Abstract
AMP-activated protein kinase (AMPK) plays a critical role in the stimulation of glucose transport in response to hypoxia and inhibition of oxidative phosphorylation. In the present study, we examined the signaling pathway(s) mediating the glucose transport response following activation of AMPK. Using mouse fibroblasts of AMPK wild type and AMPK knockout, we documented that the expression of AMPK is essential for the glucose transport response to both azide and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR). In Clone 9 cells, the stimulation of glucose transport by a combination of azide and AICAR was not additive, whereas there was an additive increase in the abundance of phosphorylated AMPK (p-AMPK). In Clone 9 cells, AMPK wild-type fibroblasts, and H9c2 heart cells, azide or hypoxia selectively increased p-ERK1/2, whereas, in contrast, AICAR selectively stimulated p-p38; phosphorylation of JNK was unaffected. Azide's effect on p-ERK1/2 abundance and glucose transport in Clone 9 cells was partially abolished by the MEK1/2 inhibitor U0126. SB 203580, an inhibitor of p38, prevented the phosphorylation of p38 and the glucose transport response to AICAR and, unexpectedly, to azide. Hypoxia, azide, and AICAR all led to increased phosphorylation of Akt substrate of 160 kDa (AS160) in Clone 9 cells. Employing small interference RNA directed against AS160 did not inhibit the glucose transport response to azide or AICAR, whereas the content of P-AS160 was reduced by ∼80%. Finally, we found no evidence for coimmunoprecipitation of Glut1 and p-AS160. We conclude that although azide, hypoxia, and AICAR all activate AMPK, the downstream signaling pathways are distinct, with azide and hypoxia stimulating ERK1/2 and AICAR stimulating the p38 pathway.
Keywords: AMP-activated protein kinase, glucose transporter 1, oxidative phosphorylation, small interfering RNA directed against AS160
two families of glucose transporters mediate the transport of glucose across plasma membranes; these include the widely expressed glucose transporters (Gluts) and the Na+-glucose cotransporters that are predominantly expressed in the intestine and kidney (29). The Glut family of transporters is composed of more than 12 members that facilitate the passive transport of glucose, fructose, and other substrates (29). Glut1, which is highly expressed in brain, retina, human red blood cells, and placenta, is also expressed at varying levels in virtually all cells and is thought to mediate much of the basal, non-insulin-dependent transport of glucose (19, 26, 29, 30). Glut2 is highly expressed in liver and β-cells of pancreatic islets, whereas Glut3 is predominantly expressed in neuronal cells (29). Glut4 is largely expressed in striated muscle (both skeletal and cardiac) and in adipocytes (29). Glucose transport mediated by Glut4 is highly responsive to insulin action by becoming translocated from intracellular membrane vesicles to the plasma membrane (12, 16).
The rate of glucose transport is regulated by a large number of agents and stimuli including insulin, hypoxia, inhibition of oxidative phosphorylation, cellular transformation, exposure to alkaline pH, serum and growth factors, and a rise in cell calcium concentration (1, 6, 8, 14, 15, 19, 20, 26, 28). In many of our previous studies we have examined the stimulation of glucose transport in response to exposure to azide, a chemical inhibitor of mitochondrial respiration, utilizing Clone 9 cells (a rat liver-derived cell line that near-exclusively expresses the Glut1 isoform) (20, 26, 33). Importantly, and in contrast to cells expressing Glut4, in which exposure to insulin, hypoxia, or chemical inhibitors of oxidative phosphorylation increases the abundance of the transporter at the cell surface (3, 27), stimulation of glucose transport in Glut1-expressing cells appears to be mediated predominantly by increased activity of Glut1 transporters preexisting in the plasma membrane (4, 19, 33).
It is well known that phosphorylation of AMP-activated protein kinase (AMPK) leads to stimulation of glucose transport in a variety of cells examined (1, 8, 13). This kinase, which appears to function as a “sensor” of the energy status of cells, becomes phosphorylated and activated in response to conditions associated with increased intracellular concentration of AMP (and decreased ATP), changes that are present during hypoxia or inhibition of oxidative phosphorylation (1, 4, 10, 20, 37). We and others have previously reported that AMPK phosphorylation and enzymatic activity are stimulated following exposure of Clone 9 cells to azide or 5′-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and that the activation of AMPK is associated with an increased rate of Glut1-mediated glucose transport (1, 4, 20). More recently, employing small interfering RNA (siRNA) technology and compound C as an inhibitor of AMPK, we have established that activation of AMPK following exposure to azide or AICAR constitutes a critical step in the glucose transport response (21). However, the steps subsequent to activation of AMPK and the signaling pathway(s) leading to stimulation of glucose transport remain largely unknown. The present study is focused on delineating some of the pathways mediating the glucose transport response.
MATERIALS AND METHODS
Materials.
Clone 9 and HepG2 cells were obtained from American Type Culture Collection (Rockville, MD). H9c2 cells were a gift from Dr. Adrea Romani (Cleveland, OH). AMPK wild-type (WT) mouse fibroblasts and AMPKα1 plus AMPKα2 knockout (AMPK KO) fibroblasts were a gift from the laboratory of Dr. Benoit Viollet (Paris, France). DMEM, trypsin-EDTA, calf serum, newborn bovine serum, bovine calf serum, and recombinant protein G-agarose were purchased from GIBCO (Grand Island, NY). Cell culture dishes were obtained from Corning Glass Works (Medfield, MA), and 3-O-methyl-d-[3H]glucose ([3H]3-OMG; 3.4 mCi/mmol) and an enhanced chemiluminescence Western blotting detection kit were obtained from Amersham Life Science (Arlington Heights, IL). Mouse anti-β-actin, peroxidase-conjugated goat anti-mouse and anti-rabbit IgG, phloretin, cytochalasin B (CB), PMSF, AICAR, U0126 ethanolate, and standard chemical reagents were obtained from Sigma (St. Louis, MO). Rabbit anti-AMPK-α-pan, rabbit monoclonal anti-phosphorylated (Thr172) AMPK (recognizing both phosphorylated α1- and α2-isoforms), mouse anti-phosphorylated p38 MAPK (Thr180/Tyr182), rabbit anti-total p38 MAPK, and rabbit anti-total AS160 were obtained from Cell Signaling Technology (Danvers, MA). Mouse anti-phosphorylated ERK1/2 (E-4) and rabbit anti-total ERK were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and the p38/SAPK2 inhibitor SB 203580 and rabbit anti-phosphorylated AS160 (Thr642) were obtained from Upstate (Temecula, CA). siRNA directed against AS160 (siAS160) was obtained from Sigma (St. Louis, MO), and Lipofectamine 2000 was obtained from Invitrogen (Carlsbad, CA). Block-iT fluorescent oligo and siRNA for nonspecific targeting of mRNAs were purchased from Dharmacon (Lafayette, CO).
Cell culture.
Clone 9 cells in 60-mm dishes were maintained in DMEM containing 5.6 mM d-glucose supplemented with 10% calf serum at 37°C in a 9% CO2-humidified chamber (pH 7.4), as described previously (20, 33). In experiments with hypoxia, cells were incubated in a sealed chamber gassed with 95% N2 and 5% CO2 (nominally <0.05% O2). HepG2 cells were maintained in MEM/F12-1 medium with 10% new born bovine serum in accordance with suggestions by the American Type Culture Collection. H9c2 cells were kept in DMEM containing 5.6 mM d-glucose with 10% bovine calf serum. AMPK WT and AMPK KO mouse fibroblasts were maintained in DMEM (5.6 mM glucose) supplemented with 10% newborn bovine serum at 37°C in a 5% CO2-humidified chamber (pH 7.4), as described previously (24).
AS160 knockdown by siRNA directed against AS160.
Seventy percent confluent Clone 9 cells in 60-mm culture dishes were incubated with fresh DMEM with no antibiotics for 2 h before transfection. Cells were transfected with an siAS160-targeted sequence of (5′-3′) UCAGGGUAAACUGUAGUAGTT (100 pmol/ml) or nonspecific scrambled siRNA as negative control by using Lipofectamine 2000 according to the manufacture's protocol. Forty-eight hours after transfection, the media were changed and the cells were treated with diluent, 10 mM azide, or 2 mM AICAR for 1 h, followed by CB-inhibitable [3H]3-OMG glucose uptake assay and Western blotting.
Transfection efficiency was monitored using the same quantity of Block-iT fluorescent oligo, and fluorescence was observed at 24–48 h after transfection by microscopy. A transfection efficiency of 90–95% was obtained at 24–48 h.
Measurement of CB-inhibitable [3H]3-OMG uptake.
Cells in triplicate 60-mm dishes were used in uptake experiments, as described previously (21). The uptake medium consisted of 1.0 ml of DMEM with 1 μCi of [3H]3-OMG and 1 μl of DMSO or CB in DMSO at a final concentration of 50 μM. Uptake was conducted for 1.0 min (4, 21, 26). [3H]3-OMG uptake was calculated as the difference between uptake in the absence and presence of CB (21, 26).
SDS-PAGE and Western blotting.
