Abstract
Alteration in cell volume of vertebrates results in activation of volume-sensitive ion flux pathways. Fine control of the activity of these pathways enables cells to regulate volume following osmotic perturbation. Protein phosphorylation and dephosphorylation have been reported to play a crucial role in the control of volume-sensitive ion flux pathways. Exposing Amphiuma tridactylu red blood cells (RBCs) to phorbol esters in isotonic medium results in a simultaneous, dose-dependent activation of both Na+/H+ and K+/H+ exchangers. We tested the hypothesis that in Amphiuma RBCs, both shrinkage-induced Na+/H+ exchange and swelling-induced K+/H+ exchange are activated by phosphorylation-dependent reactions. To this end, we assessed the effect of calyculin A, a phosphatase inhibitor, on the activity of the aforementioned exchangers. We found that exposure of Amphiuma RBCs to calyculin-A in isotonic media results in simultaneous, 1–2 orders of magnitude increase in the activity of both K+/H+ and Na+/H+ exchangers. We also demonstrate that, in isotonic media, calyculin A-dependent increases in net Na+ uptake and K+ loss are a direct result of phosphatase inhibition and are not dependent on changes in cell volume. Whereas calyculin A exposure in the absence of volume changes results in stimulation of both the Na+/H+ and K+/H+ exchangers, superimposing cell swelling or shrinkage and calyculin A treatment results in selective activation of K+/H+ or Na+/H+ exchange, respectively. We conclude that kinase-dependent reactions are responsible for Na+/H+ and K+/H+ exchange activity, whereas undefined volume-dependent reactions confer specificity and coordinated control.
Keywords: cell volume regulation, volume-dependent coordination of K loss and Na uptake, phosphoprotein, phosphatase inhibitors, phosphorylation
cell volume regulation is a fundamental physiological process whereby cells restore their normal steady-state volume in response to acute osmotic perturbation (for review see 13, 22, 23). In general, the response to cell swelling is a rapid net efflux of potassium (K+) together with chloride (Cl−) (or taurine), and water. In contrast, the response to cell shrinkage is a rapid net influx of sodium (Na+), together with Cl− and water. The flux pathways mediating net ion fluxes responsible for volume regulation vary from cell to cell but are generally characterized by the following features: 1) at normal resting cell volume, the volume-sensitive flux pathways are minimally active; 2) volume-sensitive ion flux activity is a graded function of cell volume; and 3) ion flux activity deactivates as normal volume is restored. Implicit in the above observations are the notions that volume-regulatory ion flux pathways are inducible, highly regulated, and coordinated around the cell's normal resting volume (volume set point). Although cell volume regulatory phenomena are well described, the basis for activation, control, and coordination of the volume regulatory flux pathways are poorly understood (for review see 13, 14, 17, 26, 39).
The original model proposed by Jennings and Al-Rohil (26) explaining the kinetic basis for regulation of volume-sensitive ion flux pathways, demonstrated that in rabbit red blood cells (RBCs), protein dephosphorylation events are responsible for activation of K+-Cl− cotransport in response to cell swelling. They presented compelling evidence illustrating that osmotic cell swelling decreases the activity of the kinase responsible for deactivation of K+-Cl− cotransport, yet is without effect on the activity of the opposing phosphatase. Consequently, phosphatase activity is dominant following cell swelling and the K+-Cl− cotransporter is activated. In a similar study, Parker et al. (39) presented a model accounting for the coordinated, volume-dependent regulation of swelling-induced K+-Cl− cotransport and shrinkage-induced Na+/H+ exchange in dog RBCs. In agreement with Jennings, they demonstrated that in response to cell swelling, there is a volume-dependent decrease in kinase activity, resulting in activation of K+-Cl− cotransport and deactivation of Na+/H+ exchange. In addition, following cell shrinkage, kinase activity increases and is responsible for activation of Na+/H+ exchange and deactivation of K+-Cl− cotransport. Thus the activity of the controlling kinase is an inverse function of cell volume while the activity of the controlling phosphatase is insensitive to changes in volume.
As with rabbit and dog RBCs, Amphiuma RBCs regulate volume by losing K+ in response to cell swelling and gaining Na+ in response to cell shrinkage: the K+ loss and Na+ uptake pathways are coordinated around the volume set point. In contrast to findings in the rabbit and dog, the ion flux pathways that mediate K+ loss and Na+ uptake in Amphiuma RBCs are the K+/H+ and Na+/H+ exchangers, respectively (8). Furthermore, in isotonic media, exposure of Amphiuma RBCs to phorbol 12,13-myristate acetate (PMA), results in simultaneous induction of both K+/H+ and Na+/H+ exchange (10). Hence the PMA data support the interpretation that activation of both swelling and shrinkage-induced solute fluxes are the result of phosphorylation-dependent reactions, whereas other undetermined events associated with volume perturbation are responsible for selective activation of either Na+/H+ or K+/H+ exchange. If the PMA data are a reflection of the central role of a kinase(s) in volume-dependent activation of both Na+/H+ and K+/H+ exchange and, if tonic levels of the appropriate kinase activities are significant in resting cells, then exposing Amphiuma RBCs to phosphatase inhibitors should result in the simultaneous activation of both Na+/H+ and K+/H+ exchange fluxes.
In the present study, we employed the protein phosphatase inhibitor, calyculin A (CLA), to test the hypothesis that, in Amphiuma RBCs, both the shrinkage-induced Na+/H+ exchanger and swelling-induced K+/H+ exchanger are activated as a result of phosphorylation-dependent reactions. The data presented here illustrate that exposure of Amphiuma RBCs to CLA in isotonic media results in simultaneous, robust net K+ loss and Na+ uptake. The time-course and dose-dependent activations by CLA and dose-dependent inhibition by 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) are identical for both flux pathways, and the thermodynamic driving forces for CLA-induced net Na+ and K+ fluxes identify the pathways as Na+/H+ exchange and K+/H+ exchange, respectively. In addition, we find that CLA-induced increases in both Na+ uptake and K+ loss are directly due to phosphatase inhibition, as opposed to secondary effects of changes in cell volume. Furthermore, whereas phosphatase inhibition (increased net phosphorylation) symmetrically activates both Na+/H+ and K+/H+ exchange in the absence of changes in cell volume (isotonic media), superimposing CLA exposure and cell swelling or cell shrinkage results in selective activation of K+/H+ or Na+/H+ exchange, respectively.
MATERIALS AND METHODS
General.
This project was conducted in accordance with the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals, and the University of California Davis Animal Welfare Assurance on file with the US Public Health Service. IACUC approved University of California Davis animal use and care protocol no. 07-12754.
Venous blood was drawn from adult Amphiuma tridactylum into 12-ml syringes containing heparin (Shein Pharmaceutical, Florham Park, NJ) (1 ml, 10,000 U/ml). RBCs were separated from plasma by low-speed centrifugation (1,000 g) in 15-ml conical bottom centrifuge tubes. The buffy coat (white layer) was removed by vacuum aspiration, and RBCs were washed three times in 10–15 volumes of isotonic HEPES-buffered Ringer solution (in mM: 80 NaCl, 3 KCl, 1 CaCl2, 0.5 MgCl2, 30 HEPES, 18 NaOH, and 5 glucose, pH adjusted to 7.65 ± 0.02) matched (by addition of NaCl) to the animal's plasma osmolarity (220–250 mosmol/kgH2O) as measured with a freezing-point depression osmometer (Advanced Instruments model no. 3D3). Isotonic media was aerated (with water-saturated room air) for 3 to 5 min and pH adjusted to 7.65 ± 0.02 (23°C) immediately before use. Washed RBCs were suspended at 10% hematocrit and incubated for 60 to 90 min (preincubation period) in the dark before experimental treatment. To initiate the experiments, cells were centrifuged (1,000 g) and suspended in experimental media (10% hematocrit). Unless specified, alterations in osmolarity were accomplished by varying media [NaCl]. All experimental media contained 1 mM ouabain (Sigma Chemical, St. Louis, MO), a Na/K ATPase inhibitor, unless specified. To activate or deactivate the K+/H+ and Na+/H+ exchangers, cells were exposed to experimental media of varying osmolarities ranging from hypotonic to hypertonic. Typically, hypotonic medium was 132 mosmol/kgH2O (0.55 times isotonic osmolarity; 0.55 × hypo) and hypertonic medium was 384 mosmol/kgH2O (1.6 times isotonic osmolarity; 1.6 × hyper), respectively. Given that we use very dilute solutions, we will equate mosmol/kgH2O to osmolarity (in mosmol/l H2O) throughout this article.
