Abstract
The relative importance of regulatory versus structural evolution for the evolution of different biological systems is a subject of controversy. The primacy of regulatory evolution in the diversification of morphological traits has been promoted by many evolutionary developmental biologists. For physiological traits, however, the role of regulatory evolution has received less attention or has been considered to be relatively unimportant. To address this issue for electrophysiological systems, we examined the importance of regulatory and structural evolution in the evolution of the electrophysiological function of cardiac myocytes in mammals. In particular, two related phenomena were studied: the change in action potential morphology in small mammals and the scaling of action potential duration across mammalian phylogeny. In general, the functional properties of the ion channels involved in ventricular action potential repolarization were found to be relatively invariant. In contrast, there were large changes in the expression levels of multiple ion channel and transporter genes. For the Kv2.1 and Kv4.2 potassium channel genes, which are primary determinants of the action potential morphology in small mammals, the functional properties of the proximal promoter regions were found to vary in concordance with species-dependent differences in mRNA expression, suggesting that evolution of cis-regulatory elements is the primary determinant of this trait. Scaling of action potential duration was found to be a complex phenomenon, involving changes in the expression of a large number of channels and transporters. In this case, it is concluded that regulatory evolution is the predominant mechanism by which the scaling is achieved.
Keywords: ion channel, gene regulation, ion transport, cardiac myocyte
two general principles have been advanced to describe the primary molecular mechanisms underlying the evolution of developmental systems (6, 7, 49): 1) Evolution of gene regulation (regulatory evolution) is more flexible and consequently more common than evolution of protein structure because regulatory evolution avoids the pleiotropic effects that can result from changes in protein structure and function (structural evolution). 2) Evolution of gene regulation is more likely to occur as a result of changes in cis-regulatory function rather than as a consequence of changes in the organization of transcription factor networks, again because this limits pleiotropy. In this case, the pleiotropic effects result from modifying the function of the complex interconnected networks of transcription factors.
Neither of these principles is an inviolate law; rather, they are thought to reflect the most common mechanisms used in the evolution of developmental systems. The applicability of these ideas to the evolution of physiological systems either has been brought into question (16) or has been uncertain (6). There is, in principle, no reason why these same concepts should not apply to the evolution of physiological systems, and we have chosen to address this issue by studying the evolution of the electrophysiological properties of mammalian cardiac ventricular myocytes.
A primary constraint on the electrophysiological properties of the mammalian heart is the scaling of body weight. Heart weight scales directly with body weight, and there are no significant changes in the morphology of either the heart or the cardiac myocytes (21, 30). Similarly, several fundamental physiological properties of the cardiovascular system, including mean arterial pressure and minimum diastolic pressure (11, 17), are highly constrained and remain relatively invariant across mammalian phylogeny. These constrained physiological properties reflect the core function of the system: to maintain arterial blood pressure at a level sufficient to ensure adequate organ perfusion, particularly for critical organs such as the brain.
In contrast, many of the electrophysiological properties of the heart show systematic changes with body weight. These include heart rate (17, 38, 41), action potential duration (the rate of action potential repolarization), action potential morphology, and the rate of calcium ion reuptake (3). The electrophysiological properties of the heart vary in a systematic fashion in order to compensate for the changes in the physical properties of the vasculature and the heart produced by scaling of body and heart weight. These concerted changes in electrophysiological function act to maintain the constrained physiological properties, such as mean arterial blood pressure, largely unchanged across mammalian phylogeny and are a critical factor in the ability of mammals to assume such a large range of body sizes (17, 48).
This report focuses on the electrophysiological properties of left ventricular myocytes, the primary function of which is the maintenance of arterial blood pressure. This function is both relatively simple and well understood within the context of the whole animal physiology. Ventricular myocytes are also one of the most intensively studied and best understood systems at the level of cellular physiology, with a correspondingly detailed knowledge of the molecular biology of ion channel and transport function (3, 24), and, as such, they provide an unparalleled system in which to study the evolution of electrophysiological traits. We describe the mechanisms that produce changes in one specific trait, action potential morphology, and examine the relative importance of regulatory and structural evolution in the scaling of action potential duration and calcium reuptake.
MATERIALS AND METHODS
All animal procedures were approved by the Institutional Animal Care and Use Committees of Stony Brook University, the University of Cincinnati, and the University of Utah.
Choice of species.
Mammalian species that are common experimental models for cardiac electrophysiological studies were chosen for study. The eight species used in the study encompass a broad range of body weights and show the typical allometric relationship between body size and heart rate or action potential duration observed for most terrestrial mammals (Fig. 1, A and B). These species were human (Homo sapiens), canine (Canis familiaris), ferret (Mustela putorius furo), rabbit (Oryctolagus cuniculus), guinea pig (Cavia porcellus), rat (Rattus norvegicus), Chinese hamster (Cricetus griseus), and mouse (Mus musculus).
