Abstract
Cytochrome P-450 (CYP) epoxygenases metabolize arachidonic acid to epoxyeicosatrienoic acid (EET) regioisomers, which activate several signaling pathways to promote endothelial cell proliferation, migration, and angiogenesis. Since vascular endothelial growth factor (VEGF) plays a key role in angiogenesis, we assessed a possible role of EETs in the VEGF-activated signal transduction cascade. Stimulation with VEGF increased CYP2C promoter activity in endothelial cells and enhanced CYP2C8 mRNA and protein expression resulting in increased intracellular EET levels. VEGF-induced endothelial cell tube formation was inhibited by the EET antagonist 14,15-epoxyeicosa-5(Z)-enoicacid (14,15-EEZE), which did not affect the VEGF-induced phosphorylation of its receptor or basic fibroblast growth factor (bFGF)-stimulated tube formation. Moreover, VEGF-stimulated endothelial cell sprouting in a modified spheroid assay was reduced by CYP2C antisense oligonucleotides. Mechanistically, VEGF stimulated the phosphorylation of the AMP-activated protein kinase (AMPK), which has also been linked to CYP induction, and the overexpression of a constitutively active AMPK mutant increased CYP2C expression. On the other hand, a dominant-negative AMPK mutant prevented the VEGF-induced increase in CYP2C RNA and protein expression in human endothelial cells. In vivo (Matrigel plug assay) in mice, endothelial cells were recruited into VEGF-impregnated plugs; an effect that was sensitive to 14,15-EEZE and the inclusion of small interfering RNA directed against the AMPK. The EET antagonist did not affect responses observed in plugs containing bFGF. Taken together, our data indicate that CYP2C-derived EETs participate as second messengers in the angiogenic response initiated by VEGF and that preventing the increase in CYP expression curtails the angiogenic response to VEGF.
Keywords: cytochrome P-450, matrigel plug assay
epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acid generated by cytochrome P-450 (CYP) epoxygenases. The majority of CYP enzymes are primarily expressed in the liver, but several CYP enzymes can be detected in the cardiovascular system. Of those reported to date, most is known about the cardiovascular actions of proteins belonging to the CYP4A, 2C, and 2J families. CYP4A enzymes generate the potent vasoconstrictor 20-hydroxyeicosatetraenoic acid (20-HETE), which is implicated in the regulation of myogenic tone. The 2C and 2J epoxygenases, on the other hand, generate EETs, although some enzymes can also generate 20-HETE (for review see Ref. 39).
The realization that EETs, in particular 11,12- and 14,15-EET, can activate large conductance Ca2+-activated K+ channels on vascular smooth muscle cells to elicit hyperpolarization and relaxation led to their identification as a class of “endothelium-derived hyperpolarizing factor or EDHF” (2, 13). However, although CYP epoxygenase activation and EET production is generally associated with vasodilatation, the different EETs exert a variety of membrane potential-independent effects and are now recognized as intracellular signaling molecules and have been attributed with anti-inflammatory, fibrinolytic and anti-apoptotic properties (for review see Ref 31). One of the more recently explored functions of these enzymes is their ability to promote endothelial cell proliferation (4, 16, 28, 43) as well as endothelial cell migration and degradation of the extracellular matrix (29). The mechanisms underlying these actions of the EETs have not been completely resolved but are reported to involve activation of several signaling pathways, including activation of tyrosine kinases and phosphatases (16, 22), activation of mitogen-activated protein (MAP) kinase phosphatases, and inhibition of the c-Jun NH2-terminal kinase (35) as well as transactivation of the epidermal growth factor receptor (6, 30).
The endogenous expression of CYP epoxygenases can be modulated by different stimuli, in particular, hemodynamic and physiochemical forces are thought essential to maintain CYP2C expression in endothelial cells in situ (14). Hypoxia is also an effective inducer of CYP2C8/9 expression, a mechanism that contributes to hypoxia-induced angiogenesis in human endothelial cells (29). However, nothing is known about the involvement of CYP2C epoxygenases in the signaling pathways activated by other growth factors.
The vascular endothelial growth factor (VEGF) is a key regulator of physiological and pathological angiogenesis. In vitro, VEGF induces endothelial cell proliferation and migration and is a survival factor for endothelial cells (for review see Ref. 3). Since the expression of VEGF is dependent on hypoxia (27), we postulated a link between VEGF- and CYP-epoxygenase signaling. Therefore, in the present study we assessed the effects of VEGF on CYP2C epoxygenase expression and activity in human endothelial cells. In addition, we determined the effects of interfering with CYP activity or EET levels on VEGF-induced angiogenesis in vivo.
METHODS
Materials
11,12-EET was purchased from Cayman Chemicals (Massy, France), and growth factor-reduced basement membrane matrix (Matrigel) was from BD Biosciences (San Jose, CA). The CYP2C8 antibody was from Acris (Hiddenhausen, Germany) and CYP2C8-containing bacterial supersomes (Gentest, Woburn, MA) were used as a positive control for imunoblotting. 14,15-Epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) and N-methylsulfonyl-6-(2-propargyloxyphenyl)-hexanamide (MS-PPOH) were synthesized as described (20). Sulfaphenazole, the antibody recognizing β-actin, and all other chemicals were from Sigma (Taufkirchen, Germany).
Cell Culture
Human umbilical vein endothelial cells (30) and murine lung endothelial cells (15) were isolated and cultured as described. The porcine aortic endothelial cell lines overexpressing VEGFR1 and VEGFR2 were kindly provided by Dr. J. Waltenberger (Maastricht, The Netherlands). The investigation conforms with the principles outlined in the Declaration of Helsinki (Cardiovasc Res 35: 2-41997). In some experiments subconfluent human endothelial cells were infected with adenoviruses to overexpress constitutively active AMP-activated protein kinase (AMPK) or dominant-negative AMPK as reported (10).
Immunoprecipitation and Western Blotting
For immunoprecipitation human endothelial cells were lysed in Triton X-100, left on ice for 10 min, and centrifuged at 10,000 g for 10 min. After being precleared with protein A/G Sepharose, proteins were immunoprecipitated from the cell supernatant with phosphotyrosine antibody (Santa Cruz Biotechnology, Heidelberg, Germany). For Western blot analysis, cells were lysed in Triton X-100 lysis buffer and separated by SDS-PAGE as described (30).
Reporter Gene Assay
Endothelial cells overexpressing either VEGFR1 or VEGFR2 were transiently cotransfected with the noncoding 5′ region of CYP2C9 (−2,088 to +21; kindly provided by Dr. P. Maurel, Montpellier, France), subcloned into pGL3basic (Promega, Mannheim, Germany) and a plasmid containing lacZ under the control of the cytomegalovirus (CMV) promoter. After 12 h, the cells were treated with either solvent (PBS, 0.6%) or VEGF (30 ng/ml) for 6 h. Thereafter, luciferase (Promega, Mannheim, Germany) and β-galactosidase (Tropix, Bedford, MA) activity was assayed according to the manufacturers’ protocols. Promoter activity was quantified as luciferase activity relative to that of β-galactosidase and normalized to the respective nontreated controls.