In some experiments it was necessary to activate the Na+/H+ or K+/H+ exchangers yet prevent volume regulation mediated by these ion flux pathways. Accordingly, cells were exposed to media where [Na+] and/or [K+] were chosen to maintain Na+/H+ and/or K+/H+ exchangers at thermodynamic equilibrium (nulled medium). At thermodynamic equilibrium, Na+/H+ or K+/H+ exchangers cannot mediate net fluxes of ions, regardless of the degree of transport activation. Thus in nulled media the exchange pathways cannot be used to alter cell volume in response to osmotic perturbation. The thermodynamic driving force (Δμ) for ion flux via Na+/H+ exchange is described by the following expression:
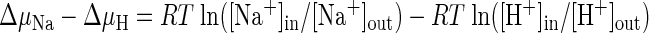 |
(See Ref. 8), where R is the gas constant and T is temperature in Kelvin, brackets denote concentration, and the subscripts “in” or “out” denote intracellular and extracellular compartments, respectively. At thermodynamic equilibrium Δμ = 0 and therefore, [Na+]in/[Na+]out = [H+]in/[H+]out. Since [Na+]in, [H+]in, and [H+]out are known, we can calculate [Na+]out necessary to maintain the Na+/H+ exchanger at thermodynamic equilibrium. An identical expression for [K+] and [H+] is used to calculate the [K+]out needed to maintain the K+/H+ exchanger at equilibrium. The desired osmolarity in nulled media was achieved by replacing Na+ and/or K+ with N-methyl-d-glucamine (NMDG).
Preparation of pharmacological compounds.
Ouabain (Sigma Chemical) was dissolved directly in experimental media to a final concentration of 1 mM. CLA (Boehringer Manheim Biochemicals, Indianapolis, IN) and EIPA (Sigma) stock solutions were prepared (at 1 mM) in DMSO (Sigma Chemical). The final concentration of DMSO in the experimental media containing EIPA was 0.1%, whereas the same value for the experimental media containing CLA was 0.25%.
Net ion flux measurements.
In net ion flux experiments, Na+, K+, Cl−, and water content were determined at appropriate intervals following initiation of flux (see Ref. 11). Briefly, 400-μl aliquots of RBCs (10% hematocrit) suspension were centrifuged (12,000 g) in preweighed 500-μl polyethylene centrifuge tubes (Stockwell Scientific, Monterrey Park, CA) for 4 min. Suspension media were sampled to determine media ion concentrations. Pellets were cleaned carefully by vacuum aspiration and weighed to determine wet cell weight. Cell pellets were subsequently lysed in 250 μl of 40 mM ZnSO4 and 5 mM MgSO4 (Mg2+ is a cofactor for endogenous nuclease to prevent DNA/hemoglobin gel formation upon cell disruption, and Zn2+ is used to precipitate protein) by mechanical disruption with a high-speed rotary tool. Lysates were centrifuged (10 min, 12,000 g) to separate the insoluble pellet from the clear supernatant. Supernatants were analyzed for Na+ and K+ by flame photometry (model 443, Instrumentation Laboratories, Boston, MA) and for Cl− by potentiometric titration with silver ions (Buchler Chloridometer, Searle Diagnostics, Fort Lee, NJ). The insoluble pellets were dried at 70°C for 18 to 24 h and water content was determined as the difference between wet and dry pellet weights on a 5-place analytic balance (Mettler-Toledo). The dry pellet weight was also used to normalize the ion content of cells to kilogram of dry cell solid (kg dcs). Each time point is thus expressed as millimole ion per kilogram dcs (i.e., mmol ion/kg dcs). The contribution of extracellular trapped ions and H2O were corrected for with an empirically determined factor (1, 11).
Unidirectional 22Na+ and 86Rb+ influx measurements.
To measure unidirectional ion influx, cells were suspended (10% hematocrit) in experimental media containing 22Na+ or 86Rb+ (5–10 μCi/ml; NEN Life Sciences Products, Boston, MA), and 100-μl aliquots were removed at specified time intervals. The aliquots were placed in 1.5-ml centrifuge tubes, and cells were separated from the supernatant by centrifugation through 900 μl of isotope free flux media layered above 400 μl of dibutyl phthalate (Sigma Chemical). Supernatants were removed by vacuum aspiration along with most of the dibutyl phthalate, and the tube was cut just above the cell pellet to minimize contamination by extracellular isotope. The isotope associated with the pellet was counted using a gamma counter (Packard Instruments, Downers Grove, IL) for 22Na+ or a beta scintillation counter (Packard Instruments) for 86Rb+. Parallel samples of the cell suspension were removed for determination of dry cell weight and media specific activity. The rates of 22Na+ and 86Rb+ uptake were calculated by linear regression analysis (r2 > 0.95), and flux rates were expressed as mmol 22Na+ or 86Rb+·kg dcs−1 × min−1. To minimize back flux, media specific activity was at least three orders of magnitude greater than that of the intracellular compartment.
RESULTS
CLA-dependent activation of Na+/H+ exchange and K+/H+ exchange in amphiuma RBCs.
We have previously reported that in Amphiuma RBCs, both Na+/H+ exchange and K+/H+ exchange pathways are activated by phosphorylation-dependent events (10). In this study we suspended Amphiuma RBCs in isotonic media containing 500 nM CLA and measured the ouabain-insensitive net fluxes of Na+ and K+. Both Na+ and K+ flux pathways were strongly activated by CLA treatment (Fig. 1), thus supporting the hypothesis that both Na+/H+ exchange and K+/H+ exchange are activated by phosphorylation-dependent events.
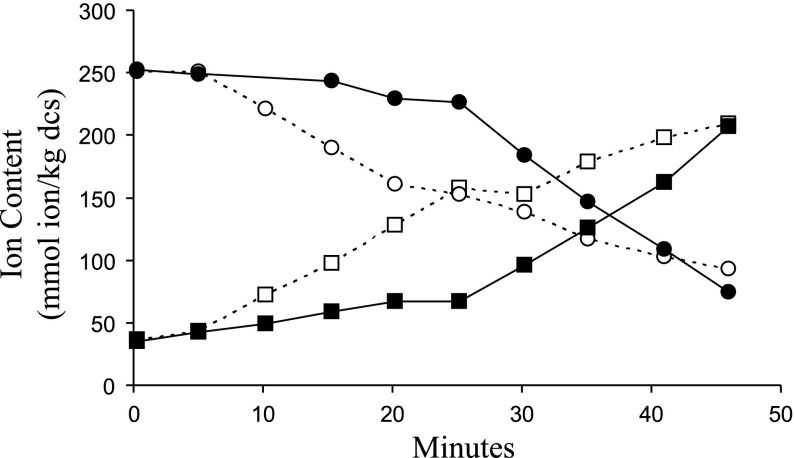
Fig. 1.
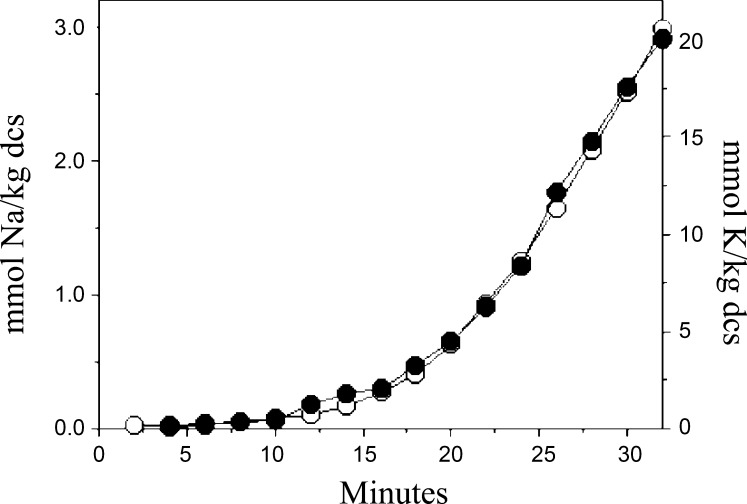
Changes in cell Na+ and K+ content following exposure to 1 μM calyculin A (CLA) in isotonic medium. Amphiuma red blood cells (RBCs) were preincubated in isotonic media in the presence of ouabain (1 mM) for 90 min. At time 0 the cells were transferred to isotonic medium + 1 μM CLA, samples were removed at the times indicated and analyzed for Na+ (squares) and K+ (circles) content. The data are from two independent experiments (solid and dashed lines), representing 4 similar results.
