Abstract
Phenotypic switching of vascular smooth muscle cells (SMCs), such as increased proliferation, enhanced migration, and downregulation of SMC differentiation marker genes, is known to play a key role in the development of atherosclerosis. However, the factors and mechanisms controlling this process are not fully understood. We recently showed that oxidized phospholipids, including 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), which accumulate in atherosclerotic lesions, are potent repressors of expression of SMC differentiation marker genes in cultured SMCs as well as in rat carotid arteries in vivo. Here, we examined the molecular mechanisms whereby POVPC induces suppression of SMC differentiation marker genes in cultured SMCs. Results showed that POVPC induced phosphorylation of ERK1/2 and Elk-1. The MEK inhibitors U-0126 and PD-98059 attenuated POVPC-induced suppression of smooth muscle (SM) α-actin and SM-myosin heavy chain. POVPC also induced expression of Krüppel-like factor 4 (Klf4). Chromatin immunoprecipitation assays revealed that POVPC caused simultaneous binding of Elk-1 and Klf4 to the promoter region of the SM α-actin gene. Moreover, coimmunoprecipitation assays showed a physical interaction between Elk-1 and Klf4. Results in Klf4-null SMCs showed that blockade of both Klf4 induction and Elk-1 phosphorylation completely abolished POVPC-induced suppression of SMC differentiation marker genes. POVPC-induced suppression of SMC differentiation marker genes was also accompanied by hypoacetylation of histone H4 at the SM α-actin promoter, which was mediated by the recruitment of histone deacetylases (HDACs), HDAC2 and HDAC5. Coimmunoprecipitation assays showed that Klf4 interacted with HDAC5. Results provide evidence that Klf4, Elk-1, and HDACs coordinately mediate POVPC-induced suppression of SMC differentiation marker genes.
Keywords: gene transcription, extracellular signal-regulated kinases 1/2, histone acetylation
phenotypic switching of smooth muscle cells (SMCs) plays a key role in the development of vascular diseases such as atherosclerosis and restenosis after percutaneous coronary interventions (8, 24). A hallmark of SMC phenotypic switching is the coordinate suppression of expression of SMC differentiation marker genes including smooth muscle (SM) α-actin, SM-myosin heavy chain (SM-MHC), and SM22α. As such, elucidation of the molecular mechanisms controlling downregulation of SMC differentiation marker genes is likely to provide important insights toward understanding of the development of vascular disease.
The promoter-enhancer regions of most SMC differentiation maker genes contain common cis-elements including multiple CC(A/T-rich)6GG (CArG) elements and a transforming growth factor-β (TGF-β) control element (8, 24). The binding factor of CArG elements is serum response factor (SRF), which activates expression of SMC differentiation marker genes by cooperating with its cofactors, myocardin, MKL1 (MRTF-A), and MKL2 (MRTF-B) (24, 25). In contrast, Krüppel-like factor 4 (Klf4) is a binding factor for the TGF-β control element and it potently represses SMC differentiation marker genes (1, 10, 11). Downregulation of SMC differentiation marker genes by platelet-derived growth factor-BB (PDGF-BB) has been shown to be mediated through these cis-elements and trans-binding factors. Indeed, PDGF-BB causes phosphorylation of Elk-1 via activation of the MEK-ERK1/2 pathway, and phosphorylated Elk-1 displaces myocardin, MKL1, and MKL2 from SRF, resulting in the transcriptional repression of SMC differentiation marker genes (20, 25). PDGF-BB also induces Klf4 expression, and siRNA-induced suppression of Klf4 partially attenuates PDGF-BB-induced downregulation of SMC differentiation marker genes (11). In addition, we have shown that PDGF-BB-induced suppression of SMC differentiation marker genes is mediated in part by the recruitment of histone deacetylases (HDACs), HDAC2, HDAC4, and HDAC5, to the promoter regions of these genes (25). Moreover, we have recently demonstrated that conditional deletion of the Klf4 gene in mice delays repression of SMC differentiation markers but accelerates neointimal formation following carotid ligation injury in vivo (26).
Oxidation of low-density lipoprotein-derived phospholipids is considered to be a key event in the early stages of development of atherosclerotic lesions. Oxidized phospholipids including 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC) and 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphocholine (PGPC) have been shown to accumulate in atherosclerotic lesions of cholesterol-fed rabbits (21). In addition, natural antibodies against these phospholipids have been found in the serum of apoE-null mice, which develop advanced atherosclerotic lesions (6). Treatment of cultured endothelial cells with oxidized phospholipids stimulates expression of a number of inflammatory cytokines and adhesion molecules resulting in enhanced monocyte binding (9). Moreover, oxidized phospholipids stimulate tissue factor expression and decrease thrombomodulin expression in endothelial cells, suggesting that these bioactive lipids may play a role in promoting thrombotic events in atherosclerosis (3, 7). Furthermore, of significant interest, increased levels of oxidized phospholipids in the plasma have been shown to correlate with the risk of coronary artery disease in humans (18). Taken together, these results provide evidence that oxidized phospholipids play an important role in the early stages of atherosclerosis.
Recently, we showed that oxidized phospholipids including POVPC and PGPC are potent repressors of expression of SMC differentiation marker genes in cultured SMCs as well as in rat carotid arteries in vivo (14). Treatment of cultured SMCs with oxidized phospholipids decreased expression of SM α-actin and SM-MHC. Exposure of rat carotid arteries to POVPC within pluronic gels also decreased expression of SMC differentiation marker genes in vivo. Although we showed that POVPC-induced suppression of these genes was mediated at least in part by the induction of Klf4 expression, the underlying molecular mechanisms have not been fully elucidated, and no studies have been completed to assess the role of alternative mechanisms for suppression of SMC differentiation marker genes, including the MEK-ERK1/2-Elk-1 pathway first identified by Olson and coworkers (20). The aims of the present studies were to determine if the MEK-ERK1/2-Elk-1 pathway contributes to POVPC-induced suppression of SMC differentiation marker genes as it does to PDGF-BB-induced suppression of these genes (20, 25). In addition, we have used the POVPC-induced suppression model to carry out the first studies examining the possibility of cooperative interaction between Klf4 and Elk-1, and how these factors induce changes in histone modification patterns involved in SMC phenotypic switching.
