at 30-min intervals for up to 4 h.
Statistical Analysis
Except as otherwise indicated, the results are expressed as means ± SE. The statistical significance was determined by ANOVA and Bonferroni posttest using GraphPad Prism 4 software (GraphPad Software, San Diego, CA). P < 0.05 was considered significant.
RESULTS
GR Recycles to the Plasma Membrane in a β-Arrestin-Dependent Manner
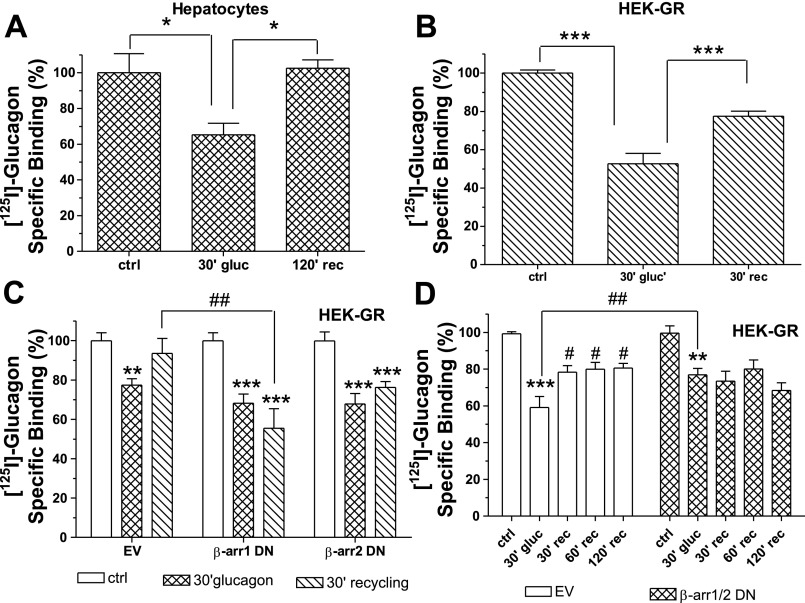
Radioligand binding assays were performed to investigate the fate of the internalized GR. In both hamster hepatocytes (Fig. 1A) and GR stably transfected HEK-293 (HEK-GR) cells (Fig. 1B), 30–40% of receptors internalized after 30-min treatment with 100 nM glucagon. In transfected HEK-293 cells, ∼70% of internalized receptors were recovered on the cell surface 30 min after the removal of glucagon (Fig. 1B) and nearly 100% after 60 min (data not shown), while 120 min were necessary to observe full recovery of GRs on the cell surface of hepatocytes (Fig. 1A). Previously, we have shown that treatment of HEK-293-GR with glucagon induced translocation of β-arrestins to the perinuclear region in living cells and that GRs colocalized with both β-arrestin1 and β-arrestin2. Overexpression or downregulation of either β-arrestin1 or β-arrestin2 did not significantly affect GR internalization (Krilov L, Nguyen A, David M, Miyazaki T, Meng J, Unson CG, and Bouscarel B, unpublished observations). To investigate the role of β-arrestins in recycling, HEK-GR cells were transfected with β-arrestin1 and β-arrestin2 dominant-negative (DN) mutants. Downregulation of at least β-arrestin1 significantly reduced GR recycling, and a trend is observed with β-arrestin2 DN (Fig. 1C). To verify whether this was a complete block or merely a delay of recycling, a time-course experiment was conducted in HEK-GR cells cotransfected with DN β-arrestin1 and DN β-arrestin2 mutants. While in control cells (HEK-GR transfected with an empty vector), the GR continued to recycle to the plasma membrane over time, reaching a plateau at 60 min, in the presence of DN β-arrestins, GR recycling was inhibited even after 120 min (Fig. 1D).
Fig. 1.
