Introduction
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVC/D) is a myocardial disease characterized by myocyte atrophy with progressive fibrofatty replacement predominantly affecting the right ventricle1-3; the usual symptoms are palpitations, syncope, or near syncope due to sustained ventricular tachycardia. ARVC/D is a well known cause of sudden cardiac death in young adults. However, patients may be asymptomatic, particularly a family member being evaluated because of genetic transmission of the disease4. Approximately 30-40% of patients with ARVC/D have been found to have a genetic abnormality of the desmosomal proteins. The most commonly reported gene mutation in ARVC/D is plakophilin-2 (PKP2)5, but mutations in desmoplakin (DSP), desmocollin- 2 (DSC2), desmoglein 2 (DSG2) and transforming growth factor-beta 3 (TGFB3) have also been reported and are usually transmitted as an autosomal dominant trait6. Plakoglobin (JUP) mutations also have been reported to be associated but are typically homozygous and cause the autosomal recessive disorder Naxos disease that is associated with skin and hair abnormalities7.
We describe a patient who had an incidental finding of a left ventricular aneurysm. Upon further evaluation, it was found that she had both right and left ventricular aneurysms. Genetic evaluation identified a monogenic abnormality in the desmosomal protein, junctional plakoglobin.
Case Report
The patient is a 25 year old woman who presented to the hospital with abdominal pain. Diagnostic evaluation included an abdominal CT scan (computed tomography) that showed a paraumbilical hernia. An incidental finding was that there was thinning of the left ventricular wall with evidence of bulging of the lateral wall of the left ventricle. Further evaluation was recommended to determine the extent and cause of the ventricular abnormality found on the CT scan. She underwent an uncomplicated laparoscopic repair of the umbilical hernia.
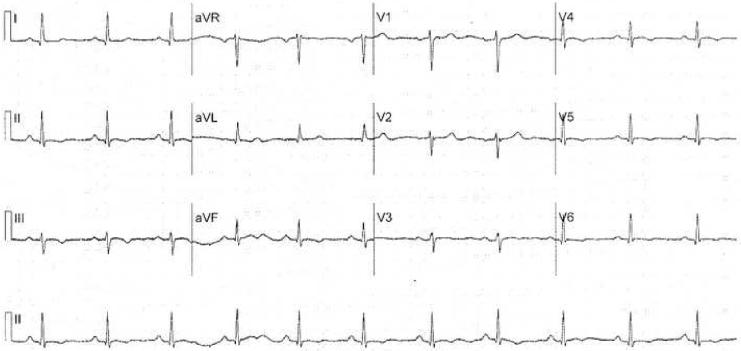
There was no history of sudden cardiac death in her immediate family. She had 4 episodes of syncope in the previous year, some of which suggested a neurocardiogenic cause. She denied any significant history of tobacco, alcohol or illegal substance abuse. She was not athletic. An electrocardiogram showed sinus rhythm at the rate of 70 beats per minute. The QRS axis was normal. The T waves were inverted in leads 2, 3 AVF and V4, V5, and V6 (Figure 1). A signal averaged ECG was normal
Figure 1.
12 lead electrocardiogram showing T wave inversion in leads 2, 3, AVF and V3-V6
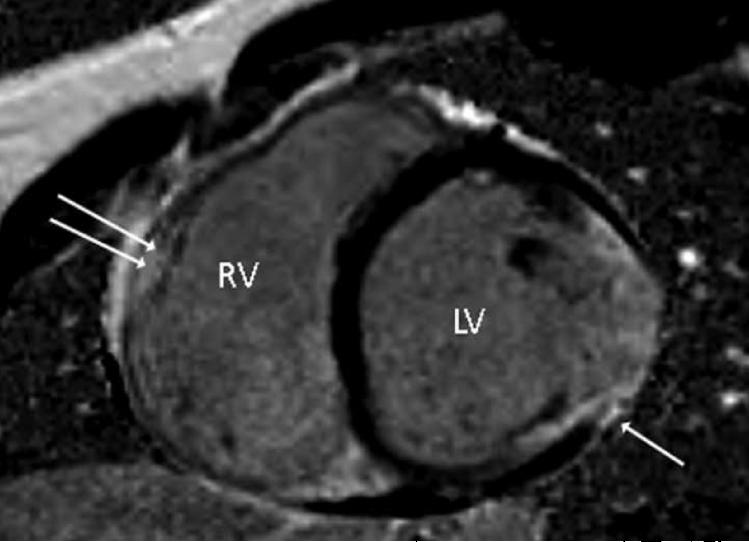
A cardiac MRI (magnetic resonance imaging) showed myocardial fibrofatty replacement both in the right and left ventricles (Figure 2). In addition, it was noted that the right ventricular wall was thin and there were multiple areas of hypokinesia and dyskinesia in the basal lateral area of the right ventricle as well as in the inferior left ventricular wall.