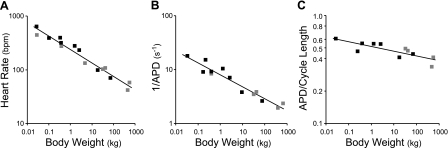
Fig. 1.
A: relationship between resting heart rate and body weight for terrestrial mammals. Data were fitted with the allometric equation y = a·Mb, where a is constant, M is the body weight, and b is the scaling coefficient (b = −0.25 ± 0.02). Black squares, species used in present study; gray squares, other species. Data were obtained from a survey of the literature (see Supplementary Material). bpm, Beats per minute. B: relationship between the inverse ventricular action potential duration (1/APD) and body weight for terrestrial mammals (scaling coefficient b = −0.22 ± 0.02). Ventricular action potential duration was estimated from uncorrected QT intervals obtained from electrocardiogram studies using conscious resting animals, where available (see Supplementary Material). C: relationship between the fraction of the cardiac cycle taken up by the action potential and body weight (scaling coefficient b = −0.04 ± 0.01). Data points were calculated from those studies in which both the heart rate and the QT interval were recorded from the same animals.
Analysis of mRNA expression.
Animals were euthanized with either halothane or pentobarbital sodium (100 mg/kg iv or ip), depending on the species. The hearts were quickly removed, and the left ventricular free wall was dissected. Total RNA was prepared with Qiagen RNeasy columns. Human RNA samples were obtained from independent commercial suppliers (Ambion or BioChain).
Complementary DNAs were prepared as previously described (32). Three independent primer pairs for each gene were used for mRNA quantitation by real-time PCR, which was performed with the SYBR Green QuantiTect PCR Kit (Qiagen). Experimental samples were analyzed in triplicate. Expression values for a given gene were the average of results from three independent sets of eight RNA samples. Real-time PCR products were sequenced to confirm that the amplicons were from the mRNA of interest.
Isolation and sequencing of genomic DNA regions from hamster and guinea pig.
Bacterial artificial chromosome (BAC) clones encompassing the Kv2.1 proximal promoter regions were identified in BAC libraries (CHORI) with a nonradioactive probe labeled with digoxigenin-11-dUTP (DIG-11-dUTP alkali-labile, Roche). Probe sequences were based on cDNA sequences or conserved regions from multiple species, and positive clones were detected with anti-DIG-AP, Fab fragments (Roche), and CDP-Star (Roche). Specific DNA fragments of interest were isolated from positive BAC clones (BACPAC Resources Center) by a combination of restriction mapping and Southern blotting, and subfragments were subcloned into pBluescript for sequencing. DNA sequences were submitted to GenBank (accession nos. EU643795 and EU643796).
Subcloning of proximal promoter regions.
Comparisons of Kv2.1 and Kv4.2 proximal promoter sequences were performed with the Vista alignment program (12), and conserved regions were used as landmarks to select orthologous sequences from the two genes, although these were not identical in length (see Supplemental Material).1 For both genes the selected sequences terminated immediately before the initiator methionine in the first exon. DNA fragments for the Kv2.1 (mouse 1,601 bp, hamster 1,680 bp, guinea pig 1,786 bp, human 1,891 bp) and Kv4.2 (mouse 2,590 bp, human 2,567 bp) genes were subcloned from BAC clones into a luciferase reporter plasmid (pGL2, Promega).
Rat neonatal myocyte transfection, culture, and luciferase assay.
Neonatal rat cardiomyocytes were isolated and cultured as described previously (51). Transfection was performed with the Rat Cardiomyocyte Nucleofector Kit (Amaxa) in a Nucleofector I device (Amaxa). Each sample included an internal control Renilla luciferase plasmid (phRL-SV40, 1,000-fold lower concentration than test plasmids). Negative (pGL2-basic) and positive (pGL2-control) controls were also included in each experiment. After electroporation the cells were plated onto fibronectin-coated 12-well plates and cultured at 37°C in 5% CO2 for 48 h. Cell survival was ∼35%. Luciferase assays were performed with the Dual Luciferase Reporter Assay Kit (Promega). Firefly and Renilla luciferase activities were measured with a Lumat luminometer (Berthold).
Myocyte electrophysiology.