Isolation of RNA and RT-qPCR
Total RNA was isolated from cultured endothelial cells using phenol and guanidine isothiocyanate (TriReagenz, Sigma, Germany). Random hexanucleotide primers were used for reverse transcription of equal amounts of RNA. The cDNA was used for real-time PCR using Taqman probes for the detection of the specific amplification products. For the detection of CYP2C8 mRNA by real-time RT-PCR the oligonucleotides used were the following: 2C8 forward: 5′-GGACTTTATCGATTGCTTCCTG-3′, reverse: 5′-CCATATCTCAGAGTGGTGCTTG-3′; FAM- and dabcyl-labeled taqman probe: 5′-TTGGCACTGTAGCTGATCTATTTGTTGCTGGA-3′ corresponding to base pair positions 878 to 899 (forward); 1000 to 1021 (reverse) and 958 to 989 (taqman probe) in the CYP2C8 cDNA (NCBI accession no.: NM_000770). RNA polymerase2 (RNA Pol2) was amplified by qRT-PCR with the primers: forward: 5′-GCACCACGTCCAATGACAT-3′, reverse: 5′-GTGCGGCTGCTTCCATAA-3′, Hex and dabcyl-labeled Taqman probe: 5′-TACCACGTCATCTCCTTTGATGGCTCCTAT-3′ corresponding to base pair positions 4301 to 4319 (forward), 4550 to 4567 (reverse) and 4381 to 4404 in RNA Pol2 cDNA (NCBI accession number NM_000937). The amount of cDNA in the samples was calculated on the basis of the amplification of a serial dilution of a plasmid (CYP2C8) or the serial dilution of the cDNA (RNA Pol2). The CYP2C8 levels were normalized to that of Pol2. At least two RT reactions were performed using each RNA preparation and at least two PCR reactions were performed with each cDNA sample.
Antisense Oligonucleotides
In some experiments, an antisense oligonucleotide approach was used to prevent the VEGF-induced upregulation of CYP2C, as described (32). Cells were treated with CYP2C sense and antisense oligonucleotides (2 μmol/l; antisense: 5′-TCCATTGAAGCCTTCTCTTCTT-3′; sense: 5′-AAGAAGAGAAGGCTTCAATGGA-3′; in both cases the three 5′ nucleotides were modified with phosphothioate; MWG-Biotech, Ebersberg, Germany) using GeneTrans II according to manufacturer's protocol (MobiTec, Göttingen, Germany). The sequence of these oligonucleotides spans the ATG and is 100% identical with human CYP2C8 and contains one mismatch to the other three human CYP2C isoforms.
Downregulation (siRNA) of the AMPK
To downregulate the AMPK α-subunit in human and murine endothelial cells specific small interfering RNAs (siRNAs) were generated (Eurogentec, Searing, Belgium). The sequence used to target the human isoform was CCAAGUGGAUAGUAGAACU-dTdT, whereas a mixture or two siRNAs were used to target the murine isoform (GGACCCAUCUUAUAGUUCA-dTdT and GGUCAUCAGUACACCAUCU-dTdT; only the coding strand of the duplex is indicated). For siRNA transfection, Gene trans II was used according to the manufacturers protocol. For the Matrigel plug assay, equal amounts (2 μmol/l) of both murine siRNAs were added to Matrigel just before implantation (see below).
Liquid Chromatography-Mass Spectrometry Measurements
Human endothelial cells, treated as described in results and harvested by scraping, and the pellets (from ∼8 × 106 cells) were suspended in 100 μl potassium phosphate buffer (0.1 mol/l, pH 7.2), hydrolyzed for 1 h in NaOH (0.5 N), and neutralized with HCl (2 mol/l) before deuterated internal standards (5-HETE-d8, 12-HETE-d8, 15-HETE-d8, 20-HETE-d6, 8,9-EET-d8, 11,12-EET-d8, and 14,15-EET-d8) were added. A liquid-liquid extraction was performed twice using ethyl acetate (0.5 ml). After evaporation of the solvent in a vacuum block under a gentle stream of nitrogen, samples were reconstituted with 50 μl of methanol-water (1:1, vol/vol) and eicosanoids were determined with a Sciex API4000 mass spectrometer operating in the multiple reaction monitoring mode. Chromatographic separation was performed on a Gemini C18 column (150 × 2 mm ID, 5-μm particle size, Phenomenex, Aschaffenburg, Germany).
In Vitro Angiogenesis Assays
Tube formation.
Primary cultures of endothelial cells were seeded on cell culture dishes coated with fibronectin and cultured in MCDB 133 medium containing 4% FCS and either solvent or VEGF (30 ng/ml). After 48 h, angiogenesis was quantified in three randomly chosen fields of view by measuring tube length with a computer-assisted microscope.
Endothelial cell spheroids.
Spheroids containing 400 cells were generated as described (26). After 24 h in the collagen gel, angiogenesis was quantified by measuring the cumulative length of all capillary like sprouts originating from the central plain of an individual spheroid using a computer assisted microscope. At least five spheroids per experimental group and experiment were analyzed.
Matrigel plug assay.
Female C57BL/6 mice (8 wk old) were lightly anesthetized with chloralhydrate (200 μl of a 4% solution injected subcutaneously) and then injected subcutaneously with 0.5 ml of Matrigel impregnated with heparin (0.0025 U/ml), VEGF (150 ng/ml), bFGF (150 ng/ml), EEZE (100 μmol/l), or combinations thereof, along the dorsal midline on each site of the spine. Fourteen days later the mice were euthanized, the Matrigel plugs were removed, embedded in tissue tech (Sakura Finetec), and frozen. Plugs were sectioned (10 μm) by cryosection and processed for staining for CD31 (BD Biosciences, San Jose, CA) or α-smooth muscle actin (Sigma). Afterwards preparations were mounted and viewed using a confocal microscope (LSM 510 META, Zeiss). Vessel formation was quantified by analyzing at least five five sections per plug. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996), assurance number A5626-01. The angiogenic response was graded by three observers who were blind to the experimental conditions. The vessel formation index scores were as follows: 0, no effect, no invaded cells; 1, few invaded endothelial cells; 2, clear invasion of endothelial cells; 3, clear invasion of endothelial cells and capillary formation; and 4, endothelial and smooth muscle cell invasion, clear vessel formation. To facilitate comparison between the different groups the scores were normalized with respect to the effects observed in the control group.
Statistics
Data are expressed as means ± SE. Statistical analysis of Matrigel plugs analyzed by ultrasound was performed using a paired t-test. For all other statistical comparisons evaluation was performed with Student's t-test for unpaired data or one-way ANOVA followed by a Bonferroni t-test. Values of P < 0.05 were considered statistically significant.