Figure 2.
Cardiac MRI with gadolinium enhancement, short axis view. Single arrow shows evidence of scar in the left ventricle, double arrow shows probable scar in the right ventricle
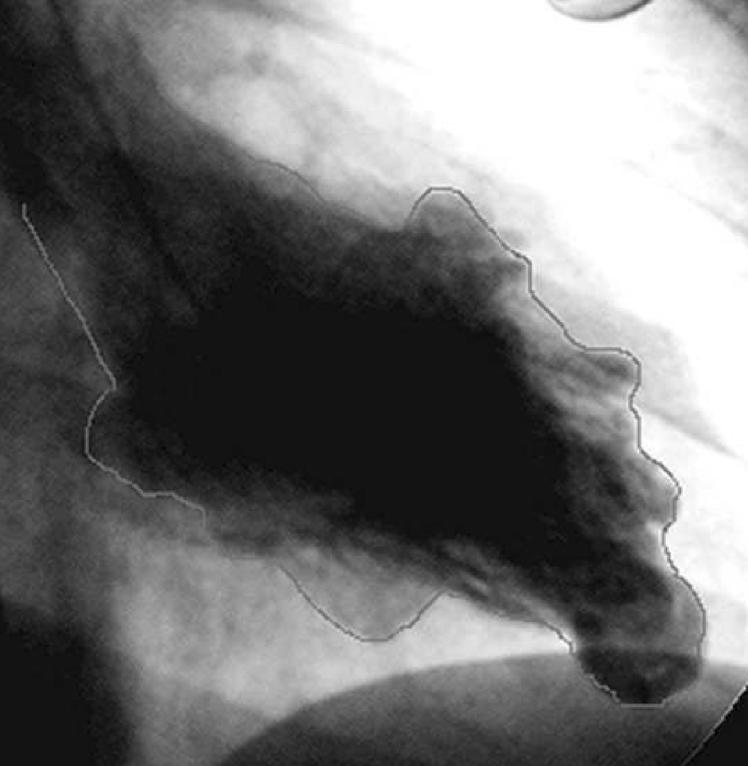
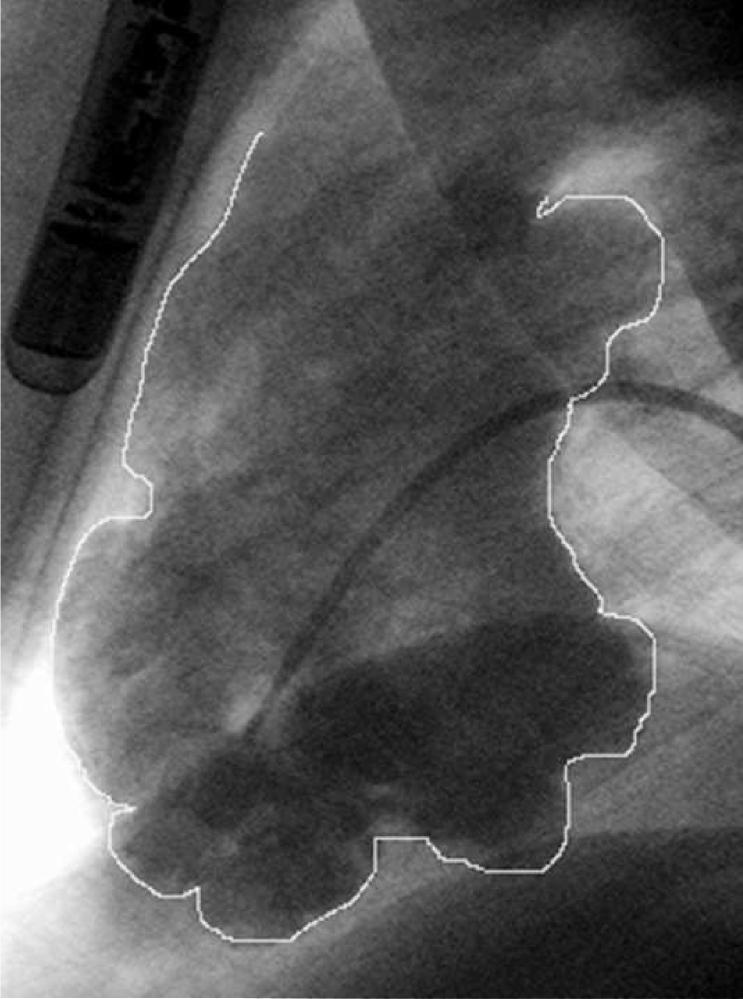
A transthoracic echocardiogram did not show evidence of ventricular aneurysms. There was only mild lateral akinesis. A repeat transthoracic echocardiogram obtained with Definity contrast revealed mid lateral wall hypokinesia of the left ventricle with a suggestion of an aneurysm. The overall biventricular function was normal. She underwent a right and left heart catheterization that showed normal coronary arteries. Both left and right end diastolic pressures were normal. The left ventricular systolic and diastolic function was normal. The angiogram showed an aneurysm in the apical region of the left ventricle (Figure 3) and multiple aneurysms in the right ventricle (Figure 4). An electrophysiology study was performed. She had no inducible arrhythmias with stimulation at the right ventricular apex and outflow tract before and with isoproterenol infusion using three extra stimuli. During telemetry monitoring, she had an 8 beat run of ventricular tachycardia at a rate of 120 bpm. A 24 hour Holter monitor showed 1 premature ventricular beat. She has had no further episodes of syncope. A loop recorder was implanted. It was interrogated 6 months later and did not record any arrhythmias. Blood was sent for genetic testing to determine whether she had a desmosomal cardiomyopathy. The results showed that she had a junctional plakoglobin mutation. Genetic testing on her 2 children is pending.
Figure 3.
Left ventricular angiogram showing prominent apical aneurysm as well as smaller outpouching in the anterior region.
Figure 4.
Right ventricular angiogram in the LAO view. There are multiple aneuryisms, particularly in the inferior region. The loop recorder is seen in the upper corner of the image.
Mutation detection of plakoglobin