Preparation of guinea pig and canine myocytes was performed as described previously (10, 44), and mouse ventricular myocytes were isolated by the same method as that used for guinea pig. For the recording of Ca2+ currents, isolated ventricular myocytes were maintained at room temperature and perfused with a Na+- and K+-free solution that contained (in mM) 137 TEA-Cl, 5.4 CsCl, 2 CaCl2, 1 MgCl2, 5 HEPES, 10 glucose, and 3 4-aminopyridine (pH = 7.4). Glass pipettes were filled with solution containing (in mM) 115 Cs-aspartate, 20 CsCl, 11 EGTA, 10 HEPES, 2.5 MgCl2, and 2 Mg-ATP (pH = 7.2) and had a resistance of 1.5–2.5 MΩ. After the membrane was ruptured, cells were clamped at −60 mV for 10 min to allow dialysis of the intracellular solution and stabilization of the Ca2+ currents before measurement of Ca2+ currents began.
Perforated patch-clamp recordings were used for action potential recordings and the dynamic clamp studies. Glass pipettes were backfilled with a pipette solution containing (in mM) 110 K-aspartate, 20 KCl, 8 NaCl, 10 HEPES, 2.5 MgCl2, and 0.1 CaCl2 and 240 mg/ml amphotericin B (pH adjusted to 7.2 with KOH). Cells were studied once stable series resistances <7 MΩ were achieved. All action potential and contraction experiments were performed at 34°C.
Dynamic clamp experiments were performed as previously described (10, 44). A modified version of the Windows-based DynClamp software was used in the dynamic clamp studies (27). Voltage sampling of the dynamic clamp software and output of the current injection command were through an Axon Digidata 2100 A/D board. The transient outward potassium current (Ito) was defined as a rapidly and fully inactivating outward current, formulated as described previously (10).
Myocyte contraction was imaged with a charge-coupled device camera, and cell length and shortening were measured with a video edge detector (Crescent Electronics).
Expression of KCNH2 and KCNQ1 channels.
The human KCNH2 and KCNQ1 cDNA clones have been described previously (36, 37). Full-length guinea pig cDNA clones were derived with a combination of rapid amplification of cDNA ends (RACE) and standard PCR as described previously (40) (accession nos. EF204534 and EF204535). Channel expression and recording with Xenopus oocytes were performed as described previously (36, 37). The decaying phase of tail current traces was fitted to one [KCNH2 or slow delayed rectifier potassium current (IKs)] or two (KCNQ1) exponential functions. Peak tail current amplitudes were normalized to the maximum value, and the resulting data were fitted to a Boltzmann function.
RESULTS
Scaling of ventricular action potential duration.
The relationship between body weight and the resting heart rate of terrestrial mammals was fitted with an allometric equation with a scaling coefficient of −0.25 ± 0.02 (Fig. 1A), similar to values obtained in earlier studies with independent data sets (17, 41). Ventricular action potential durations for a range of terrestrial mammals were estimated from QT intervals obtained from electrocardiogram studies (Fig. 1B). The inverse action potential duration, which corresponds approximately to the overall rate of repolarization following the upstroke of the action potential, scales continuously over a wide range of body sizes and has a scaling coefficient of −0.22 ± 0.02.
The scaling of heart rate and action potential duration are similar but not perfectly matched. There is a modest increase in the fraction of the cardiac cycle taken up by the action potential in smaller mammals, so that this value displays a weak dependence on body mass (Fig. 1C). This is consistent with the observation that the diastolic interval decreases as a fraction of the cardiac cycle in smaller mammals (29). Because the coronary blood supply to the left ventricle is only active during diastole, there is a strong constraint on the minimum fraction of the cardiac cycle that must be devoted to diastole in order to ensure adequate perfusion of the myocardium, which must be one important factor maintaining the relatively tight linkage between heart rate and action potential duration.
Discontinuities in scaling of ventricular action potential morphology.
Although ventricular action potential duration scales over a large range of mammalian body weights without any obvious discontinuities (Fig. 1B), at the cellular level there are marked discontinuities in the electrophysiological mechanisms underlying action potential repolarization in the ventricles of mammals of different weights. In species the size of guinea pigs and larger, action potential repolarization depends primarily on three potassium currents: the IKs and the rapid delayed rectifier potassium current (IKr), which have relatively slow kinetics of activation, and the inward rectifier potassium current (IK1) (24). In smaller species, repolarization is primarily dependent on two relatively large and rapidly activating potassium currents, Ito and the ultrarapid potassium current (IKur). These differences in repolarization mechanism are reflected in the morphology of the ventricular action potential.