In several repetitions of the experiment described above we observed that the time necessary for onset of CLA-induced ion flux was variable (between 5 and 30 min). Representative experiments are shown in Fig. 1. However, we noted that within any given experiment, net Na+ and K+ fluxes were stimulated with identical time dependence (Fig. 1, solid and dashed lines), and that net Na+ and K+ fluxes were of equivalent magnitude yet opposite in direction. The data in one of the experiments presented in Fig. 1 (solid lines) demonstrate a net Na+ uptake of 170 mmol/kg dcs and a net K+ loss of 158 mmol/kg dcs, whereas the net Na+ uptake and K+ loss depicted in the other experiement (Fig. 1; dashed lines) are 172 and 180 mmol/kg dcs, respectively (representative of four similar results). Hence, CLA induces equivalent net Na+ uptake and K+ loss in Amphiuma RBCs. We also noted that cell water content (cell volume) is not affected by CLA treatment in isotonic media. Figure 2 illustrates that when cells are transferred from isotonic medium to isotonic medium + 1 μM CLA the cell water content is not significantly altered (1.75 vs. 1.83 l H2O/kg dcs) during a 30-min period, the time required to maximally activate the CLA-induced Na+ and K+ net fluxes (see Fig. 1). In contrast, when cells are transferred from isotonic to hypotonic or hypertonic medium cell volume significantly increases from 1.75 to 2.88 l H2O/kg dcs or decreases from 1.75 to 1.22 l H2O/kg dcs in the first 5 min (Fig. 2). These data further support the notion of equivalent net fluxes of inorganic ions across the cell membrane.
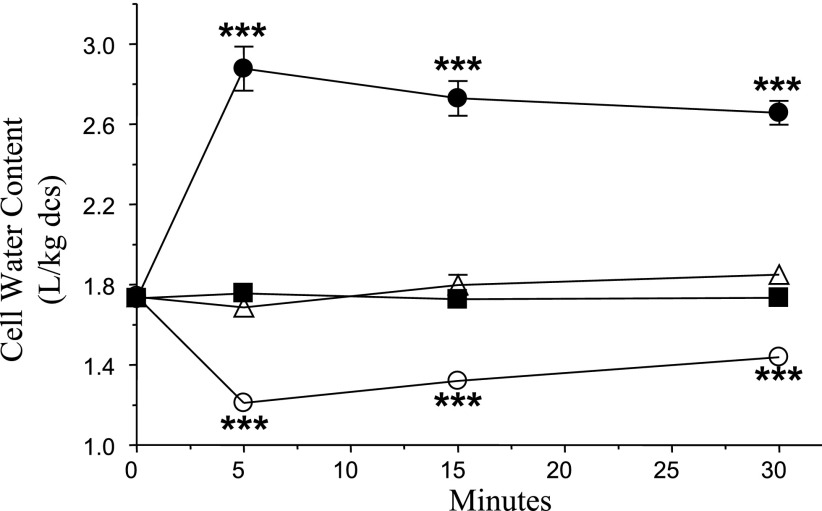
Fig. 2.
Cell water content in Amphiuma RBCs in control and experimental media. Amphiuma RBCs were transferred at time 0 from isotonic medium to either isotonic (▪), isotonic + 1 μM CLA (▵), hypertonic (○) or hypotonic medium (•). Aliquots were removed at specified times, and water content was determined gravimetrically, normalized to dried whole cell solids (dcs). Significant differences (***P < 0.001) were seen in water content for cells in hypertonic or hypotonic media over 30 min relative to the control condition. No significant difference in cell water content was seen in cells treated with CLA versus untreated cells in isosmotic media (n = 5 means ± SE).
Time-dependent induction of CLA-induced Na+ and K+ fluxes.
To determine the basis for the variability in the time of onset for activation of Na+ and K+ fluxes in response to CLA (Fig. 1), and to better illustrate the identical time dependence of Na+ and K+ flux activity, we measured the uptake of radioactive isotopic tracers (22Na+ and 86Rb+) sampled at paired time points following treatment of RBCs with CLA. The time-dependent 22Na+ and 86Rb+ uptake data were superimposed on a double-Y plot for direct comparison of the kinetics of activation. Within individual experiments, the time-dependent activation of CLA-induced 22Na+ and 86Rb+ uptake were virtually identical (Fig. 3). This remarkably identical time dependence suggests that Na+ and K+ flux pathways are under tight control by a common regulatory event.
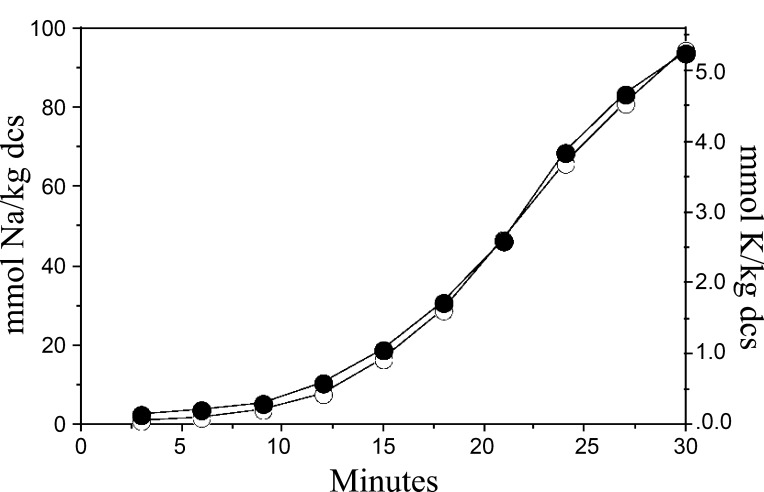
Fig. 3.
Time course for CLA-induced unidirectional Na+ and Rb+ uptake by Amphiuma RBCs in isotonic media. To initiate the experiment, cells were transferred at time 0 to 22Na+ or 86Rb+ containing isotonic media + 1 μM CLA and sampled at 3-min intervals during the flux period to measure Na+ (○) and Rb+ (•) uptake. Similar results were obtained in 4 experiments.
In 22Na+ and 86Rb+ uptake experiments, peak CLA-induced Na+ and K+ flux magnitudes were large relative to those typical of volume-perturbed cells (Table 1). Briefly, for untreated RBCs in isotonic media (240 mosM) unidirectional Na+ uptake is 0.23 ± 0.06 mmol Na+·kg dcs−1·min−1 and is increased 57-fold to 13.10 ± 0.75 mmol Na+·kg dcs−1·min−1 in hypertonic media (384 mosM). By comparison, the Na+ uptake for CLA-treated cells in isotonic media was 31.7 ± 2.6 mmol Na+·kg dcs−1·min−1, an increase of more than 100-fold above that of untreated RBCs isotonic media. Similarly, the K+ (86Rb+) uptake for untreated RBCs in isotonic media is 0.04 ± 0.02 mmol K+·kg dcs−1·min−1 and is increased roughly eightfold to 0.33 ± 0.075 mmol K+·kg dcs−1·min−1 in hypotonic media (132 mosM). However, the K+ (86Rb+) uptake rate for CLA-treated RBCs in isotonic media was 2.80 ± 0.46 mmol K+·kg dcs−1·min−1, a 70-fold increase relative to untreated cells in isotonic media. The increase in 86Rb+ uptake rate in response to CLA was nearly an order of magnitude greater than that due to volume-activated K+/H+ exchange. Hence, the magnitudes of the Na+/H+ and K+/H+ exchange induced by CLA exposure are far greater than those observed in response to volume perturbation.
Table 1.
Unidirectional Na and Rb uptake rates in Amphiuma RBCs in media of different tonicities and in isotonic medium plus CLA
| Condition | Na+ Uptake Rate, mmol Na·kg dcs−1·min−1 | Rb+ Uptake Rate, mmol Rb·kg dcs−1·min−1 |
|---|---|---|
| Isotonic | 0.23±0.06 (n = 4) | 0.0402±0.02 (n = 5) |
| Hypertonic | 13.10±0.75‡ (n = 4) | 0.023±0.005 (n = 3) |
| Hypotonic | 0.33±0.08 (n = 4) | 0.33±0.08*(n = 4) |
| Isotonic + 500 nM CLA | 31.7±2.6‡ (n = 3) | 2.80±0.46† (n = 8) |
Comparison of maximum unidirectional Na+ and Rb+ uptake rates in Amphiuma red blood cells (RBCs) in isotonic, anisotonic, and isotonic media containing 500 nM calyculin (CLA). Uptake rates were measured by suspending cells in media containing 86Rb+ or 22Na+ and sampling at 15-s to 2-min intervals for 1 to 10 min. Data are means ± SE; n is the number of animals. dcs, Dry cell solid.
Significantly different from the respective uptake in isotonic media with P < 0.05;
significantly different from the respective uptake in isotonic media with P < 0.01;
significantly different from the respective uptake in isotonic media with P < 0.001.
Concentration dependence of CLA-stimulated 22Na+ and 86Rb+ fluxes.