Cells were lysed in Nonidet P-40 (NP-40)-containing buffer (5 mM NaCl, 25 mM Tris, 25 mM sodium fluoride, 10 mM sodium pyrophosphate, 0.5 mg/l leupeptin, 1 mg/l aprotinin, 1 mM sodium orthovanadate, 0.1 mM PMSF, and 0.5% NP-40; pH 7.5). In experiments on phosphorylation of AS160, cells were directly lysed in SDS sample buffer. Proteins were separated by 8 or 10% SDS-PAGE. Polyvinylidene difluoride membranes were blocked using 5% nonfat milk and incubated with rabbit anti-AMPK-α-pan (63 kDa) for measurement of total AMPK, rabbit anti-phosphorylated (Thr172) AMPK for measurement of α1- plus α2-isoform phosphorylation (p-AMPK; 63 kDa), mouse anti-phosphorylated p38 (p-p38; 43 kDa) or rabbit anti-total p38 (43 kDa), mouse anti-phosphorylated ERK1/2 (p-ERK1/2; 42 and 44 kDa) or rabbit anti-total ERK (42 and 44 kDa), or rabbit anti-phosphorylated AS160 for measurement of phosphorylated AS160 (p-AS160; 160 kDa). Mouse anti-β-actin (42 kDa) antibody was used to verify equal loading of the gels. The secondary antibody was a 1:1,000 to 1:4,000 (vol/vol) dilution of horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse in Tris-buffered saline-Tween 20 (50 mM Tris, 150 mM NaCl, and 0.05% Tween 20, pH 7.5). Blots were developed using enhanced chemiluminescence reagents, and immunoreactive bands were visualized on Kodak X-Omat film; the intensity of the bands was determined by densitometry. Multiple exposures of blots were developed to ensure linearity of the signals. Each experiment was repeated at least three times. In the case of ERK1/2, the abundance of ERK1 and ERK2 bands was added together. In all experiments measuring the relative abundance of p-AMPK, p-ERK1/2, and p-p38, the density of the bands was normalized against the total abundance of each of the proteins.
Immunoprecipitation of p-p38.
Clone 9 cells were treated with diluent, 10 mM azide, or 2 mM AICAR for 1 h and then lysed in the NP-40 buffer. Postnuclear cell lysates from each condition were immunoprecipitated overnight using mouse monoclonal anti-phospho-p38 MAPK antibody (1:100) with prewashed protein G-Sepharose. Samples were centrifuged at 3,000 rpm for 1 min at 4°C, and the pellets were washed three times with 10 volumes of ice-cold 0.5% NP-40 buffer. The final pellet was treated with SDS sample buffer. Membranes were probed with rabbit anti-total p38.
Immunoprecipitation of AS160 and p-AS160.
Clone 9 cells and HepG2 cells were treated with diluent, 10 mM azide, or 2 mM AICAR for 1 h and then lysed in the NP-40 buffer. Postnuclear cell lysates from each condition were immunoprecipitated overnight using mouse monoclonal anti-Glut1 antibody (1:10) and prewashed protein G-Sepharose. Samples were washed three times with 0.5% NP-40 buffer and lysed with SDS sample buffer. Immunoprecipitated products were employed for Western blotting using rabbit polyclonal anti-phospho-AS160 or rabbit polyclonal anti-total AS160 antibody. HepG2 cell lysates (following the same treatments) were immunoprecipitated with rabbit anti-total AS160 antibody, and the immunoprecipitated products were employed for Western blotting using mouse anti-Glut1 antibody.
Statistical analysis.
Values are means ± SE. Data are expressed in terms of natural logarithmic scale, and differences between each treatment and control were computed. To test for differences between each treatment and control, we conducted a Student's t-test for single mean. One-way analysis of variance was conducted to test for differences among the three treatments, followed by the Bonferroni adjustment factor for multiple comparison tests. All tests were conducted with a 0.05 level of significance. Analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC).
RESULTS
Effect of azide and AICAR on glucose transport and p-AMPK in AMPK WT and AMPK KO mouse fibroblast cells.
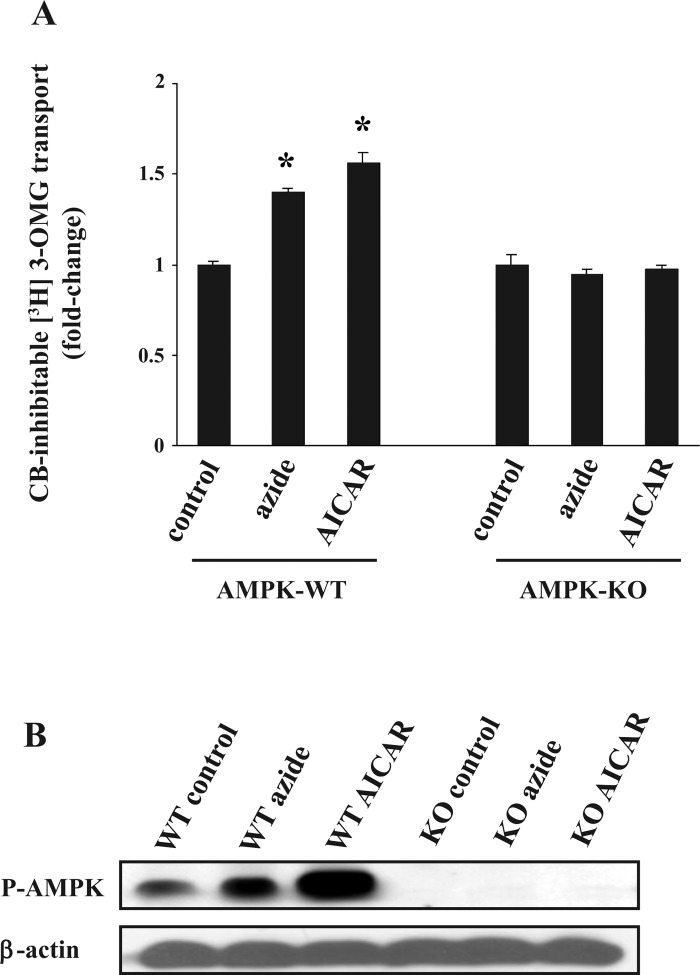
To further establish the critical role of AMPK in the glucose transport response to azide and AICAR, we used AMPK WT and AMPK KO mouse fibroblasts in which both the α1- and α2-isoforms of the enzyme are inactivated (24). Treatment of AMPK WT cells with azide or AICAR stimulated glucose transport (Fig. 1A). In contrast, AMPK KO fibroblasts manifested no change in the rate of glucose transport in response to either azide or AICAR. The rate of transport in AMPK KO cells under control conditions was similar to the rate in AMPK WT cells. Treatment with either azide or AICAR increased the phosphorylation of AMPK in WT fibroblasts; the abundance of p-AMPK increased 1.5 ± 0.1- and 2.3 ± 0.1-fold in cells treated with azide and AICAR, respectively, whereas p-AMPK was absent in AMPK KO cells (Fig. 1B).
Fig. 1.
Effect of azide and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) on glucose transport and on the abundance of phosphorylated AMP-activated protein kinase (p-AMPK) in wild-type (WT) and AMPK knockout (KO) fibroblasts. A: glucose transport. Confluent WT and AMPK KO fibroblasts in triplicate 6-cm culture dishes were serum-starved for 24 h. Cells were treated with diluent, 10 mM azide, or 2 mM AICAR for 1 h before measurement of cytochalasin B (CB)-inhibitable 3-O-methyl-d-[3H]glucose ([3H]3-OMG) transport in 3 independent experiments. Cells were lysed in 0.5 ml of H2O and used for radioactive counting. In each experiment, the rate of transport was normalized against that of untreated WT cells, and the results were averaged. The basal rate of glucose transport in WT and AMPK KO fibroblasts were not significantly different. *P < 0.05 compared with WT control (ANOVA). B: p-AMPK. Cells were treated as described in A, and lysates were subjected to SDS-PAGE. The abundance of p-AMPK was determined in the immunoblots. The abundance of p-AMPK increased 1.5 ± 0.1- and 2.3 ± 0.1-fold in WT cells treated with azide or AICAR, respectively. Blots were reprobed with antibodies against β-actin. Two additional independent experiments yielded similar results.