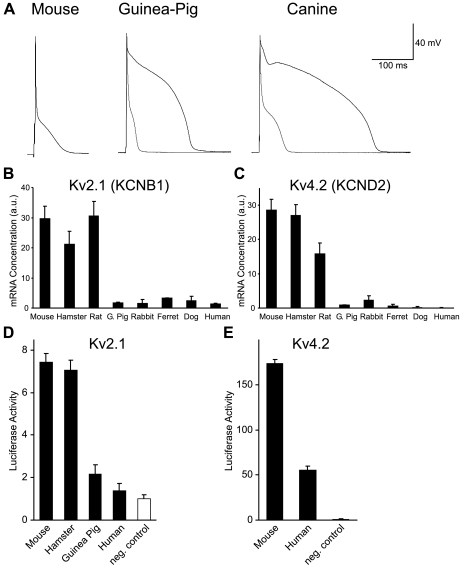
In large and intermediate-sized mammals, such as canine and guinea pig, action potentials have a “spike and dome” morphology, whereas action potentials in small mammals, such as the mouse, have a “triangular” morphology, which is distinguished by the lack of a prominent plateau phase (Fig. 2A). The presence of a large Ito in the myocytes of small mammals precludes the development of the high plateau phase observed in larger animals. The critical role of Ito expression levels in determining action potential morphology can be shown experimentally by adding a large Ito to either guinea pig or canine myocytes with dynamic clamp (gray traces in Fig. 2A). After addition of this current, the action potential assumes a morphology that is similar to that of the mouse action potential.
Fig. 2.
A: comparison of ventricular action potentials recorded from mouse, guinea pig, and canine ventricular myocytes. For guinea pig and canine cells the waveform following the addition of a mathematically modeled transient outward potassium current (Ito; ≈50 pA/pF) with dynamic clamp is shown in gray. B and C: comparison of Kv2.1 (KCNB1; B) and Kv4.2 (KCND2; C) mRNA expression in the left ventricular wall of 8 mammalian species. Values are means ± SD (n = 3). a.u., Arbitrary units. D: comparison of the functional properties of mouse, hamster, guinea pig, and human Kv2.1 proximal promoter regions. E: comparison of the functional properties of mouse and human Kv4.2 proximal promoter regions. For promoter analysis, orthologous regions of genomic DNA (see materials and methods) were subcloned into a luciferase reporter gene construct, and gene activity was determined by assaying luciferase activity following transfection into cultured rat neonatal cardiac myocytes. Data values are expressed relative to the background level of luciferase activity following transfection of the control plasmid (pGL2-basic). Values are means ± SD (n = 3–6).
Regulation of rapidly activating repolarizing currents.
The discontinuity in action potential repolarization mechanism is produced by abrupt changes in the level of expression of the Ito and IKur channels, and this is reflected in the expression of two genes that encode these channels (Fig. 2, B and C). The species-dependent expression pattern of the genes underlying the main component of the Ito channel (Kv4.2) and one component of the rapidly activating delayed rectifier potassium channel IKur (Kv2.1) resemble a step function, with both Kv2.1 and Kv4.2 mRNA being abundantly expressed in mouse, hamster, and rat ventricles but uniformly low in all larger species (Fig. 2, B and C).
A second channel type that also contributes to IKur, the Kv1.5 channel, was tested, but expression of Kv1.5 mRNA was poorly correlated with expression of the channel, suggesting that regulation of this channel is posttranscriptional or dependent on a yet-to-be identified accessory subunit or that the mRNA is expressed at significant levels in cells other than myocytes in the ventricle wall (Refs. 5, 47; Rosati and McKinnon, unpublished data). The Kv4.2 α-subunit makes the primary contribution to Ito expression in small rodents (13). In larger animals expression of Ito is highly variable, is relatively small when present (34), and has little or no effect on action potential duration in these species (44). The small Ito found in these species is encoded by the Kv4.3 gene (9), which displays highly variable species-dependent expression patterns, with no correlation to body size (data not shown).
Comparison of mouse, hamster, guinea pig, and human Kv2.1 proximal promoter function.
In principle, the changes in Kv2.1 mRNA expression in different species (Fig. 2B) can be produced by changes in either the cis- or trans-regulatory elements controlling gene expression. To address this issue the transcriptional activity of the proximal promoter regions of the mouse, hamster, guinea pig, and human Kv2.1 genes were compared in cultured rat myocytes.
There are clear differences in the transcriptional activity of the Kv2.1 proximal promoters from different species that closely reflect the differences in mRNA expression (Fig. 2D). This result suggests that the differences in Kv2.1 mRNA expression observed in vivo are mediated by changes in the functional properties of cis-regulatory elements found in the Kv2.1 gene.
Given the highly distributed nature of mammalian gene regulation, it is notable how well the different in vivo expression patterns of the Kv2.1 gene are retained by the proximal promoter regions when expressed in vitro. In addition to the large difference between the mouse/hamster and guinea pig/human expression levels, the guinea pig/human promoters are essentially turned “off” in cardiac myocytes in vitro, as they are in vivo.
Comparison of mouse and human Kv4.2 proximal promoter function.
There were also clear differences in the transcriptional activity of the mouse and human Kv4.2 proximal promoter regions (Fig. 2E). For the Kv4.2 gene, although there are large differences between the mouse and human constructs, expression of the human promoter is significantly above the background levels seen in vivo, and expression relative to the Kv2.1 constructs was also anomalously high. Both results suggest that the Kv4.2 promoter construct lacks one or more repressor elements found in the native gene.