Glucagon receptor (GR) recycles to the plasma membrane in a β-arrestin-dependent manner: A: hamster hepatocytes were incubated without (control, ctrl) and with 100 nM glucagon (gluc) for 30 min (30′) at 37°C, washed, and then incubated or not at 37° for 120 min recycling (rec). GR expression on the cell surface was determined by radioligand binding (method A). The results shown are means ± SE of 4 independent experiments. *Significantly different: P < 0.05. B: human embryonic kidney (HEK)-293 cells transfected with GR (HEK-GR) were incubated without (ctrl) and with 100 nM glucagon for 30 min at 37°C. For recycling, the cells were washed and then returned to 37°C for 30 min. GR expression on the cell surface was determined by radioligand binding (method B). The results shown are means ± SE of 4 independent experiments. ***Significantly different: P < 0.001. C: HEK-GR cells were transfected with β-arrestin1 (β-arr1) and β-arrestin2 (β-arr2) dominant-negative (DN) mutants or with an empty vector (EV). Recycling was assessed as described in B. Data shown represent means ± SE of 3 independent experiments. **Significantly different from control: P < 0.01; ***significantly different from control: P < 0.001; ##significantly different: P < 0.01. D: HEK-GR cells were transiently cotransfected with both β-arrestin1 and β-arrestin2 DN mutants or empty vector. After 30-min treatment with 100 nM glucagon at 37°C, the cells were washed and incubated up to 120 min at 37°C. The cell surface GR expression level was determined by radioligand binding (method B). Data represent means ± SE of 4 independent experiments. **Significantly different from control: P < 0.01; ***significantly different from control: P < 0.001; #significantly different from 30-min glucagon; ##significantly different: P < 0.05. The data were analyzed by one-way ANOVA and Bonferroni posttest using Prism software (GraphPad Software, San Diego, CA).
Carboxyl Terminus Is Required for GR Internalization and Recycling
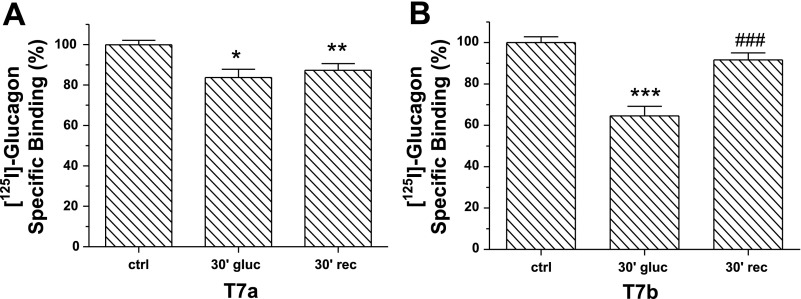
Human GR truncated at the COOH terminus has a significantly reduced internalization rate compared with its wild-type counterpart (5). The role of the rat GR COOH terminus in receptor internalization and trafficking has not been studied thus far. HEK-293 cells were transfected with GR truncation mutants T7a and T7b lacking the last 70 and 42 COOH-terminal amino acids, respectively (27). Control cells were transfected with wild-type GR. Treatment with glucagon for 30 min caused a small (20%) but significant decrease in the number of T7a receptors at the cell surface (Fig. 2A), Under the same conditions, ∼40% of T7b receptors were internalized (Fig. 2B). Furthermore, at 30 min after removal of glucagon, only T7b receptors significantly recycled to the plasma membrane (Fig. 2B) and to the same extent as the wild-type counterpart (Fig. 1B).
Fig. 2.
Role of the COOH terminus in GR internalization and recycling: HEK-293T cells transfected with T7a (A) or T7b (B) containing plasmids were incubated in the absence (control) and presence of 100 nM glucagon for 30 min at 37°C. For recycling, the cells were washed and placed at 37°C for an additional 30 min. Surface GR was determined by radioligand binding assay (method B). The results shown are means ± SE of 3–4 independent experiments. The data were analyzed by one-way ANOVA and Bonferroni posttest. *,**,***Significantly different from respective control: P < 0.05, P < 0.01, and P < 0.001, respectively. ###Significantly different from 30-min glucagon: P < 0.001.