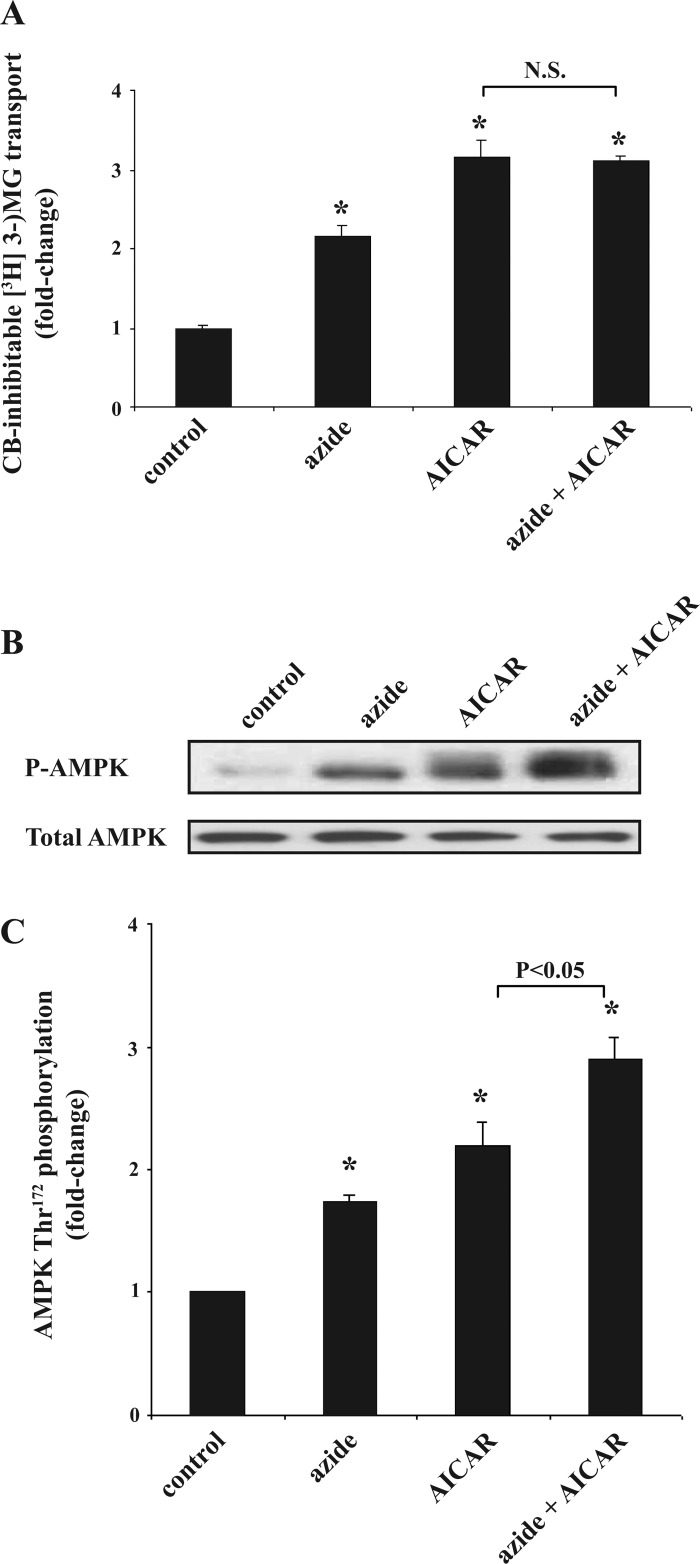
Effect of azide plus AICAR on glucose transport and p-AMPK abundance in Clone 9 cells.
We explored whether the glucose transport response to azide and AICAR is additive in Clone 9 cells. In dose-response studies, we found that 10 mM azide and 2 mM AICAR resulted in maximal stimulation of glucose transport (1). Higher concentration of azide was associated with cell toxicity and decreased glucose transport. Exposure to azide or AICAR alone stimulated the rate of glucose uptake, and simultaneous exposure to both agents resulted in no further increase in the rate of glucose transport (Fig. 2A). Employing anti-phosphorylated (Thr172) AMPK, which recognizes phosphorylation of both α1- and α2-isoforms of AMPK, we found that the content of p-AMPK (normalized against total AMPK) increased in both azide- and AICAR-treated cells, and simultaneous expose to both agents resulted in a higher abundance of p-AMPK in a near-additive manner (Fig. 2, B and C). The content of total AMPK was unchanged.
Fig. 2.
Effect of azide plus AICAR on glucose transport and p-AMPK abundance in Clone 9 cells. A: glucose transport. Confluent Clone 9 cells in triplicate 6-cm dishes were serum-starved for 24 h. Cells were treated with diluent, 10 mM azide, 2 mM AICAR, or both for 1 h before measurement of CB-inhibitable [3H]3-OMG. The experiment was repeated 3 times, and the results were averaged. Rates of transport were normalized against the control. *P < 0.05 compared with control. There was no significant difference (N.S.) between the rate of transport in AICAR- vs. AICAR + azide-treated cells (t-test; P > 0.05). B and C: p-AMPK and total AMPK abundance. Cell lysates treated in parallel with those described in A were subjected to immunoblotting to measure the relative content of cell p-AMPK and total AMPK. Results of the blots were analyzed using densitometry, and phosphorylation of AMPK is expressed as the degree of (fold) change compared with values in control cells after normalization against total AMPK. *P < 0.05 compared with control. p-AMPK in AICAR- vs. AICAR + azide-treated cells was significantly different. The content of total AMPK remained constant.
Selective phosphorylation of ERK1/2 by azide and p38 by AICAR in Clone 9 cells.
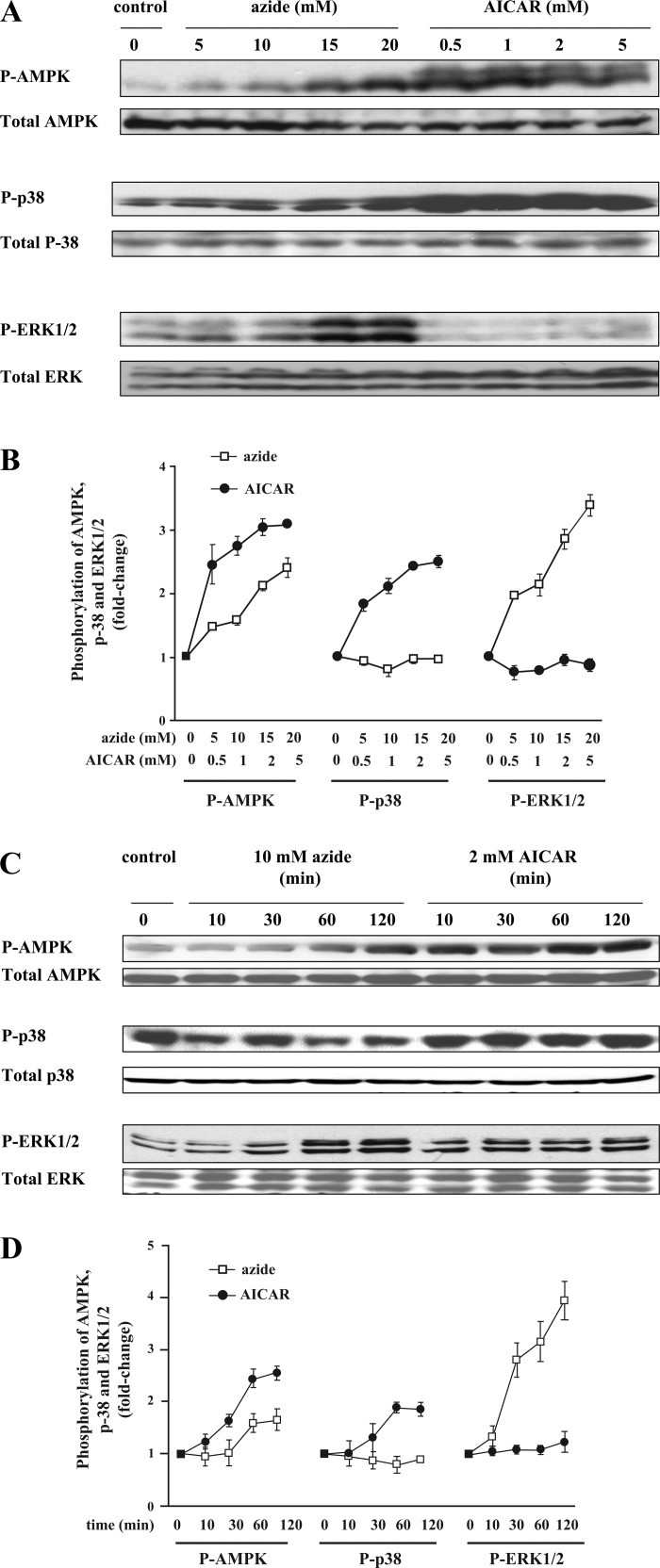
We tested whether p38 MAP kinase is activated in Clone 9 cells treated with azide, as has been reported with AICAR (38). Consistent with previous studies, cell content of p-AMPK increased in response to treatment with either AICAR or azide, whereas the abundance of total AMPK remained unchanged (Fig. 3A). In AICAR-treated cells, there was significant phosphorylation of p38 even at the lowest concentration of AICAR used (Fig. 3B). In marked contrast, there was no phosphorylation of p38 in response to any concentration of azide. ERK1/2 became phosphorylated in response to azide but not to AICAR.
Fig. 3.
AICAR stimulates phosphorylation of p38 and azide activates phosphorylation of ERK1/2 in a dose- and time-dependent manner. A and B. dose response. Confluent Clone 9 cells were treated with diluent; 5, 10, 15, or 20 mM azide; or 0.5, 1.0, 2, or 5 mM AICAR for 1 h in 3 independent experiments. Cell lysates prepared in Nonidet P-40 (NP-40)-containing buffer were subjected to immunoblotting to measure the relative content of p-AMPK, AMPK, phosphorylated p38 (p-p38), total p38, phosphorylated ERK1/2 (p-ERK1/2), and total ERK. In each experiment, the densitometry value of each phosphoprotein in the different conditions was normalized against relative amounts of each protein. The results of 3 independent experiments were averaged and are expressed as fold change compared with the value of the control. C and D: time course. Confluent Clone 9 cells were treated with diluent, 10 mM azide, or 2 mM AICAR for 10, 30, 60, or 120 min. Cell lysates were subjected to immunoblotting to measure the relative content of cell p-AMPK, total AMPK, p-p38, total p38, p-ERK1/2, and total ERK. In each experiment, the densitometry value of each phosphoprotein was normalized against the relative abundance of each protein. The experiment was repeated 3 times, and the results were averaged and are expressed as fold change.