Function and regulation of slowly activating repolarizing currents.
The changes in Ito and IKur expression are clear examples of regulatory evolution. The scaling of action potential duration in larger species could, however, involve structural evolution modifying the function of one or more of the other currents that are required for action potential generation in these animals. Guinea pig and larger species depend predominantly on two voltage-gated potassium currents, IKs and IKr, for action potential repolarization. Therefore, of all the channels involved in this process, these channels would seem to be the most obvious candidates for structural evolution. The α-subunits of the IKs and IKr channels are encoded by the KCNQ1 and KCNH2 genes, respectively. If, for example, the activation rate of the KCNQ1 channel scaled between human and guinea pig, this change in channel function could contribute significantly to the decrease in action potential duration observed in guinea pigs.
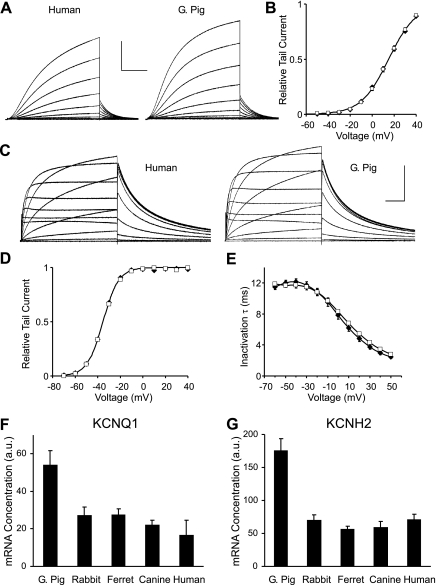
Because it is difficult to accurately record the kinetic properties of IKs and IKr in large mammals because of the relatively small size of the currents, the properties of the guinea pig and human KCNQ1 and KCNH2 channels were compared with a heterologous expression system. No significant difference in either the rate or voltage dependence of KCNQ1 channel activation was observed, either when the channel was coexpressed with its normal auxiliary subunit, KCNE1 (Fig. 3, A and B), or when it was expressed alone (data not shown). The only detectable difference between the channels from the two species was an ∼20% slower deactivation rate for the guinea pig channel compared with the human channels. This small difference was observed when the channels were expressed alone and when they were coexpressed with KCNE1. For IKr, no detectable differences were found in comparisons of the kinetic properties of human and guinea pig KCNH2 channels, and the two currents were indistinguishable (Fig. 3, C–E).
Fig. 3.
KCNQ1 and KCNH2 function and expression in Xenopus oocytes. A: current traces of human (left) and guinea pig (right) KCNQ1 channels coexpressed with KCNE1 (current scale: 4 and 2.5 μA, respectively, time scale: 2 s). B: activation curve determined from tail currents for human and guinea pig KCNQ1 (n = 10). Data points for human and guinea pig overlap. Data were fitted with Boltzmann curves [half-maximal potential (V1/2) = 14.9 ± 0.2 and 15.0 ± 0.3 mV, slope (k) = −12.2 ± 0.2 and −12.6 ± 0.3, respectively]. C: current traces of human (left) and guinea pig (right) KCNH2 channels (current scale 0.4 μA, time scale 1 s). D: activation curves (determined from tail currents) and inactivation time constants for human and guinea pig KCNH2 channels (n = 14 and 21, respectively). Data points for human and guinea pig activation curve overlap. Data were fitted with Boltzmann curves (V1/2 = −34.9 ± 0.3 and −35.3 ± 0.3 mV, k = −7.2 ± 0.2 and −7.0 ± 0.2, respectively). E: inactivation time constants (τ) for human and guinea pig KCNH2 channels. B, D, and E: values are means ± SE; □, human; ⧫, guinea pig. F and G: comparison of KCNQ1 (F) and KCNH2 (G) gene expression in the left ventricular free wall of guinea pig and larger species. Values are means ± SD (n = 3).
In contrast to the conserved channel function, it is known that guinea pig myocytes express significantly higher levels of IKs and IKr than larger species (22), and this is likely to be one important factor contributing to the decreased action potential duration in these species. Both KCNQ1 and KCNH2 gene expression were found to be significantly (P < 0.01) increased in guinea pig compared with larger species (Fig. 3, F and G), suggesting that regulatory evolution of KCNQ1 and KCNH2 gene expression may contribute to the scaling of action potential duration. Regulation of these currents is complex, however, and it has been shown previously that KCNH2 mRNA and protein are expressed at high levels in small rodents (50, 28), whereas the IKr is very small, so other factors in addition to expression of the α-subunits are likely to contribute to the regulation of these currents.
Function and regulation of L-type calcium current.