MATERIALS AND METHODS
Reagents and plasmid constructs.
POVPC was purchased from Cayman Chemical (Ann Arbor, MI). PD-98059 and U-0126 were obtained from Calbiochem (Darmstadt, Germany) and Cell Signaling Technology (Beverly, MA), respectively. Expression plasmids for FLAG-tagged HDAC2 and FLAG-tagged HDAC5 were provided by Dr. Stuart L. Schreiber (Harvard University, Cambridge, MA) and Dr. Edward Seto (H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL), respectively. An expression plasmid for FLAG-tagged Klf4 was described previously (10). Expression plasmids for Myc-tagged Klf4 and Myc-tagged Elk-1 were constructed by inserting mouse Klf4 cDNA and human Elk-1 cDNA, respectively, into pcDNA3.1(+)/myc-His (Invitrogen, Carlsbad, CA). Expression plasmids for Myc-tagged Klf4 (aa 1-350) and Myc-tagged Klf4 (aa 1-155) were constructed from Myc-tagged Klf4 expression plasmid by restriction enzyme digestion.
Cell culture and transient transfection.
All animal use procedures employed in these studies were approved by the University of Virginia Animal Care and Use Committee. Rat aortic SMCs were cultured as described previously (23, 25). Mouse aortic SMCs deficient for Klf4 and control SMCs were described previously (14, 26). Rat and mouse SMCs were grown to confluence in DMEM-Ham's F-12 medium with 10% fetal bovine serum (Hyclone, Logan, UT). Confluent SMCs were incubated in serum-free DMEM-Ham's F-12 medium supplemented with 5 μg/ml transferrin, 6.25 ng/ml sodium selenite, 1 μm/l insulin, and 0.2 mmol/l l-ascorbic acid for 4 days and were treated with 10 μg/ml POVPC or vehicle. Transfection of DNA plasmids was performed using FuGene6 (Roche Diagnostics, Indianapolis, IN).
Real-time RT-PCR.
Total RNA prepared from cultured SMCs was used for real-time RT-PCR. Primer and probe sequences for SM α-actin, SM-MHC, Klf4, and 18S rRNA were described previously (11, 23).
Western blot analysis and immunofluorescence.
Western blot analysis and immunofluorescence staining were performed as described previously (25). Antibodies used were as follows: ERK1/2 (Cell Signaling Technology), phospho-ERK1/2 (E10, Cell Signaling Technology), Elk-1 (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-Elk-1 (Cell Signaling Technology), Klf4 (raised against mouse Klf4 from amino acid 15 to 29, ASGPAGREKTLRPAG, Chemicon International, Temecula, CA), SRF (Santa Cruz Biotechnology), MKL1 (Santa Cruz Biotechnology), HDAC2 (Zymed, South San Francisco, CA), HDAC5 (Cell Signaling Technology), GAPDH (6C5, Chemicon International), Myc (9E11, Santa Cruz Biotechnology), and FLAG (M2, Sigma).
Quantitative chromatin immunoprecipitation assays.
Quantitative chromatin immunoprecipitation (ChIP) assays were performed as previously described (25). Antibodies used were as follows: Elk-1, Klf4, SRF, MKL1, acetyl histone H4 (Chemicon International), HDAC2, HDAC4 (Cell Signaling Technology), and HDAC5. Real-time PCR was performed to amplify the promoter region of the SM α-actin gene that contains CArG elements and a TGF-β control element. Data represent the relative enrichment of immunoprecipitated DNA as compared with input DNA. Sequential ChIP assays were performed as described previously (2).
Coimmunoprecipitation assays.
Coimmunoprecipitation assays were performed in COS cells as described previously (16, 25).
Statistical analyses.
Data are presented as means ± SE. Statistical analyses were performed by one-way ANOVA with a post hoc Fishers protected least significant differences test or unpaired t-test. P < 0.05 was considered significant.
RESULTS
MEK inhibitors attenuated POVPC-induced suppression of SMC differentiation marker genes in cultured SMCs.
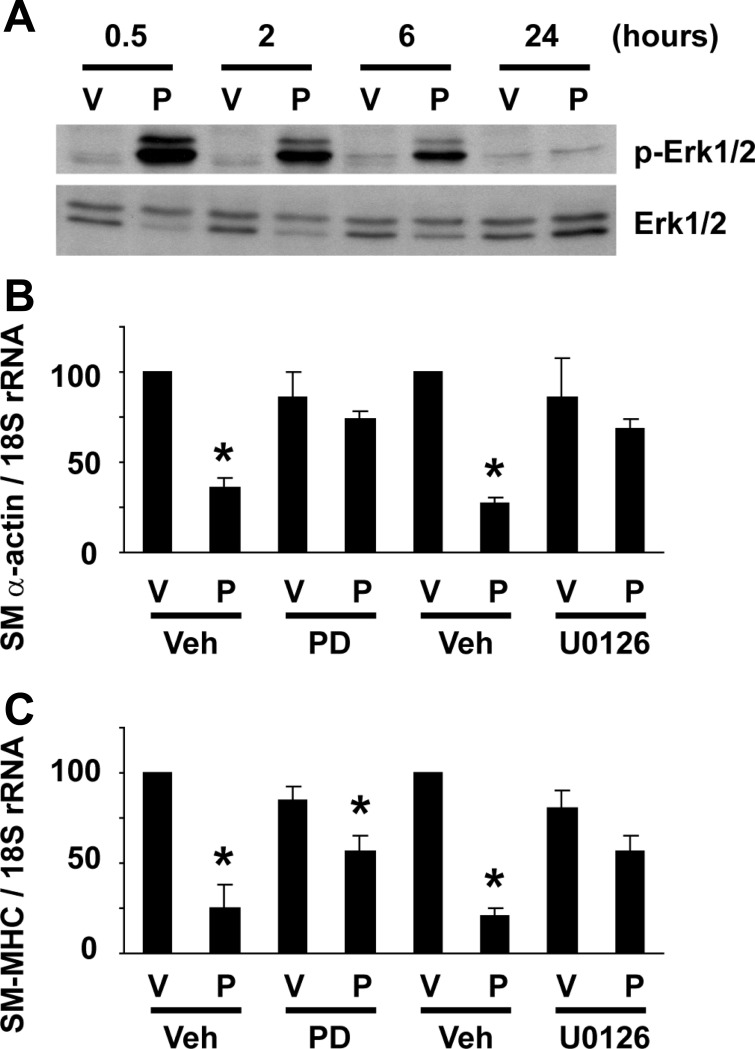
The initial aim of the present studies was to determine if the MEK-ERK1/2 pathway is involved in POVPC-induced suppression of SMC differentiation marker genes. We first examined if POVPC activates ERK1/2 in cultured rat aortic SMCs. As shown in Fig. 1A, POVPC treatment at 10 μg/ml induced ERK1/2 phosphorylation at 30 min, 2 h, and 6 h, but not at 24 h, suggesting that POVPC elicits a transient activation of the MEK-ERK1/2 pathway. Effects of MEK inhibitors, PD-98059 and U-0126, on POVPC-induced suppression of SMC differentiation marker genes were then tested. Cultured SMCs were pretreated with 10 μmol/l PD-98059, 10 μmol/l U-0126, or vehicle for 30 min, and POVPC was added to the media. Although treatment with POVPC alone for 24 h decreased expression of SMC differentiation marker genes including SM α-actin and SM-MHC by 65% and 74%, respectively, MEK inhibitors partially attenuated POVPC-induced suppression of SMC differentiation marker genes (Fig. 1, B and C). Results indicate that POVPC-induced suppression of SMC differentiation marker genes is mediated in part by the MEK-ERK1/2 pathway.