Using 10 mM azide and 2 mM AICAR, we found that the abundance of p-AMPK (normalized against total AMPK) was significantly increased in azide- and AICAR-treated cells, reaching a near-maximal level at 60 min (Fig. 3C). Again, we noted that azide selectively increased the abundance of p-ERK1/2, whereas exposure to AICAR exclusively increased the abundance of p-p38; results of repeated experiments are shown in Fig. 3D (all values were normalized against the total amount of the specific protein being examined). We found no evidence that JNK phosphorylation occurred following exposure to azide or AICAR.
To verify the above results using an alternative approach, we immunoprecipitated p-p38 from Clone 9 cells treated with AICAR, azide, and their combination and developed the immunoblots using antibodies directed at total p-38. Consistent with the above observations, exposure to AICAR, but not azide, increased the abundance of p-p38 (data not shown).
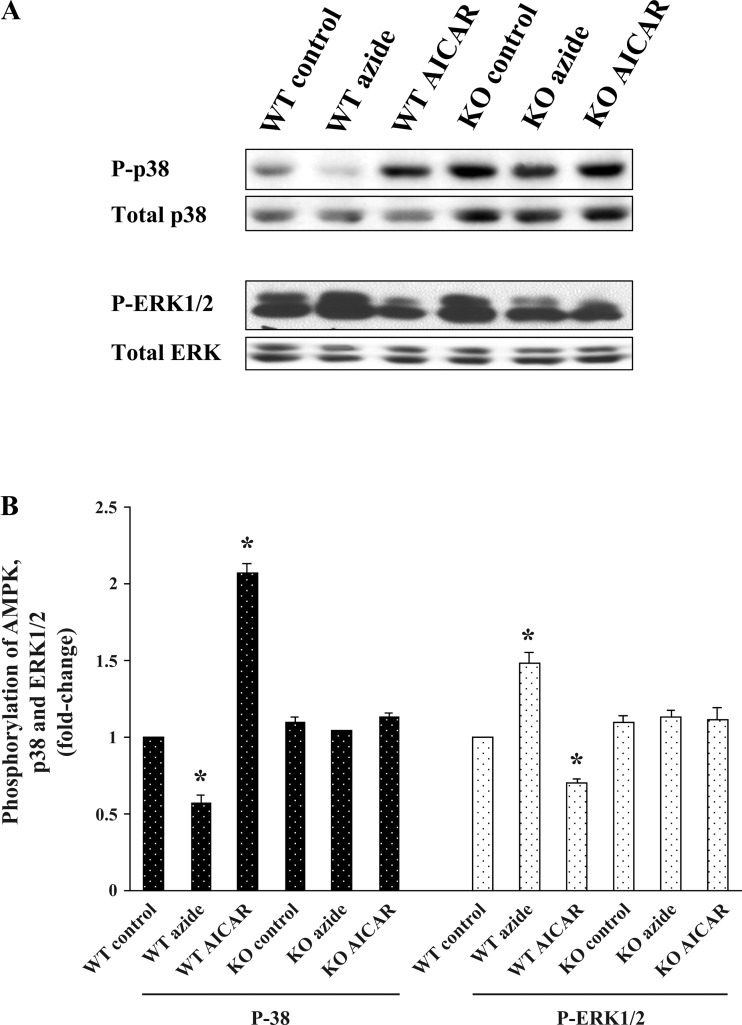
Selective phosphorylation of ERK1/2 by azide and of p38 by AICAR in mouse fibroblasts.
To explore for possible generality of the divergent signaling pathways observed in Clone 9 cells, we examined the response to azide and AICAR in the mouse AMPK WT and AMPK KO fibroblast cell lines. In WT cells, phosphorylation of ERK1/2 increased selectively in azide-treated cells (1.4 ± 0.1-fold), and phosphorylation of p38 increased 2.1 ± 0.1-fold only in AICAR-treated cells, whereas the abundances of total AMPK, ERK1/2, and p38 in the WT cells remained unchanged (Fig. 4, left 3 lanes). In KO cells, the abundance of p-p38 was increased, as was the content of total p38. However, the ratio of p-p38 to p38 in KO cells was the same as that in WT cells. In addition, the selective activation of p-ERK1/2 and p-p38 was totally absent in KO cells in response to azide or AICAR treatment (Fig. 4, right 3 lanes). It should be noted that the increased abundance of p-p38 and p-38 in KO cells under control conditions is not associated with an increase in the basal level of glucose transport (see Fig. 1). Quantitation of three separate experiments is shown in Fig. 4B.
Fig. 4.
Effect of azide and AICAR on the abundance of p-p38 and p-ERK1/2 in mouse fibroblasts. A and B: confluent AMPK WT and KO mouse fibroblasts were treated with diluent, 10 mM azide, or 2 mM AICAR for 1 h. Cell lysates were subjected to immunoblotting to measure the relative content of the phospho- and total proteins. The experiment was repeated 3 times; a representative blot is shown. Results of the blots were analyzed using densitometry and normalized against total p38 and total ERK1/2. Phosphorylation of p38 and ERK1/2 is expressed as fold change compared with values in individual control cells. *P < 0.05 compared with individual control. P-p38 increased response to AICAR treatment, and p-ERK1/2 increased response to azide treatment in WT mouse fibroblasts, whereas there was no change in phosphoprotein in KO mouse fibroblast exposed to either azide or AICAR.
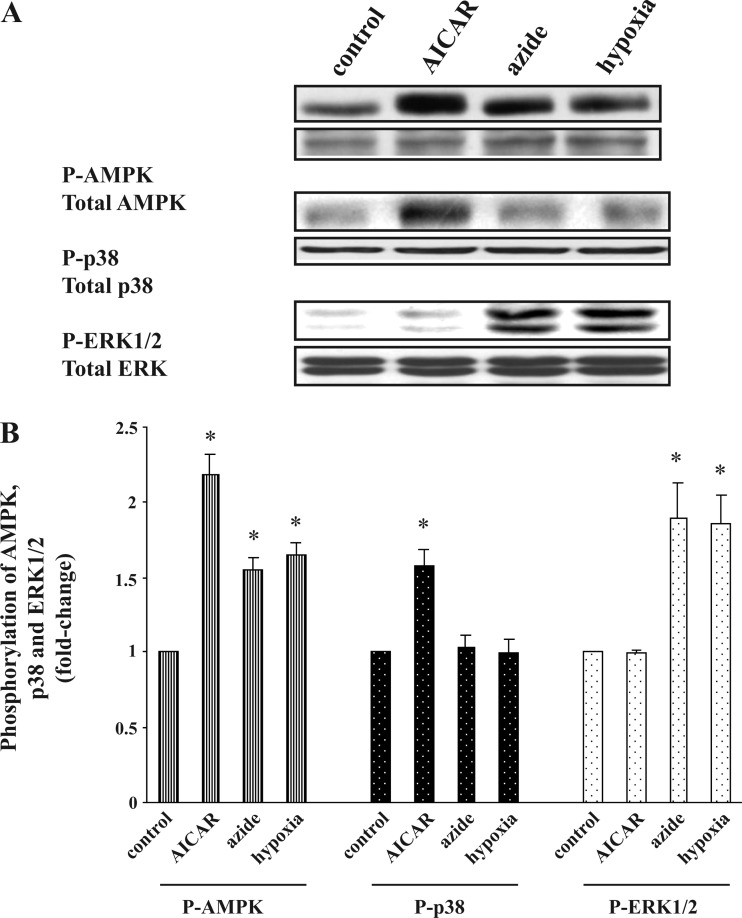
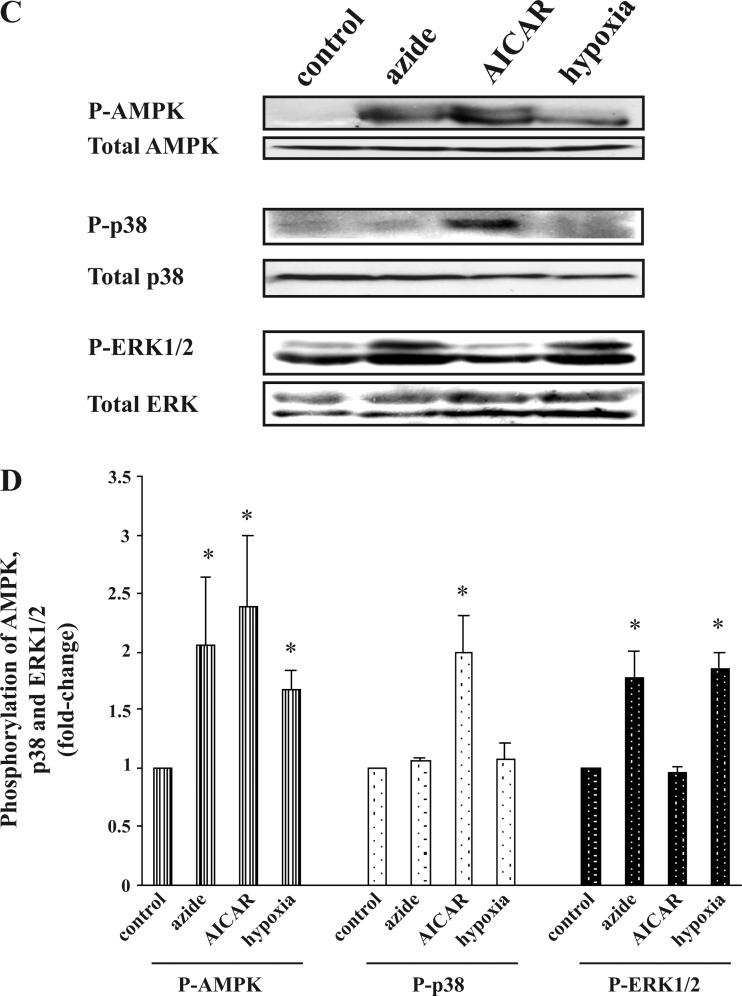
Effect of hypoxia on glucose transport, p-AMPK, p-ERK1/2, and p-p38 in Clone 9 and H9C2 cells.
