Abstract
A complex rearrangement mutation in the mouse titin gene leads to an in-frame 83-amino acid deletion in the N2A region of titin. Autosomal recessive inheritance of the titin muscular dystrophy with myositis (Ttnmdm/mdm) mutation leads to a severe early-onset muscular dystrophy and premature death. We hypothesized that the N2A deletion would negatively impact the force-generating capacity and passive mechanical properties of the mdm diaphragm. We measured in vitro active isometric contractile and passive length-tension properties to assess muscle function at 2 and 6 wk of age. Micro-CT, myosin heavy chain Western blotting, and histology were used to assess diaphragm structure. Marked chest wall distortions began at 2 wk and progressively worsened until 5 wk. The percentage of myofibers with centrally located nuclei in mdm mice was significantly (P < 0.01) increased at 2 and 6 wk by 4% and 17%, respectively, compared with controls. At 6 wk, mdm diaphragm twitch stress was significantly (P < 0.01) reduced by 71%, time to peak twitch was significantly (P < 0.05) reduced by 52%, and half-relaxation time was significantly (P < 0.05) reduced by 57%. Isometric tetanic stress was significantly (P < 0.05) depressed in 2- and 6-wk mdm diaphragms by as much as 64%. Length-tension relationships of the 2- and 6-wk mdm diaphragms showed significantly (P < 0.05) decreased extensibility and increased stiffness. Slow myosin heavy chain expression was aberrantly favored in the mdm diaphragm at 6 wk. Our data strongly support early contractile and passive mechanical aberrations of the respiratory pump in mdm mice.
Keywords: N2A, cytoskeleton mechanics, chest wall mechanics
muscular dystrophy with myositis (mdm) is an early-onset autosomal recessive mouse disease that arose spontaneously at The Jackson Laboratory. It was reported near the same time as the discovery of the genetic defect in Duchenne's muscular dystrophy (DMD) (3, 20, 24). DMD is thought to be due to the absence of dystrophin, leading to destabilization of the sarcolemmal membrane and membrane fragility (26). A number of dystrophies are caused primarily by pathogenetic etiologies, whereby force transmission pathways between the extracellular matrix and cytoskeleton are disrupted. We have demonstrated, in a number of these mouse muscular dystrophy models (16, 19, 21, 25), that complete loss of membrane and extracellular structural elements (such as α7-integrin, merosin, α-sarcoglycan, and dystrophin) is associated with alterations in diaphragm passive length-tension relationships and active isometric contractile properties. Only recently, the mdm mutation was mapped precisely as a complex rearrangement and LINE insertion into the N2A region of the mouse titin gene (5). Titin mutations leading to muscular dystrophies in humans (10, 11) represent a minority of etiologies whereby myofibrillar proteins are the principal culprits. Titin mutations leading to muscular dystrophies are the result of subtle mutations that do not cause complete loss of the protein itself, inasmuch as titin is vital for normal development. Histopathology of tibial muscle in human muscular dystrophy, in particular, has been reported to be similar to that in mdm mice, inasmuch as the anterior tibial muscles are severely affected (10). However, the age of onset is much later, and mutations identified in humans are to date associated with the m-line region of titin.
Titin is a giant myofilament (22) and is critical for myo fibrillogenesis (6, 30) and sarcomeric integrity during contraction (4); it confers passive elastic properties to striated muscle (31). It is also implicated as a structural scaffolding element (12) that serves important mechanosensitive signaling functions (23). The titin mdm (Ttnmdm) mutation leads to aberrant splicing of four critical skeletal muscle-specific exons encoding 83 amino acids in the N2A region of the I band. The mutation does not appear to affect the expression level or size of the titin protein, inasmuch as changes in the apparent molecular weight were indistinguishable by SDS-PAGE (5). The m-line and N2A titin mutations abolish one of many putative binding sites for calpain-3, a muscle-specific protease; the loss of calpain-3 is also linked to limb girdle muscular dystrophy 2A. However, transgenic calpain-3-knockout mice with homozygous titin N2A deletion demonstrated that the absence of calpain-3 does not prevent or mitigate natural progression of the severe muscular dystrophy phenotype (15).
The physiological importance of the titin N2A region is further underscored by the severe disease it causes in the mdm mouse model. The mdm mouse model is a pertinent clinical phenotype, because it exhibits a clinical course similar to that of DMD, in that mdm mice have an early-onset, rapidly progressive muscle-wasting disease that ends in premature death likely due to respiratory insufficiency. Despite these similarities, the pathogenetic mechanism of the DMD model is clearly distinct from that of the mdm model. The mdm model is ripe with opportunities, in the context of a severe early-onset muscular dystrophy, to elucidate the importance of titin's N2A region and the larger role that titin may play in maintaining normal skeletal muscle function. The diaphragm, in particular, is the principal pressure-generating skeletal muscle that drives ventilation. Alteration of its mechanical properties could contribute to compromised respiratory function. The extent to which mechanical dysfunction of the diaphragm plays a role in facilitating premature death is still debated but is generally recognized to be an important factor leading to significant comorbidities, i.e., pneumonia and hypercapnia (9, 13).
Heretofore, no data on the effect of the Ttnmdm mutation on the diaphragm were available. We utilized high-resolution micro-CT of the thorax, cross-sectional histology, and myosin heavy chain (MHC) Western blots to assess diaphragm structure. In vitro contractile and passive length-tension properties of the costal diaphragm were measured before and after the onset of overt muscle histopathology. We hypothesized that the titin N2A deletion in the mdm mouse diaphragm would have a deleterious impact on the force-generating capacity and altered passive mechanical properties, independent of major histopathology. Our data show significant structural and functional aberrations of the respiratory pump in the mdm mouse that may be attributable to a critical function of titin's N2A region.
METHODS
Animals.
Mice from the B6.B6C3Fe-Ttnmdm-J/Cx strain were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of Baylor College of Medicine approved animal protocols in advance. Heterozygous (Ttnmdm/+) breeder pairs were used to generate homozygous wild-type (Ttn+/+) and homozygous mutant (Ttnmdm/mdm) mice. All experimental mice were fed ad libitum under 14-h day light cycles. Genotyping was performed on tail snips before experimentation utilizing the following primers: 5-GGAGTGACTGAACAATGAACG-3 (Ttn55F, forward) and 5-GACCAGACTGGAATTCTAAGG-3 (Ttn55R, reverse) for Ttn+/+ and 5-AGGACCAACAGAGCTGACTG-3 (Ttn58F, forward) and 5-CCTCTTTTCCAATTTGAGGC-3 (mdm1R, reverse) for Ttnmdm. In total, for all experiments, results from wild-type, heterozygous mutant (heterozygous), and homozygous mutant (mdm) mice are reported at 2- and 6-wk time points. Age (mean ± SE) at 2 wk was 17.92 ± 1.34, 16.56 ± 1.65, and 18.14 ± 1.40 days for wild-type (n = 39), heterozygous (n = 21), and mdm (n = 33) mice, respectively. Age (mean ± SE) at 6 wk was 45.91 ± 1.343, 46.25 ± 3.29, and 42.83 ± 1.55 days for wild-type (n = 18), heterozygous (n = 5), and mdm (n = 26) mice, respectively.
Micro-CT imaging.
A pair of littermates, one mdm and one wild-type, were scanned weekly from 2 to 5 wk of age using micro-CT (FLEX X-O X-SPECT SPECT/CT, Gamma Medica, Northridge, CA). Additional scans were performed at 2 and 6 wk in three wild-type and three mdm mice. Mice were anesthetized by isoflurane inhalation, placed in the supine position, and secured to the CT scanner bed. Three-dimensional CT reconstruction models of skeleton, lung, and airway were created utilizing Amira 3.1.1.
Mice and tissue preparation.
Mice were deeply anesthetized by injection (0.5–0.7 ml/kg ip) with a combination rodent anesthetic provided by Baylor College of Medicine Center for Comparative Medicine [Rodent III: ketamine (37.6 mg/ml) + xylazine (1.92 mg/ml) + acepromazine (0.38 mg/ml)]. The muscle was excised and immediately immersed in a muscle bath containing a modified Krebs-Ringer solution (in mM: 137 NaCl, 5 KCl, 1 NaH2PO4, 24 NaHCO3, 2 CaCl2, and 1 MgSO4, pH 7.4) bubbled with 95% O2-5% CO2.
In vitro isometric contractility.