RESULTS
Effect of VEGF on CYP2C Expression and Activity
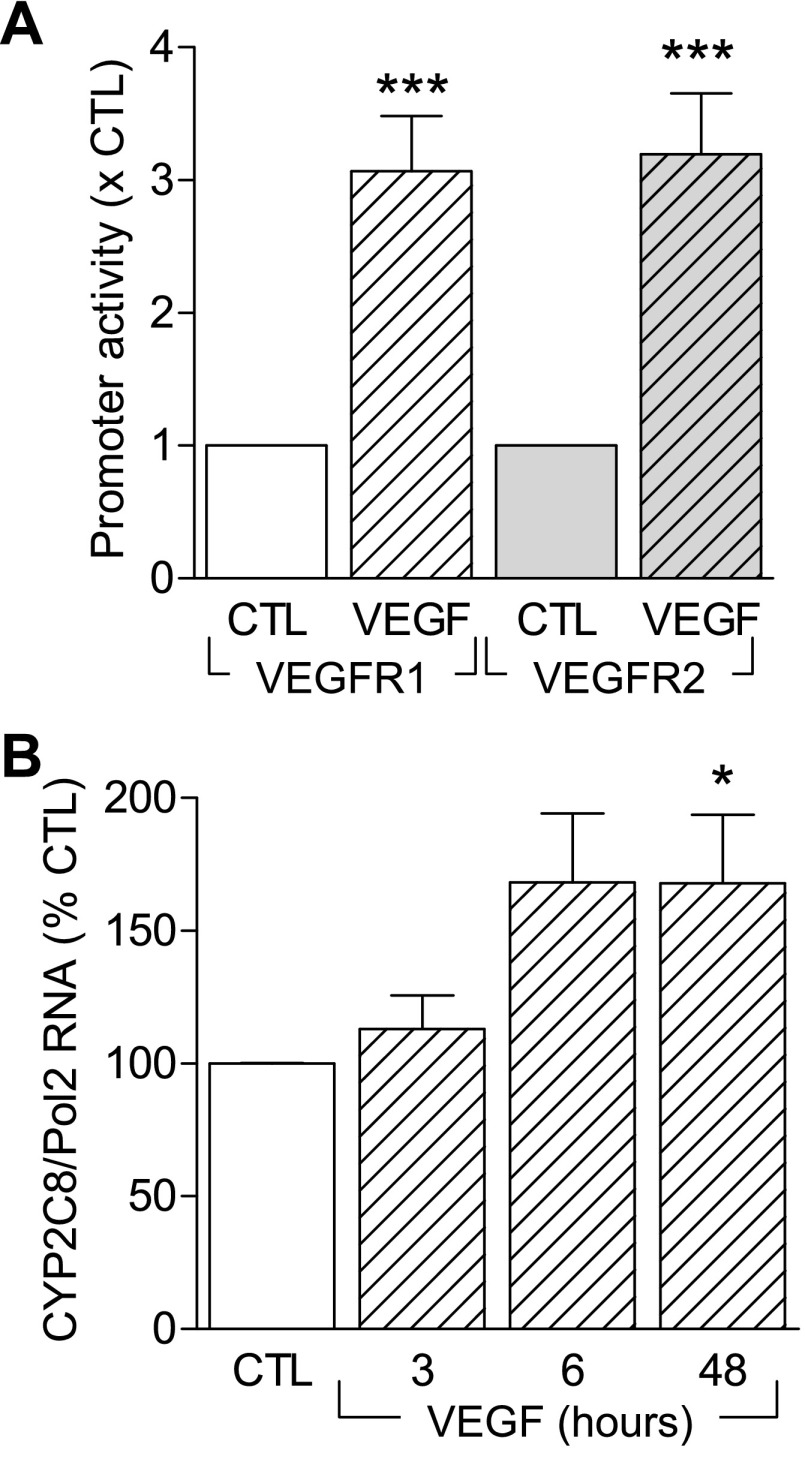
To assess the effects of VEGF on CYP2C expression we measured the activity of a reporter construct as well as levels of endogenous CYP2C RNA and protein. VEGF treatment (30 ng/ml, 6 h) of porcine aortic endothelial cells overexpressing either VEGFR1 or VEGFR2 and transfected with a CYP2C promoter-luciferase construct elicited a significant increase in the activity of the reporter gene construct (Fig. 1A). The increase in promoter activity in the cell line was paralleled by a VEGF-induced increase in CYP2C8 mRNA (Fig. 1B) in primary cultures of human endothelial cells.
Fig. 1.
Effect of vascular endothelial growth factor (VEGF) on the cytochrome P-450 (CYP) CYP2C gene in endothelial cells. A: CYP2C9 promoter activity in porcine endothelial cells overexpressing VEGFR1 or VEGFR2 were transfected with the promoter construct 14 h before stimulation with VEGF (30 ng/ml, 6 h). B: time course showing the effect of VEGF (30 ng/ml, 3–48 h) on the expression of CYP2C mRNA in human umbilical vein endothelial cells. Bar graphs summarize data obtained in 7–13 independent experiments. *P < 0.05, ***P < 0.001 vs. control (CTL).
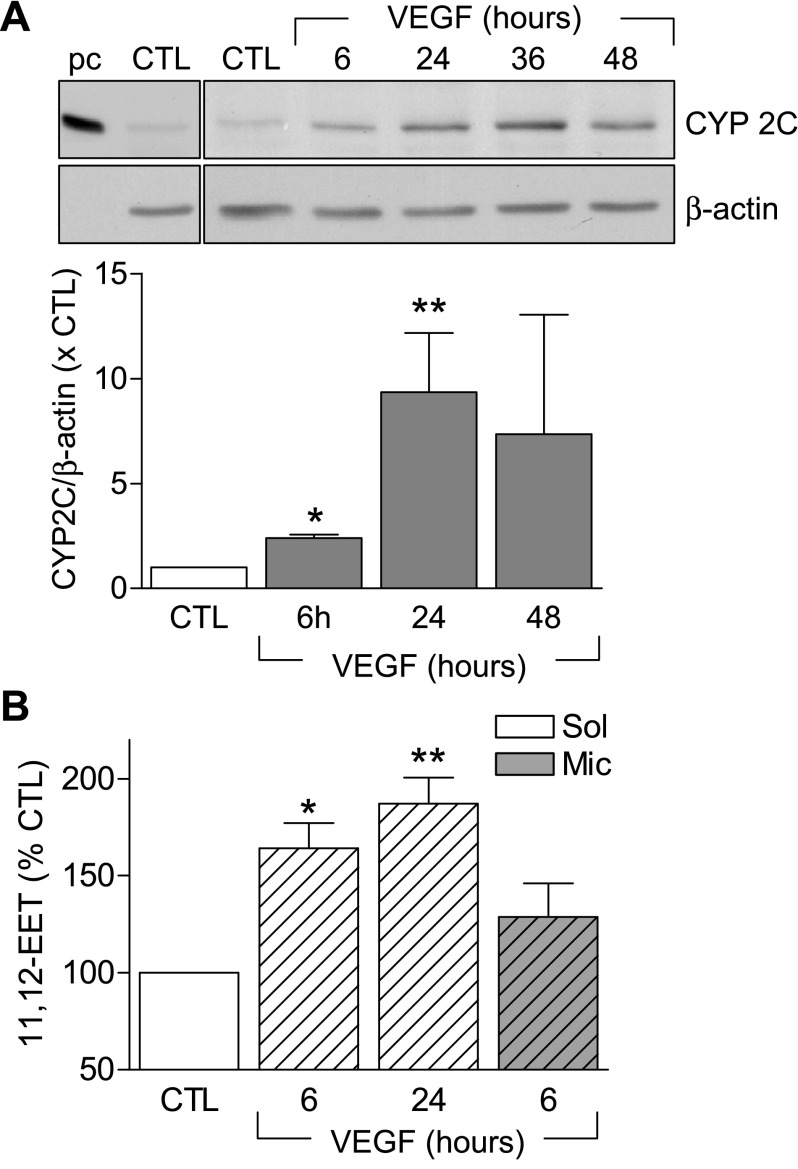
As reported previously, although CYP2C protein can easily be detected in native endothelial cells, its expression decreases rapidly following cell isolation (12, 18). Indeed, only low levels of CYP2C protein were detectable in the human umbilical vein endothelial cells studied (Fig. 2A). Simulation of the endothelial cells with VEGF, however, induced the expression of CYP2C (Fig. 2A) as well as CYP epoxygenase activity and the generation of 11,12-EET (Fig. 2B), which was sensitive to the epoxygenase inhibitor, miconazole.