Fig. 1.
MEK inhibitors attenuated 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC)-induced suppression of smooth muscle cell (SMC) differentiation marker genes. A: cultured SMCs were treated with 10 μg/ml POVPC (P) or vehicle (V) for indicated times. Expression of ERK1/2 and phosphorylated ERK1/2 (p-ERK1/2) was examined by Western blotting. Representative pictures are shown from three independent experiments. B and C: cultured smooth muscle cells (SMCs) were treated with 10 μmol/l PD-98059 (PD), 10 μmol/l U-0126, or vehicle (Veh) 30 min before treatment with POVPC or vehicle for 24 h. Expression of smooth muscle (SM) α-actin (B) and SM-myosin heavy chain (SM-MHC) (C) was determined by real-time RT-PCR. Values represent means ± SE. *P < 0.05 compared with vehicle-treated SMCs (n = 4).
POVPC elicited simultaneous binding of Klf4 and Elk-1 to the promoter region of the SM α-actin gene.
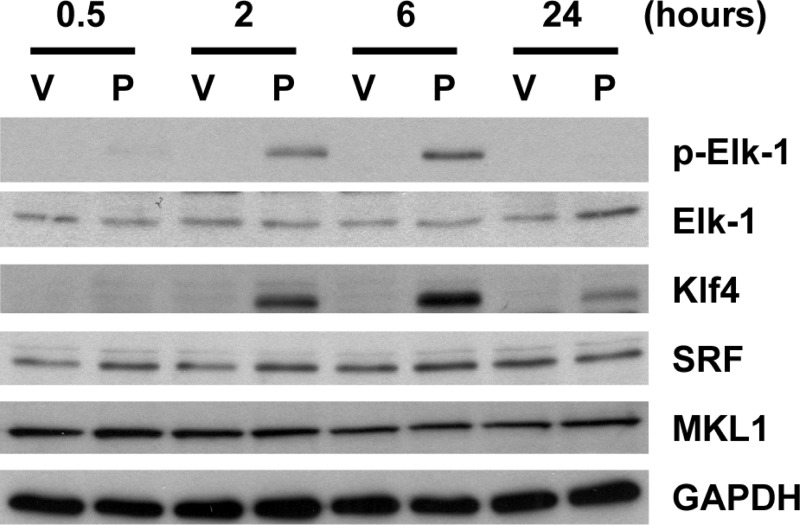
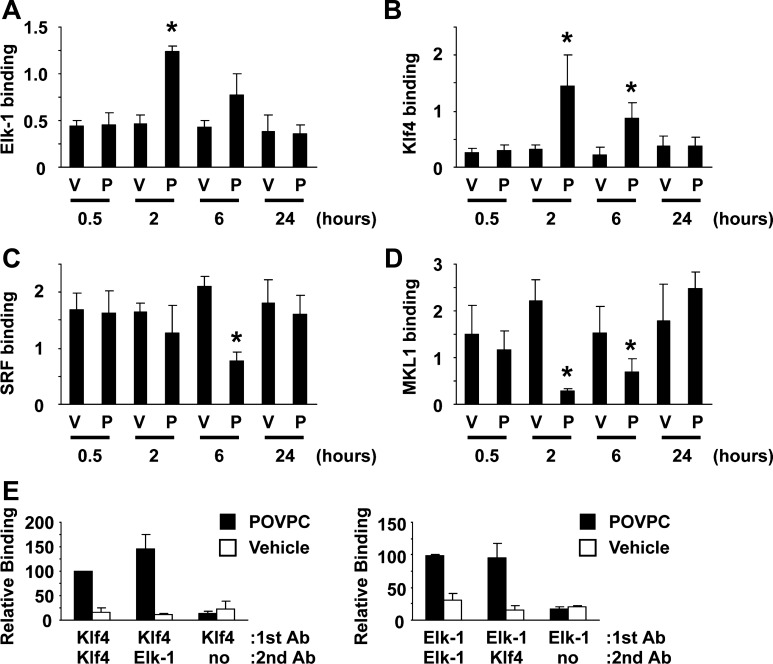
Since Elk-1 is a downstream target of the MEK-ERK1/2 pathway, we tested if POVPC activates Elk-1 in cultured SMCs. Results of Western blot analysis showed that treatment with POVPC caused phosphorylation of Elk-1 at 2 and 6 h (Fig. 2). Results of Western blot analysis also showed that Elk-1 phosphorylation occurred with a similar time course as induction of Klf4 expression. In contrast, expression of SRF and MKL1 was unaltered by POVPC treatment. Binding of Elk-1, Klf4, SRF, and MKL1 to the promoter region of the SM α-actin gene was examined by ChIP assays. Consistent with the kinetics of Elk-1 phosphorylation and Klf4 induction, POVPC treatment caused increased binding of Elk-1 and Klf4 to the promoter region of the SM α-actin gene at 2 and 6 h in cultured SMCs (Fig. 3, A and B). SRF binding was transiently decreased at 6 h, and MKL1 binding was decreased at 2 and 6 h following POVPC treatment (Fig. 3, C and D). On the basis of the results of previous studies, MKL1 binding has been shown to correlate with the binding of the myocardin family including myocardin and MKL2 (20, 25). To determine if Elk-1 and Klf4 simultaneously occupy the same chromosomal fragment containing the SM α-actin promoter or if proteins bind to independent alleles within a given cell or different cells, we then performed sequential ChIP assays. Results showed that Elk-1 and Klf4 simultaneously bound to the SM α-actin promoter following POVPC treatment (Fig. 3E). Collectively, our results suggest that POVPC-induced suppression of SMC differentiation marker genes is mediated by the simultaneous recruitment of two repressors, phosphorylated Elk-1 and Klf4, to the promoter regions of these genes as well as the release of the activators, SRF, and myocardin family members from these promoters.