We used a temperature-controlled in vitro biaxial mechanical apparatus to assess force-generating capacity of the mouse diaphragm at 37°C. Biaxial loading subjects the muscle to mechanical stresses not only in the direction of the muscle fibers, but also in the direction transverse to the fibers. The left costal diaphragm from 2- and 6-wk-old mice and their controls was clamped between two stainless steel clamps along the y-axis (0.8 cm) and allowed to equilibrate for 5 min. Diaphragms were loaded to optimal length as determined by isometric twitch response. Subsequently, isometric contractile force-frequency responses were measured as previously described (10). Briefly, a 50-g thin-film load cell transducer (model LQB-630-100g, Cooper Instruments, Warrenton, VA) and a 250-g force transducer (model FORT250, World Precision Instruments, Sarasota, FL) were used to measure force produced by passive stretching or active isometric contraction. Two platinum probes were placed in the muscle bath directly above and beneath the diaphragm for electrical stimulation. Stimulation was delivered via a stimulus isolation unit and a square-pulse stimulator (models S48K and SIU5, Grass, West Warwick, RI) at supermaximal voltage (8 V), 0.5-ms pulses, and tetanic train duration of 500 ms. Maximum tetanic stress generation was measured at stimulation frequencies of 10, 30, 50, 60, 100, and 150 Hz, with 90 s of recovery between any consecutive stimulations. All data for the contractile property protocols were acquired at 300 Hz using a data acquisition board (model Lab-PC-1200/AI, National Instruments, Austin, TX) and LabVIEW 7.0 software. Muscle thickness was computed as follows: mass of diaphragm muscle ÷ [density (1.06 g/cm3) × unstressed surface area of muscle]. Stress (N/cm2) was calculated as follows: muscle tension (N/cm) ÷ unstressed diaphragm thickness (cm).
Measurement of passive length-tension relationships.
Passive length-tension relationships were measured using the left costal diaphragms, as previously described (21). Briefly, two pairs of markers made of silk surgical thread (0.2 mm) were sutured to the surface of the muscle in a centrally located position away from the insertions in a square pattern at 1-mm intervals on the membranes of two neighboring muscle fibers. The markers were sutured onto the abdominal surface of the midcostal region of the left hemidiaphragm. The left hemidiaphragm was then clamped along the x-axis in the biaxial apparatus, and a closed-circuit television camera was positioned directly over the muscle sheet. We preconditioned each muscle with five uniaxial lengthening-shortening cycles with the peak tension of 5 g/cm. The left hemidiaphragm was then subjected to a series of passive lengthening and passive shortening maneuvers along the axial direction of the muscle fibers and orthogonal to the long axis of the muscle fibers. Marker positions were recorded to VHS tape and digitized after experiments. Two-dimensional surface strain was calculated using Matlab and plotted against applied passive muscle force (in g). Extension ratios were calculated as a fraction of unstressed strains. Passive tension was calculated as follows: force (in g) ÷ tissue clamp width (0.8 cm).
Stress-relaxation analysis.
As previously described (21), the costal diaphragm was passively lengthened to a length that is equivalent to about optimal muscle length, and the muscle passive force was allowed to relax asymptotically at constant length for 5 min. Recorded force data were then fitted to a standard linear solid model of viscoelasticity using a nonlinear least-squares fit function in Matlab. The standard linear model of viscoelasticity is a simple mechanical model that describes the muscle as a parallel combination of a dashpot with coefficient of viscosity η1 and a linear spring with spring constant μ1, with a second linear spring with spring constant μ0 parallel to the first spring and the dashpot. The dashpot consists of a closed cylinder holding a piston immersed in viscous damping fluid, and the fit of the piston and cylinder is not tight. The piston moves slowly in response to mechanical applied load, and the greater the load, the faster the piston moves. The dashpot relaxation time is the time necessary for the dashpot, or the shock-absorbing quality of the muscle, to essentially be relaxed, and at this point, the muscle is characterized as perfectly elastic.
Histology and morphometry.
Immediately after excision of the diaphragm, the right costal muscle was dissected free from its attachments at the rib cage and fixed overnight in 10% neutral buffered formalin. Muscles were embedded in paraffin, cut into 5-μm-thick transverse sections, and stained with hematoxylin-eosin for blinded histopathological assessment. To visualize collagen, we used Masson's trichrome stain. Stained slides were viewed under a bright-field microscope (AxioPlan2 Microscope, Carl Zeiss Microimaging, Thornwood, NY), and images were digitally captured (CoolSnap HQ CCD, Photometrics, Tucson, AZ). Images were then analyzed for number of central nuclei and diaphragm thickness. Each hemidiaphragm section was imaged along the entire length of the muscle strip in the transverse section at ×40 magnification. All images for each hemidiaphragm were analyzed (ImageJ, http://rsb.info.nih.gov/ij/index.html) for number of centrally located nuclei where myofibers were readily discernible and free of artifacts. At least 550 myofibers were counted for each age × genotype group. Histological measurements of thickness were done using ImageJ on each diaphragm, where abdominal and thoracic epimysium were visible and myofiber cross sections were clearly defined. At least three measurements per diaphragm were made utilizing perpendicular measurements to the abdominal surfaces. Cross-sectional diameters of 1,500 diaphragm myofibers in eight control (4 young and 4 old) and six mdm (2 young and 4 old) mice were measured as Feret's diameters, i.e., the longest distance between any two points on the perimeter of the region. Analysis was performed using ImageJ. The boundary of the region was traced using the polygon tool, and Feret's diameter was calculated using ImageJ. The inclusion and exclusion criteria for the muscle fibers to be measured were as follows: all had clearly defined edges, whether they were bordering other muscle fibers or the boundaries of the muscles themselves. Nevertheless, extremely warped or irregularly shaped cells (i.e., those that were exceedingly noncircular) were not counted, even if their edges were clearly defined. The entirety of the cells also needed to be inside the frames of the cross-sectional images to be measured; no extrapolation was conducted. Furthermore, there were no impurities within the measured cells; cells were free of histological artifacts. The variance coefficient for each age × genotype group was calculated using the standard deviation of Feret's diameter and dividing it by the mean of Feret's diameter for each respective group.
MHC Western blot.
The MHC preparation and electrophoretic separation were performed essentially as described by Talmadge and Roy (29) and modified by Kohn and Myburgh (18). Monoclonal anti-skeletal myosin fast and monoclonal anti-myosin skeletal slow antibodies (Sigma, St. Louis, MO; 1:200 dilution) were used as primary antibodies.
Statistical analysis.
ANOVA was performed on all data sets where means and standard error of the means are shown. Genotype and age were defined as two factors, with three levels for genotype and two levels for age. A multiple-comparison test was performed where appropriate. When wild-type and heterozygous groups showed no significant difference, they were defined together as a control group. Matlab (Mathworks, Natick, MA), Sigma Plot, and Sigma Stat (Systat Software, San Jose, CA) were utilized for data analysis and statistical treatments.
RESULTS
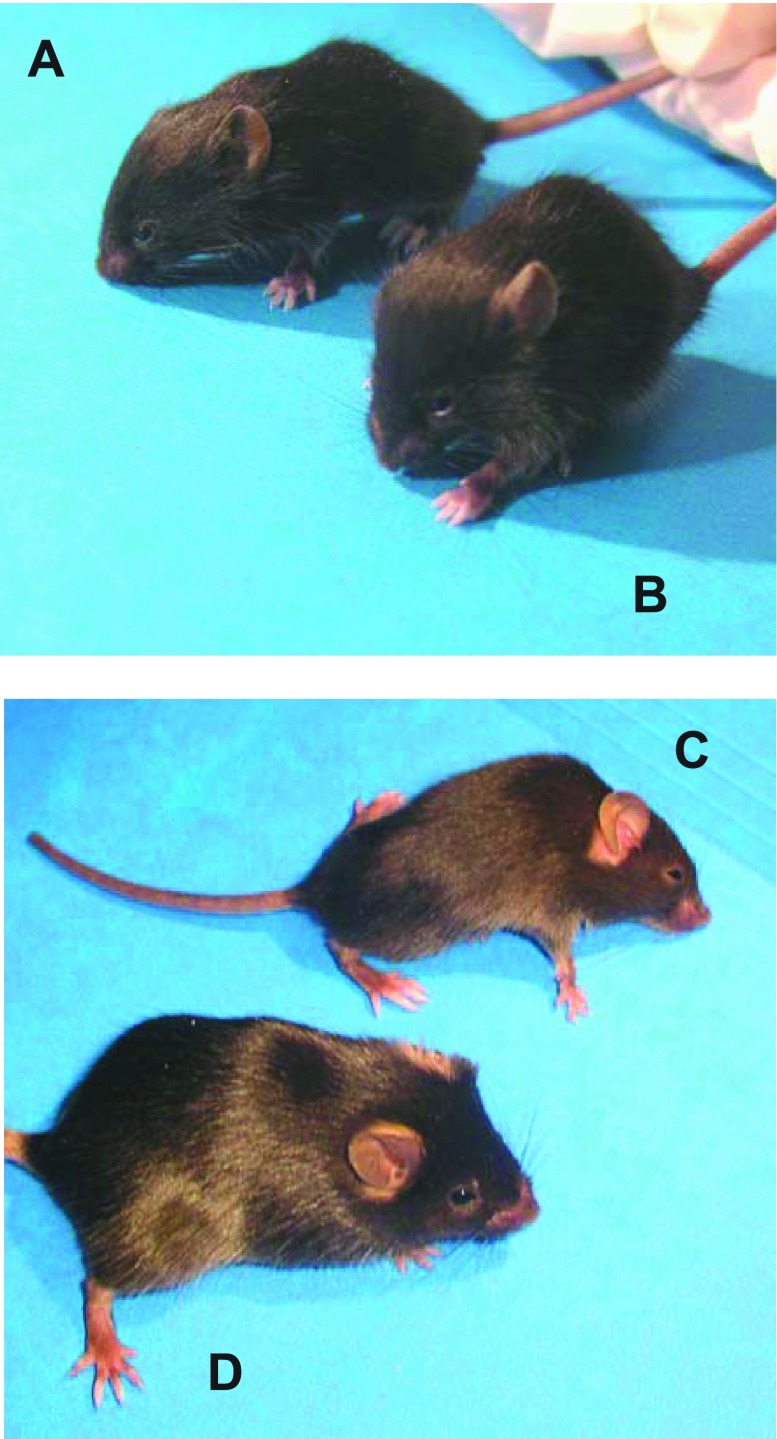
Differences in physical characteristics of the mdm mouse were observed as early as 2 wk of age and progressed to a striking muscular dystrophy phenotype by 6 wk of age (Fig. 1). On visual inspection, 2-wk-old mdm mice did not appear to be different from their wild-type or heterozygous littermates (Fig. 1, A and B). The 6-wk-old mdm mouse, however, was dramatically affected by the homozygous mutation: it showed decreased ambulation (stiff gait), hunched back, and reduced size compared with normal littermates (Fig. 1, C and D).