To further characterize the biochemical control of CLA-induced Na+/H+ and K+/H+ exchange, we examined the CLA concentration dependence of 22Na+ and 86Rb+ influx activation. The dose-response relationships between 22Na+ or 86Rb+ unidirectional uptake rates and CLA concentration were obtained from RBCs suspended at varied concentrations of CLA in the presence of ouabain. The data illustrate that the concentration dependence of CLA-induced 22Na+ and 86Rb+ fluxes are virtually identical and that the CLA concentration required for half-maximal stimulation of 22Na+ and 86Rb+ uptake is roughly 40 nM for either flux pathway (Fig. 4). Taken together, the results in Figs. 2–4 suggest that CLA-induced Na+ and K+ fluxes are under tight functional control by the same rate-limiting, phosphorylation-dependent event. However, the fact that both Na+/H+ exchange and K+/H+ exchange are volume regulatory flux pathways raises the question of whether activation of one of the pathways is secondary to incipient changes in volume induced by activation of the other pathway. In most reports, shrinkage-activated Na+/H+ exchange in RBCs is activated by agents that promote phosphorylation, whereas in contrast, activation of volume regulatory K+ flux (K-Cl cotransport) appears to be activated by agents that promote dephosphorylation (see 13, 17, 28, 39). Therefore K+/H+ exchange may be activated in response to cell swelling, secondary to CLA-induced net Na+ influx via the Na+/H+ exchange.
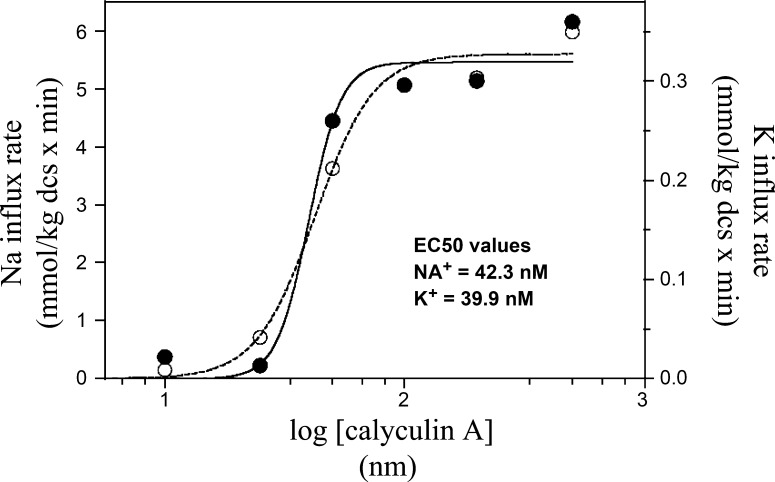
Fig. 4.
CLA concentration dependence of unidirectional Na+ (○) and Rb+ (•) uptake in isotonic medium. To initiate flux, cells were transferred to 22Na+- and 86Rb+-containing medium at the CLA concentration indicated in the ordinate axis. Both Na+ and Rb+ unidirectional uptake rates were measured and expressed as mmol·kg dcs−1·min−1. The data were fit to a sigmoidal dose-response function by nonlinear regression to obtain values for the half-maximal stimulatory concentration (EC50) of CLA for Na+ (dashed line) and K+ (solid line) flux. EC50 values for CLA in this experiment were 42.3 nM for Na+ flux and 39.9 nM for K+ flux. Similar results were obtained in 3 experiments.
Nature of CLA-induced activation of Na+ and K+ flux pathways.
We conducted experiments to test the hypothesis that the Na+ and K+ fluxes observed during CLA treatment are mediated by the Na+/H+ and K+/H+ exchangers. To this end, the CLA-stimulated unidirectional 22Na+ and 86Rb+ uptake were measured in RBCs suspended in medium designed to maintain the Na+/H+ and K+/H+ exchangers at thermodynamic equilibrium (thermodynamically nulled medium; see materials and methods). Consequently, any net ion transport via Na+/H+ exchange or K+/H+ exchange would not be possible as cells are suspended in medium chosen to set the thermodynamic driving force for both pathways at zero. In contrast, any alteration in the activity of these pathways would be detected by measuring unidirectional ion fluxes. Under these conditions, we observed a symmetrical, CLA-dependent stimulation of both 22Na+ and 86Rb+ unidirectional uptake (Fig. 5). We concluded that CLA-mediated activation of both Na+ and K+ fluxes are directly due to phosphatase inhibition-induced activation of both Na+/H+ and K+/H+ exchange. Consequently, our data suggest that activation of both Na+/H+ exchange and K+/H+ exchange are phosphorylation-dependent events. It is noteworthy that the time-dependent activation of 22Na+ and 86Rb+ uptake by RBCs in nulled media (Fig. 5) is virtually identical to that of RBCs in physiological media (Fig. 3). Consequently, the data presented in Fig. 5 further supports the notion that CLA-induced activation of either of these ion flux pathways is not secondary to incipient changes in cell volume.
Fig. 5.
CLA-induced unidirectional 22Na+ (○) and 86Rb+ (•) uptake in nulled isotonic medium containing 0.5 μM CLA. Cells were preincubated in ouabain (1 mM)-containing isotonic media for 90 min. At time 0 cells were placed in nulled isotonic media + 500 nM CLA, and samples were removed at the times indicated to measure 22Na+ and 86Rb+ unidirectional uptake. Similar results were obtained in 3 experiments.
Inhibition of CLA-stimulated 22Na and 86Rb fluxes by an NHE1 inhibitor.
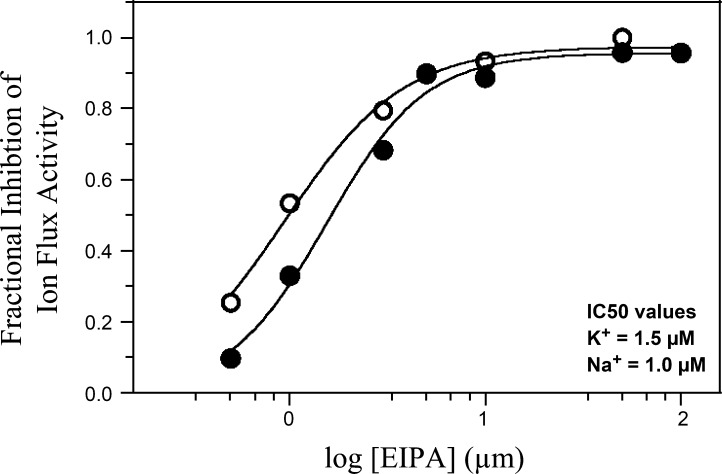
Previous work in our laboratory has demonstrated that the plasma membrane Na+/H+ exchanger in Amphiuma RBCs is a homolog of the mammalian Na+/H+ exchanger NHE1 isoform. The molecular identity of the K+/H+ exchange pathway has, however, not been established. Whereas NHE1 is not known to perform K+/H+ exchange, at least three other mammalian Na/H exchanger isoforms (NHE6, NHE7, and NHE9) mediate K/H exchange in addition to Na/H exchange (21, 36). Given the tight functional coupling of CLA-induced Na+ and K+ fluxes in Amphiuma RBCs, CLA-induced Na+ and K+ fluxes may be mediated by the same transport pathway. To test this hypothesis, we measured the dose-dependent inhibition of CLA-induced 22Na+ and 86Rb+ fluxes by the NHE1 transport inhibitor EIPA. RBCs were preincubated in 0.5 μM CLA in thermodynamically nulled isotonic media in the presence of ouabain. At the end of the incubation period RBCs were transferred to ouabain-containing nulled isotonic media at varied concentrations of EIPA, with either 22Na+ or 86Rb+. Initial rates of isotope uptake were determined as a function of EIPA concentration. The EIPA dose-response data for fractional inhibition of 22Na+ and 86Rb+ fluxes are shown in Fig. 6. The half-maximal inhibitory concentration (IC50) of EIPA was 1.5 μM for 86Rb+ flux and 1.0 μM for 22Na+ flux. Thus the IC50 values with EIPA are similar for both Na+ and K+ flux pathways. Similar results were obtained for inhibition by amiloride (data not shown). Whereas this does not establish that Na+ and K+ fluxes are mediated by the same transport pathway, the virtually identical IC50 values for EIPA inhibition of CLA-induced 22Na+ or 86Rb+ fluxes are suggestive that both 22Na+ and 86Rb+ fluxes are mediated by the Na+/H+ exchanger in Amphiuma RBCs and reflect different operational modes of the same transport protein.
Fig. 6.
Inhibition of CLA-induced unidirectional 22Na+ (○) and 86Rb+ (•) uptake by 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) in nulled isotonic medium. Cells were preincubated in ouabain (1 mM)-containing nulled isotonic media + 500 nM CLA for 35 min. At time 0 cells were placed in isotope-containing nulled isotonic media and sampled over a 5-min interval to measure 22Na+ (○) and 86Rb+ (•) unidirectional uptake rates. The half-maximal inhibitory concentration (IC50) for EIPA was 1.0 μM for 22Na+ and 1.5 μM for 86Rb+. Similar results were obtained in 3 experiments.
Implications for the control of Na+/H+ and K+/H+ exchange by volume-dependent kinases and phosphatases.