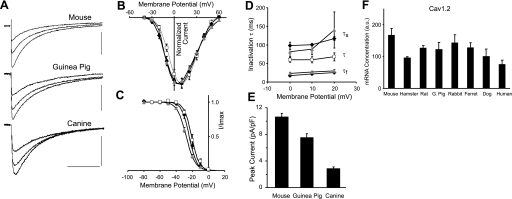
One other channel for which changes in kinetic properties could have an impact on action potential duration, particularly in species with a spike and dome action potential morphology, is the L-type calcium current (ICa,L). The kinetic properties of ICa,L were determined with voltage-clamp recordings in three species, mouse, guinea pig, and canine, and the biophysical properties of the currents were found to be quite similar (Fig. 4A). The normalized current-voltage (I-V) curves for the three species were very similar (Fig. 4B). The steady-state inactivation curves were also similar, although the mouse curve was shifted 4 mV more negative relative to the guinea pig curve, which overlapped with the canine curve (Fig. 4C). The rate of inactivation for the mouse and guinea pig currents was best described by two time constants, which were indistinguishable for the two species (Fig. 4D). The relatively small canine current could not be reliably fitted with two time constants. In this case, the single fitted time constant was intermediate between those of mouse and guinea pig. Qualitatively, there is very little difference in the inactivation properties of the three currents (Fig. 4A).
Fig. 4.
Calcium current function and expression in ventricular myocytes. A: L-type calcium current (ICa,L) traces recorded from mouse, guinea pig, and canine myocytes (current scale 5, 4, and 2 pA/pF, respectively; time scale 80 ms). Holding potential was −50 mV, and currents were elicited by voltage steps to 20, 30, and 40 mV. B: normalized current-voltage (I-V) relations for mouse, guinea pig, and canine ICa,L in ventricular myocytes. C: steady-state inactivation for mouse, guinea pig, and canine ICa,L. Data were fitted with Boltzmann curves (V1/2 = −27.7 ± 0.9, −23.9 ± 1.4, −20.9 ± 1.6 mV, k = 5.6 ± 0.1, 4.7 ± 0.2, 4.7 ± 0.1, n = 7, 14, 8, respectively). I/Imax, fraction of maximum peak current. D: inactivation time constant τ for ICa,L. For mouse and guinea pig the data were fitted with a biexponential curve with 2 time constants [fast (τf) and slow (τs)], whereas for canine an exponential curve with a single time constant (τ) was fitted (n = 5, 5, and 6, respectively). B–D: data points are means ± SD; gray triangle, mouse; black diamond, guinea pig; open square, canine. E: comparison of peak calcium current density in mouse, guinea pig, and canine ventricular myocytes. Values are means ± SE for mouse, guinea pig, and canine (n = 31, 14, and 19, respectively). F: comparison of Cav1.2 (CACNA1C) mRNA expression in the left ventricular wall of 8 mammalian species. Values are means ± SD (n = 3).
In contrast to the relatively constant biophysical properties, there were significant changes in peak calcium current expression levels. In this case, however, the change in expression level is unrelated to regulation of action potential duration because there was a significant increase in calcium current density with decreasing body weight for the three species tested (Fig. 4E). If scaling of action potential duration was the sole determinant of ICa,L expression levels, it would be expected that the calcium current would either remain constant or decline in smaller species, because this current will generally act to lengthen action potential duration.
Expression of Cav1.2 (CACNA1C) mRNA, which encodes the α-subunit of the predominant L-type calcium channel in ventricular myocytes (31, 42), was relatively constant (Fig. 4F), with the changes between species being significantly less than was seen for ICa,L density. Expression of several cardiac calcium channel auxiliary subunit genes, β2, α2δ-1, and α2δ-2, which have been shown to affect calcium current expression (1, 42), showed no consistent dependence on body weight for the species tested (data not shown). Therefore, the mechanism by which ICa,L expression is modified between different species remains uncertain.
Interactions between ICa,L density and maintenance of excitation-contraction coupling.
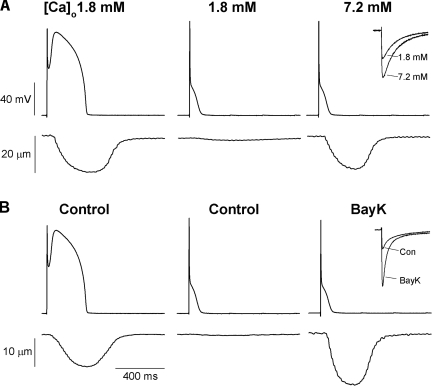
Given that ICa,L shows systematic changes in magnitude with body size that cannot be explained by regulation of action potential duration, it is likely that the changes in ICa,L magnitude reflect constraints imposed by the maintenance of excitation-contraction coupling. This possibility was examined in canine ventricular myocytes with action potential clamp (Fig. 5). A normal canine action potential waveform elicits a robust contraction in canine myocytes, as expected. In contrast, a mouse action potential waveform fails completely to elicit a contraction in these cells. To determine whether the failure of the mouse action potential to elicit a contraction in canine myocytes was due simply to the smaller size of ICa,L in canine myocytes, ICa,L was increased by one of two independent methods: an increase in external calcium concentration or treatment with the calcium channel activator BAY K 8644. In both cases, the increased magnitude of ICa,L restored excitation-contraction coupling, suggesting that constraints related to the maintenance of excitation-contraction coupling drive the increased ICa,L density in mouse myocytes.