Fig. 2.
POVPC phosphorylated Elk-1 and induced Krüppel-like factor 4 (Klf4) expression in cultured SMCs. Cultured SMCs were treated with POVPC or vehicle for indicated times. Expression of Elk-1, phosphorylated Elk-1 (p-Elk-1), Klf4, serum response factor (SRF), MKL1, and GAPDH was examined by Western blotting. Representative pictures are shown from three independent experiments.
Fig. 3.
POVPC caused simultaneous binding of Elk-1 and Klf4 to the SM α-actin promoter. A–D: cultured SMCs were treated with POVPC or vehicle for indicated times. Association of Elk-1 (A), Klf4 (B), SRF (C), and MKL1 (D) with the promoter region of the SM α-actin gene was determined by chromatin immunoprecipitation (ChIP) assays. Values represent means ± SE. *P < 0.05 compared with vehicle-treated SMCs (n = 3). E: sequential ChIP assays were performed in cultured SMCs treated with POVPC or vehicle for 2 h (n = 3). Ab, antibody.
Induction of Klf4 and phosphorylation of Elk-1 were not interdependent.
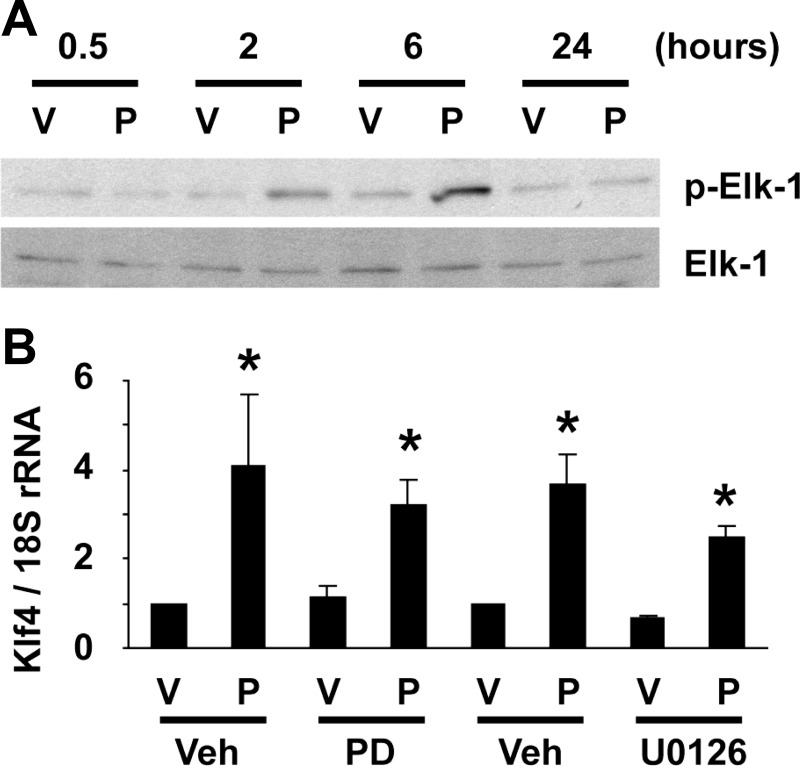
We next tested if Klf4 induction and Elk-1 phosphorylation were interdependent. First, POVPC-induced phosphorylation of Elk-1 was examined in cultured mouse SMCs deficient for Klf4. POVPC treatment caused Elk-1 phosphorylation at 2 and 6 h in Klf4-null SMCs (Fig. 4A), indicating that Elk-1 phosphorylation is not impaired or delayed even in the absence of Klf4. Next, induction of Klf4 expression was examined in cultured rat aortic SMCs in the presence of MEK inhibitors. MEK inhibitors, PD-98059 and U-0126, both did not affect POVPC-induced expression of Klf4 (Fig. 4B). These results clearly demonstrate that Klf4 induction and Elk-1 phosphorylation can occur independent of each other.
Fig. 4.
Phosphorylation of Elk-1 and induction of Klf4 were not interdependent. A: Klf4-null SMCs were treated with POVPC or vehicle for indicated times. Expression of Elk-1 and p-Elk-1 was examined by Western blotting. Representative pictures are shown from three independent experiments. B: cultured SMCs were treated with PD-98059 (PD), U-0126, or vehicle 30 min before treatment with POVPC or vehicle for 24 h. Expression of Klf4 was determined by real-time RT-PCR. Values represent means ± SE. *P < 0.05 compared with vehicle-treated SMCs (n = 4).
Klf4 and Elk-1 cooperatively mediated POVPC-induced suppression of SMC differentiation marker genes.