Fig. 1.
Two- and 6-wk-old control mice (A and D) and age-matched mice with muscular dystrophy with myositis (mdm; B and C). At 2 wk, mdm mouse was phenotypically indistinguishable from its wild-type littermate (A and B). Progression of disease was marked at 6 wk, manifesting in severe wasting of hindlimb muscles, dorsal kyphosis, decreased ambulation, and decreased body mass (C).
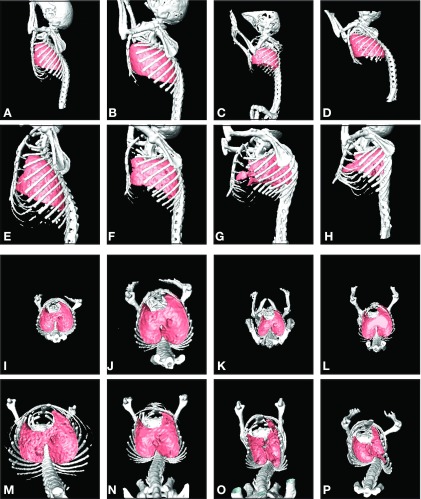
To gauge the temporal nature of the disease and to understand how the mechanical environment of the diaphragm is altered with disease, we imaged the thorax of mdm mice with high-resolution micro-CT over 5 wk (Fig. 2). At 2 wk of age, except for differences in size, the thoraces of the mdm and wild-type littermates were virtually indistinguishable. At this age, across all genotypes, the spinal curvatures were similar and rib cage configurations were symmetrical. By 3 wk of age, a subtle increase in thoracic spine curvature was observed in the mdm mice. By 5 and 6 wk of age, the skeletal malformations and lung alterations were dramatic. A ∼90° bend in the thoracic vertebrae of mdm mice caused a marked dorsal kyphosis. The lumbar region was also affected: a lateral deviation was observed, and the natural lumbar concavities were abolished. The rib cage of the mdm mouse at 6 wk was warped into asymmetry. The asymmetry distorted the natural circumferential boundaries of the lower margins of the rib cage where the costal diaphragm inserts. This rib cage distortion led to increased anterior-posterior dimensions and narrowed lateral dimension at the level of the insertion of the diaphragm on the chest wall. These cumulative thoracic changes concomitantly altered the shape of the lungs. The reach of the lower lobes of the lung into costodiaphragmatic recesses was abolished in mdm mice. The tracheal and main stem bronchi appeared unobstructed.
Fig. 2.
Three-dimensional surface reconstructions of thoracic skeleton and lungs from high-resolution micro-CT. Images are representative of control and mdm mice at 2 and 6 wk of age (n = 3 for each genotype and age group). A, E, I, and M (column 1) represent wild-type mice for comparison with images of mdm mice (columns 2, 3 and 4). Sagittal views are shown for 2-wk-old (A–D) and 6-wk-old (E–H) mice. Corresponding axial views are also shown for the same 2-wk-old (I–L) and 6-wk-old (M–P) mice. Note normal spinal curvature, symmetric thoracic rib cage configuration, and normal lung shape in 2-wk-old mdm compared with wild-type mice. Note severe scoliosis and dorsal kyphosis, in combination with asymmetric distortion of the thoracic rib cage, namely, lateral constriction and anterior-posterior lengthening, in addition to decreased skeletal and lung mass in 6-wk-old mdm mice. Lung shapes in 6-wk-old mdm mice and appeared to be distorted concomitantly with rib cage changes and appeared smaller than controls.
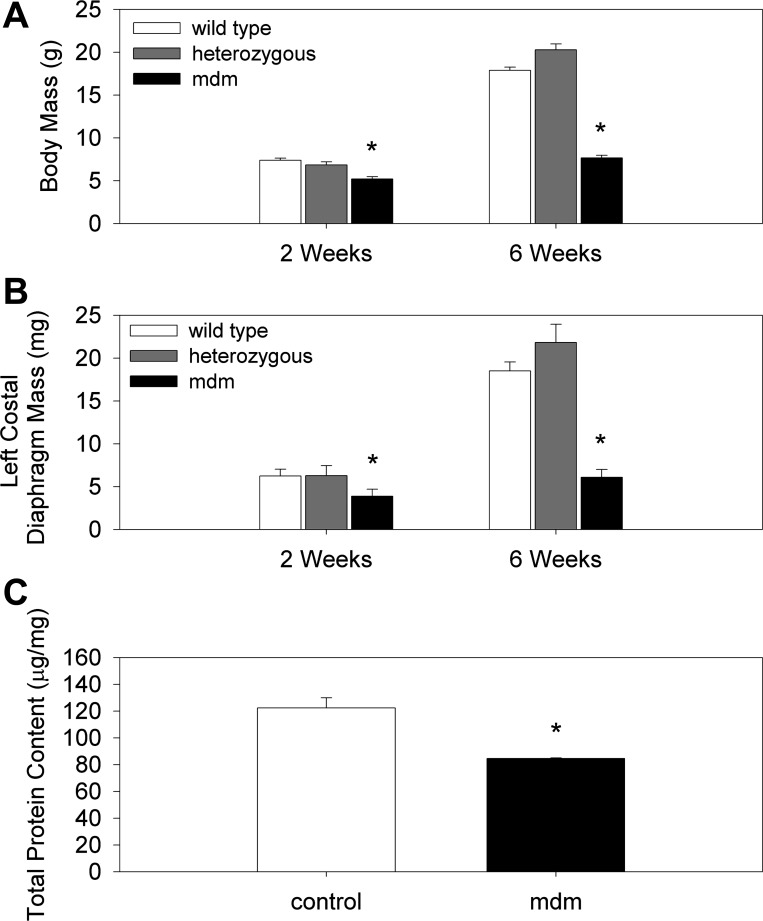
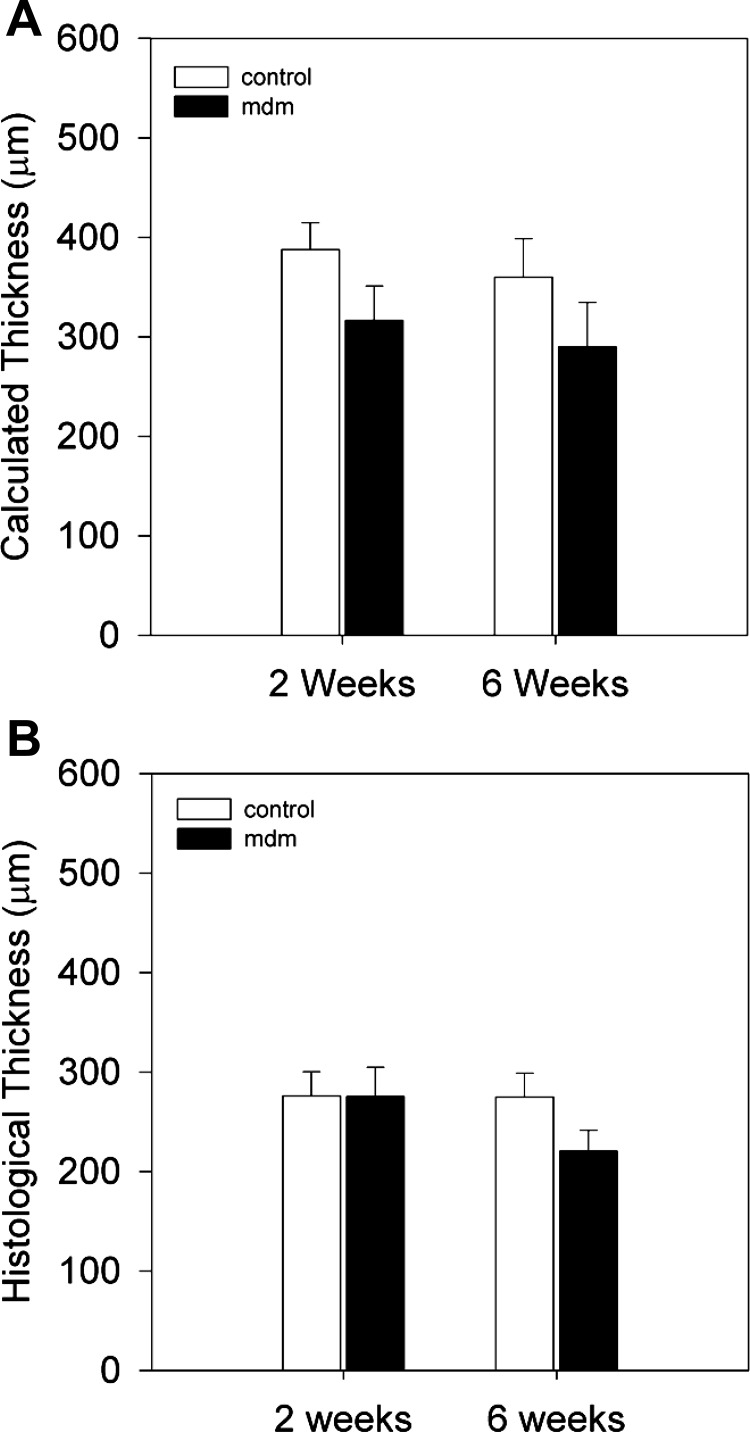
Another prominent aspect of the mdm mouse and the earliest detectable feature is a dramatic failure to grow. At 2 wk of age, significant reductions in whole body mass, costal diaphragm mass, and costal diaphragm total protein content were apparent (Fig. 3). For example, compared with wild-type mice at 2 wk of age the mdm mice demonstrated 29% and 38% reductions in whole body (5.20 ± 0.277 vs. 7.39 ± 0.254 g) and costal diaphragm (3.89 ± 0.82 vs. 6.24 ± 0.80 mg) mass, respectively. The costal diaphragm total protein content was similarly reduced by 31% in mdm vs. wild-type mice (84.62 ± 0.372 vs. 122.38 ± 7.60 μg/mg) at 2 wk of age. These differences in whole body and costal diaphragm mass were much greater at 6 wk of age, inasmuch as the wild-type mice grew in size, whereas mdm mice did not. Mean costal diaphragm thickness