Given the apparent divergent pathways downstream to activation of AMPK, we wondered which pathway is involved in the response to hypoxia. Consistent with our previous study (20), incubation of Clone 9 cells under hypoxic conditions for 1 h resulted in a 1.7 ± 0.1-fold increase in the rate of glucose transport. Azide (10 mM) and hypoxia respectively increased the abundance of p-AMPK by 1.6 ± 0.1- and 1.7 ± 0.2-fold and increased the cellular content of p-ERK1/2 by 1.9 ± 0.2- and 1.9 ± 0.1-fold (Fig. 5A). Similar results were obtained in H9c2 cardiac myocyte cells (Fig. 5B). However, neither Clone 9 nor H9c2 cells exposed to hypoxia showed an increase in the abundance of p-p38. (All the above results were normalized against the abundance of the protein under examination.) The above results suggest not only that the selective signaling pathway found in Clone 9 cells and fibroblasts is present in the cardiac myocyte cell line but also that the response to hypoxia is similar to the response to azide but not the response to AICAR.
Fig. 5.
Effect of hypoxia on the abundance of p-AMPK, p-p38, and p-ERK1/2 in Clone 9 and H9C2 cells. A and B: Clone 9 cells. Confluent Clone 9 cells were exposed to hypoxia for 1 h, while cells treated with diluent, 10 mM azide, or 2 mM AICAR served as controls. C and D: H9C2 cells. Confluent H9C2 cells were exposed to hypoxia for 1 h, while cells treated with diluent, 10 mM azide, or 2 mM AICAR served as controls. The experiment was repeated 3 times; a representative blot is shown. Results of the blots were analyzed using densitometry and normalized against total AMPK, total p38, and total ERK1/2. Phosphorylation of AMPK, p38, and ERK1/2 is expressed as fold change compared with values in control cells. *P < 0.05 compared with individual control.
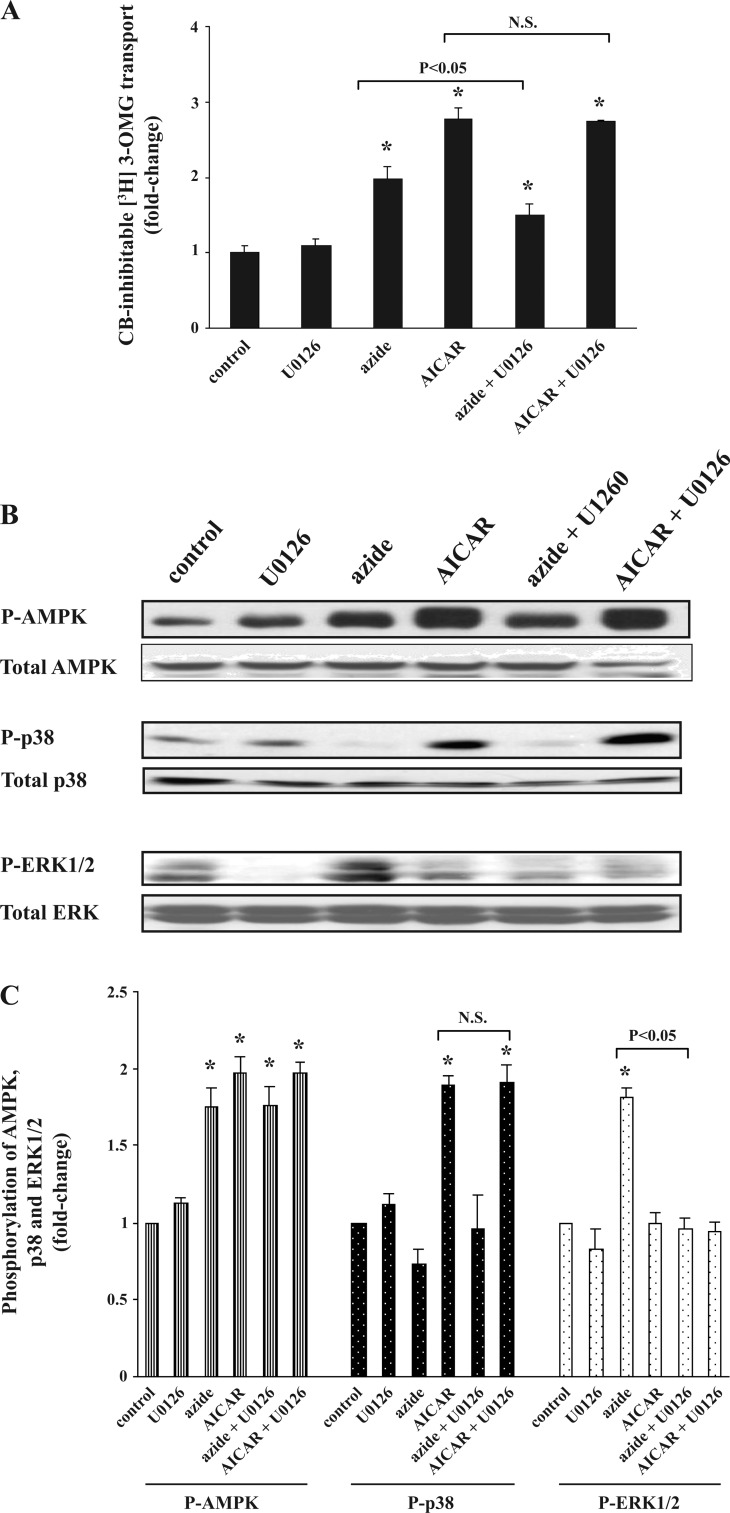
Effect of U0126 on azide-induced phosphorylation of ERK1/2 and stimulation of glucose transport in Clone 9 cells.
Use of U0126 as an inhibitor of MEK1 and MEK2, both of which are upstream of ERK1/2, decreased the stimulation of glucose transport in response to azide by ∼50%, whereas the AICAR-induced stimulation of glucose transport was unaffected (Fig. 6A). U0126 had no significant effect on phosphorylation of AMPK in response to AICAR or azide (Fig. 6, B and C). However, U0126 significantly reduced the abundance of p-ERK1/2 in cells treated with azide and had no effect on AICAR-induced increase in p-p38.
Fig. 6.
Effect of U0126 on azide- or AICAR-induced stimulation of glucose transport, p-AMPK, p-p38, and p-ERK1/2. A: glucose transport. Confluent Clone 9 cells in triplicate 60-mm dishes were pretreated with diluent or 10 μM U0126 for 20 min before being treated with diluent, 10 mM azide, or 2 mM AICAR for 1 h. Uptakes in control and treated cells were performed in parallel. The experiment was repeated 3 times using triplicate dishes for each condition, and the results were averaged. Values are expressed as fold change (±SE) compared with control. *P < 0.05 compared with control (ANOVA). Glucose transport was lower in cells treated with both U0126 and azide compared with azide alone (t-test; P < 0.05). U0126 had no effect on glucose transport in AICAR-treated cells. B and C: abundance of p-AMPK, p-p38, and p-ERK1/2. Cells were treated as described in A, lysed in NP-40-containing buffer, and used for immunoblotting. In each experiment, the densitometry value of phosphorylation of each protein was normalized against the control, and the results of 3 independent experiments were averaged. *P < 0.05 compared with control. Cells exposed to U0126 plus azide had decreased abundance of p-ERK1/2 compared with azide alone (P < 0.05). U0126 had no effect on p-p38 in AICAR-treated cells.