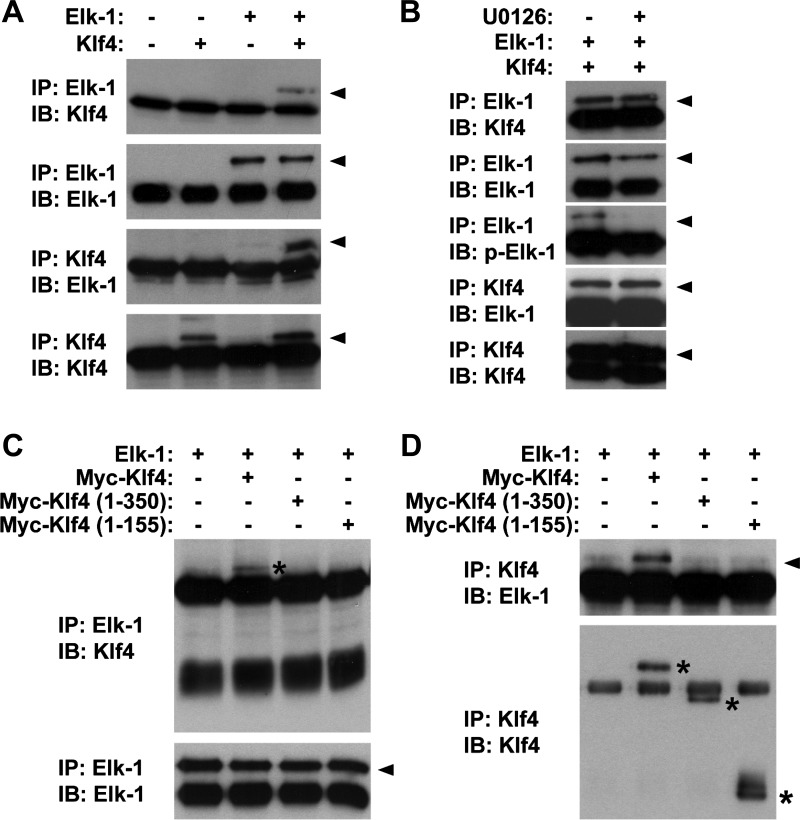
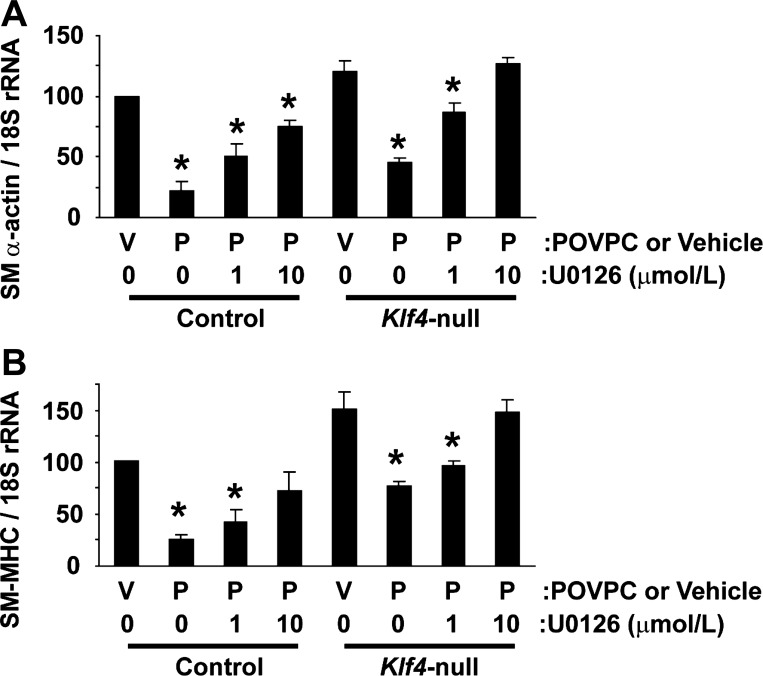
Results thus far have shown that, although POVPC induces Klf4 expression and Elk-1 phosphorylation independently, these proteins simultaneously bind to the same chromosomal fragment of SMC differentiation marker genes. These results raised the possibility that these pathways converge and cooperatively repress expression of SMC differentiation marker genes at the promoter level. We tested this possibility by performing the following two experiments. First, coimmunoprecipitation assays were done to test the possible interaction between Elk-1 and Klf4. Results showed that Elk-1 and Klf4 physically interacted with each other (Fig. 5A). The interaction was seen even in the presence of U-0126 at 10 μg/ml (Fig. 5B), suggesting that phosphorylation of Elk-1 is not necessary for Klf4 binding. We determined the domain of Klf4 responsible for Elk-1 interaction using Klf4 deletion mutants. Although full-length Klf4 (aa 1-483) interacted with Elk-1, Klf4 deletion mutants including Klf4 (aa 1-350) and Klf4 (aa 1-155) did not interact with Elk-1 (Fig. 5, C and D), indicating that the domain from amino acid 351 to the carboxyl terminus (amino acid 483) of Klf4 protein is responsible for Elk-1 interaction. Second, combinatorial blockade of the MEK-ERK1/2-Elk-1 pathway and the Klf4 pathway was examined in cultured mouse SMCs. Results showed that combination of blockade of Klf4 induction, which was achieved by Klf4 gene ablation, and blockade of Elk-1 phosphorylation, which was mediated by the MEK inhibitor U-0126, completely abolished POVPC-induced suppression of SMC differentiation marker genes including SM α-actin and SM-MHC (Fig. 6). Taken together, results suggest that POVPC-induced suppression of SMC differentiation marker genes is coordinately mediated by the combination of the MEK-ERK1/2-Elk-1 pathway and the Klf4 pathway.
Fig. 5.
Klf4 physically interacted with Elk-1. COS cells were cotransfected with expression plasmids for Elk-1 and Klf4, or empty plasmid, and coimmunoprecipitation assays were performed. A: cell lysates were immunoprecipitated (IP) with anti-Klf4 antibody or anti-Elk-1 antibody and were immunoblotted (IB) with anti-Klf4 antibody or anti-Elk-1 antibody. B: interaction between Klf4 and Elk-1 was examined in COS cells in the presence or absence of 10 μmol/l U-0126. C and D: COS cells were cotransfected with Elk-1 expression plasmid and expression plasmid for wild-type Myc-tagged Klf4, Myc-tagged Klf4 (aa 1-350), or Myc-tagged Klf4 (aa 1-155). Cell lysates were immunoprecipitated with anti-Elk-1 antibody or anti-Klf4 antibody and were immunoblotted with anti-Elk-1 antibody or anti-Klf4 antibody. Arrowheads and asterisks indicate specific bands.