After informed consent, blood for DNA extraction and lymphoblastoid cell line immortalization was obtained, as approved by the Baylor College of Medicine Institutional Review Board (IRB).
Genomic DNA samples were amplified by PCR using primers designed to amplify the coding exons to all desmosome-encoding genes. The PCR products were sequenced using Big Dye Terminator version 3.1 chemistry (Applied Biosystems, Foster City, CA) and analyzed using an ABI 3730 DNA sequencer (Applied Biosystems).
Genetic Analysis
A heterozygous nucleotide substitute (G475>T) in the proband was identified. The detected nucleotide change was not found in an ethnic matched control group of 250 healthy and unrelated subjects (500 control chromosomes). The mutation has not been reported elsewhere. The heterozygous point substitution results in a predicted p. V159L amino acid substitution and is located within the first armadillo repeat domain of plakoglobin, which is known to mediate the interactions with desmosomal cadherins.8 This variant occurs in a residue that is highly conserved among species. The proband was also investigated for mutations of other desmosome-encoding genes, including plakophilin-2, desmoplakin, desmoglein-2 and desmocollin-2 and no mutations were identified.
Discussion
This patient had a unique presentation of ARVC/D. A ventricular aneurysm was an incidental finding on an abdominal CT scan. Other unusual features are that both right and left ventricular function was normal despite the presence of multiple biventricular aneurysms. Also instructive was that the aneurysms were not detected by a 2-D high quality echocardiogram. A second echocardiographic study was done after it was known that the aneurysms were present and it was interpreted with this knowledge but did not show the extent of the disease despite the aneurysms being clearly visible both on MRI and angiography. This illustrates that complete evaluation of suspected abnormalities of both right and left ventricles in patients suspected of ARVC/D may require several imaging modalities.