Fig. 2.
Effect of VEGF on the expression and activity of CYP2C in endothelial cells. A: time course showing the effect of VEGF (30 ng/ml, 6–48 h) on the expression of CYP2C protein. CYP2C8-containing bacterial supersomes were used as a positive control (pc). B: 11,12-epoxyeicosatrienoic acid (EET) production in human umbilical vein endothelial cells. Some experiments were performed in the presence of miconazole (Mic, 3 μmol/l). Bar graphs summarize data obtained in 3–10 independent experiments. *P < 0.05, **P < 0.01 vs. control (CTL).
Role of CYP in VEGF-Induced Endothelial Cell Tube Formation
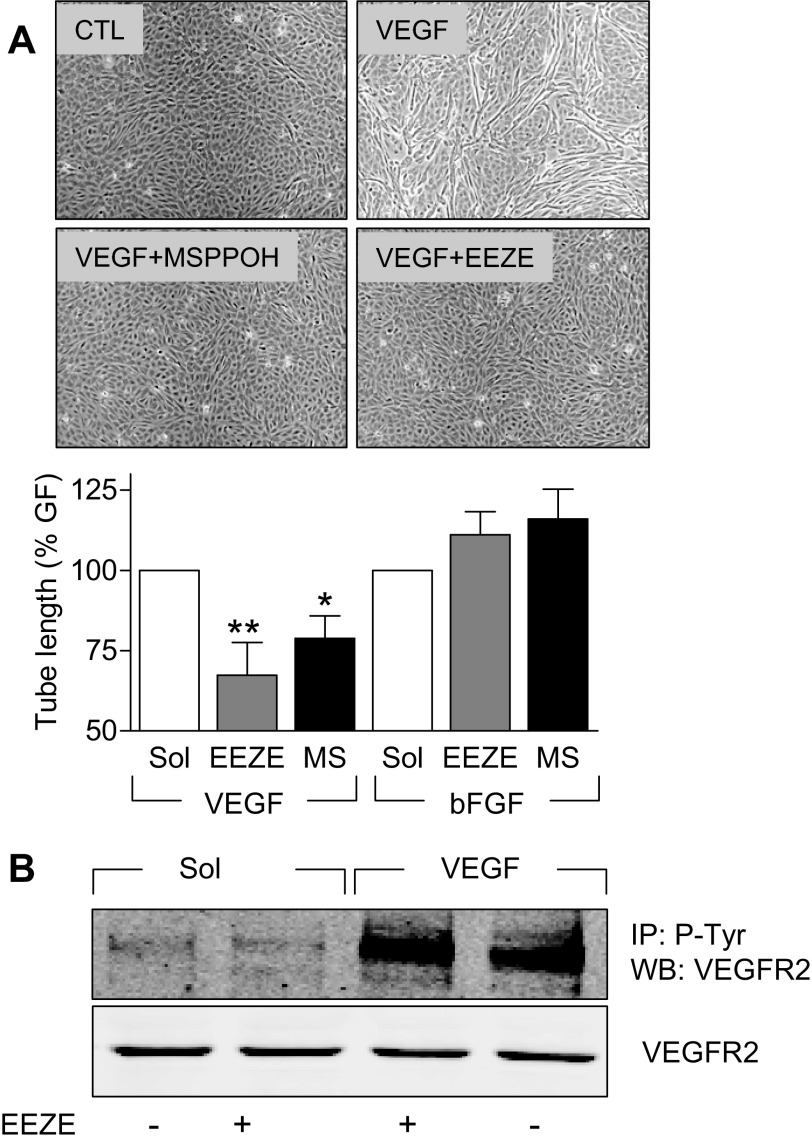
To assess the involvement of CYP epoxygenases in VEGF-induced endothelial cell tube formation, human umbilical vein endothelial cells were seeded onto fibronectin-coated culture dishes and treated with VEGF (30 ng/ml, 48 h) in the absence and presence of the EET antagonist 14,15-EEZE (10 μmol/l) or the CYP epoxygenase inhibitor MS-PPOH (10 μmol/l). Whereas endothelial cell tubes were apparent after 48 h in the VEGF-treated cultures, the number of tubes formed was significantly attenuated in cells treated with either the EET antagonist or the CYP inhibitor (Fig. 3A). To determine whether the effects observed were specific to VEGF-activated signaling, we repeated the experiments using bFGF as an angiogenic stimulus. In the latter case, interfering with CYP generation and EET activity was without effect on the ability of the growth factor to stimulate tube formation (Fig. 3A).
Fig. 3.
Effect of CYP inhibition and EET antagonism on VEGF-induced endothelial cell tube formation and receptor phosphorylation. A: endothelial cell tube formation on fibronectin-coated culture dishes was assessed after 48 h in response to VEGF (30 ng/ml) or bFGF (30 ng/ml) in the absence and presence of solvent (Sol; DMSO, 0.01%), the EET antagonist 14,15-epoxyeicosa-5(Z)-enoicacid (EEZE, 10 μmol/l), or the CYP2C inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl)-hexanamide (MS-PPOH) (MS, 10 μmol/l). Representative photographs show the effect of VEGF in the absence and presence of MS-PPOH and EEZE. Bar graph summarizes data obtained in 3–7 independent experiments. *P < 0.05, **P < 0.01 vs. Sol. B: lack of effect of EEZE on the VEGF (30 ng/ml, 20 min)-induced phosphorylation of the VEGFR2 receptor in phospho-tyrosine immunoprecipitates (IP) from VEGFR2 overexpressing porcine endothelial cells.
To ensure that the inhibitory effects observed using EEZE did not result from any direct interference between the EET antagonist and VEGF signaling, we assessed its effect on the VEGF-induced phosphorylation of VEGFR2. In VEGFR2 expressing endothelial cells, there was a low but detectable basal phosphorylation of the receptor, which was increased significantly by the application of VEGF (30 ng/ml, 20 min). 14,15-EEZE failed to affect the tyrosine phosphorylation of VEGFR2 under basal conditions or following stimulation with VEGF (Fig. 3B).