was also assessed by combined measurement of diaphragm muscle mass and unstressed surface area, as well as by histology (Fig. 4). Histological measurements of diaphragm thickness were smaller than calculated thickness. This difference is likely due to a shrinking artifact as part of the dehydration steps in the histological process. Calculated thickness was largest (387 μm) in 2-wk control diaphragms (317 ± 34.4 and 387 ± 27.2 μm in mdm and control, respectively) and smallest (290 μm) in 6-wk mdm diaphragms (290 ± 44.5 and 360 ± 38.5 μm in mdm and control, respectively; Fig. 4A). Histological thickness measurements were much smaller but over a tighter range of values, with a maximum of 275 μm for 2-wk mdm diaphragms and a minimum of 220 μm for 6-wk mdm diaphragms (220.40 ± 21.02 and 274.69 ± 24.27 μm at 6 wk for mdm and control, respectively; Fig. 4B). Differences between diaphragm thicknesses were not statistically significant between control and mdm mice within either age group for either thickness measurement methodology (P = 0.141 for calculated thickness and P = 0.063 for histological thickness). Given that costal diaphragm masses were significantly different, yet diaphragm thickness were relatively preserved, in mdm mice compared with controls, we also measured the unstressed diaphragm lengths of costal diaphragm bundles from the central tendon to the rib cage insertions. We found shorter length from tendon to insertions for mdm than for control diaphragm (2.5 ± 0.4 vs. 3.6 ± 0.5 mm). This difference between mdm (n = 4) and control (n = 8) diaphragm unstressed lengths at 6 wk of age was significant (P = 0.073).
Fig. 3.
Whole body mass, costal diaphragm mass, and total protein content. A and B: mean whole body mass and left costal diaphragm mass of control and mdm mice at 2 and 6 wk (n > 20 for all groups). Decreases in total body mass (A) and left costal diaphragm mass (B) were statistically significant at 6 wk compared with controls. Reduction in diaphragm mass was slightly (10%) greater than whole body weight loss at 2 and 6 wk. C: total protein content per unit mass of muscle was significantly reduced (31%) in mdm compared with control mice at 2 wk (n = 3 per genotype). *P < 0.05 vs. age-matched controls.
Fig. 4.
Thickness calculated from surface area and mass of excised left costal diaphragms (A) and from histological tissue sections (B). A: calculated thickness for control (n = 6) and mdm (n = 6) mice at 2 and 6 wk. Although mean values were ∼20% less for mdm than wild-type mice, these differences were not statistically significant. B: mean histological thickness of mdm (n = 6, 0.0317 and 0.0290 cm at 2 and 6 wk, respectively) and wild-type (n = 6, 0.0407 and 0.0362 cm at 2 and 6 wk, respectively) diaphragm. Differences in mean calculated thickness between genotype groups were not statistically different in either age group.
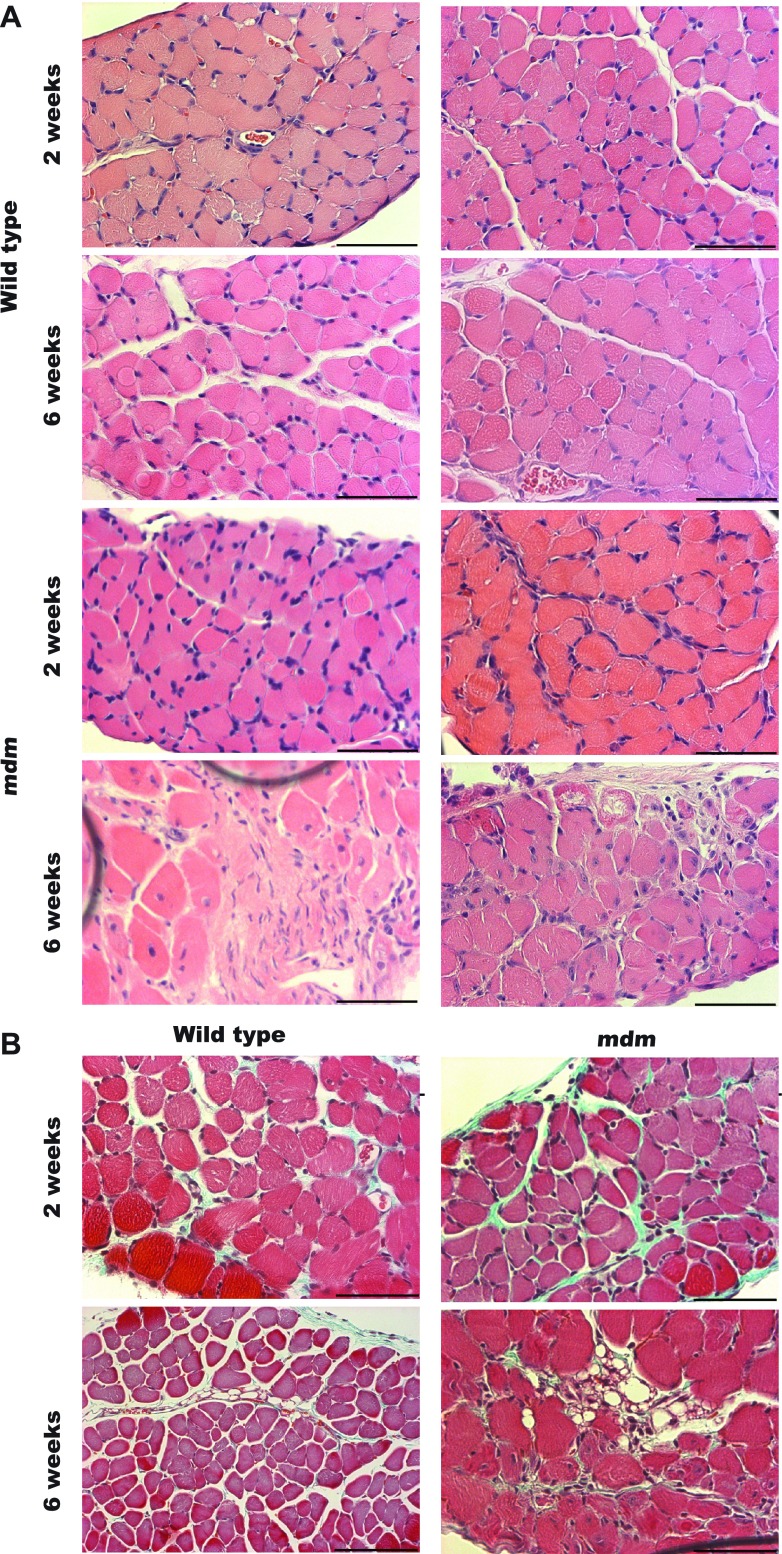
At 2 and 6 wk, we examined costal diaphragm histological sections with hematoxylin-eosin and Masson's trichrome staining. At 2 wk (Fig. 5), we found that the mdm diaphragm was free of the classic histopathological hallmarks of muscular dystrophy. We assessed each hemidiaphragm in its entirety for histopathological signs of muscular dystrophy, including centrally located nuclei, increased variation in myofiber diameter, hypertrophy, and fatty or connective tissue infiltration (Fig. 6). These features were most notable in the mdm diaphragms at 6 wk. At 2 wk, mdm diaphragms also began to show signs of dystrophic features, but to a far lesser extent. At 6 wk, mdm diaphragms tended to have focal regions of severe histopathology interspersed with healthy fibers throughout the hemidiaphragm. Additionally, endomysial connective tissue thickening was not diffusively prominent.