Fig. 5.
Action potential clamp recordings from canine myocytes and corresponding unloaded contraction. Top: imposed action potential voltage-clamp waveform. Bottom: unloaded cell shortening. A: left: canine action potential waveform in the presence of normal external calcium concentration ([Ca]o). Center: mouse action potential waveform in the presence of normal external calcium. Note the failure to initiate a contraction. Right: mouse action potential waveform in the presence of 7.2 mM external calcium restores the contraction (inset shows a comparison of peak calcium current recorded under voltage clamp in the control and 7.2 mM external calcium solutions). B: experimental design similar to that in A using 0.5 μM BAY K 8644 (BayK) to increase calcium current (inset). Again, note the recovery of contraction with the mouse action potential waveform when the calcium current is experimentally enhanced.
Scaling of calcium reuptake.
There is a tight linkage between the duration of the calcium transient triggered by an action potential and the duration of myocyte contraction (3). As a consequence, one important constraint on the scaling of cardiac electrophysiological function is that the duration of the calcium transient must also decrease with decreasing body size. There are two transporter systems responsible for the rapid uptake of calcium ions from the cytosol, the sarcoplasmic reticulum (SR) Ca2+-ATPase pump and the Na+/Ca2+ exchanger (3). The primary change with body weight is seen in the activity of the Ca2+-ATPase pump, and this also appears to be an example of regulatory evolution. The activity of the SR Ca2+-ATPase pump increases significantly with decreasing body size because of an increase in the density of Ca2+-ATPase pump proteins rather than a change in the specific activity of the pump (15, 19, 43, 45). Because of this increase in Ca2+-ATPase pump activity, the relative activity of the two transport systems changes significantly with body size. In small mammals the Ca2+ uptake process is dominated by the Ca2+-ATPase pump, whereas in larger mammals there is a greater role for the Na+/Ca2+ exchanger, although the Ca2+-ATPase pump is still the dominant uptake mechanism (2, 3).
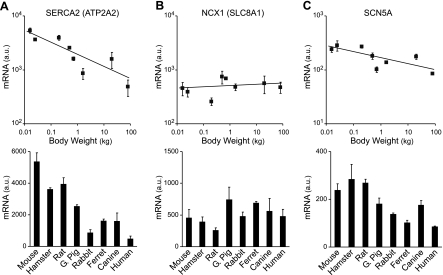
These body mass-dependent changes in calcium handling appear to be determined at the level of transcription. Expression of SERCA2 (ATP2A2) mRNA, which encodes the SR Ca2+-ATPase pump, scales steeply with body weight, with a scaling factor similar to that of inverse action potential duration (Fig. 6A). Expression of the NCX1 gene, which encodes the Na+/Ca2+ exchanger, is essentially independent of body size, although there is some species-dependent variation in expression levels (Fig. 6B). This result is consistent with the observation that the level of Na+/Ca2+ exchanger activity does not vary systematically with body mass (39).
Fig. 6.
Comparison of SERCA2, NCX1, and SCN5A mRNA expression in the left ventricular wall of 8 mammalian species. Top: dependence on body weight. Bottom: same data replotted as histograms (means ± SD, n = 3). A: dependence of SERCA2 (ATP2A2) mRNA expression levels on body weight (scaling coefficient b = −0.25 ± 0.03). B: dependence of NCX1 (SLC8A1) mRNA expression levels on body weight (slope of fitted line was not significantly different from 0). C: dependence of SCN5A mRNA expression levels on body weight (scaling coefficient b = −0.11 ± 0.02).
Unlike the action potential, there are no discontinuities in the scaling of the calcium reuptake transporters. This most likely reflects the fact that the rate of calcium uptake can scale in a simple linear way in proportion to the abundance of the molecular components that underlie the process. Calcium ion pumping obeys the law of mass action, and linear scaling of the concentration of one of the key reactants, the Ca2+-ATPase pump, produces an appropriate effect, an increased rate of Ca2+ uptake.
Regulation of sodium channel.