The differential diagnosis includes the possibility of congenital left ventricular aneurysms. A recent comprehensive review of the subject of congenital left aneurysms and diverticula noted that 111 cases have been reported9. These frequently involve the left ventricle but occasionally the aneurysms may be seen in both ventricles. It is possible that some of these patients with aneurysms thought to be congenital may have a desmosomal defect.
This patient has a biventricular cardiomyopathy due to a mutation of the gene encoding for the cell- cell adhesion protein, plakoglobin (JUP). A recessive mutation of the gene encoding plakoglobin has been shown to cause Naxos disease, a cardiocutaneous syndrome characterized by ARVC/D and abnormalities of hair and skin7. A dominant (heterozygous) mutation of the gene encoding plakoglobin has recently been reported in a German family with ARVC/D who had no cutaneous abnormalities.10 The clinical phenotype described herein is novel and suggests that other similar subjects with ventricular aneurysms could be at risk for arrhythmias, and, potential sudden death.
The traditional clinical characterization of ARVC/D is that it causes disease in right ventricular function and wall motion abnormalities with minimal or no involvement of the left ventricle. This definition is incorporated in the diagnostic Task Force Criteria11. This view has now been broadened. In a recent review of 200 probands and relatives with ARVC/D, 5% had left dominant disease and 56% showed a biventricular pattern.12
It is a challenge to select appropriate treatment for this patient who has not had sustained ventricular tachycardia. The fact that the electrophysiological study did not induce ventricular arrhythmias is not reassuring since this test has not been decisive in risk stratification.13 She has a loop recorder implanted to determine the cause of the syncopal episodes.
Acknowledgments
This work was supported in part by grant U01-HL 65594 from the National Institute of Health, Bethesda, Maryland
We would like to thank Dr. Dennis Citron for the patient referral, Dr. Samuel L. Butman who performed the cardiac catheterization and Dr. Vincent Sorrell for assistance with the MRI illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marcus FI, Fontaine G. Arrhythmogenic right ventricular dysplasia/cardiomyopathy. A Review. PACE. 1995;18:1298–1314. doi: 10.1111/j.1540-8159.1995.tb06971.x. [DOI] [PubMed] [Google Scholar]
- 2.Sen-Chowdhry S, Lowe MD, Sporton SC, et al. Arrhythmogenic right ventricular cardiomyopathy: clinical presentation, diagnosis, and management. Am J. of Med. 2004;117:685–695. doi: 10.1016/j.amjmed.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia a United States experience. Circulation. 2005;112:3823–3832. doi: 10.1161/CIRCULATIONAHA.105.542266. [DOI] [PubMed] [Google Scholar]
- 4.Hamid MS, Norman M, Quraishi A, et al. Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden criteria. J. Am Coll Cardiol. 2002;40:1445–1450. doi: 10.1016/s0735-1097(02)02307-0. [DOI] [PubMed] [Google Scholar]
- 5.Gerull B, Heuser A, Wichter T, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nature Genetics. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 6.Sen-Chowdhry S, Syrris P, McKenna WJ, et al. Role of genetic analysis in the management of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J. Am Coll Cardiol. 2007;50:1813–1821. doi: 10.1016/j.jacc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 7.McKoy G, Protonotarios N, Crosby A, et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 8.Witcher LL, Collins R, Puttagunta S, et al. Desmosomal cadherins binding domains of plakoglobin. J Biol Chem. 1996;27:10904–9. doi: 10.1074/jbc.271.18.10904. [DOI] [PubMed] [Google Scholar]
- 9.Ohlow MA. Congenital left ventricular aneurysms and diverticula: Definition, pathophysiology, clinical relevance and treatment. Cardiology. 2006;106:63–72. doi: 10.1159/000092634. [DOI] [PubMed] [Google Scholar]
- 10.Asimaki A, Syrris P, Wichter T, et al. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J. Human Genetics. 2007;81:964–973. doi: 10.1086/521633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Br. Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. on behalf of the Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology, supported by the Schoepfer Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen-Chowdhry, Syrris P, Ward D, et al. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710–1720. doi: 10.1161/CIRCULATIONAHA.106.660241. [DOI] [PubMed] [Google Scholar]
- 13.Buja G, Estes M, Wichter T, et al. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: Risk stratification and therapy. Progress in Cardiovascular Disease. 2008;50:282–293. doi: 10.1016/j.pcad.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]