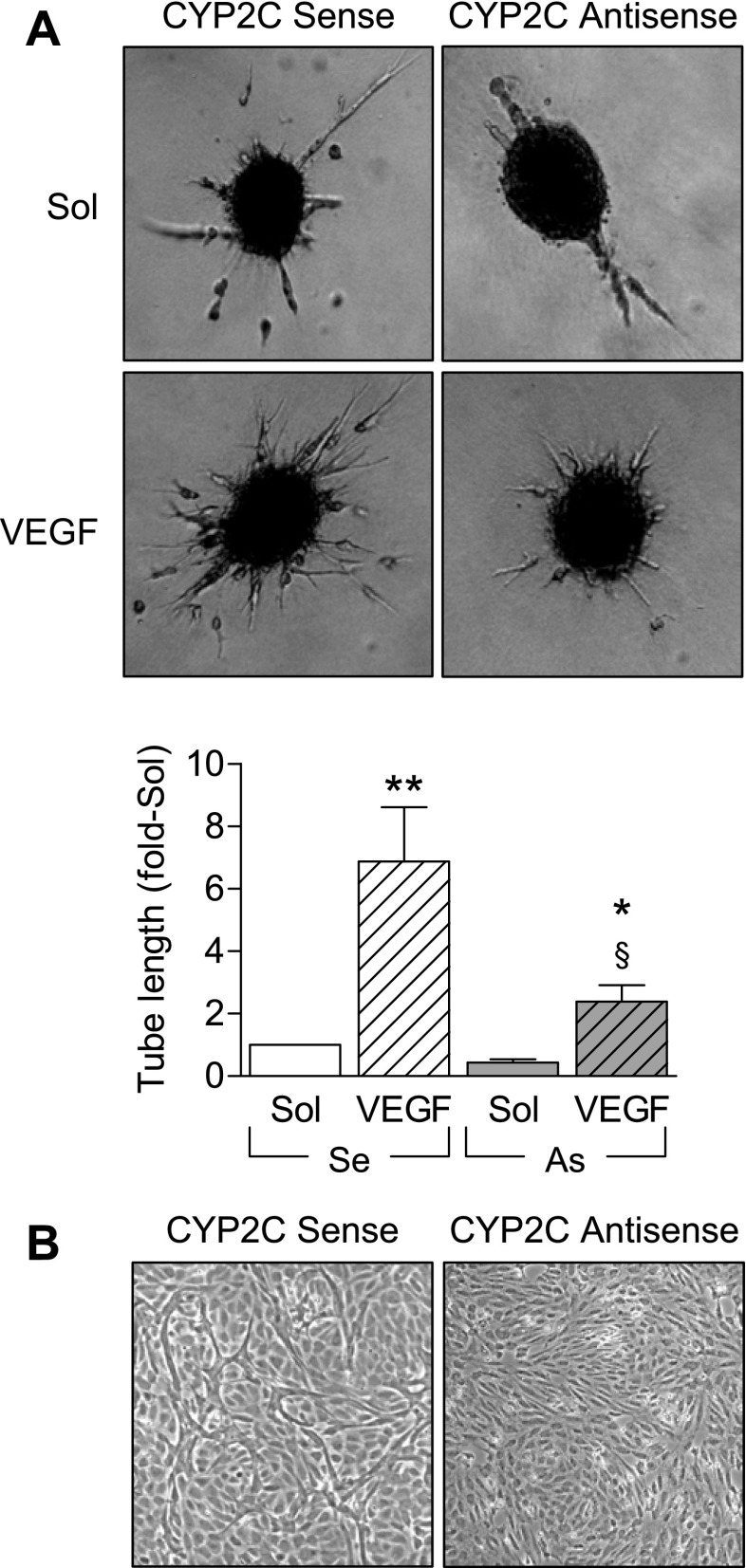
To more directly assess the impact of CYP2C on VEGF-induced endothelial cell sprouting in vitro, we performed a collagen-based spheroid assay and combined it with a previously described antisense oligonucleotide approach (13) to prevent the VEGF-induced increase in CYP2C expression. VEGF (30 ng/ml, 24 h) stimulated endothelial cell sprouting (Fig. 4); however, transfecting endothelial cells with CYP2C antisense oligonucleotides 14 h before generating the spheroids markedly attenuated VEGF-induced sprouting. CYP2C sense oligonucleotides used as a control were without effect on the response to VEGF (Fig. 4). Similarly, CYP2C antisense oligonucleotides also prevented VEGF-induced tube formation (Fig. 4B).
Fig. 4.
Effect of inhibiting CYP2C expression on VEGF-induced endothelial cell sprouting and tube formation. A: sprouting activity in endothelial cell spheroids treated with solvent (PBS, 0.3%) or VEGF (30 ng/ml, 24 h). Experiments were performed using cells transfected with CYP2C sense (Se) or antisense (As) oligonucleotides 14 h before spheroid generation. Bar graph summarizes data obtained in 3–5 independent experiments. *P < 0.05, **P < 0.01 vs. Sol, §P < 0.05 vs. VEGF stimulation in cells treated with Se oligonucleotides. B: effect of CYP2C sense or antisense oligonucleotides on endothelial cell tube formation assessed after 48 h stimulation with VEGF (30 ng/ml). The images shown are representative of data obtained in three experiments, each performed using a different cell batch.
Role of the AMPK in the VEGF-Induced Induction of CYP2C
The AMPK has been implicated in both VEGF-induced angiogenesis (33) as well as the induction of CYP expression by phenobarbital (37). We therefore determined the involvement of the AMPK in the VEGF-induced upregulation of CYP2C expression.
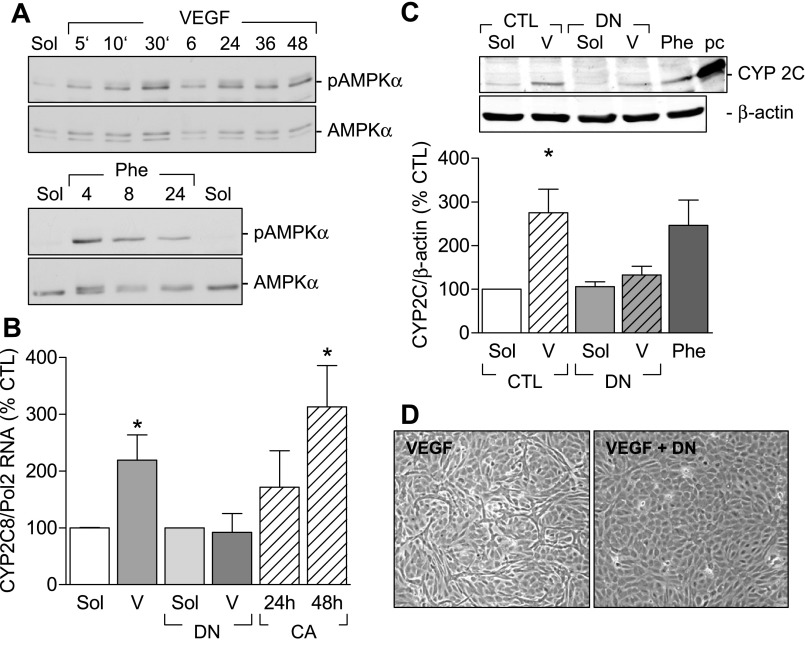
VEGF induced a rapid (within 5 min) phosphorylation of the AMPK on Thr172. The response was biphasic with an initial peak at 10 min (813 ± 55% of control, n = 4, P < 0.001) followed by a return to basal levels and a secondary but sustained AMPK activation after 24 h (593 ± 120% of control, n = 4, P < 0.01; Fig. 5A). The overexpression of constitutively active AMPKα increased CYP2C RNA levels in endothelial cells, whereas the overexpression of a dominant negative AMPKα mutant prevented the VEGF-induced increase in CYP2C RNA (Fig. 5B). The dominant negative AMPKα mutant also prevented the VEGF-induced increase in CYP2C protein (Fig. 5C) as well as endothelial cell tube formation (Fig. 5D). The CYP inducer phenobarbital (1 mmol/l) stimulated the phosphorylation of AMPKα in endothelial cells (Fig. 5A) and increased endothelial CYP2C expression (Fig. 5C).