Cytoskeleton Is Involved in GR Trafficking
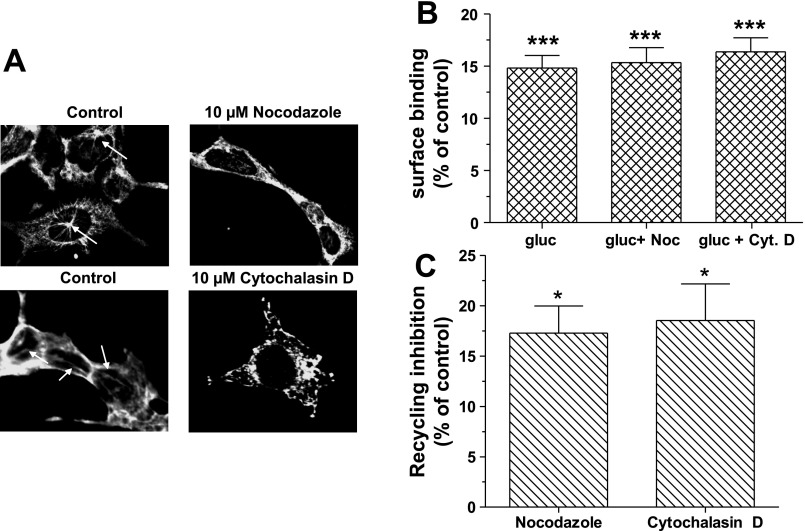
Recent studies suggest that actin acts as a spatial organizer of endocytic hot spots and as a carrier of endocytic vesicles (8). To investigate the possible interaction of GR with actin filaments, HEK-Flag-GR cells were stained with the F-actin-specific label phalloidin (Fig. 3A). In the absence of glucagon (control), GR and F-actin were colocalized at the plasma membrane (Fig. 3A, merged). As early as 5 min after glucagon (100 nM) treatment, the distribution of F-actin at the plasma membrane became discontinuous and punctuate. GR translocated from the plasma membrane to the cytosol and at 15 and 30 min after treatment, colocalized with F-actin (Fig. 3A, merged). In HEK-Flag-GR total cell lysate, GR coprecipitated with β-actin, a major constituent of actin filaments, in control cells and in cells treated with 100 nM glucagon for 10 min. At 20 min of glucagon treatment, the association of β-actin and GR was not detectable (Fig. 3B). Likewise, a time-dependent association of GR and β-actin was noted in hepatocytes when GR was immunoprecipitated from the plasma membrane (Fig. 3C). To further investigate the involvement of the cytoskeleton in GR trafficking, actin filaments and microtubules were pharmacologically disrupted. As shown in Fig. 4A, 30-min treatment with 10 μM nocodazole and 10 μM cytochalasin D disrupted microtubule organizing centers (arrows) and actin filaments (arrows), respectively. While disruption of actin filaments or microtubules did not affect GR internalization (Fig. 4B), it significantly reduced GR recycling by up to 20% (Fig. 4C).
Fig. 3.
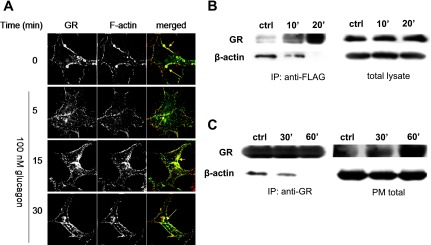
Actin cytoskeleton is involved in GR trafficking. A: HEK-Flag-GR cells were treated with 100 nM glucagon for the indicated period of time. GR was labeled with anti-FLAG antibody (green), and F-actin was labeled with Texas Red-phalloidin (red). The images shown are representative of 3 independent experiments. B and C: HEK-Flag-GR cells and hamster hepatocytes, respectively, were treated with 100 nM glucagon for the indicated time. Total Flag-GR (B) and plasma membrane GR (PM; C) were immunoprecipitated (IP) using anti-FLAG antibody and anti-GR antibody (ST-18), respectively, followed by immunoblot analysis of GR and β-actin as described in materials and methods. The images shown are representative of 3 independent experiments.
Fig. 4.
Role of cytoskeleton in GR internalization and recycling. A: HEK-GR cells were treated with either 10 μM nocodazole or 10 μM cytochalasin D for 30 min at 37°C. The cells were permeabilized and immunostained with either the mouse anti-β-tubulin antibody or the mouse anti-β-actin antibody. The arrows indicate starlike structures of microtubules (top) and actin filaments (bottom). The images shown are representative of 3 independent experiments: B: HEK-GR cells were treated with either 10 μM nocodazole (Noc) or 10 μM cytochalasin D (Cyt D) for 30 min at 37°C, followed by 30-min incubation in the absence (control) and presence of 100 nM glucagon at 37°C. Surface binding was determined by radioligand binding assay (method B). The data shown are means ± SE of 3 independent experiments. C: after glucagon treatment, the cells were washed and incubated without (control) and with either 10 μM nocodazole or 10 μM cytochalasin D and returned to 37°C for 30 min. Surface binding was determined by radioligand binding assay (method B). Data represent means ± SE of 3 independent experiments. Data were analyzed by one-way ANOVA and Bonferroni posttest. *Significantly different from control: P < 0.05. ***Significantly different from control: P < 0.001.