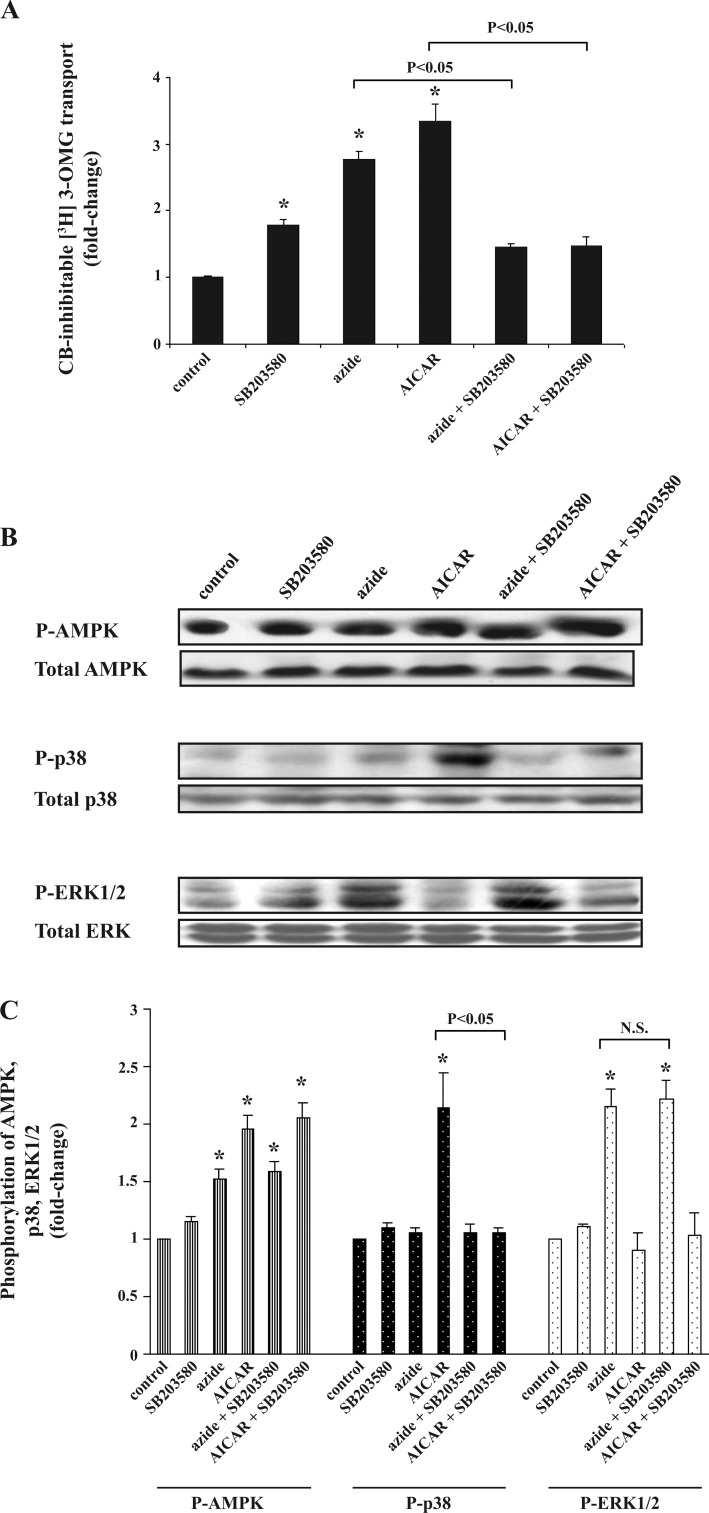
Effect of SB 203580 on AICAR-induced phosphorylation of p38 and stimulation of glucose transport in Clone 9 cells.
Exposure to SB 203580 (an inhibitor of p38) led to a substantial reduction in the stimulation of glucose transport in response to AICAR (Fig. 7A). SB 203580 also inhibited the glucose transport response to azide; the reasons for this finding are unknown. Exposure to SB 203580 alone also stimulated glucose uptake, perhaps due to nonspecific effects. SB 203580 had a profound negative effect on the abundance of p-p38 in response to AICAR, whereas the agent had no effect on the increase in the abundance of p-ERK1/2 in response to azide (Fig. 7, B and C).
Fig. 7.
Effect of SB 203580 on azide- or AICAR-induced stimulation of glucose transport, p-AMPK, p-p38, and p-ERK1/2. A: glucose transport. Confluent Clone 9 cells in triplicate 60-mm dishes were serum-starved for 24 h before the experiment. Cells were pretreated with 10 μM SB 203580 or diluent for 60 min before being treated 10 mM azide or 2 mM AICAR for 1 h. Uptakes in control and all treated cells were performed in parallel. The experiment was repeated 2 times using triplicate dishes for each condition, and the results were averaged. Values are expressed as fold change (±SE) compared with basal values. *P < 0.05 compared with control (ANOVA). Glucose uptake was significantly different in cells treated with both SB 203580 and AICAR vs. AICAR alone and in cells treated with both SB 203580 and azide vs. azide alone (t-test; P < 0.05). Exposure to SB 203580 alone also stimulated the rate of glucose transport. B and C: abundance of p-AMPK, p-p38, and p-ERK1/2. Cell treated as described in A were lysed in NP-40-containing buffer and used for immunoblotting. In each experiment, the densitometry value of each phosphoprotein in each condition was normalized against the control, and the results of 3 independent experiments were averaged. *P < 0.05 compared with control. Cells preexposed to SB 203580 and AICAR had decreased abundance of p-p38 compared with cells treated with AICAR alone (P < 0.05). SB 203580 had no effect on the effect of azide on p-ERK1/2.
Effects of azide, AICAR, and hypoxia on phosphorylation of AS160 in Clone 9 cells.
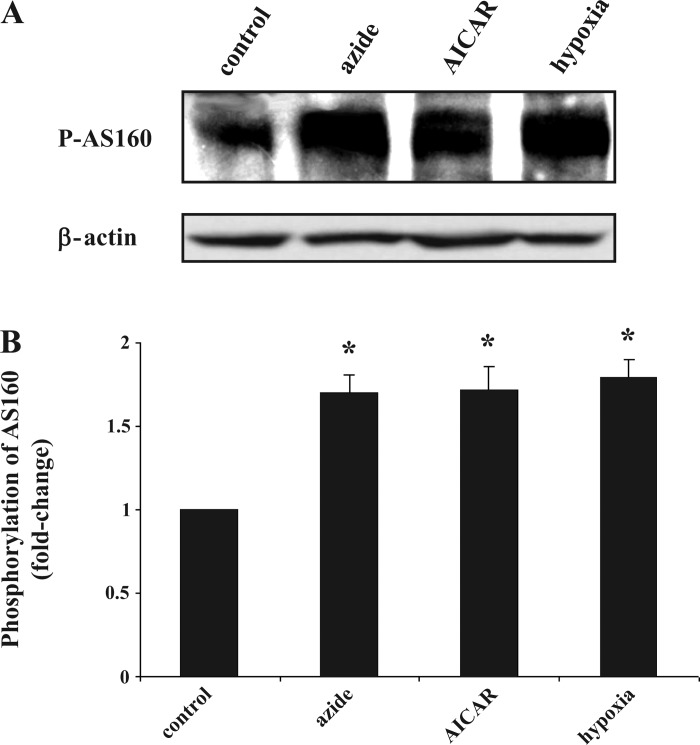
Recent reports suggest that AS160 and its phosphorylation status are a central point of convergence for insulin signaling and for AICAR-stimulated AMPK signaling pathways in the stimulation of Glut4-mediated glucose transport (18, 22, 36). We examined whether activation of AMPK by azide, hypoxia, or AICAR is also associated with phosphorylation of this novel regulator. There was increased phosphorylation of AS160 in Clone 9 cells by all three stimuli (Fig. 8A).
Fig. 8.
Effect of azide, hypoxia, and AICAR on the abundance of phosphorylated Akt substrate of 160 kDa (p-AS160). A: Western blot of p-AS160. Confluent Clone 9 cells were treated with diluent, 10 mM azide, hypoxia, or 2 mM AICAR for 1 h. Cell lysates were subjected to immunoblotting to measure the relative content of p-AS160; β-actin was used as a control for loading of the gel. B: quantification of p-AS160. Results of 3 independent experiments were averaged and are expressed as fold change compared with control. *P < 0.05 compared with control (n = 3).
We next explored the possibility that AS160 is associated with Glut1, especially upon exposure to hypoxia, azide, or AICAR. Employing Clone 9 cells, we immunoprecipitated Glut1 and examined the presence of AS160 or p-AS160 in the pellet. In three independent experiments, we found no evidence of AS160 in the Glut1 immunoprecipitates (data not shown). Because of limitations in the availability of immunoprecipitating antibodies against rat AS160, we employed HepG2 cells. In three independent experiments, we found no Glut1 in immunoprecipitates of AS160 in control cells or in cells exposed to hypoxia, azide, or AICAR (data not shown).
Effect of downregulation of AS160 expression on the glucose transport response in Clone 9 cells.