Fig. 5.
Involvement of the AMPK in VEGF-induced CYP2C expression. A: representative Western blots showing the time-dependent (5 min to 48 h) effect of VEGF and phenobarbital (Phe) on AMPK phosphorylation on Thr172. Identical results were obtained in three additional experiments. B: effect of AMPKα mutants on CYP2C RNA expression. Human endothelial cells were infected with either a control virus or a dominant negative AMPKα (DN) adenovirus 48 h before stimulation with either solvent (Sol) or VEGF (V) (30 ng/ml) for 6 h. CYP 2C mRNA was assessed by RT-qPCR. In some experiments endothelial cells were infected with a constitutively active AMPKα mutant (CA), and CYP2C RNA levels were assessed after 24 and 48 h. Effect of the dominant negative AMPKα mutant on the VEGF (V)-induced increase in CYP2C protein expression (C) and tube formation (D) is shown. Phenobarbital (Phe) and a positive control (pc; CYP2C8 overexpression) were used. Bar graphs summarize data obtained in 3–4 independent experiments. *P < 0.05 vs. CTL.
Effect of 14,15-EEZE on VEGF-Induced Angiogenesis In Vivo
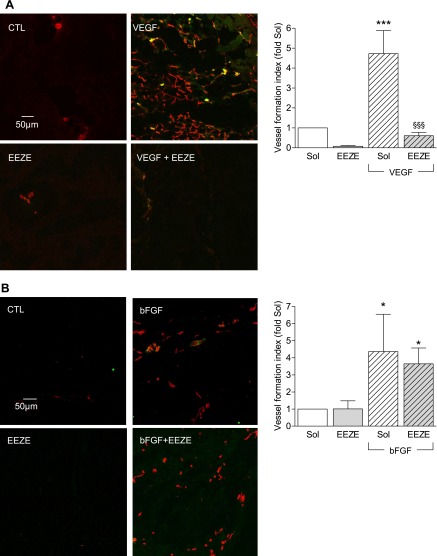
To assess the role of CYP-derived EETs on VEGF-induced angiogenesis in vivo, we determined the endothelial cell content of Matrigel plugs that were impregnated with either solvent, VEGF (150 ng/ml), the EET antagonist 14,15-EEZE (10 μmol/l), or a combination of both and injected dorsally on each side of the spine in 8-wk-old female C57/BL6 mice. Under the experimental conditions employed, endothelial cells (CD31) and a few sparse smooth muscle cells/pericytes (α-actin) were detected in the VEGF-impregnated plugs after 14 days. Only a few endothelial cells and no smooth muscle cells/pericytes were detected in the solvent- or 14,15-EEZE-containing plugs. However, 14,15-EEZE almost completely abolished the angiogenic effect of VEGF when both were applied in combination (Fig. 6A).
Fig. 6.
Effect of 14,15-EEZE on VEGF- and bFGF-induced angiogenesis in vivo. A: endothelial cell migration into Matrigel plugs impregnated with either solvent (DMSO, 0.1%, CTL), VEGF (150 ng/ml), the EET antagonist 14,15-EEZE (100 μmol/l), or the combination of both VEGF and EEZE. Images were taken after sectioning and immunostaining for CD31 (red) and α-actin (green). B: endothelial cell migration into Matrigel plugs impregnated with either solvent (Sol), bFGF (150 ng/ml), the EET antagonist 14,15-EEZE (100 μmol/l), or the combination of both bFGF and EEZE. Images were taken after sectioning and immunostaining for CD31 (red) and α-actin (green) and scored as the vessel formation index (see methods). Bar graphs summarize data obtained in 4–10 independent experiments. *P < 0.05, ***P < 0.001 vs. the appropriate solvent (Sol) in the absence of EEZE, §§§P < 0.001 vs. VEGF.
To demonstrate that the effect of 14,15-EEZE was specific for VEGF, the same assay was repeated using Matrigel plugs impregnated with bFGF (150 ng/ml) alone or in combination with 14,15-EEZE. Whereas bFGF clearly induced endothelial cell invasion of the plugs, this effect was insensitive to the EET antagonist (Fig. 6B).
Effect of AMPK Downregulation on VEGF-Induced Angiogenesis
Because our data indicated that the VEGF-induced increase in CYP2C expression is dependent on the activation of the AMPK, we next assessed the consequences of AMPK downregulation on endothelial cell tube formation in vitro in human endothelial cells and endothelial cell invasion of Matrigel in vivo in mice.