Fig. 5.
Histological staining of 2- and 6-wk diaphragms. A: hematoxylin-eosin staining of wild-type and mdm diaphragms (n = 3 for each age × genotype group) at 2 and 6 wk. Rows 1 and 2 are images of wild type diaphragms. Rows 3 and 4 are images of mdm diaphragms. Columns 1 and 2 show examples of the same age × genotype groups. At 2 wk, histopathological signs were largely absent in mdm diaphragm. At 6 wk, mdm diaphragms showed marked foci of necrosis, scar tissue, and variation in myofiber size. B: small foci of increased perimysial connective tissue deposition in 2-wk mdm diaphragm stained with Masson's trichrome. At 6-wk, large endomysial and perimysial connective tissue deposition was noted in localized foci of diaphragm necrosis. Scale bar, 60 μm.
Fig. 6.
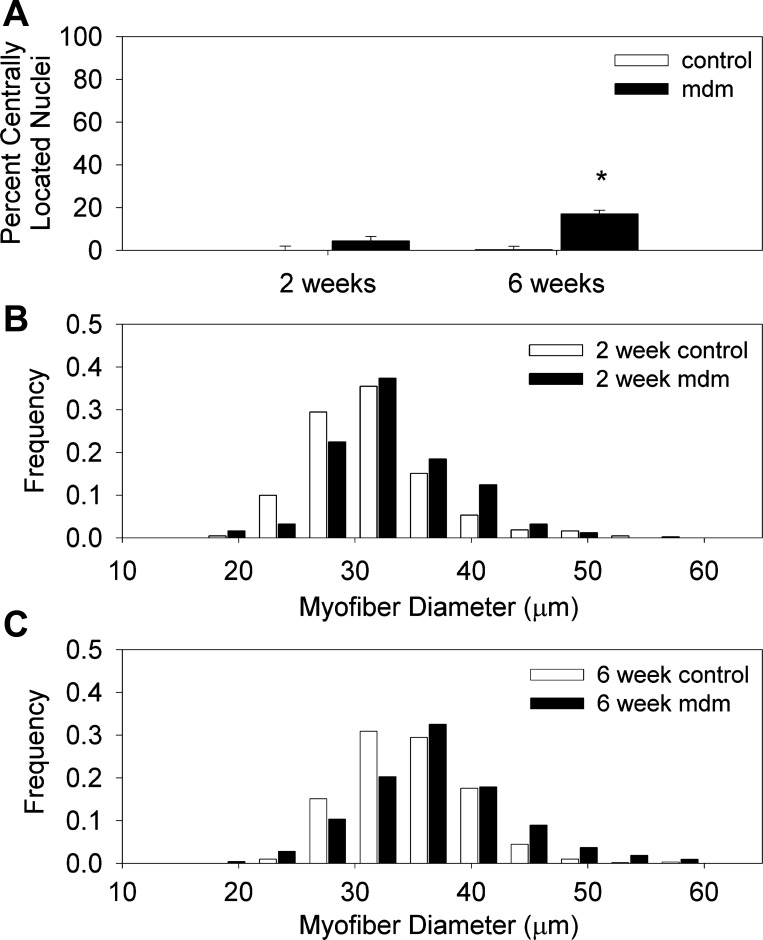
Myofiber morphometry of 2- and 6-wk mdm diaphragms. A: percentage of myofibers with centrally located nuclei was significantly greater at 6 wk. Total number of individual myofibers for 2-wk control (553) and mdm (596) and 6-wk control (1,842) and mdm (651) diaphragms was ≥500 per group (n = 3 for each age × genotype group; *P < 0.05). B and C: myofiber Feret's diameter frequency plots in 2-wk control and mdm and 6-wk control and mdm diaphragms. Feret's diameter values are as follows: 31.52 ± 0.266, 33.00 ± 0.35, 34.91 ± 0.20, and 36.79 ± 0.38 for 2-wk control, 2-wk mdm, 6-wk control, and 6-wk mdm, respectively. Corresponding variance coefficients were 179.24, 166.75, 143.93, and 178.07, respectively. Mean Feret's diameters of 2- and 6-wk mdm diaphragms were significantly increased compared with age-matched controls (P < 0.01). Total number of individual myofibers for 2-wk control (431) and mdm (249) and 6-wk control (608) and mdm (212) diaphragms was ≥200 per group. For frequency histograms, 8 control (4 each at 2 and 6 wk of age) and 6 mdm (2 at 2 wk of age and 4 at 6 wk of age) diaphragms were analyzed.
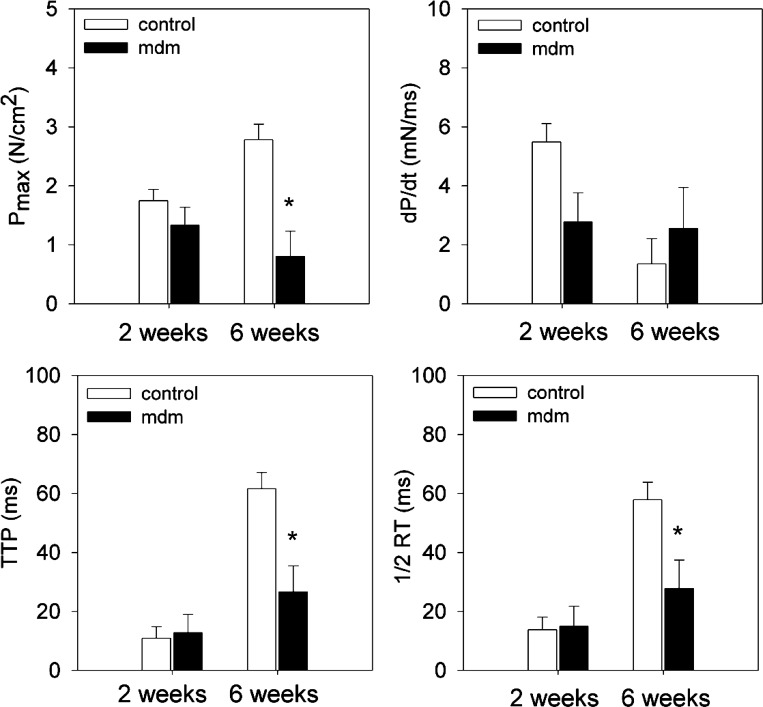
After assessing the morphological and structural changes of the mdm phenotype, we measured twitch and tetanic contractile function of excised costal diaphragms in 2- and 6-wk-old control and mdm mice. Twitch characteristics are reported for control and mdm diaphragms are shown in Fig. 7. Maximum twitch stress (0.80 ± 0.43 and 2.78 ± 0.26 N/cm2 at 6 wk for mdm and control, respectively), time-to-peak stress (26.7 ± 8.8 and 61.7 ± 5.39 ms at 6 wk for mdm and control, respectively), and half-relaxation time (27.8 ± 9.7 and 57.9 ± 5.91 ms at 6 wk for mdm and control, respectively) were significantly reduced in the mdm diaphragm at 6 wk, but not at 2 wk. Rate of force development was not different in either age group.
Fig. 7.
In vitro isometric twitch characteristics of left costal diaphragm at 2 and 6 wk. Maximum twitch stress (Pmax), time to peak (TTP) twitch, and half-relaxation time (1/2RT) were significantly reduced at 6 wk (n = 10 control and 3 mdm), but not at 2 wk (n = 7 per genotype). Pmax, TTP, and 1/2RT in mdm diaphragms were reduced by 71%, 57%, and 52%, respectively. Rate of force development (dP/dt) was not different for either age group. *Significantly different from age-matched controls (P < 0.05).
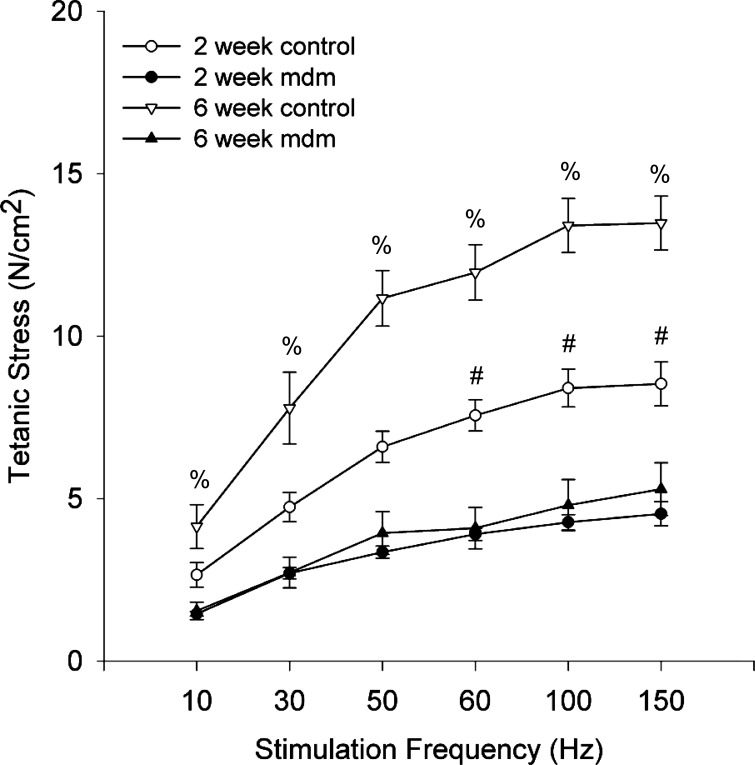
Supramaximal tetanic stimulations were used to obtain the force-frequency response curves of costal diaphragm muscles (Fig. 8). The tetanic stress (N/cm2) vs. stimulation frequency (Hz) response shown in Fig. 8 demonstrates profound and early contractile reductions in mdm diaphragms relative to their age-matched controls. At 2 wk, the contractile stress generation was reduced relative to age-matched controls, but statistical difference was only achieved at stimulation frequencies ≥60 Hz (4.53 ± 0.38 and 8.53 ± 0.68 N/cm2 at 2 wk at 150 Hz for mdm and control, respectively). In contrast, tetanic stress was significantly reduced, by an average of 64%, across all stimulation frequencies in 6-wk mdm diaphragms relative to their age-matched controls (5.29 ± 0.81 and 13.48 ± 0.83 N/cm2 at 6 wk at 150 Hz for mdm and control, respectively). The force-frequency response curve was nonlinear for mdm and control groups, but to a greater extent for the 6-wk control group.