Fig. 6.
Klf4 and Elk-1 cooperatively mediated POVPC-induced suppression of SMC differentiation marker genes. Klf4-null SMCs and control SMCs were treated with U-0126 or vehicle 30 min before treatment with POVPC or vehicle for 24 h. Expression of SM α-actin (A) and SM-MHC (B) was determined by real-time RT-PCR. Values represent means ± SE. *P < 0.05 compared with vehicle-treated SMCs (n = 3).
POVPC-induced suppression of SMC differentiation marker genes was accompanied by HDAC-mediated hypoacetylation of histone H4 at the SMC promoter.
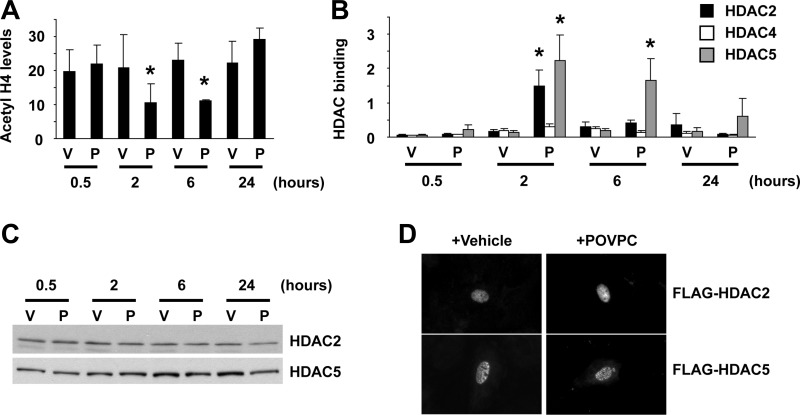
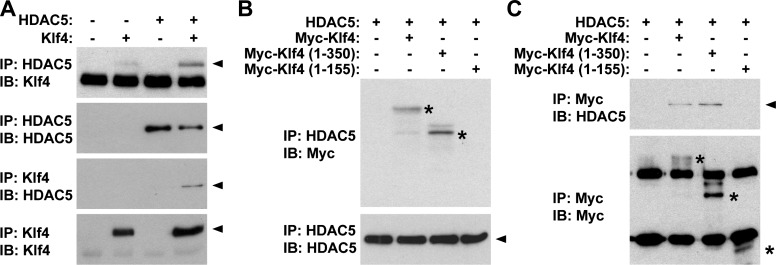
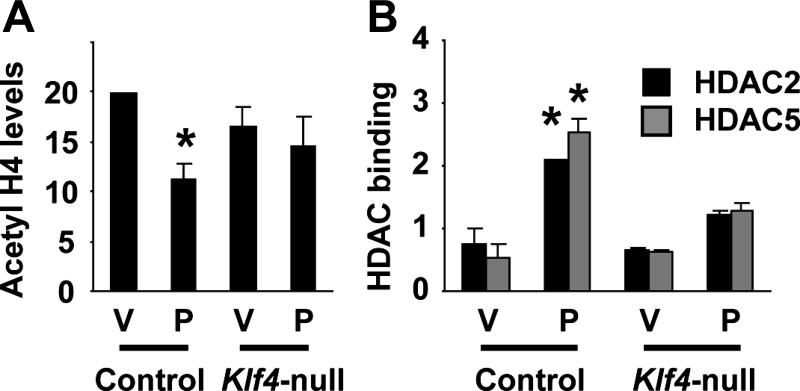
To determine if epigenetic control mechanisms also contribute to POVPC-induced suppression of SMC differentiation marker genes, we examined if POVPC treatment induces changes in histone acetylation status at the promoter regions of SMC differentiation marker genes. As shown in Fig. 7A, treatment with POVPC induced hypoacetylation of histone H4 at the promoter region of the SM α-actin gene in cultured rat aortic SMCs. Because results of previous studies have shown that a subset of HDACs including HDAC2, HDAC4, and HDAC5 are capable of inhibiting expression of SMC differentiation marker genes (25), association of these HDACs with the SM α-actin promoter was examined by ChIP assays. Results showed that POVPC increased the association of HDAC2 and HDAC5 with the promoter region of the SM α-actin gene in cultured SMCs (Fig. 7B). However, expression levels and intracellular localization of these HDACs were unaltered (Fig. 7, C and D), suggesting that POVPC causes the recruitment of HDAC2 and HDAC5 to the promoter regions of SMC differentiation marker genes within the nucleus. We hypothesized that POVPC induces expression of Klf4 which recruits HDAC2 and HDAC5 to the promoter regions of SMC differentiation marker genes by protein-protein interactions. Because the interaction between HDAC2 and Klf4 has been demonstrated by multiple investigators previously (8, 13), the interaction between Klf4 and HDAC5 was examined in the present studies. Coimmunoprecipitation assays revealed that Klf4 interacted with HDAC5 (Fig. 8A). We also determined the domain of Klf4 responsible for HDAC5 interaction. Although deletion from amino acid 351 to the carboxyl terminus [Klf4 (aa 1-350)] did not affect HDAC5 interaction, a further deletion mutant from amino acid 151 to the carboxyl terminus [Klf4 (aa 1-155)] failed to interact with HDAC5 (Fig. 8, B and C). Results indicate that the domain of Klf4 protein from amino acid 156 to amino acid 350 is responsible for HDAC5 interaction. Finally, we performed ChIP assays to determine histone acetylation levels and HDAC binding at the SM α-actin promoter in Klf4-null cultured SMCs. Although POVPC treatment caused decreased acetyl histone H4 levels and increased binding of HDAC2 and HDAC5 at the SM α-actin promoter in control SMCs, these effects were attenuated in Klf4-null SMCs (Fig. 9). Results provide evidence that HDAC2 and HDAC5 as well as Klf4 coordinately mediate POVPC-induced suppression of SMC differentiation marker genes.
Fig. 7.