Our results with CLA treatment of Amphiuma RBCs indicate that inhibition of phosphatases by CLA unmasks phosphorylation events that are responsible for the induction of Na+/H+ and K+/H+ exchange pathways and that in the absence of CLA treatment, tonic phosphatase activity suppresses both Na+/H+ and K+/H+ exchange, resulting in virtually no activity in isotonic media. Since under normal physiological conditions the Na+/H+ and K+/H+ exchange pathways perform opposing regulatory roles with respect to acute changes in cell volume, this then further suggests that during osmotic cell shrinkage tonic protein phosphatase activity is suppressed permitting selective activation of Na+/H+ exchange, and that during osmotic cell swelling tonic phosphatase activity is suppressed permitting selective activation of K+/H+ exchange. Alternatively, a simpler explanation for the control of these volume-sensitive pathways includes a shrinkage-sensitive kinase responsible for Na+/H+ activation, a swelling-sensitive kinase mediating K+/H+ exchange activation, and a tonically active, CLA-sensitive phosphatase (active in isotonic medium) that opposes the activity of both volume-sensitive kinases. The question remains as to what is the mechanism that confers selective activation of Na+/H+ exchange during osmotic cell shrinkage or selective activation of K+/H+ exchange during osmotic cell swelling. In the context of the phosphatase/kinase model, a shrinkage-activated kinase must exist that activates Na+/H+ exchange, but that is suppressed in swollen cells, and a complementary swelling-activated kinase must exist that activates K+/H+ exchange, but that is suppressed in shrunken cells. In addition, both volume-sensitive kinases must be tonically active in unstimulated cells in isotonic media, such that inhibition of opposing phosphatase(s) by CLA treatment results in simultaneous activation of both flux pathways.
Selective activation of CLA-induced net Na+ or K+ fluxes by osmotic alterations in cell volume.
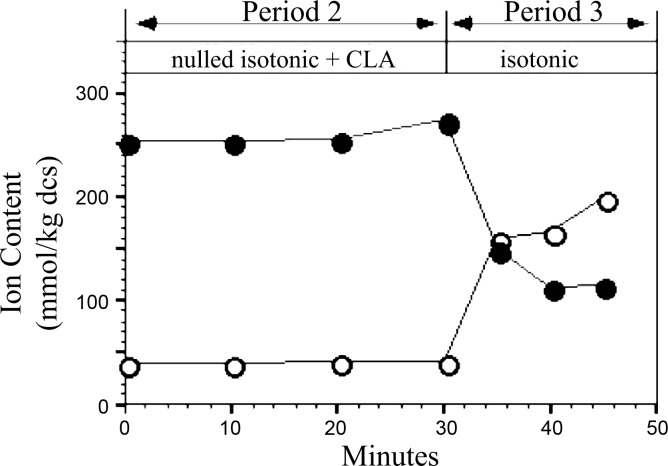
To better study the CLA-insensitive activation events and given the observed variability in the time required for CLA to activate the Na+ and K+ flux pathways (Fig. 1), we devised a way to consistently and fully inhibit phosphatase activity with CLA before suspension in flux media and the initiation of flux measurement. To prevent net ion flux during CLA pretreatment, RBCs were placed in media thermodynamically nulled with respect to Na+/H and K+/H+ exchange pathways. Consistent with the assumptions of the nulled condition, no net influx of Na+ or net efflux of K+ occurs over 30 min for RBCs in CLA-containing nulled media (Fig. 7, period 2). In addition, robust CLA-dependent activation of net ion flux is observed upon subsequent transfer to normal (non-nulled) physiological media (Fig. 7, period 3). Therefore, the use of nulled media during CLA exposure eliminates the variability in the time required for CLA-dependent activation of net Na+ and K+ flux, allowing the experimenter to dictate the time of onset of net ion flux following CLA treatment.
Fig. 7.
Effect of CLA on Na+ (○) and K+ (•) content of cells in isotonic media. Cells were incubated in normal isotonic media + 1 mM ouabain for 90 min (period 1) before suspension in thermodynamically nulled isotonic medium containing 1 mM ouabain and 1 μM CLA for 30 min (period 2). Subsequently, the cells were transferred to CLA-free normal isotonic medium at time = 30 min (period 3). Samples were removed at the times indicated and analyzed for Na+, K+, and water content. Similar results were obtained in 4 experiments.
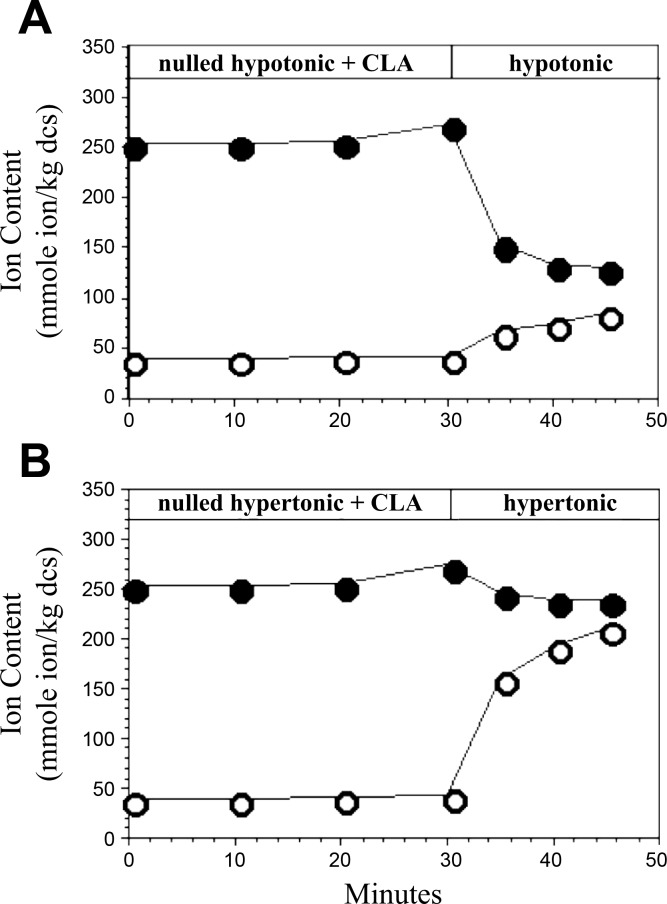
To determine whether changes in cell volume confer specificity (for Na+/H+ exchange in hypertonic media or K+/H+ exchange in hypotonic media) in the presence of CLA, we superimposed volume perturbations and CLA exposure (Fig. 8). Employing a protocol analogous to that used for Fig. 7, cells were suspended in hypotonic or hypertonic nulled media for 60 min with the inclusion of CLA in the latter 30 min before flux measurement in CLA-free hypotonic (Fig. 8A) or hypertonic (Fig. 8B) media, respectively. Figure 8A illustrates that for RBCs in hypotonic media, CLA treatment results in net K+ loss that is nearly identical to that of CLA-treated cells in isotonic media (Fig. 7), whereas Na+ uptake is greatly reduced. Conversely, for RBCs in hypertonic media, CLA treatment results in net Na+ uptake (Fig. 8B) that is nearly identical to that of CLA-treated cells in isotonic media (Fig. 7), whereas K+ loss is greatly reduced. Taken together, these data suggest that for each flux pathway a separate volume-sensitive kinase exists, opposed by a CLA-sensitive phosphatase that is responsible for suppression of either Na+/H+ or K+/H+ exchange, respectively, in the absence of an appropriate volume stimulus. Upon treatment with CLA, this phosphatase is suppressed permitting induction of Na+/H+ exchange and K+/H+ exchange pathways by unmasking their respective volume regulatory kinases.
Fig. 8.
Selective activation of net Na+ or K+ flux in Amphiuma RBCs by CLA in anisotonic media. Shown are the Na+ (○) and K+ (•) content of cells exposed to CLA in thermodynamically nulled hypotonic (A) or hypertonic (B) media for 30 min, then transferred to CLA-free, hypotonic (A) or hypertonic (B) media at time = 30 min. The data were generated from the same batch of cells as those in Fig. 7. Similar results were obtained in 3 experiments.
DISCUSSION
Overview.
The data presented in this study address the hypothesis that in Amphiuma RBCs, both swelling-induced K+/H+ exchange and shrinkage-induced Na+/H+ exchange are activated by phosphorylation-dependent events. We found that when Amphiuma RBCs in isotonic media are exposed to CLA, robust Na+/H+ and K+/H+ exchange pathways are activated simultaneously. This indicates that phosphorylation is involved in the activation of both volume-sensitive ion flux pathways. These results also are consistent with the notion that in resting cells (isotonic media) there is tonic kinase activity that can activate Na+/H+ and K+/H+ exchange. Since CLA stimulates Na+/H+ and K+/H+ exchange with the same time course and dose dependence, the data suggest that in unstimulated cells both flux pathways are suppressed by a CLA-sensitive phosphatase. This is further supported by the observation that activation of Na+/H+ or K+/H+ exchange during CLA treatment is not secondary to incipient changes in cell volume resulting from net Na+ uptake or K+ loss, since in thermodynamically nulled media, where net transport and therefore transport-induced volume changes are not possible, CLA stimulates both 22Na+ and 86Rb+ unidirectional fluxes. Furthermore, we found that superimposition of shrinkage or swelling and CLA exposure results in a preferential stimulation of Na+/H+ or K+/H+ exchange, respectively. In summary, phosphorylation is involved in the activation of both Na+/H+ and K+/H+ exchange functions in Amphiuma RBCs, yet events specific to cell swelling or shrinkage, are responsible for selective activation of K+/H+ or Na+/H+ exchange.