GR Colocalizes With Rab4 and Rab11
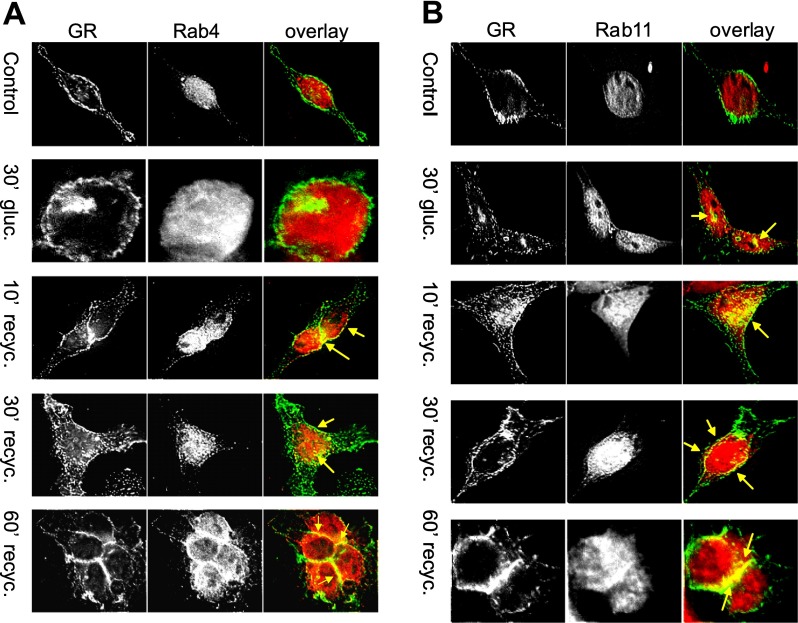
To elucidate the recycling route used by the GR, HEK-Flag-GR cells were immunostained with anti-Rab4 and anti-Rab11 antibodies (Fig. 5, A and B). In control cells, GR was localized predominantly at the plasma membrane, whereas Rab4 and Rab11 were distributed uniformly throughout the cytosol (Fig. 5, A and B, control overlay). After 30 min of treatment with 100 nM glucagon, GR accumulated inside the cell and colocalized with Rab11 (arrows, Fig. 5B) but not with Rab4 (Fig. 5A). At 10 min of recycling, GR colocalized with both Rab4 and Rab11 in the cytosol. As recycling progressed, Rab4 and Rab11 translocated toward the plasma membrane. At 30 and 60 min of recycling, colocalization of GR with Rab4 and Rab11 was predominantly observed in the perimembrane region, at cell-cell junctions, and at the plasma membrane (Fig. 5, A and B).
Fig. 5.
GR colocalizes with Rab4 (A) and Rab11 (B). HEK-Flag-GR cells were treated with 100 nM glucagon for 30 min, washed, further incubated for up to 60 min at 37°C, fixed, and permeabilized. GR was immunostained with anti-FLAG antibody (green), anti-Rab4 (red), or anti-Rab11 (red). Images shown are representative of 3 independent experiments. Recyc, recycling.