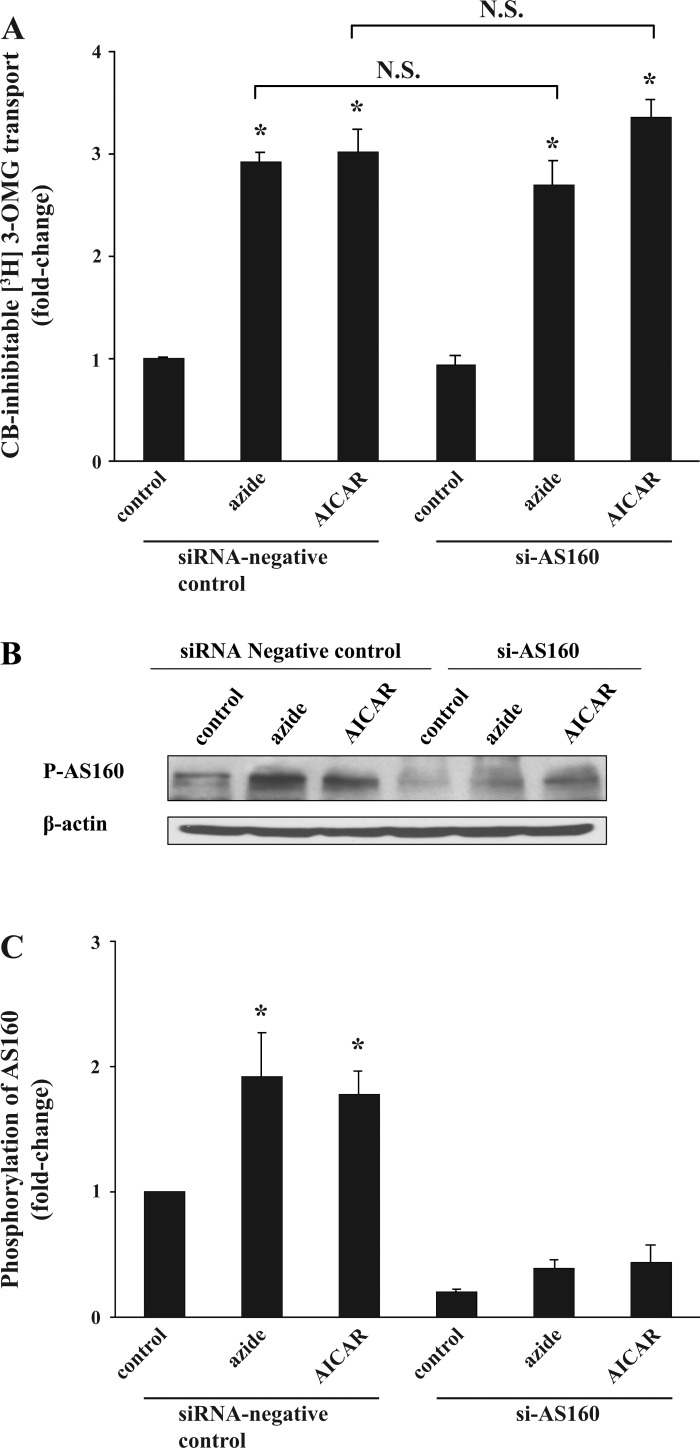
To further investigate the role of AS160 in azide- or AICAR-induced glucose transport, we decreased the expression of AS160 by application of siAS160. Forty-eight hours after transfection with 100 pmol/ml siAS160 or nonspecific scrambled siRNA, the acute effect of 10 mM azide and 2 mM AICAR on 3-OMG-inhibitable glucose transport was measured. Treatment with azide or AICAR for 1 h stimulated the rate of glucose transport in control-transfected cells by 2.9 ± 0.1- and 3.0 ± 0.2-fold, respectively, and the stimulation of transport by these agents was not affected in cells treated with siAS160 (2.7 ± 0.2- and 3.4 ± 0.2-fold, respectively; P > 0.05) (Fig. 9A). Samples of lysates assayed for p-AS160 content in two independent experiments showed an average decrease of 81, 80, and 75% in basal, azide-, and AICAR-treated cells, respectively (Fig. 9B). (Antibody against p-AS160 was employed in this experiment because of the poor quality of the blots using the anti-total AS160 antibody against the rat protein; nevertheless, the same degree of depression of AS160 was observed using the latter antibody.) The abundance of β-actin (used as a control) remained unchanged.
Fig. 9.
Effect of small interfering RNA directed against AS160 (siAS160) on glucose transport in response to azide and AICAR and on p-AS160. A: glucose transport. Seventy percent confluent Clone 9 cells in triplicate dishes were transfected with 5 μl/ml Lipofectamine 2000 containing 100 pmol/ml siAS160 or scrambled siRNA. Forty-eight hours after transfection, cells were treated with diluent, 10 mM azide, or 2 mM AICAR for 1 h before measurement of CB-inhibitable 3-OMG transport. The experiment was repeated another time, and the results were averaged. *P < 0.05 compared with control siRNA. The stimulation of transport in response to azide or AICAR was not decreased by exposure to the siRNA (P > 0.05). B and C: p-AS160. Cells lysates were subjected to immunoblotting for p-AS160. Similar results were obtained in a second independent experiment. The content of β-actin remained constant. Results of the blots were analyzed using densitometry and normalized against the respective control. *P < 0.05 compared with control.
DISCUSSION
The present study was prompted by our previous report that activation of AMPK is critical for the stimulation of glucose transport in response to exposure to AICAR or following inhibition of oxidative phosphorylation by azide (21). This conclusion was based on results of experiments using Clone 9 cells (a nontransformed rat liver-derived cell line expressing Glut1), siRNA technology, and compound C as an inhibitor of AMPK (21). However, the steps subsequent to activation of AMPK that lead to stimulation of Glut1-mediated glucose transport remained unknown. In the present study, we have made a number of novel observations that extend the previous findings and help enlighten some of the mediating pathways in the above response. 1) In contrast to WT mouse fibroblasts, fibroblasts devoid of both AMPK α1- and α2-isoforms showed no stimulation of glucose transport in response to either azide or AICAR, verifying the essential role of AMPK in this response. 2) The stimulatory effect of azide and AICAR on glucose transport was not additive, whereas their effect on the abundance of p-AMPK was near-additive. 3) Although azide and AICAR both lead to increased phosphorylation of AMPK that is associated with stimulation of AMPK activity (1, 4, 32, 34, 35), the signaling pathways appear to be divergent (in Clone 9 cells, mouse WT fibroblasts, and H9C2 cells); azide selectively resulted in phosphorylation of ERK1/2, whereas exposure to AICAR led to phosphorylation of p38. 4) Similar to azide, hypoxia selectively phosphorylated ERK1/2 in both Clone 9 cells and H9c2 cells. 5) Exposure of Clone 9 cells to hypoxia, azide, or AICAR resulted in phosphorylation of AS160. However, Glut1 was not physically associated with AS160 (or p-AS160), and reduced expression of AS160 by siRNA directed against the protein did not diminish the glucose transport response to either azide or AICAR. Finally, 6) the results raise the possibility that cells contain different functional pools of AMPK, one responsive to azide and hypoxia and the other responsive to AICAR. It is worth emphasizing that the above results involve Glut1- and not Glut4-mediated glucose transport.
We took advantage of the availability of mouse fibroblasts in which both α-isoforms of AMPK have been deleted (24). These cells afforded us the opportunity to rigorously test the hypothesis that stimulation of AMPK is critical for stimulation of glucose transport in response to azide and AICAR. The results indicated that in the absence of phosphorylation of AMPK, the rate of glucose transport was completely unaffected by the above stimuli. In contrast, WT fibroblasts showed a positive glucose transport response to both azide and AICAR.
Exposure to azide or AICAR alone stimulated the rate of glucose uptake, and simultaneous exposure to both agents was not additive and resulted in no further increase in the rate of glucose transport compared with AICAR alone. Interestingly, simultaneous exposure to both agents resulted in a higher abundance of p-AMPK than exposure to either agent alone in a near-additive manner. These findings raise the possibility of potential heterogeneity in cell AMPK distribution; alternatively, cells may contain different functional pools of AMPK, one responsive to azide and the other to AICAR. One source of this presumed heterogeneity could result from the possibility that α1- and α2-isoforms of AMPK respond differentially to the above two stimuli. However, the available evidence is not in keeping with this premise for two reasons: 1) we and others have reported that the α1-isoform of AMPK is by far the predominant isoform of AMPK in Clone 9 cells (4), and more importantly, 2) suppression of AMPKα2 mRNA and protein content using siRNA technology did not alter the glucose transport response of these cells to either azide or AICAR, whereas siRNA directed toward the α1-isoform significantly inhibited the response to either agent (21).
We also made the novel observation that stimulation of AMPK by exposure to azide or hypoxia leads to a selective stimulation of the ERK1/2 pathway, whereas AMPK stimulation by AICAR leads to selective activation of the p38 pathway in three cell lines, namely, Clone 9, H9c2 cells, and fibroblasts. Neither agent stimulated the phosphorylation of JNK. It has been reported that exposure of L6 cells to AICAR increases the phosphorylation of ERK1/2 (9). In agreement with this report, we found an ∼1.7-fold increase in phosphorylation of ERK1/2 by AICAR in L6 cells; however, exposure to AICAR resulted in an ∼1.9-fold increase in p-p38 (data not shown). In further experiments employing inhibitors of ERK1/2 and p38 pathways, we made two critical observations, namely, 1) although U0126 and SB 203580 were observed to selectively inhibit the phosphorylation of these two mitogen-activated protein kinases (MAPKs), they had no effect on phosphorylation of AMPK in response to either azide or AICAR, suggesting that these mediators are “downstream” to activation of AMPK, and 2) phosphorylation of ERK1/2 and p38 in response to azide and AICAR, respectively, appeared to be responsible for the observed stimulation of glucose transport in response to the above stimuli. Finally, we found that the signaling pathway following the activation of AMPK in response to hypoxia was similar to the pathway that is stimulated in response to azide but not to AICAR.