POVPC induced hypoacetylation of histone H4 at the SM α-actin promoter by recruiting histone deacetylases (HDAC) HDAC2 and HDAC5. A and B: cultured SMCs were treated with POVPC or vehicle for indicated times. Acetyl histone H4 levels at the SM α-actin promoter (A) and the association of HDAC2, HDAC4, and HDAC5 with the SM α-actin promoter (B) were determined by ChIP assays. Values represent means ± SE. *P < 0.05 compared with vehicle-treated SMCs (n = 3). C: cultured SMCs were treated with POVPC or vehicle for indicated times. Expression of HDAC2 and HDAC5 was examined by Western blotting. Representative pictures are shown from three independent experiments. D: cultured SMCs were transfected with expression plasmids for FLAG-tagged HDAC2 and FLAG-tagged HDAC5 and were treated with POVPC or vehicle for 2 h. Intracellular localization of HDAC2 and HDAC5 was examined by anti-FLAG antibody.
Fig. 8.
Klf4 interacted with HDAC5. A: COS cells were cotransfected with expression plasmids for HDAC5 and Myc-tagged Klf4, or with empty plasmid, and coimmunoprecipitation assays were performed. Cell lysates were immunoprecipitated with anti-Klf4 antibody or anti-HDAC5 antibody and were immunoblotted with anti-Klf4 antibody or anti-HDAC5 antibody. B and C: COS cells were cotransfected with HDAC5 expression plasmid and with expression plasmid for wild-type Myc-tagged Klf4, Myc-tagged Klf4 (aa 1-350), or Myc-tagged Klf4 (aa 1-155). Cell lysates were immunoprecipitated with anti-Myc antibody or anti-HDAC5 antibody and were immunoblotted with anti-Myc antibody or anti-HDAC5 antibody. Arrowheads and asterisks indicate specific bands.
Fig. 9.
POVPC-induced hypoacetylation of histone H4 at the SM α-actin promoter was attenuated in Klf4-null SMCs. Control and Klf4-null cultured mouse SMCs were treated with POVPC or vehicle for 2 h, and acetyl histone H4 levels at the SM α-actin promoter (A) and the association of HDAC2 and HDAC5 with the SM α-actin promoter (B) were determined by ChIP assays. Values represent means ± SE. *P < 0.05 compared with vehicle-treated SMCs (n = 2).
DISCUSSION
Results of the present studies provide evidence that POVPC-induced suppression of SMC differentiation marker genes is mediated by cooperative interaction of MEK-ERK1/2-Elk-1 and Klf4 pathways. Indeed, simultaneous blockade of both pathways completely abolished POVPC-induced suppression of SM α-actin and SM-MHC in cultured SMCs. In addition, we provide novel evidence showing that Elk-1 and Klf4 physically interact and simultaneously bind to the same chromosomal fragment of the SM α-actin promoter. We also demonstrated that POVPC-induced suppression of SMC differentiation marker genes is accompanied by hypoacetylation of histone H4 at the SM α-actin promoter, and we provide evidence that this is mediated by the recruitment of HDAC2 and HDAC5. Induction of Klf4 is likely to play a role in the recruitment of these HDACs to the promoter, because the present studies and previous studies have shown the physical interaction between Klf4 and these HDACs (8, 13). As such, the present studies clarify several critical mechanisms that contribute to suppression of SMC differentiation marker genes in response to oxidized phospholipids and provide new insights as to potential mechanisms that may contribute to the development of atherosclerosis.
Results of the present studies showing interaction between Elk-1 and Klf4 indicate that these proteins cooperatively suppress expression of SMC differentiation marker genes in response to oxidized phospholipids. Of interest, phosphorylation of Elk-1 was not required for this interaction. In contrast, results of previous studies showed the interaction between phosphorylated Elk-1 and SRF on CArG elements within the promoter regions of SMC differentiation marker genes following PDGF-BB treatment (20, 25). In this case, phosphorylation of Elk-1 at tyrosine 159 was required for this protein to compete with the myocardin family for SRF binding. The difference in the requirement of phosphorylation of Elk-1 for protein-protein interactions suggests that Elk-1 binds to SRF and Klf4 through different domains. In addition, because Liu et al. (11) previously showed that Klf4 was able to bind to SRF by coimmunoprecipitation assays, it is possible that Elk-1, Klf4, and SRF form a triad to repress SMC differentiation marker gene expression in response to multiple stimuli such as PDGF-BB and oxidized phospholipids. However, on the basis of our present and previous observations that overexpression of Klf4 or treatment with PDGF-BB or oxidized phospholipids dramatically reduced SRF binding to CArG elements within the promoter regions of SMC differentiation marker genes (11), it is likely that this complex is present within the nucleoplasm rather than being bound to DNA and thus would not be detected by electrophoretic mobility shift assays. Indeed, consistent with this, Mack et al. (12) were unable to detect the presence of Elk-1 within CArG-SRF complexes by electrophoretic mobility shift assays using nuclear extracts from cultured SMCs.
Recently, we showed that conditional deletion of the Klf4 gene in mice transiently delayed injury-induced suppression of SMC differentiation markers following carotid ligation-injury (26). Indeed, downregulation of expression of SM α-actin and SM22α was inhibited at day 3, but not at day 7 after vascular injury in Klf4-deficient mice. We also provided evidence that Klf4 transiently associated with the promoter regions of SMC differentiation marker genes as well as the p21WAF/Cip1 gene, but not with the c-fos gene in phenotypically modulated SMCs (26). On the basis of the results of our present studies, it is possible that combinatorial knockout of multiple transcription factors including Klf4 and Elk-1 may elicit profound and sustained inhibition of suppression of SMC differentiation markers, as compared with the transient delay caused by the deletion of the single transcription factor, Klf4. In addition, multiple additional repressor pathways have also been shown to contribute to repression of SMC differentiation marker genes in response to vascular injury and during disease states including atherosclerosis in vivo (8). For example, mutation of a G/C-rich element in the SM22α promoter abolished downregulation of the SM22α gene transcriptional activity in response to carotid wire-injury as well as in atherosclerotic lesions in apoE knockout mice (15, 19), although the mechanisms that regulate the activity of the G/C-rich element in vivo remain to be elucidated. Substitution of two degenerate CArG elements within the SM α-actin promoter with the c-fos consensus CArG elements also significantly attenuated downregulation of SM α-actin gene transcription after wire-injury through mechanisms associated with altered SRF binding affinity to CArG elements (5). Taken together, the preceding studies and our present studies support a model wherein repression of SMC differentiation marker genes is coordinately regulated by a combination of multiple transcription factors, cofactors, and cis-regulatory elements. Although the reasons for the apparent redundancy and cooperativity between different repressor pathways for SMC differentiation marker genes are not known, it is interesting to speculate that they evolved as a protective mechanism to optimize the effective repair of mechanical, immunological, or other forms of vascular injury. However, further studies are needed to determine the relative importance of these various pathways in vivo during vascular repair and/or in disease states.