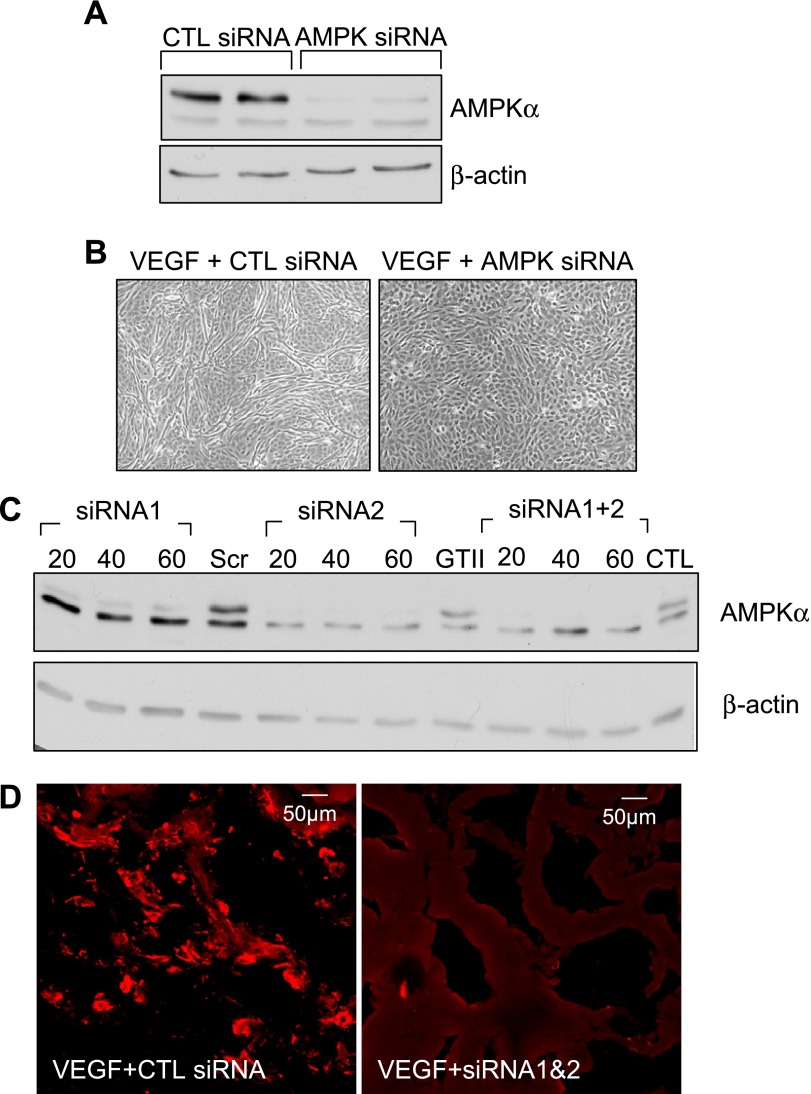
The siRNA used almost abrogated AMPKα expression in human endothelial cells (Fig. 7A) and prevented the VEGF-induced formation of tube-like structures (Fig. 7B). The same procedure was repeated using murine lung endothelial cells in which we compared the ability of two siRNAs to downregulate the AMPK (Fig. 7C). Both of the siRNA tested downregulated the AMPK with one of them (siRNA2) being more effective.
Fig. 7.
Effect of AMPK downregulation on VEGF-stimulated angiogenesis. Effect of AMPKα small interfering RNA (siRNA) on the expression of the kinase (A) and VEGF-induced tube formation (B) in human endothelial cells in vitro is shown. Effectiveness of the AMPKα siRNA on the expression of the kinase in cultured mouse lung endothelial cells (C) and on the invasion of endothelial cells into VEGF-impregnated Matrigel plugs in vivo (D) is shown. The results shown are representative of data obtained in 3–6 additional experiments.
To determine whether the AMPK siRNA could also affect angiogenesis in vivo, Matrigel plugs were then impregnated with either solvent or VEGF together with either a control siRNA (GFP) or a mixture of the AMPK siRNA1 and siRNA2 and implanted in mice for a total of 7 days. Little or no PECAM-1 was detected in plugs impregnated with solvent (data not shown). Moreover, whereas PECAM-1-positive endothelial cells could be detected in plugs treated with VEGF and the control siRNA, PECAM-1 staining was sparse in plugs treated with VEGF and the AMPK siRNA (Fig. 7D).
DISCUSSION
The results of the present investigation demonstrate that stimulation of endothelial cells with VEGF results in an increase of CYP2C protein as well as of 11,12-EET levels. This activation of CYP2C and subsequent EET release seems to be dependent on the AMPK and to be an essential component of the signaling cascade initiated by VEGF as endothelial cell sprouting was abolished by CYP2C downregulation (antisense oligonucleotides). In addition, the CYP epoxygenase inhibitor MS-PPOH and EET antagonist 14,15-EEZE selectively inhibited VEGF-, but not FGF-mediated, angiogenesis.
Although interest in the CYP epoxygenases expressed in the vasculature was focused on the role played by the EETs in the regulation of membrane potential, these eicosanoids are now recognized as signaling molecules, able to stimulate several steps in the angiogenic process, including endothelial cell proliferation, migration, and tube formation (31). However, the majority of the studies performed to date have relied on overexpression systems, and little is known about the involvement of CYP epoxygenases/EETs in angiogenic pathways stimulated by classical growth factors. Because we recently reported that EETs are involved in hypoxia-induced angiogenesis (29), it seemed logical to hypothesize a potential link between VEGF and EET signaling, since the induction of VEGF by hypoxia and the role of the latter growth factor in hypoxia-induced cell migration is well documented (11). The results of our study demonstrate that endothelial cell stimulation with VEGF increases CYP2C expression and EET production and that preventing the induction or inhibiting the activity of CYP2C significantly attenuates the angiogenic response to VEGF. These observations fit well with a recent report indicating that another CYP-derived metabolite of arachidonic acid, namely 20-HETE, can be activated by VEGF in pulmonary arteries (23).
The CYP2C enzyme sensitive to VEGF was identified as CYP2C8, and we were able to show that both CYP2C8 mRNA and protein increased in the cells studied. One limitation of the present study was that we were only able to analyze the effects of VEGF on the activity of a CYP2C9 and not a CYP2C8 promoter construct. However, the enzymes are highly homologous and both have been implicated in angiogenesis. Moreover, we have frequently found similar effects (Ca2+ signaling and angiogenesis) in native endothelial cells, which express CYP2C8 and in cultured endothelial cells overexpressing CYP2C9 (18, 41). The stereochemistry of the EETs produced by these two enzymes is however quite different, thus it will be important to determine whether different effects in angiogenesis and cell signaling in general are observed using different EET enantiomers. Which of the EETs is responsible for the effects reported also remains to be clarified, and although we have repeatedly been able to reproduce the effects of CYP activation/overexpressing using 11,12-EET and not 14,15-EET (30, 41), this finding is not shared by others (28).
The importance of VEGF and its receptors in vascular development has been demonstrated in various knockout animals. For example, the deletion of either VEGFR1 (38) or VEGFR2 (19) has proven to be lethal due to abnormalities in vessel formation, therefore, it appears both receptors are essential for the development of the vasculature in the mouse embryo. Analysis of VEGFR signaling has led to the conclusion that although affinity for VEGF binding is approximately 10-fold higher for VEGFR1 than for VEGFR2, it is activation of the latter that is believed to convey the VEGF-mediated effects in endothelial cells (21). We observed however that VEGF was able to increase the activity of the CYP2C9 promoter in cells expressing either VEGFR1 or VEGFR2. Although EETs have been suggested to act as second messengers in the EGF-activated signaling cascade (5), not all growth factors are able to upregulate CYP epoxygenases, and we found that although the EET antagonist 14,15-EEZE almost abolished VEGF-induced endothelial tube formation in vitro and in vivo, it did not affect the angiogenesis induced by bFGF.
In the search to identify the molecular mechanism(s) underlying the VEGF-induced increase in CYP2C expression, we analyzed the role of the AMPK as VEGF has previously been demonstrated to activate the kinase in endothelial cells (36), and activation of AMPK has been linked to endothelial cell migration and angiogenesis (33, 34). Moreover, activation of the AMPK by phenobarbital is reported to increase the expression of CYP2B in chicken and human hepatocytes (1, 37). Consistent with these reports, both VEGF and phenobarbital stimulated the phosphorylation of the AMPK and increased in CYP2C expression in endothelial cells. Moreover, the overexpression of a dominant-negative AMPK mutant significantly reduced VEGF-induced CYP2C expression, and AMPK downregulation markedly attenuated VEGF-induced angiogenesis in vitro and in vivo. Although we found this involvement of AMPK in the VEGF-induced CYP2C expression, the remaining steps that involved in this process have not been elucidated, but LKB1 (serine/threonine kinase 11) is likely to be involved (1).