An unexpected finding was that SB 203580 significantly inhibited the glucose transport response to azide as well as to AICAR. We also noted that exposure to SB 203580 alone stimulated the rate of glucose transport. The reasons for these findings are not clear and probably reflect well-known nonspecific actions of this inhibitor, a situation that limits analysis of results of experiments using this inhibitor (5, 25). Other investigators have noted the structural similarities between Glut1 and Glut4 and that SB 203580 can inhibit Glut4 function (2, 31). Although it is possible that this agent has a similar effect on Glut1, our finding that addition of SB 203580 alone to control cells stimulated transport makes this possibility less likely.
The nonadditive nature of the glucose transport response to azide plus AICAR raises the possibility of a common pathway in which the signals generated by azide and AICAR converge. We considered AS160 as a potential candidate. AS160 is a novel Rab GTPase-activating protein with two phosphotyrosine domains, a COOH-terminal Rab-GAP domain, and six phosphorylation motifs (RXRXXS*/T*) targeted by Akt and other kinases (7). Interestingly, in addition to insulin, AICAR increases the phosphorylation of AS160 in rat skeletal muscle (11, 22, 36). Furthermore, under basal conditions, AS160 in skeletal muscle is attached to intracellular Glut4-containing vesicles, and phosphorylation of AS160 results in its binding to 14-3-3 protein, leading to dissociation of p-AS160 from the vesicles (18). Finally, in a cell-free system, AS160 is phosphorylated by activated AMPK (36). On the basis of these findings, we examined whether AS160 becomes phosphorylated in response to azide and hypoxia similar to that reported for AICAR. The results indicated that this is indeed the case: phosphorylation of AS160 was significantly increased in response to azide, hypoxia, and AICAR. This finding raises the intriguing possibility that AS160 is either in the presumed final common pathway leading to increased glucose uptake or might itself serve as the point of convergence in the signaling pathways that are stimulated by azide, hypoxia, and AICAR. However, our results demonstrate that AS160 (or p-AS160) is not physically associated with Glut1 (similar to Glut4-expressing tissues) and that decreased expression of AS160 with the use of siRNA did not diminish the glucose transport response to the above stimuli. Hence, the phosphorylation of AS160 that occurs in response to AMPK activation may not play a mediating role in Glut1-mediated glucose transport response. This result is not totally unexpected, since in contrast to Glut4-expressing cells, the major mode of stimulation of glucose transport in cells expressing Glut1 is through activation of transporters preexisting in the plasma membrane (15). On the other hand, it should be noted that in the case of insulin signaling in 3T3-L1 adipocytes leading to Glut4 translocation, a minor amount (∼5–10%) of AS160 (Rab GAP TBC1D4/AS160) is sufficient for the glucose transport response (18). This raises the possibility that the ∼80% decrease in AS160 content was not sufficient to inhibit the glucose transport response in our experiments.
The present study has illuminated some of the signaling steps that occur following the inhibition of oxidative phosphorylation and activation of AMPK that lead to stimulation of Glut1-mediated glucose transport. In addition, it appears that activation of ERK1/2 and p38 pathways by azide and hypoxia on the one hand and AICAR on the other, respectively, is essential in the glucose transport response. Nevertheless, the absolute necessity of phosphorylation of these mediators, and the steps leading to the apparent activation of Glut transporters residing in the plasma membrane, is unclear at present and requires further study.
NOTE ADDED IN PROOF
In the Articles in PresS version of this manuscript, the images representing some of the lanes in Figs. 3, 4, and 9 were not from the same blot or were rearranged from the same blot. We regret this error. Here, in this final published version, the new figures are from the same experiment and blot, and the fields from within the same experimental treatment group are shown as a composite image within the same panel.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-61994.
Supplementary Material
Acknowledgments
We thank Bridget Christopher and Dr. Kimberly Martin for generous help in the glucose transport assays. AMPK wild-type and AMPK knockout fibroblasts were the generous gifts of Dr. Benoit Viollet.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abbud W, Habinowski S, Zhang JZ, Kendrew J, Elkairi FS, Kemp BE, Witters LA, Ismail-Beigi F. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch Biochem Biophys 380: 347–352, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Antonescu CN, Huang C, Niu W, Liu Z, Eyers PA, Heidenreich KA, Bilan PJ, Klip A. Reduction of insulin-stimulated glucose uptake in L6 myotubes by the protein kinase inhibitor SB203580 is independent of p38MAPK activity. Endocrinology 146: 3773–3781, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Asahi Y, Hayashi H, Wang L, Ebina Y. Co-localization of the translocated GLUT4 with rearranged actin by insulin treatment in CHO cells and L6 myotubes. J Med Invest 46 :192–199, 1999. [PubMed] [Google Scholar]
- 4.Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer Lee GD, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic, and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115: 2433–2442, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Begonja AJ, Geiger J, Rukoyatkina N, Rauchfuss S, Gambaryan S, Walter U. Thrombin stimulation of p38 MAP kinase in human platelets is mediated by ADP, and thromboxane A2 and inhibited by cGMP/cGMP-dependent protein kinase. Blood 109: 616–618, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Behrooz A, Ismail-Beigi F. Induction of GLUT1 mRNA in response to azide and inhibition of protein synthesis. Mol Cell Biochem 187: 33–40, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem 276: 9519–9525, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV Jr, Farese RV. Activation of the ERK pathway, and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-β-d-riboside (AICAR)-stimulated glucose transport. J Biol Chem 277: 23554–23562, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol 4: 315–324, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Deshmukh A, Coffey VG, Zhong ZH, Chibalin AV, Hawley HA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes 55: 1776–1782, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Emoto M, Langille SE, Czech MP. A role for kinesin in insulin-stimulated GLUT4 glucose transporter translocation in 3T3-L1 adipocytes. J Biol Chem 276: 10677–10682, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Fryer LG, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J 363: 167–174, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakimian J, Ismail-Beigi F. Enhancement of glucose transport in Clone 9 cells by exposure to alkaline pH: studies on potential mechanisms. J Membr Biol 120: 29–39, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Hamrahian AH, Zhang JZ, Elkhairi FS, Prasad R, Ismail-Beigi F. Activation of Glut1 glucose transporter in response to inhibition of oxidative phosphorylation. Arch Biochem Biophys 368: 375–379, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol 85: 1218–1222, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, Ebina Y, James DE. IRS1-independent defects define major nodes of insulin resistance. Cell Metab 7: 421–433, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howlett KF, Sakamoto K, Garnham A, Cameron-Smith D, Hargreaves M. Resistance exercise and insulin regulate AS160 and interaction with 14-3-3 in human skeletal muscle. Diabetes 56: 1608–1614, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Ismail-Beigi F Metabolic regulation of glucose transport. J Membr Biol 135: 1–10, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Jing M, Ismail-Beigi F. Role of AMP-activated protein kinase in stimulation of glucose transport in response to inhibition of oxidative phosphorylation. Am J Physiol Cell Physiol 290: C484–C491, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Jing M, Ismail-Beigi F. Critical role of 5′-AMP-activated protein kinase in the stimulation of glucose transport in response to inhibition of oxidative phosphorylation. Am J Physiol Cell Physiol 292: C477–C487, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55: 2067–2076, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin-, and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol 26: 5336–5347, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin alphaIIb beta3. Blood 107: 965–972, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercado CL, Loeb JN, Ismail-Beigi F. Enhanced glucose transport in response to inhibition of respiration in Clone 9 cells. Am J Physiol Cell Physiol 257: C19–C28, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated AICAR protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol Endocrinol Metab 273: E1107–E1112, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Mitani Y, Dubyak GR, Ismail-Beigi F. Induction of GLUT1 mRNA in response to inhibition of oxidative phosphorylation: role of increased [Ca2+]i. Am J Physiol Cell Physiol 270: C235–C242, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Mueckler M Facilitative glucose transporters. Eur J Biochem 219: 713–725, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence, and structure of a human glucose transporter. Science 229: 941–945, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Ribé D, Yang J, Patel S, Koumanov F, Cushman SW, Holman GD. Endofacial competitive inhibition of glucose transporter-4 intrinsic activity by the mitogen-activated protein kinase inhibitor SB203580. Endocrinology 146: 1713–1717, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG. Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase, and a model substrate. J Mol Biol 317: 309–323, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Shetty M, Loeb JN, Vikstrom K, Ismail-Beigi F. Rapid activation of GLUT-1 glucose transporter following inhibition of oxidative phosphorylation in Clone 9 cells. J Biol Chem 268: 17225–17232, 1993. [PubMed] [Google Scholar]
- 34.Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, Van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31: 162–168, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jørgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic, and regulatory subunits. Diabetes 55: 2051–2058, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol 20: 6704–6711, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi X, Han J, Zhang JZ. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J Biol Chem 276: 41029–41034, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.