Fig. 8.
Mean costal diaphragm force-frequency response curves. At 2 wk, mdm diaphragm (n = 5) generated less tetanic stress than control (n = 9), with significance reached at 60, 100, and 150 Hz. At 6 wk, maximum tetanic stress was reduced 64% in mdm diaphragms (n = 5) relative to controls, with significance at all stimulation frequencies (#P < 0.05). All force-frequency response curves were nonlinear over the range of stimulation frequencies. %Statistically different from 2-wk and 6-wk mdm (P < 0.05).
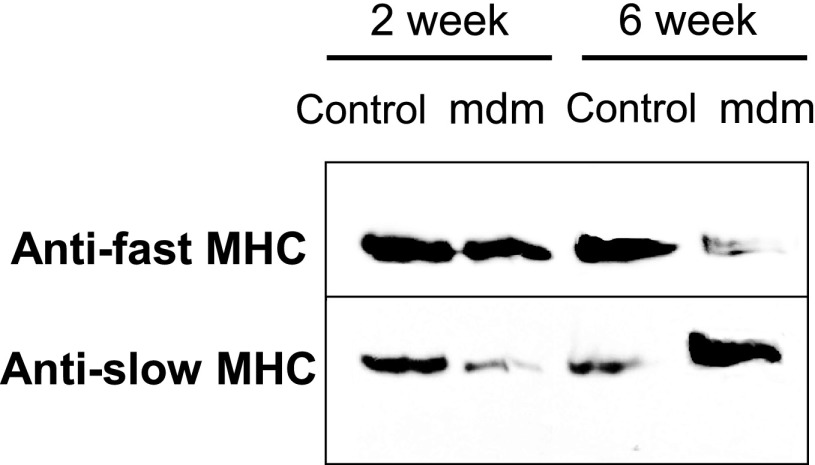
The decreased contractile force-generating capacity and faster twitch characteristics of the mdm diaphragms led us to question whether the expression of slow and fast MHC isoforms was altered. MHC isoforms were extracted from costal diaphragms of mdm and control mice at 2 and 6 wk of age. Western blots were performed with fast and slow antibodies at 2 and 6 wk (Fig. 9). At 2 wk, fast MHC protein was more prominent than the slow isoform, especially in mdm diaphragms. At 6 wk, Western blot results for mdm diaphragms were exactly opposite those for controls, showing more of the slow than the fast MHC.
Fig. 9.
Western blot of myosin heavy chain (MHC) isoforms. Anti-fast and anti-slow MHC primary antibodies were used to detect changes in extracted myosin myofilaments from wild-type (control) and mdm diaphragms at 2 and 6 wk (n = 3 for all age × genotype groups). At 2 wk, anti-fast MHC appeared similar in control and mdm diaphragms. Anti-slow MHC appeared to be slightly decreased in mdm diaphragms. At 6 wk, relative proportions of anti-fast MHC and anti-slow MHC detection were reversed in mdm diaphragm. Anti-slow MHC signal was much stronger in mdm diaphragm.
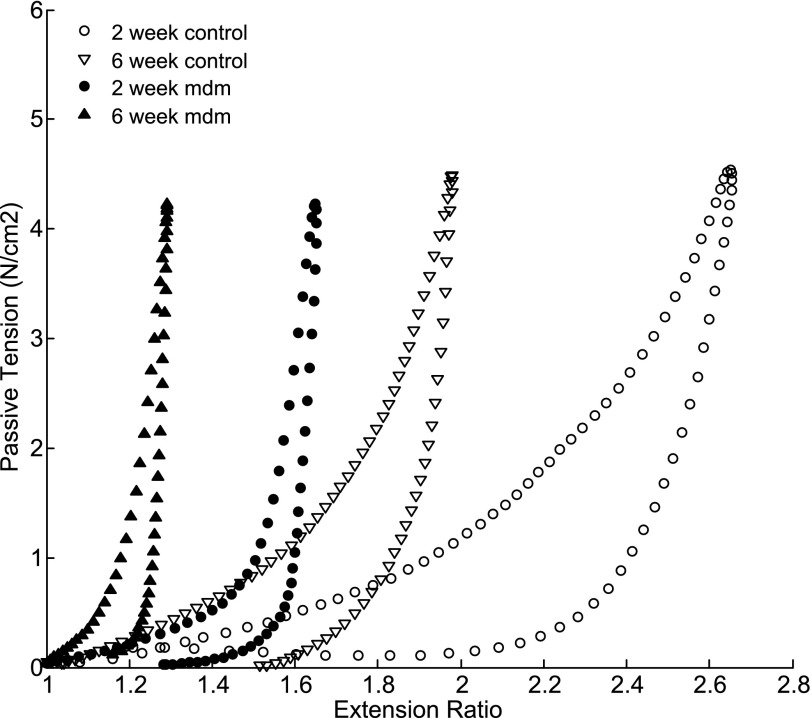
Passive length-tension relationships were measured from mdm and control mice. A series of stretching and shortening cycles were utilized to characterize the length-tension relationship of the left costal diaphragm. Diaphragms were stretched successively to 1.11, 2.22, and 4.44 g/cm of passive tension. The mean extension ratios were calculated for each mouse diaphragm from each of the three stretch cycles. Representative stretch cycles were plotted as passive tension vs. extension ratio (Fig. 10). All length-tension relationships were strongly nonlinear and demonstrated hysteresis typical of skeletal muscle. The data show a leftward shift in the length-tension relationship of the mdm diaphragm at 2 and 6 wk. Furthermore, in contrast to controls, 2- and 6-wk mdm diaphragms demonstrated a much steeper rise in tension at 55% and 15% strains(ɛ = 1 − λ, where ɛ is tension and λ is extensibility), respectively. In both genotype groups, we observed a leftward shift of 6-wk diaphragm extension ratios relative to the 2-wk measurements, with a 70% difference in controls and a 50% difference in mdm diaphragms.
Fig. 10.
Passive length-tension relationship plotted as tension vs. extension ratio at 2 wk. Representative length-tension relationships compare control and mdm diaphragm extensibilities in response to applied mechanical loading and unloading. Passive length-tension relationships were shifted leftward for mdm diaphragm vs. age-matched control.
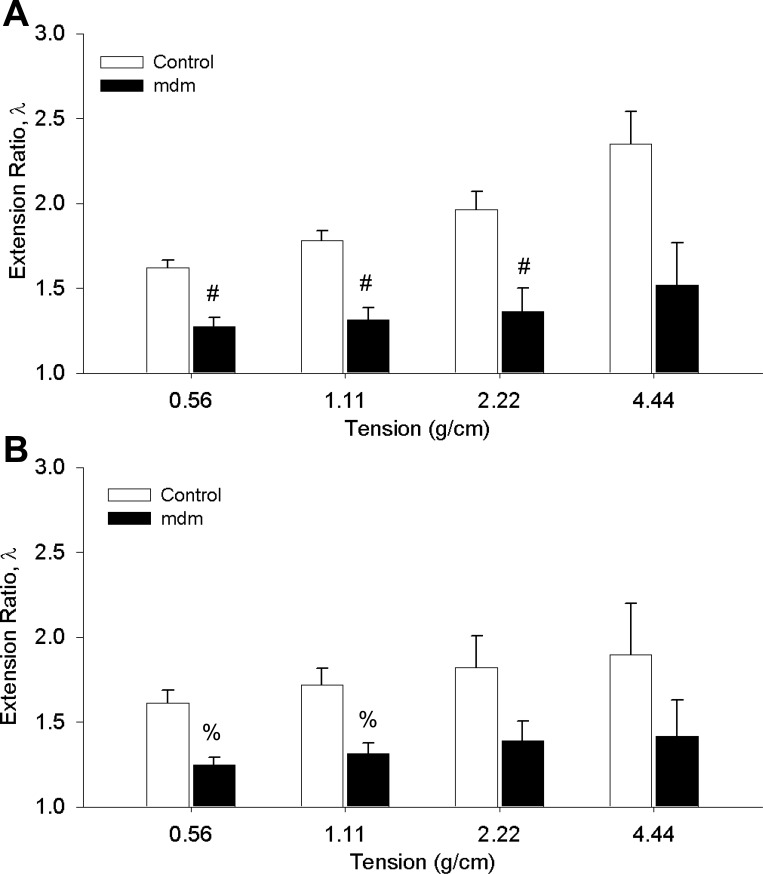
The mean extension ratios in control and mdm groups were also calculated at four levels of passive tension (Fig. 11). At 2 wk, the difference in means of the extension ratios (λ) was significant up to 2.22 g/cm (1.9 and 1.36 for wild-type and mdm, respectively). At 6 wk, the difference in means of the extension ratios was significant up to a passive tension of 1.11 g/cm (1.7 and 1.32 for wild-type and mdm, respectively). With the assumption that behavior over the range of 1.11–2.22 g/cm is linear, the compliance ratio, i.e., the reciprocal of slope over the linear region of the mdm group relative to controls, can be calculated. At 2 and 6 wk, mdm compliance ratios relative to age-matched controls were 0.58 and 0.27, respectively. These compliance ratios indicate decreased compliance in mdm diaphragms relative to controls. In contrast to the altered extensibility and compliance, we did not detect significant differences in viscoelastic properties in mdm diaphragm: mean relaxed elastic modulus = 0.57 ± 0.025 and 0.61 ± 0.034 at 2 wk for control and mdm, respectively, and 0.60 ± 0.044 and 0.60 ± 0.044 at 6 wk for control and mdm, respectively.