The VEGF-activated signaling cascade leading to angiogenesis has previously been linked to an increase in the formation of reactive oxygen species (9). Although the NADPH oxidase has been implicated as the source of these radicals (40), it should be noted that the activation of CYP2C epoxygenases also results in the generation of superoxide anions (O2•−) in sufficient amounts to alter the bioavailability of nitric oxide and the expression of adhesion molecules (17). To verify that CYP2C-derived EETs rather than reactive oxygen species were involved in mediating VEGF-induced angiogenesis, we employed the EET antagonist 14,15-EEZE. In contrast to the epoxygenase inhibitors MS-PPOH and sulfaphenazole, as well as the CYP2C antisense treatment, which attenuate the production of all the CYP2C products (EETs, 20-HETE, and O2•−), 14,15-EEZE antagonizes only the effects of EETs without interfering with those of 20-HETE (20) or the production of O2•− by CYP2C enzymes (U. R. Michaelis, unpublished observations). We found that 14,15-EEZE inhibited VEGF-induced tube formation in vitro and angiogenesis in vivo, indicating that EETs and not reactive oxygen species were responsible for the effects observed. Moreover, we were unable to detect an effect of VEGF on 20-HETE formation, indicating that 20-HETE does not contribute to angiogenesis under the conditions studied.
Taken together our data indicate the potential importance of 11,12-EET to (patho)physiological angiogenic processes. At this point it is important to point out that CYP enzymes are not only expressed in endothelial cells and CYP2C as well as CYP2J enzymes have been detected in different tumor tissues (24, 42) and can induce tumor growth as well as promote metastasis (25). It is therefore tempting to speculate that CYP enzymes might represent a new target for the treatment of tumor growth and therefore cancer therapy. Pharmacological inhibitors of some CYP isoforms have been identified as promising anti-cancer agents (7, 8); however, the majority of work published to date has focused on the consequences of CYP inhibition on the bioavailability of anti-cancer agents rather than determining the consequences of CYP inhibition per se.
GRANTS
This work was supported by the Deutsche Forschungsgemeinschaft (SFB-TR 23, A6), National Institutes of Health Grant GM-31278, and the Robert A. Welch Foundation.
Acknowledgments
The authors are indebted to Isabel Winter and Mechtild Piepenbrock for expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Blattler SM, Rencurel F, Kaufmann MR, Meyer UA. In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase. Proc Natl Acad Sci USA 104: 1045–1050, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Cebe-Suarez S, Zehnder-Fjallman A, and Ballmer-Hofer K. The role of VEGF receptors in angiogenesis: complex partnerships. Cell Mol Life Sci 63: 601–615, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem 273: 29254–29261, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Chen JK, Wang DW, Falck JR, Capdevila J, Harris RC. Transfection of an active cytochrome P450 arachidonic acid epoxygenase indicates that 14,15-epoxyeicosatrienoic acid functions as an intracellular messenger in response to epidermal growth factor. J Biol Chem 274: 4764–4769, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Chen JK, Capdevila J, Harris RC. Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells. Proc Natl Acad Sci USA 99: 6029–6034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ Jr, and Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol 2: 263–270, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Chun YJ, Kim S. Discovery of cytochrome P450 1B1 inhibitors as new promising anti-cancer agents. Med Res Rev 23: 657–668, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem 277: 3101–3108, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dixit M, Bess E, Fisslthaler B, Hartel FV, Noll T, Busse R, Fleming I. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res 77: 160–168, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, and vis-Smyth T. The biology of vascular endothelial growth factor. Endocr J 18: 4–25, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Fisslthaler B, Hinsch N, Chataigneau T, Popp R, Kiss L, Busse R, Fleming I. Nifedipine increases cytochrome P4502C expression and endothelium- derived hyperpolarizing factor-mediated responses in coronary arteries. Hypertension 36: 270–275, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Fisslthaler B, Popp R, Michaelis UR, Kiss L, Fleming I, Busse R. Cyclic stretch enhances the expression and activity of coronary endothelium-derived hyperpolarizing factor synthase. Hypertension 38: 1427–1432, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci 118: 4103–4111, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Fleming I, Fisslthaler B, Michaelis UR, Kiss L, Popp R, Busse R. The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signalling pathways and proliferation in vascular cells. Pflügers Arch 442: 511–518, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res 88: 44–51, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol 27: 2612–2618, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem 276: 3222–3230, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Hoebel BG, Graier WF. 11,12-Epoxyeicosatrienoic acid stimulates tyrosine kinase activity in porcine aortic endothelial cells. Eur J Pharmacol 346: 115–117, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs ER, Zhu D, Gruenloh S, Lopez B, Medhora M. VEGF-induced relaxation of pulmonary arteries is mediated by endothelial cytochrome P-450 hydroxylase. Am J Physiol Lung Cell Mol Physiol 291: L369–L377, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res 65: 4707–4715, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Jiang JG, Ning YG, Chen C, Ma D, Liu ZJ, Yang S, Zhou J, Xiao X, Zhang XA, Edin ML, Card JW, Wang J, Zeldin DC, Wang DW. Cytochrome P450 epoxygenase promotes human cancer metastasis. Cancer Res 67: 6665–6674, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Korff T, Dandekar G, Pfaff D, Fuller T, Goettsch W, Morawietz H, Schaffner F, Augustin HG. Endothelial ephrinB2 is controlled by microenvironmental determinants and associates context-dependently with CD31. Arterioscler Thromb Vasc Biol 26: 468–474, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 270: 13333–13340, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, Harder DR. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol 284: H215–H224, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci 118: 5489–5498, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J 17: 770–772, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther 111: 584–595, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Michaelis UR, Xia N, Barbosa-Sicard E, Falck JR, Busse R, Fleming I. Role of cytochrome P450 2C epoxygenases in hypoxia-induced cell migration and angiogenesis in retinal endothelial cells. Invest Ophthalmol Vis Sci 49: 1242–1247, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 278: 31000–31006, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279: 1304–1309, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of Cyclin D1. J Biol Chem 277: 15671–15676, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun 354: 1084–1088, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Wolf CR. AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J Biol Chem 280: 4367–4373, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 292: C996–C1012, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91: 1160–1167, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Webler AC, Popp R, Korff T, Michaelis UR, Urbich C, Busse R, Fleming I. Cytochrome P450 2C9-induced angiogenesis is dependent on EphB4. Atherioscer Thromb Vasc Biol 28: 1123–1129, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Yokose T, Doy M, Taniguchi T, Shimada T, Kakiki M, Horie T, Matsuzaki Y, Mukai K. Immunohistochemical study of cytochrome P450 2C and 3A in human non- neoplastic and neoplastic tissues. Virchows Arch 434: 401–411, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic acid. Stroke 33: 2957–2964, 2002. [DOI] [PubMed] [Google Scholar]