Role of phosphorylation in the activation of swelling-sensitive ion flux pathways.
Consistent with the observation that CLA stimulates K+/H+ exchange, we previously reported that the phorbol ester, PMA induces K+/H+ exchange in Amphiuma RBCs (10). The finding that phosphorylation is involved in activation of K+/H+ exchange (a swelling-induced pathway) in Amphiuma RBCs is at odds with what is reported regarding activation of swelling-induced, Cl−-dependent K+ loss for rabbit, dog, and sheep RBCs (14, 26, 39). In other studies of this kind, swelling-induced K+ flux pathways are activated by dephosphorylation and deactivated by phosphorylation (13, 17). More specifically, the volume regulatory K+-Cl+ cotransport pathway is deactivated by treatment of cells with the potent phosphatase inhibitors okadaic acid (OkA) or CLA, whereas the reciprocal volume regulatory pathway (Na+/H+ exchange) is activated in response to OkA or CLA treatment. To characterize the role of phosphorylation in the control of volume regulatory ion transport, Jennings and Al-Rohil (26) employed a two-state relaxation kinetic model to analyze the rates of swelling-induced activation and shrinkage-induced deactivation of the K+-Cl− cotransporter in rabbit RBCs. They found that phosphatase inhibitors: fluoride, orthovanadate, and inorganic phosphate decreased the rate of activation of swelling-induced K+-Cl− cotransport and concluded that swelling-dependent activation involves net dephosphorylation. Jennings provided additional evidence in support of this view by demonstrating that the more specific phosphatase inhibitors OkA (27) and CLA (47) decrease the rate of swelling-induced K+-Cl− cotransport. This pattern of swelling-induced activation of K+-Cl− cotransport by dephosphorylation and deactivation by phosphorylation has also been demonstrated in human, sheep, and dog RBCs (14, 26, 28, 39).
In contrast to the evidence above, our results with CLA and PMA treatment of Amphiuma RBCs suggest that phosphorylation is involved in the swelling-dependent induction of K+/H+ exchange. Whereas at odds with studies of K+-Cl− cotransporter activation in mammalian RBCs, the notion that phosphorylation is involved in activation of swelling-induced, volume regulatory, solute efflux pathways is not unique to Amphiuma RBCs. For example, the swelling-induced Cl− conductance in frog proximal tubule cells is inhibited by the protein kinase C inhibitor, PKC-ps, and increased by PMA (42). PKC has also been shown to play a role in the activation of the swelling-sensitive Cl− currents in HeLa cells (20). Finally, Tilly and coworkers (49) demonstrated that 86Rb+ efflux, via swelling-activated K+ channels, is inhibited by the tyrosine kinase inhibitors herbimycin A and genistein. Therefore, the results of our studies with Amphiuma RBC K+/H+ exchange, while at odds with studies of swelling-dependent K+-Cl− cotransport in mammalian RBCs, are consistent with results obtained in studies of other swelling-induced transport pathways where phosphorylation-dependent events are responsible for activation of swelling-induced solute efflux.
Role of phosphorylation in the activation of shrinkage-sensitive NHE1.
It is generally accepted that mammalian NHE1 is activated as a result of phosphorylation-dependent reactions. The Amphiuma RBC Na+/H+ exchanger is a highly conserved homologue (79% amino acid identity) of the human NHE1 protein (34) that performs the classic “housekeeping” functions of intracellular pH and cell volume regulation (8, 12). Given these similarities, the following discussion will focus on reports that support the notion that NHE1 activity is phosphorylation dependent.
Our observation that volume-induced Na+ uptake in Amphiuma RBCs is phosphorylation dependent is consistent with observations by others suggesting that phosphorylation is involved in the activation of Na+/H+ exchange for nearly every cell type studied (5, 32, 41, 43, 44). Several groups have reported increased NHE1 activity and phosphorylation of NHE1 in response to phorbol esters or OkA, as in hamster fibroblasts and A431 human epidermoid cells (43). Other studies with mammalian fibroblasts demonstrate that direct phosphorylation of NHE1 is necessary for NHE1 activity in response to growth factor/serum stimulation (48), or prolonged acidification (33). Thus far, all such reports include phosphorylation of NHE1 exclusively on serine residues (not threonine or tyrosine). Our laboratory has also shown that the Winter flounder RBCs NHE1 homologue is phosphorylated on serine residues in response to PKA agonists but not in response to CLA treatment or osmotic cell shrinkage (24). Hence our data suggest that the phosphorylation-dependent event controlling the volume-dependent activation of NHE1 is not increased net phosphorylation of the NHE1 protein. This is supported by the observations of two other laboratories that net phosphorylation of NHE1 is not increased in response to osmotic cell shrinkage in human foreskin fibroblasts (35), Chinese hamster ovary cells (18), or human bladder carcinoma cells.
Whereas there is good agreement that phosphorylation is involved in the activation of NHE1, several other studies are at odds with the notion that direct phosphorylation of NHE1 protein is the basis for NHE1 activity. In fibroblasts, reduced NHE1 activity following ATP depletion (5) is not associated with a decrease in NHE1 protein phosphorylation (16). Finally, whereas there are many reports that growth factor, serum treatment (50), or intracellular acidification (33) stimulates NHE1 activity with a concomitant increase in NHE1 phosphorylation, there are similar reports that stimulation of NHE1 activity does not depend entirely on direct phosphorylation of NHE1. This is consistent with studies showing that phosphorylation of human serine-703 is necessary for serum-dependent stimulation of NHE1 in fibroblasts, i.e., mutation of serine to alanine at residue 703 (S703A) abolishes the growth factor-stimulated component of NHE1 activity (48). Yet the S703A mutation does not prevent activation of NHE1 in response to intracellular acidification. Several other treatments that alter NHE1 activity do not require direct phosphorylation or dephosphorylation of the NHE1 protein, e.g., ATP depletion (5), or calmodulin binding (4, 15, 31), suggesting that ancillary protein(s) mediate phosphorylation-dependent regulation of NHE1 activity. Data consistent with this view have been presented by several independent studies (2–4, 15, 29–31, 37, 38, 46, 51). Thus, whereas the vast majority of reports are consistent with the view that Na+/H+ exchange activity is phosphorylation-dependent, it is not clear whether phosphorylation is direct or through an NHE1-associated regulatory protein.
Alkali metal/H+ exchanger hypothesis.
Since our initial discovery of volume regulatory Na+/H+ and K+/H+ exchange in Amphiuma RBCs 28 years ago (8), no protein has been identified as being responsible for K+/H+ exchange in these cells. In early studies, we presented evidence that volume-induced K+/H+ exchange and Na+/H+ exchange pathways in Amphiuma RBCs are mediated by the same transport moiety (9). From recent findings that certain mammalian NHE isoforms (NHE6, NHE7, and NHE9) mediate K+/H+ exchange (21, 36), we suspect that certain as of yet undefined conditions (either subtle deviations in the NHE1 protein structure or of the associated regulatory pathways) permit the Amphiuma RBC NHE1 homologue to perform K+/H+ exchange in response to osmotic cell swelling. To recapitulate our previous findings, when osmotically swollen Amphiuma RBCs are treated with the stilbene compound 4,4′-diisothiocyanatostilbene-2,2′-disulfonate (DIDS) (25–100 μM), both Na+/H+ exchange and K+/H+ exchange become activated. Activation of Na+/H+ exchange by DIDS treatment in hypotonic media is the result of a direct chemical modification of NHE1 or of some other regulatory protein by DIDS and is not secondary to incipient changes in cell volume or to net proton influx via K+/H+ exchange. In a subsequent study we demonstrated that PMA induces Na+/H+ exchange and K+/H+ exchange with identical dose dependence in Amphiuma RBCs and further that exposure of Amphiuma RBCs to the Ca2+ ionophore A23187 activates both exchange pathways (10). The data presented in the current study are consistent with the notion that the Na+/H+ exchanger can be induced to perform K+/H+ exchange in that CLA activates both Na+ and K+ fluxes with identical time course, dose dependence, and inhibitor sensitivity (Figs. 2–6). Consistent with the latter observation, our earlier work shows that PMA-induced Na+/H+ exchange and K+/H+ exchange are similarly inhibited by amiloride (10). Hence accumulating evidence supports the hypothesis of an alkali metal Na+(K+)/H+ exchanger in Amphiuma RBCs that is reciprocally regulated by cell volume to selectively perform either Na+/H+ or K+/H+ exchange. Despite this evidence, we have failed to observe swelling-induced net K+ efflux in our mammalian (AP-1) cell-based expression system following functional expression of the Amphiuma NHE1 protein (P. M. Cala, unpublished observations). Thus the molecular identity of the Amphiuma K+/H+ exchanger remains elusive.