Fig. 11.
Extension ratios vs. passive lengthening at 4 tension levels for control (n = 9) and mdm (n = 10) diaphragms. Significant decreases in extensibility were detected at 3 levels of tension at 2 wk (A) and 2 levels of tension at 6 wk (B). P < 0.05. Extensibility was measured from 3 successive stretch cycles in each diaphragm and averaged for each age group. #,%Statistically different from control (P < 0.05).
DISCUSSION
Our study demonstrates early and persistent diaphragm muscle weakness and decreased muscle extensibility in the setting of progressively marked spinal and thoracic rib cage deformities in the mdm mouse. Morphometric analysis of mdm mice also showed that whole body mass, diaphragm muscle mass, and total protein content are severely decreased. Histology revealed modest increases in centrally occurring nuclei and mean myofiber diameter. Finally, we found that slow MHC isoforms were aberrantly favored as the disease progresses.
Previous studies demonstrated that pathology in the mdm soleus and anterior tibialis starts in the first 2 wk (14), and others show that pathology is absent in the soleus at 24 days (33). To our knowledge, no studies have examined mdm diaphragm muscle structure or function. Histopathology of the skeletal muscle has the potential to dramatically alter mechanical properties after secondary processes, such as adipose infiltration, endomysial and perimysial fibrosis, and necrosis, set in. Histopathological analysis showed that the mdm diaphragm was essentially free of severe histopathology at 2 wk of age. Three to 4 wk later, the classic histopathological signs of muscular dystrophy were recapitulated in localized spots within the costal diaphragm. Evidence of regenerating myofibers was detected by an increase in centrally located nuclei, foci of necrosis, an increase in fiber size variation, and myofiber hypertrophy in mdm diaphragms. However, histopathology is apparently more severe in distal hindlimb muscles of mdm than in the diaphragm, according to published data (14) on soleus and anterior tibialis of mdm mice at 60 days. Heimann et al. (14) reported >80% centrally located nuclei of mdm hindlimb muscles, whereas we found only 20% in our diaphragm studies. They also found marked atrophy in hindlimb muscles (40% reduction in cross-sectional areas compared with controls), whereas we found a modest increase in mean Feret's diameter in the mdm diaphragm fibers. Taken together, it is apparent that histopathology is not as severe in the diaphragm as in the hindlimb muscles. This stands in contrast to the mdx mouse model, in which the diaphragm is the most affected muscle histologically.
In contrast to the histopathology, muscle wasting is a prominent and progressive feature in mdm costal diaphragm. Despite very small differences in total body mass at 2 wk of age, total protein content decreased 31% and wet mass decreased 38% in mdm costal diaphragm compared with age-matched controls. The decreased protein content could be due to increased water or collagen content in the mdm diaphragms. However, we did not detect significant increases in connective tissue by Masson's trichrome staining at 2 wk. At 6 wk of age, mdm diaphragm wet mass was further reduced by 67% compared with age-matched controls. Although the mdm diaphragm muscle mass progressively decreased, myofiber diameter appeared to modestly, but statistically significantly, increase in individual myofibers that remained at both ages. Decreased total protein content and decreased diaphragm muscle mass in the presence of myofiber hypertrophy can only be remedied if the total number of myofibers and/or the length of myofibers are decreased. We found a decrease in costal diaphragm muscle length, as measured from insertion to origin in the midcostal region. In mdm diaphragms, we found an average 30% decrease compared with age-matched controls. The likely result is that mdm diaphragms lose mass by changes in the physical dimensions of the muscle (i.e., total muscle surface area), but, at the cellular level, individual myofibers compensate for the lost mass by increasing myofiber diameter.
The diaphragm functions principally as an inspiratory pump by displacing volume through transdiaphragmatic pressure (Pdi) generation. Two key determinants are important for generation of Pdi: the shape of the diaphragm (curvature) and active contractile stress as related by Laplace's law. Reduction in curvature or reduction in contractile force production of the diaphragm leads to reductions in the pressure-generating capacity of the diaphragm (1, 2). Our micro-CT data show that the lower costal margins of the mdm rib cage, where the diaphragm inserts, are distorted in an anterior-posterior direction. Furthermore, the caudal surface of the lung appears flattened, and the reach of the lower lobes into the costodiaphragmatic recesses is abolished by 6 wk. These two features suggest that curvature of the costal diaphragm is reduced, leading to a deleterious alteration in the mechanical loading environment of the respiratory pump as the disease progresses. These alterations may contribute to respiratory pump dysfunction and reduction of the ability of the diaphragm to convert diaphragm muscle contractile force to Pdi.
The contribution of contractile force generation by the diaphragm to Pdi is necessary for respiratory pump function. As such, we measured the isometric twitch and tetanic force-generating properties of the costal diaphragm to assess its contractile function. In the mdm costal diaphragm, twitch stress kinetics (time to peak stress and half-relaxation time) and muscle stress generation (maximum twitch stress and maximum tetanic stress) were decreased. Although the decreased mdm twitch parameters were not significantly different at 2 wk, they were significantly different by 6 wk. Also, at 2 wk in the mdm costal diaphragm, the force-frequency response curve was dramatically depressed. Maximum tetanic stress generation measured in response to increasing stimulation frequencies demonstrated significant weakness in 2-wk mdm diaphragms at 60, 100, and 150 Hz. At 6 wk, tetanic stress generation was decreased at every stimulation frequency in two of three controls. For example, at 150-Hz stimulation, the 6-wk mdm diaphragm generated mean tetanic stress of 5.3 N/cm2 vs. 13.5 N/cm2 in age-matched controls. These data showing dramatic muscle weakness stand in contrast to soleus muscle data, which show no loss of contractile stress generation (14, 33). Within a given genotype, the differences between 2- and 6-wk force-frequency responses were significant only for the control group. The isometric contractile force-frequency response curves of the mdm diaphragm at 2 and 6 wk were essentially the same. This means that contractile deficits start early and are persistent in the mdm diaphragm, thereby blunting the normal developmental enhancement of contractile force-generating capacity that would be expected over this time in control mice (17). According to the literature, the average stress values for twitch and tetanic stimulations in 6-wk control diaphragms are below the reported values in similar experiments. This discrepancy is likely attributable to differences in calculation of cross-sectional areas. However, all statistical comparisons between groups are still valid, inasmuch as all experiments and data were calculated by the same methodology in the present study. It is unlikely that muscle histopathology alone can explain muscle weakness.
The alterations in the twitch kinetics and failure of the mdm diaphragms to develop increased contractile strength from 2 to 6 wk similar to controls led us to examine MHC expression. However, the MHC isoforms are developmentally regulated in the diaphragm and correlate with increases in specific force (7). Shifts and/or delays in myofiber MHC switching have been reported in human patients with DMD (32) and mdx mice (27). Indeed, after isolation of myofilaments from costal diaphragms, Western blotting showed that mdm diaphragms contained a relative majority of the slow MHC at 6 wk, but not at 2 wk. These data suggest that the fast MHC isoforms are selectively lost as the disease progresses, as seen in other muscular dystrophies. Although muscle weakness could have many explanations other than myosin isoform differences, we did expect that faster twitch kinetics would be associated with an increased level of fast MHC expression. Curiously, this was not the case at 6 wk, when twitch kinetics were significantly different. The actual fiber type compositions may be more complex, inasmuch as embryonic, neonatal, and other developmental myosin isoforms are likely upregulated as part of the regenerative/repair process. This shift in MHC isoforms unmasks a preferential bias of myofiber MHC composition in the setting of advanced muscular dystrophy. This may be due to the fact that slow oxidative fibers have higher satellite cell density and frequency than their fast glycolytic counterparts and, therefore, may be overrepresented in repair/regenerative stages of the disease (8).