GR Is Degraded in Lysosomes
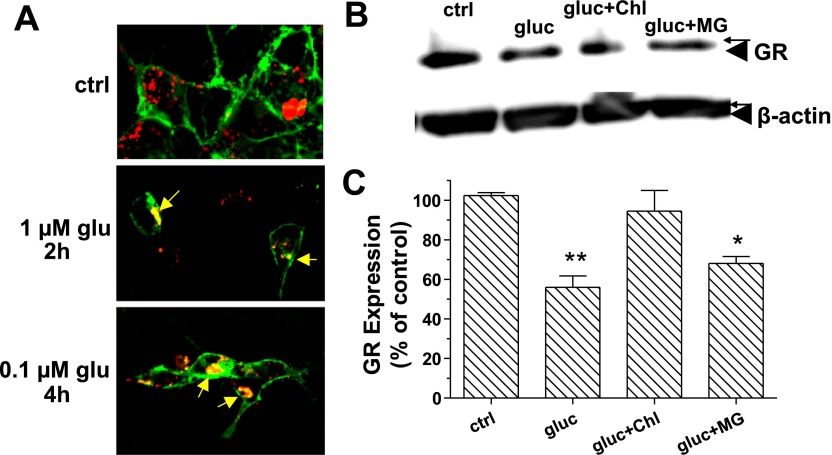
Prolonged treatment of cells with specific agonist frequently results in degradation of the respective receptors. In a preliminary binding study (data not shown), it was determined that in HEK-GR cells, glucagon triggered GR degradation as early as 3 h after treatment, and the level of GR remained stable for up to 8 h. Lysosomes were labeled with Lysotracker Red in live HEK-293 cells transfected with GR-GFP (Fig. 6A). Upon treatment with 1 μM glucagon for 2 h or with 0.1 μM glucagon for 4 h, we observed colocalization of GR and lysosomes (Fig. 6A, 2 h and 4 h). No colocalization was noted in the absence of glucagon (control). Western blot analysis of the total cell lysate (HEK-GR cells) was conducted to quantify the amount of GR in cells. To prevent de novo protein synthesis, the cells were pretreated with 25 μM cyclohexamide for 1 h before incubation of the cells with 100 nM glucagon for 5 h in serum-free media (Fig. 6, B and C). Densitometric analysis indicated that 5-h glucagon treatment reduced the amount of GR by ∼40%, in the presence and absence of 20 μM proteosomal inhibitor MG-132. In contrast, in the presence of 200 μM lysosomal inhibitor chloroquine, the GR expression level was similar to that of control (Fig. 6, B and C). These results indicate that prolonged treatment with glucagon targets GRs to degradation at least partially in lysosomes.
Fig. 6.
GR is degraded in lysosomes. A: HEK-293 cells were transfected with GR-green fluorescent protein. Lysosomes were labeled with Lysotracker Red as described in materials and methods and treated with the indicated concentration of glucagon (glu) for up to 4 h. Images were taken every 30 min. Representative images from 5–7 independent experiments are shown. B: HEK-GR cells were serum starved for 30 min and then treated with 25 μM cyclohexamide, without and with either 20 μM MG-132 (MG) or 200 μM chloroquine (Chl) for 30 min at 37°C, followed by 100 nM glucagon for 5 h at 37°C. Total lysates were prepared, and Western blot analysis was conducted as described in materials and methods. The image shown is representative of 3 independent experiments. C: protein levels for the anti-GR and anti-β-actin bands were determined by densitometric analysis using STORM phosphoimager and ImageQuant software. The amount of GR protein was normalized to the amount of β-actin in each lane, and the obtained values are expressed as percentage of control (nontreated cells). Data shown are means ± SE of 3 independent experiments. The data were analyzed by one-way ANOVA and Bonferroni posttest. *Significantly different from control: P < 0.05. **Significantly different from control: P < 0.01.
DISCUSSION
Results of the present study show that internalized GR recycles to the plasma membrane in both hepatocytes and transfected HEK-293 cells. We noted that expression of β-arrestin1 and β-arrestin2 DN mutants diminishes recycling of the GR. This is in agreement with the study of Vines et al. (29), who demonstrated that β-arrestins are required for recycling of the N-formyl peptide receptor. In contrast, other studies have suggested that stable interactions with β-arrestins abrogate receptor GPCR recycling (17).
The COOH terminus plays a critical role in desensitization of many GPCRs and mediates postendocytic sorting (24). The expression and function of the rat GR truncation mutants T7a and T7b are similar to their wild-type counterpart in terms of affinity for glucagon and ability to stimulate cAMP production (27). Here we show that the T7a mutant, which lacks the entire COOH terminus (last 70 amino acids), leads to a significant although reduced GR internalization but prevents GR recycling. Under the same conditions, the GR T7b mutant, which lacks approximately half of the COOH terminus (last 40 amino acids), internalized and recycled to the same level as the wild-type counterpart. It is of interest to mention that the human GR lacking the last 56 or 62 amino acids of the COOH terminus had reduced receptor internalization compared with its wild-type counterpart, while deletion of only the last 24 amino acids of the COOH terminus did not have a great impact on receptor internalization (5). Mutation of seven serine residues to alanine in the COOH terminus of the human GR significantly reduced GR internalization (5), suggesting that phosphorylation plays a critical role in ligand-induced GR internalization. On the basis of the amino acid sequence of the rat GR carboxyl terminus (27), results of the present study are suggesting a maximum of three serines and one threonine to be important in the rat GR internalization. Therefore, at least part of the GR COOH terminus plays a critical role in receptor internalization. Clearly, further studies are required to identify the serine(s) and/or threonine(s) that are phosphorylated and therefore responsible for rat GR internalization induced by glucagon, as well as the functional domains of the COOH terminus involved in recycling and interaction with the cytoskeleton. However, dephosphorylation of GR is not required for recycling (data not shown), as was also observed with the related parathyroid hormone receptor (7).