Coordination of volume-sensitive pathways around the volume set point.
It has long been known that the volume-sensitive ion flux pathways are coordinated around the volume set point in many cell types (8, 19, 40, 45). Yet, the basis for this coordination is not understood. The Jennings and Al-Rohil (26) two-state kinetic model provided the first means of analysis for volume-dependent control and coordination of K+-Cl+ cotransport. Subsequently, Parker et al. (40) employed the model to study the coordination of the shrinkage-induced Na+/H+ exchange and swelling-induced K+-Cl− cotransport in dog RBCs. Specifically, Parker and co-workers (39) found that during cell shrinkage there is rapid activation of Na+/H+ exchange and rapid deactivation of K+-Cl− cotransport. Conversely, during cell swelling, K+-Cl− cotransport activation and Na+/H+ deactivation occurs slowly. Furthermore, they found that OkA stimulates Na+/H+ exchange yet inhibits K+-Cl− cotransport. Thus they inferred that phosphorylation is necessary for Na+/H+ exchange activation during cell shrinkage, and in agreement with Jennings, dephosphorylation is necessary for swelling-induced K+-Cl− cotransport. They also inferred that the reciprocal behavior, both in the rates of activation/deactivation and transport activity of Na+/H+ exchange and K+-Cl− cotransport suggested that in dog RBCs, Na+/H+ exchange and K+-Cl− cotransport are controlled by a common regulatory system. Based on their findings with protein phosphatase inhibitors, they reasoned that the phosphorylation state of this common regulator determines whether Na+/H+ exchange or K+-Cl− cotransport activity is manifest. The appeal of the model they proposed is its simplicity: a single kinase/phosphatase system explains the observed coordination of volume-sensitive solute efflux and influx pathways around the volume set point. More complex activation schemes have since been devised to explain the control of KCC1 by various stimuli (6, 7, 25), and admittedly the two-state model of activation is oversimplified. However, under a restricted set of experimental conditions the two-state model is adequate for the analysis of relative changes in the rate-limiting activation or deactivation events in response to acute cell volume perturbation.
In contrast to dog RBCs, our results from Amphiuma RBCs indicate that phosphorylation is involved in activation of both shrinkage-induced Na+ flux and swelling-induced K+ flux. That is, both Na+/H+ and K+/H+ exchange are stimulated by CLA or PMA (10) in isotonic media. However, it is evident that a unique signal, specific to the type of osmotic perturbation (cell swelling or shrinkage) is responsible for the selective induction of either K+/H+ exchange following cell swelling or Na+/H+ exchange following cell shrinkage (Fig. 8, A and B). Specifically, we demonstrate that while CLA activates both pathways in isotonic medium (Figs. 1 and 7), superimposition of swelling and CLA results in a preferential activation of K+/H+ exchange (Fig. 8A), whereas superimposition of shrinkage and CLA exposure results in a preferential activation of Na+/H+ exchange (Fig. 8B). The Amphiuma RBC data, unlike those of dog RBCs, are not compatible with an absolute reciprocal relationship between the shrinkage and swelling-sensitive pathways. That is, in Amphiuma RBCs, both volume-sensitive pathways (Na+/H+ and K+/H+ exchangers) are activated by phosphorylation, yet some other signal related to shrinkage or swelling is responsible for selective activation of Na+/H+ or K+/H+ exchange, respectively.
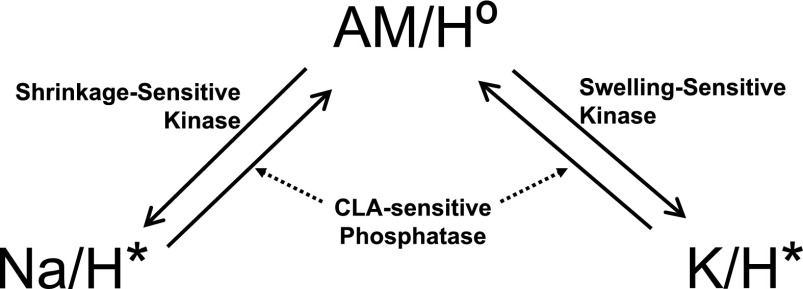
Based on the data gathered so far we propose a model for the control of the volume-sensitive Na+/H+ and K+/H+ exchangers in Amphiuma RBCs (Fig. 9). Briefly, in isotonic medium a CLA-sensitive phosphatase masks the activity of opposing shrinkage and swelling-sensitive kinases. Under this condition (isotonic medium) the alkali metal/H+ exchanger remains minimally active (AM/H°; Fig. 9). When cells are exposed to hypertonic medium, increased shrinkage-sensitive kinase activity results in activation of the alkali metal/H+ exchange in Na+/H+ exchange mode (Na/H*; Fig. 9). In contrast, when cells are exposed to hypotonic medium, increased swelling-sensitive kinase activity results in activation of the alkali metal/H exchange in K+/H+ exchange mode (K/H*; Fig. 9).
Fig. 9.
Potential model for the activation and deactivation of the volume-sensitive Na+/H+ and K+/H+ exchangers in Amphiuma RBCs. In this scheme, AM/H° represents the alkali metal/H+ exchanger at minimal activity. Na/H* and K/H* represent the alkali metal/H+ exchanger activated in the Na+/H+ and K+/H+ exchanger mode.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Research Grant R01 HL-21179 (to P. M. Cala). A. Ortiz-Acevedo was supported by a National Research Service Award (5 F31 GM-18985-02) from the National Institutes of Health.
Acknowledgments
We thank Baudouin Bulaya and Elizabeth Nguyen for excellent technical help.
The present address of A. Ortiz-Acevedo is: Dept. of Natural Sciences, PO Box 2500, University of Puerto Rico, Utuado, Puerto Rico 00641. The present address of R. R. Rigor is Laboratory of Pharmacology and Chemistry, National Institute of Environmental Heath Sciences/National Institutes of Health, 111 T. W. Alexander Dr., Research Triangle Park, NC 27709. The present address of H. M. Maldonado is Departamento de Farmacología, Universidad Central del Caribe, Bayamón, Puerto Rico 00960.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adorante JS, Cala PM. Activation of electroneutral K flux in Amphiuma red blood cells by N-ethylmaleimide. Distinction between K/H exchange and KCl cotransport. J Gen Physiol 90: 209–227, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aharonovitz O, Demaurex N, Woodside M, Grinstein S. ATP dependence is not an intrinsic property of Na+/H+ exchanger NHE1: requirement for an ancillary factor. Am J Physiol Cell Physiol 276: C1303–C1311, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Aharonovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S. Intracellular pH regulation by Na(+)/H(+) exchange requires phosphatidylinositol 4,5-bisphosphate. J Cell Biol 150: 213–224, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertrand B, Wakabayashi S, Ikeda T, Pouysségur J, Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J Biol Chem 269: 13703–13709, 1994. [PubMed] [Google Scholar]
- 5.Bianchini L, Woodside M, Sardet C, Pouyssegur J, Takai A, Grinstein S. Okadaic acid, a phosphatase inhibitor, induces activation and phosphorylation of the Na+/H+ antiport. J Biol Chem 266: 15406–15413, 1991. [PubMed] [Google Scholar]
- 6.Bize I Theoretical validation for a model of KCC regulation in human erythrocytes. Blood Cells Mol Dis 27: 121–126, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bize I, Guvenc B, Buchbinder G, Brugnara C. Stimulation of human erythrocyte K-Cl cotransport and protein phosphatase type 2A by n-ethylmaleimide: role of intracellular Mg++. J Membr Biol 177: 159–168, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Cala PM Volume regulation by Amphiuma red blood cells: The membrane potential and its implications regarding the nature of the ion-flux pathways. J Gen Physiol 76: 683–708, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cala PM Volume regulation by Amphiuma red blood cells: strategies for identifying alkali metal/H+ transport. Fed Proc 44: 2500–2507, 1985. [PubMed] [Google Scholar]
- 10.Cala PM Volume-sensitive alkali metal-H transport in Amphiuma red blood cells. In: Current Topics in Membranes and Transport. New York: Academic, 1986, p. 79–99.