Another critical question with implications for respiratory function is how the passive mechanical properties of the mdm diaphragm is affected. We expected that a mutation in titin could affect the passive length-tension relationship. Titin is the principal source of differential passive elasticity in striated muscles, and it was thought to serve as a dual-stage spring to confer viscoelastic and passive properties to the muscle (31). In the diaphragm, in particular, it has been shown that titin contributes to the majority of passive tension within the physiological range (28). The titin N2A deletion disrupts flexible Ig and PEVK domains within the I band, but the deletion is exceedingly small relative to the full-length protein, 83 residues of >33,000. Interestingly, our data demonstrated that, for the same amount of passive tension applied to the mdm diaphragm, there is much less lengthening of the muscle, especially at 6 wk. These data show that the mdm diaphragm has decreased passive extensibility and a stronger nonlinearity of the length-tension relationship in the supraphysiological range (tension >1.11 g/cm). The differences in passive extensibility were most significant between 0.56 and 2.22 g/cm of passive tension. We also expected alterations of titin's dual-stage viscoelastic spring-like properties to be reflected in the relaxed elastic modulus. However, within physiological loading range, we were not able to detect significant changes in the relaxed elastic modulus or other viscoelastic time parameters in the mdm mouse diaphragm (data not shown). Given these changes in passive mechanical properties within the in vivo physiological loading range, we speculate that the subtle N2A deletion does not directly change intrinsic extensibility of titin but, rather, may indirectly lead to a decreased number of PEVK domain repeats. A reduced number of PEVK domain repeats could lead to decreased passive muscle extensibility. The leftward shift of length-tension curves has the functional consequence of requiring more force to shorten and lengthen the mdm diaphragm. Therefore, diaphragm muscle weakness could further compromise the ability of the less extensible diaphragm to displace volume and, consequently, alters ventilation.
In summary, our study provides new information on the mechanical loading environment, morphometric features, and physiological function of the respiratory pump in the mdm mouse. Our data demonstrate dramatic muscle weakness as early as 2 wk in the mdm diaphragm that does not coincide with severe histopathology. In fact, compared with other studies on the distal hindlimbs, we found that the diaphragm is somewhat protected from secondary histopathological processes, but not from muscle wasting or weakness. These results also stand in contrast to the mdx mouse model, in which skeletal muscle pathology is mostly restricted to the diaphragm. Furthermore, the early mdm diaphragm muscle weakness belies dramatic and progressive skeletal deformations affecting the thorax. The combination of these alterations most likely contributes to compromised respiratory function and decreased reserve respiratory capacity. Our data suggest that as the disease worsens, respiratory muscle weakness may play a critical role in hastening an early death. It is clear that the titin N2A deletion in the mdm mouse disrupts a major homeostatic function of the giant myofilament titin. Further investigation is needed to explore how titin's N2A region can interact with intracellular molecular ligands to impact such processes as MHC switching, muscle hypertrophy, muscle protein content, contractile function, and primary protein structural properties of diaphragm myofilaments/cytoskeleton involved in passive elasticity. Our improved understanding of these relationships between skeletal muscle function and titin structure may have important implications for other severe early-onset muscular dystrophies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-63134 (to A. M. Boriek), Muscular Dystrophy Association Grant 3674, and National Science Foundation Grant CBET-0650686.
Acknowledgments
The authors thank Brian Batas, Daniela Rimer, Jennifer Vega, Cristina Velasco, and Deshen Zhu for invaluable technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Boriek AM, Liu S, Rodarte JR. Costal diaphragm curvature in the dog. J Appl Physiol 75: 527–533, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Boriek AM, Rodarte JR, Reid MB. Shape and tension distribution of the passive rat diaphragm. Am J Physiol Regul Integr Comp Physiol 280: R33–R41, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81: 1189–1192, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuda N, Granzier HL, Ishiwata S, Kurihara S. Physiological functions of the giant elastic protein titin in mammalian striated muscle. J Physiol Sci 58: 151–159, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Garvey SM, Rajan C, Lerner AP, Frankel WN, Cox GA. The muscular dystrophy with myositis (mdm) mouse mutation disrupts a skeletal muscle-specific domain of titin. Genomics 79: 146–149, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Gautel M, Mues A, Young P. Control of sarcomeric assembly: the flow of information on titin. Rev Physiol Biochem Pharmacol 138: 97–137, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Geiger PC, Cody MJ, Macken RL, Bayrd ME, Fang YH, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J Appl Physiol 90: 380–388, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Gibson MC, Schultz E. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec 202: 329–337, 1982. [DOI] [PubMed] [Google Scholar]
- 9.Gozal D Pulmonary manifestations of neuromuscular disease with special reference to Duchenne muscular dystrophy and spinal muscular atrophy. Pediatr Pulmonol 29: 141–150, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Hackman P, Vihola A, Haravuori H, Marchand S, Sarparanta J, De Seze J, Labeit S, Witt C, Peltonen L, Richard I, Udd B. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Genet 71: 492–500, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haravuori H, Vihola A, Straub V, Auranen M, Richard I, Marchand S, Voit T, Labeit S, Somer H, Peltonen L, Beckmann JS, Udd B. Secondary calpain 3 deficiency in 2q-linked muscular dystrophy: titin is the candidate gene. Neurology 56: 869–877, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi C, Ono Y, Doi N, Kitamura F, Tagami M, Mineki R, Arai T, Taguchi H, Yanagida M, Hirner S, Labeit D, Labeit S, Sorimachi H. Multiple molecular interactions implicate the connectin/titin N2A region as a modulating scaffold for p94/calpain 3 activity in skeletal muscle. J Biol Chem 283: 14801–14814, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Heckmatt JZ Respiratory care in muscular dystrophy. Br Med J 295: 1014–1015, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heimann P, Menke A, Rothkegel B, Jockusch H. Overshooting production of satellite cells in murine skeletal muscle affected by the mutation “muscular dystrophy with myositis” (mdm, Chr 2). Cell Tissue Res 283: 435–441, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Huebsch KA, Kudryashova E, Wooley CM, Sher RB, Seburn KL, Spencer MJ, Cox GA. Mdm muscular dystrophy: interactions with calpain 3 and a novel functional role for titin's N2A domain. Hum Mol Genet 14: 2801–2811, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jannapureddy SR, Patel ND, Hwang W, Boriek AM. Merosin deficiency leads to alterations in passive and active skeletal muscle mechanics. J Appl Physiol 94: 2524–2533, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BD, Wilson LE, Zhan WZ, Watchko JF, Daood MJ, Sieck GC. Contractile properties of the developing diaphragm correlate with myosin heavy chain phenotype. J Appl Physiol 77: 481–487, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Kohn TA, Myburgh KH. Electrophoretic separation of human skeletal muscle myosin heavy chain isoforms: the importance of reducing agents. J Physiol Sci 56: 355–360, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Khandelwal N, Malya R, Reid MB, Boriek AM. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J 18: 102–113, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Lane PW Muscular Dystrophy With Myositis (mdm). Bar Harbor, ME: Jackson Laboratory, 1985.
- 21.Lopez MA, Mayer U, Hwang W, Taylor T, Hashmi MA, Jannapureddy SR, Boriek AM. Force transmission, compliance, and viscoelasticity are altered in the α7-integrin-null mouse diaphragm. Am J Physiol Cell Physiol 288: C282–C289, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama K, Matsubara S, Natori R, Nonomura Y, Kimura S. Connectin, an elastic protein of muscle. Characterization and function. J Biochem (Tokyo) 82: 317–337, 1977. [PubMed] [Google Scholar]
- 23.Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol 333: 951–964, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature 323: 646–650, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Patel ND, Jannapureddy SR, Hwang W, Chaudhry I, Boriek AM. Altered muscle force and stiffness of skeletal muscles in α-sarcoglycan-deficient mice. Am J Physiol Cell Physiol 284: C962–C968, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrof BJ, Stedman HH, Shrager JB, Eby J, Sweeney HL, Kelly AM. Adaptations in myosin heavy chain expression and contractile function in dystrophic mouse diaphragm. Am J Physiol Cell Physiol 265: C834–C841, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol 126: 461–480, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75: 2337–2340, 1993. [DOI] [PubMed] [Google Scholar]
- 30.van der Ven PF, Bartsch JW, Gautel M, Jockusch H, Furst DO. A functional knock-out of titin results in defective myofibril assembly. J Cell Sci 113: 1405–1414, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Wang K, McCarter R, Wright J, Beverly J, Ramirez-Mitchell R. Viscoelasticity of the sarcomere matrix of skeletal muscles. The titin-myosin composite filament is a dual-stage molecular spring. Biophys J 64: 1161–1177, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webster C, Silberstein L, Hays AP, Blau HM. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52: 503–513, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Witt CC, Ono Y, Puschmann E, McNabb M, Wu Y, Gotthardt M, Witt SH, Haak M, Labeit D, Gregorio CC, Sorimachi H, Granzier H, Labeit S. Induction and myofibrillar targeting of CARP, and suppression of the Nkx2.5 pathway in the MDM mouse with impaired titin-based signaling. J Mol Biol 336: 145–154, 2004. [DOI] [PubMed] [Google Scholar]