Actin filaments and microtubules are established regulators of protein endocytosis and trafficking (1), and their importance in GPCR signal transduction has been addressed recently (10, 11). While, under the conditions tested, disruption of the actin cytoskeleton and microtubules with cytochalasin D and nocodazole, respectively, did not impair GR internalization, it significantly but modestly reduced GR recycling. We also observed that GR colocalized with actin filaments and coprecipitated with β-actin in a time-dependent manner, suggesting a dynamic correlation with receptor trafficking. β-Actin coprecipitated with endogenous GR in hepatocytes as well. The observed reorganization of actin filaments by glucagon may be mediated by effectors of GR signaling known to bind F-actin, such as G protein-coupled receptor kinase, Gs, and PKC. The observed inhibition of recycling by actin depolymerization is in agreement with what was noted for the chemokine receptors CXCR1 and CXCR2 (30) and the β2-adrenergic receptor (6). In summary, our results suggest a possible involvement of both actin filaments and microtubules in GR recycling.
The GTPases Rab4 and Rab11 are present in early endosomes, as well as in recycling endosomes (23, 31). Rab4 regulates rapid recycling of proteins from Rab4/Rab5-positive early endosomes, whereas Rab11 controls slow recycling from perinuclear and pericentriolar recycling endosomes (reviewed in Ref. 21). We found that GR colocalizes both with Rab4 and Rab11, suggesting simultaneous usage of multiple recycling pathways, as proposed for the β2-adrenergic receptors (15).
The molecular mechanisms of postendocytic sorting of GPCRs are still poorly understood. Our results indicate that short-term stimulation with glucagon (30 min) results in GR internalization followed by rapid recycling, whereas long-term glucagon treatment (5 h) targets GRs for degradation at least in part in lysosomes.
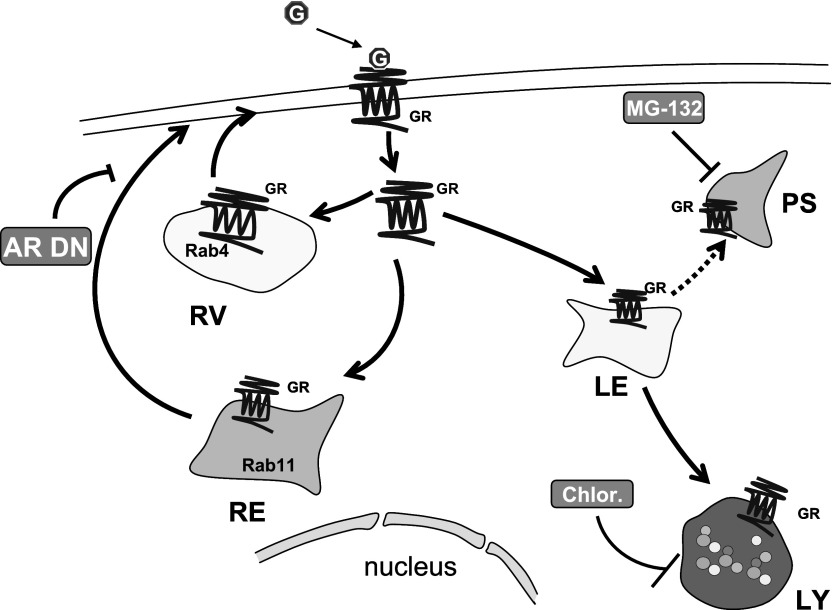
In conclusion, the results of the present study allow us to propose a model of GR trafficking in HEK-293 cells (Fig. 7). Internalized GRs are either recycled to the plasma membrane via Rab4- and Rab11-positive vesicles or targeted for degradation in lysosomes. Recycling is independent of receptor dephosphorylation, but it is diminished by cytoskeleton disruption and downregulation of β-arrestins. Furthermore, the receptor's COOH terminus is required for its internalization and recycling.
Fig. 7.