- 11.Cala PM, Hoffmann KS. Alkali metal/proton exchange. Methods Enzymol 173: 330–346, 1989. [DOI] [PubMed] [Google Scholar]
- 12.Cala PM, Maldonado HM. pH regulatory Na/H exchange by Amphiuma red blood cells. J Gen Physiol 103: 1035–1053, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cossins AR, Gibson JS. Volume-sensitive transport systems and volume homeostasis in vertebrate red blood cells. J Exp Biol 200: 343–352, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Dunham PB, Klimczak J, Logue PJ. Swelling activation of K-Cl cotransport in LK sheep erythrocytes: a three-state process. J Gen Physiol 101: 733–765, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goss G, Orlowski J, Grinstein S. Coimmunoprecipitation of a 24-kDa protein with NHE1, the ubiquitous isoform of the Na+/H+ exchanger. Am J Physiol Cell Physiol 270: C1493–C1502, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Goss GG, Woodside M, Wakabayashi S, Pouyssegur J, Waddell T, Downey GP, Grinstein S. ATP dependence of NHE-1, the ubiquitous isoform of the Na+/H+ antiporter. Analysis of phosphorylation and subcellular localization. J Biol Chem 269: 8741–8748, 1994. [PubMed] [Google Scholar]
- 17.Grinstein S, Furuya W, Bianchini L. Protein kinases, phosphatases and the control of cell volume. News Physiol 7: 232–237, 1992. [Google Scholar]
- 18.Grinstein S, Woodside M, Sardet C, Pouyssegur J, Rotin D. Activation of the Na+/H+ antiporter during cell volume regulation. Evidence for a phosphorylation-independent mechanism. J Biol Chem 267: 23823–23828, 1992. [PubMed] [Google Scholar]
- 19.Haas M, McManus TJ. Effect of norepinephrine on swelling-induced potassium transport in duck red cells. Evidence against a volume-regulatory decrease under physiological conditions. J Gen Physiol 85: 649–667, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy SP, Goodfellow HR, Valverde MA, Gill DR, Sepulveda V, Higgins CF. Protein kinase C-mediated phosphorylation of the human multidrug resistance P-glycoprotein regulates cell volume-activated chloride channels [published erratum appears in EMBO J 1995 Apr 18;14(8):1844]. EMBO J 14: 68–75, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill JK, Brett CL, Chyou A, Kallay LM, Sakaguchi M, Rao R, Gillespie PG. Vestibular hair bundles control pH with (Na+, K+)/H+ exchangers NHE6 and NHE9. J Neurosci 26: 9944–9955, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann EK, Pedersen SF. Sensors and signal transduction in the activation of cell volume regulatory ion transport systems. Contrib Nephrol 123: 50–78, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann EK, Pedersen SF. Sensors and signal transduction pathways in vertebrate cell volume regulation. Contrib Nephrol 152: 54–104, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Holt ME, King SA, Cala PM, Pedersen SF. Regulation of the Pleuronectes americanus Na+/H+ exchanger by osmotic shrinkage, beta-adrenergic stimuli, and inhibition of Ser/Thr protein phosphatases. Cell Biochem Biophys 45: 1–18, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Jennings ML Volume-sensitive K(+)/Cl(−) cotransport in rabbit erythrocytes. Analysis of the rate-limiting activation and inactivation events. J Gen Physiol 114: 743–758, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings ML, Al-Rohil N. Kinetics of activation and inactivation of swelling-stimulated K+/Cl- transport The volume-sensitive parameter is the rate constant for inactivation. J Gen Physiol 95: 1021–1040, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennings ML, Schulz RK. Okadaic acid inhibition of KCl cotransport. Evidence that protein dephosphorylation is necessary for activation of transport by either cell swelling or N-ethylmaleimide. J Gen Physiol 97: 799–817, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauf PK, Bauer J, Adragna NC, Fujise H, Zade-Oppen AM, Ryu KH, Delpire E. Erythrocyte K-Cl cotransport: properties and regulation. Am J Physiol Cell Physiol 263: C917–C932, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Liu Y, Alvarez BV, Casey JR, Fliegel L. A Novel Carbonic Anhydrase II Binding Site Regulates NHE1 Activity. Biochemistry 45: 2414–2424, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Liu Y, Kay CM, Muller-Esterl W, Fliegel L. The Na+/H+ exchanger cytoplasmic tail: structure, function, and interactions with tescalcin. Biochemistry 42: 7448–7456, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Lin X, Barber DL. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc Nat Acad Sci USA 93: 12631–12636, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mairbaurl H, Herth C. Na+-K+-2Cl- cotransport, Na+/H+ exchange, and cell volume in ferret erythrocytes. Am J Physiol Cell Physiol 271: C1603–C1611, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Malo ME, Li L, Fliegel L. Mitogen-activated protein kinase-dependent activation of the Na+/H+ exchanger is mediated through phosphorylation of amino acids Ser770 and Ser771. J Biol Chem 282: 6292–6299, 2007. [DOI] [PubMed] [Google Scholar]
- 34.McLean LA, Zia S, Gorin FA, Cala PM. Cloning and expression of the Na+/H+ exchanger from Ampiuma RBCs: resemblance to mammalian NHE1. Am J Physiol Cell Physiol 276: C1025–C1037, 1999. [DOI] [PubMed] [Google Scholar]
- 35.McSwine RL, Li J, Villereal ML. Examination of the role for Ca2+ in regulation and phosphorylation of the Na+/H+ antiporter NHE1 via mitogen and hypertonic stimulation. J Cell Physiol 168: 8–17, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch 447: 549–565, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Pang T, Hisamitsu T, Mori H, Shigekawa M, Wakabayashi S. Role of calcineurin B homologous protein in pH regulation by the Na+/H+ exchanger 1: tightly bound Ca2+ ions as important structural elements. Biochemistry 43: 3628–3636, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Pang T, Su X, Wakabayashi S, Shigekawa M. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J Biol Chem 276: 17367–17372, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Parker JC, Colclasure GC, McManus TJ. Coordinated regulation of shrinkage-induced Na/H exchange and swelling-induced [K-Cl] cotransport in dog red cells. Further evidence from activation kinetics and phosphatase inhibition. J Gen Physiol 98: 869–880, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker JC, McManus TJ, Starke LC, Gitelman HJ. Coordinated regulation of Na/H exchange and [K-Cl] cotransport in dog red cells. J Gen Physiol 96: 1141–1152, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen SF, Kramhoft B, Jorgensen NK, Hoffmann EK. Shrinkage-induced activation of the Na+/H+ exchanger in Ehrlich ascites tumor cells: mechanisms involved in the activation and a role for the exchanger in cell volume regulation. J Membr Biol 149: 141–159, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Robson L, Hunter M. Role of cell volume and protein kinase C in regulation of a Cl- conductance in single proximal tubule cells of Rana temporaria. J Physiol 480: 1–7, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sardet C, Counillon L, Franchi A, Pouyssegur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science 247: 723–726, 1990. [DOI] [PubMed] [Google Scholar]
- 44.Sardet C, Fafournoux P, Pouysségur J. Alpha-thrombin, epidermal growth factor, and okadaic acid activate the Na+/H+ exchanger, NHE-1, by phosphorylating a set of common sites. J Biol Chem 266: 19166–19171, 1991. [PubMed] [Google Scholar]
- 45.Sarkadi B, Mack E, Rothstein A. Ionic events during the volume response of human peripheral blood lymphocytes to hypotonic media. I. Distinctions between volume-activated Cl- and K+ conductance pathways. J Gen Physiol 83: 497–512, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva NL, Haworth RS, Singh D, Fliegel L. The carboxyl-terminal region of the Na+/H+ exchanger interacts with mammalian heat shock protein. Biochemistry 34: 10412–10420, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Starke LC, Jennings ML. K-Cl cotransport in rabbit red cells: further evidence for regulation by protein phosphatase type 1. Am J Physiol Cell Physiol 264: C118–C124, 1993. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi E, Abe J, Gallis B, Aebersold R, Spring DJ, Krebs EG, Berk BC. p90(RSK) is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem 274: 20206–20214, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Tilly BC, van, den Berghe N, Tertoolen LG, Edixhoven MJ, de JH. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem 268: 19919–19922, 1993. [PubMed] [Google Scholar]
- 50.Wakabayashi S, Bertrand B, Shigekawa M, Fafournoux P, Pouysségur J. Growth factor activation and “H(+)-sensing” of the Na+/H+ exchanger isoform 1 (NHE1). Evidence for an additional mechanism not requiring direct phosphorylation. J Biol Chem 269: 5583–5588, 1994. [PubMed] [Google Scholar]
- 51.Wakabayashi S, Ikeda T, Iwamoto T, Pouysségur J, Shigekawa M. Calmodulin-binding autoinhibitory domain controls “pH-sensing” in the Na+/H+ exchanger NHE1 through sequence-specific interaction. Biochemistry 36: 12854–12861, 1997. [DOI] [PubMed] [Google Scholar]