Klf4 has been shown to interact with multiple proteins implicated in the regulation of gene transcriptional activity. For example, the amino-terminal domain from amino acid 1 to amino acid 109 interacts with CREB-binding protein (CBP) (4), whereas the carboxyl terminus of Klf4 from amino acid 350 to amino acid 483 binds to p53 (27). The carboxyl-terminal domain also contains three contiguous cysteine and histidine-type (C2H2) zinc fingers that are common for the Klf family and confer DNA binding (17). Our results showed that the domain from amino acid 156 to amino acid 350 of mouse Klf4 protein is responsible for HDAC5 interaction. HDAC5 binding of Klf4 is not likely to be a common feature of the Klf family, because the domain is not conserved among different family members. Of interest, our results are consistent with results of previous studies by Yet et al. (22) in that they mapped a repression domain in human Klf4 protein to amino acids 188 to 388, whereas amino acids 91 to 117 of human Klf4 confer an activation function, as determined by fusing human Klf4 to the DNA-binding domain of GAL4. Klf4 is a transcription factor that is capable of both activating and repressing gene transcriptional activity, although the underlying mechanisms have not been determined yet. It is of interest to speculate that Klf4 exerts bifunctional effects by changing the partner proteins. Further studies are needed to determine whether CBP and HDAC5 are able to bind to Klf4 simultaneously, or binding of these proteins to Klf4 is mutually exclusive.
In summary, we provide evidence that Klf4, Elk-1, and HDACs coordinately repress expression of SMC differentiation marker genes in response to oxidized phospholipids. Further studies are needed to determine the role of these factors in the development of atherosclerosis in experimental animal models as well as in humans. A major limitation in the field is that no membrane receptors for oxidized phospholipids have yet been defined, thus precluding development of pharmacologic inhibitors. However, results of the present studies have defined key downstream effector pathways required for at least some of the potent biological effects of these molecules that may contribute to development of therapeutic agents and/or aid in the identification of upstream signaling molecules and pathways.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants P01-HL-19242, R01-HL-38854, R01-HL-087867, and R37-HL-57353 (to G. K. Owens) and by American Heart Association National Scientist Development Grant 0635253N (to T. Yoshida).
Acknowledgments
We thank Diane Raines and Rupande Tripathi for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Krüppel-like transcription factors bind a transforming growth factor β control element required for expression of the smooth muscle cell differentiation marker SM22α in vivo. J Biol Chem 275: 37798–37806, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125: 315–326, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and CA++/NFAT. Blood 99: 199–206, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Geiman DE, Ton-That H, Johnson JM, Yang VW. Transactivation and growth suppression by the gut-enriched Krüppel-like factor (Krüppel-like factor 4) are dependent on acidic amino acid residues and protein-protein interaction. Nucleic Acids Res 28: 1106–1113, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrix JA, Wamhoff BR, McDonald OG, Sinha S, Yoshida T, Owens GK. 5′ CArG degeneracy in smooth muscle α-actin is required for injury-induced gene suppression in vivo. J Clin Invest 115: 418–427, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hörkkö S, Miller E, Dudl E, Reaven P, Curtiss LK, Zvaifler NJ, Terkeltaub R, Pierangeli SS, Branch DW, Palinski W, Witztum JL. Antiphospholipid antibodies are directed against epitopes of oxidized phospholipids: recognition of cardiolipin by monoclonal antibodies to epitopes of oxidized low density lipoprotein. J Clin Invest 98: 815–825, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishii H, Tezuka T, Ishikawa H, Takada K, Oida K, Horie S. Oxidized phospholipids in oxidized low-density lipoprotein down-regulate thrombomodulin transcription in vascular endothelial cells through a decrease in the binding of RARβ-RXRα heterodimers and Sp1 and Sp3 to their binding sequences in the TM promoter. Blood 101: 4765–4774, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Kawai-Kowase K, Owens GK. Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol 292: C59–C69, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci USA 96: 12010–12015, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Sinha S, Owens G. A transforming growth factor-β control element required for SM α-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J Biol Chem 278: 48004–48011, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem 280: 9719–9727, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Mack CP, Thompson MM, Lawrenz-Smith S, Owens GK. Smooth muscle α-actin CArG elements coordinate formation of a smooth muscle cell-selective, serum response factor-containing activation complex. Circ Res 86: 221–232, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Noti JD, Johnson AK, Dillon JD. The leukocyte integrin gene CD11d is repressed by gut-enriched Kruppel-like factor 4 in myeloid cells. J Biol Chem 280: 3449–3457, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res 101: 792–801, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest 106: 1139–1147, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang Y, Yoshida T, Amendt BA, Martin JF, Owens GK. Pitx2 is functionally important in the early stages of vascular smooth muscle cell differentiation. J Cell Biol 181: 461–473, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem 271: 20009–20017, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med 353: 46–57, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22α promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res 95: 981–988, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428: 185–189, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Watson AD, Leitinger N, Navab M, Faull KF, Hörkkö S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 272: 13597–13607, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Yet SF, McA'Nulty MM, Folta SC, Yen HW, Yoshizumi M, Hsieh CM, Layne MD, Chin MT, Wang H, Perrella MA, Jain MK, Lee ME. Human EZF, a Krüppel-like zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J Biol Chem 273: 1026–1031, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, Sinha S, Dandré F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 92: 856–864, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res 96: 280–291, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, Gan Q, Shang Y, Owens GK. Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am J Physiol Cell Physiol 292: C886–C895, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Krüppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res 102: 1548–1557, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, Biggs JR, Kraft AS, Yang VW. The gut-enriched Krüppel-like factor (Krüppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem 275: 18391–18398, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]