Schematic representation of GR trafficking: Upon binding glucagon (G), the GR is internalized. After short-term stimulation with glucagon, the GR recycles to the plasma membrane through Rab4- and Rab11-positive vesicles in a β-arrestin (AR)-dependent manner. Prolonged stimulation directs GR to degradation in lysosomes (LY) and possibly proteosomes (PS). RV, recycling vesicle; RE, recycling endosome; Chlor, chloroquine; LE, late endosome.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-56108.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Apodaca G Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2: 149–159, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Authier F, Desbuquois B, De Galle B. Ligand-mediated internalization of glucagon receptors in intact rat liver. Endocrinology 131: 447–457, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Bockaert J, Marin P, Dumuis A, Fagni L. The ‘magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett 546: 65–72, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bouscarel B, Gettys TW, Fromm H, Dubner H. Ursodeoxycholic acid inhibits glucagon-induced cAMP formation in hamster hepatocytes: a role for PKC. Am J Physiol Gastrointest Liver Physiol 268: G300–G310, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Buggy JJ, Heurich RO, MacDougall M, Kelley KA, Livingston JN, Yoo-Warren H, Rossomando AJ. Role of the glucagon receptor COOH-terminal domain in glucagon-mediated signaling and receptor internalization. Diabetes 46: 1400–1405, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401: 286–290, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Chauvin S, Bencsik M, Bambino T, Nissenson RA. Parathyroid hormone receptor recycling: role of receptor dephosphorylation and beta-arrestin. Mol Endocrinol 16: 2720–2732, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 422: 37–44, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Durrbach A, Raposo G, Tenza D, Louvard D, Coudrier E. Truncated brush border myosin I affects membrane traffic in polarized epithelial cells. Traffic 1: 411–424, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Gundersen GG Evolutionary conservation of microtubule-capture mechanisms. Nat Rev Mol Cell Biol 3: 296–304, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281: 26391–26399, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Ikegami T, Cypess AM, Bouscarel B. Modulation of glucagon receptor expression and response in transfected human embryonic kidney cells. Am J Physiol Cell Physiol 281: C1396–C1402, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Kyle ME, Nakae D, Sakaida I, Miccadei S, Farber JL. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem 263: 3784–3789, 1988. [PubMed] [Google Scholar]
- 14.Merlen C, Fabrega S, Desbuquois B, Unson CG, Authier F. Glucagon-mediated internalization of serine-phosphorylated glucagon receptor and Gsalpha in rat liver. FEBS Lett 580: 5697–5704, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Moore RH, Tuffaha A, Millman EE, Dai W, Hall HS, Dickey BF, Knoll BJ. Agonist-induced sorting of human beta2-adrenergic receptors to lysosomes during downregulation. J Cell Sci 112: 329–338, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 274: 32248–32257, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem 276: 19452–19460, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275: 17201–17210, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 64: 106–110, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Seachrist JL, Anborgh PH, Ferguson SS. beta 2-Adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem 275: 27221–27228, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Seachrist JL, Ferguson SS. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74: 225–235, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85: 4053–4059, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149: 901–914, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsao P, Cao T, von Zastrow M. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol Sci 22: 91–96, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Unger RH Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism 27: 1691–1709, 1978. [DOI] [PubMed] [Google Scholar]
- 26.Unson CG Expression of glucagon receptors in tetracycline-inducible HEK293S GnTI-stable cell lines: an approach toward purification of receptor protein for structural studies. Biopolymers 90: 287–296, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Unson CG, Cypess AM, Kim HN, Goldsmith PK, Carruthers CJ, Merrifield RB, Sakmar TP. Characterization of deletion and truncation mutants of the rat glucagon receptor. Seven transmembrane segments are necessary for receptor transport to the plasma membrane and glucagon binding. J Biol Chem 270: 27720–27727, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Van Deurs B, Holm PK, Kayser L, Sandvig K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosomes and preexisting lysosomes. Eur J Cell Biol 66: 309–323, 1995. [PubMed] [Google Scholar]
- 29.Vines CM, Revankar CM, Maestas DC, LaRusch LL, Cimino DF, Kohout TA, Lefkowitz RJ, Prossnitz ER. N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J Biol Chem 278: 41581–41584, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Zaslaver A, Feniger-Barish R, Ben Baruch A. Actin filaments are involved in the regulation of trafficking of two closely related chemokine receptors, CXCR1 and CXCR2. J Immunol 166: 1272–1284, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117, 2001. [DOI] [PubMed] [Google Scholar]