There is a modest dependence of SCN5A (Nav1.5) mRNA expression on body weight (Fig. 6C), and there is a significant difference in the average expression values for the three smallest species, which express large Ito and IKur currents, compared with the five largest species (P < 0.01). Because sodium channel expression appears to be mediated predominantly by transcriptional regulation (4, 26), this may produce an increase in sodium current expression in these smaller species. The relatively fast activation times of the large Ito and IKur result in partial overlap of these currents with the activation period of the sodium current, and the increased SCN5A expression may represent a compensatory mechanism, possibly to maintain action potential peak height (25).
DISCUSSION
Despite the large number of studies on the physiology and molecular biology of cardiac myocyte electrophysiology, this is the first study to our knowledge on the mechanisms by which cardiac electrophysiology evolves within the mammalian lineage. There are two basic ways in which the electrophysiological properties of any electrically excitable cell can evolve, either by changes in channel and transporter expression levels or by changes in protein sequence and function. Although these are not mutually exclusive alternatives, the results described in this report suggest that regulation of expression levels is the primary means by which ventricular electrophysiological function has been modified during the course of mammalian evolution.
Evolution of action potential morphology.
Mammalian ventricular myocytes can display one of two different action potential morphologies: either a spike and dome morphology or a triangular morphology. The spike and dome action potential morphology, which is observed in most mammals, is evolutionarily ancient. The IKs and IKr channels, which produce action potential repolarization in these species, are also the major repolarizing currents in the hearts of nonmammalian vertebrates, which have similar spike and dome action potential morphologies (20, 46). Expression of either Ito or IKur has not been described in the ventricular myocytes of nonmammalian vertebrates. The triangular waveform appears to be an innovation that is restricted to small rodents with very rapid heart rates and reflects the acquisition of a novel trait in these species. The shift to the triangular waveform morphology is produced by the increased expression of two rapidly activating potassium currents, Ito and IKur. The experimental evidence presented in this report suggests that the switch in this particular physiological trait is due primarily to regulatory evolution modifying the expression of the Kv4.2 and Kv2.1 genes, with little or no role for structural evolution in determining this trait. It has been shown previously that artificially increasing Kv4 α-subunit mRNA expression is, by itself, sufficient to produce the expected change in action potential morphology (18, 53).
The analysis of Kv4.2 and Kv2.1 proximal promoter function described in this report suggests that the evolution of cis-regulatory function is likely to be the predominant factor contributing to the changes in Kv4.2 and Kv2.1 gene expression seen in small mammals. We cannot, however, exclude a role for changes in transcription factor network function without a complete analysis of the expression levels of the transcription factors that are important for regulating expression of these genes, which currently remain, in large part, unknown.
Evolution of action potential duration.
The evolution of action potential duration is a complex phenomenon, involving a relatively large number of different genes (shown in part in Table 1). In addition, the trait grades smoothly across the entire mammalian phylogeny and requires a complex set of changes including graded changes in the expression of multiple genes, in addition to the “on” or “off” changes in Kv2.1 and Kv4.2 gene expression. The complex nature of this evolution is illustrated by the paradoxical changes in calcium channel expression, with expression levels of this current being primarily constrained by the role of this current in excitation-contraction coupling rather than in regulating action potential duration.
Table 1.
Comparison of cardiac channel and transporter deduced amino acid sequences
| Current or Transporter | Primary Subunit |
Percentage Amino Acid Sequence Identity |
||||||
|---|---|---|---|---|---|---|---|---|
| Human-Mouse | Human-Guinea Pig | Rat-Mouse | ||||||
| Universally important for mammalian ventricular myocyte function | ||||||||
| INa | SCN5A | 94 | ||||||
| ICa,L | CaV1.2 (CACNA1C) | 94 | ||||||
| IK1 | Kir2.1 (KCNJ2) | 98 | ||||||
| IK1 | Kir2.2 (KCNJ12) | 96 | ||||||
| Ca2+-ATPase | SERCA2 (ATP2A2) | 99 | ||||||
| Na+/Ca2+ exchanger | NCX1 (SLC8A1) | 95 | ||||||
| Functionally important only in larger mammals | ||||||||
| IKs | KCNQ1 | 90 | 91 | |||||
| IKr | KCNH2 | 96 | 96 | |||||
| Functionally important only in small mammals | ||||||||
| Ito,f | Kv4.2 (KCND2) | 99 | 100 | |||||
| IKur | Kv2.1 (KCNB1) | 94 | 97 | |||||
| IKur | Kv1.5 (KCNA5) | 86 | 96 | |||||
INa, sodium channel current; ICa,L, L-type calcium current; IK1, inward rectifier potassium current; IKs, IKr, slow and rapid delayed rectifier potassium currents; Ito,f, transient outward potassium current; IKur, ultrarapid potassium current.
Scaling of action potential duration does not appear to require significant changes in the function of any of the main proteins controlling cellular electrophysiology. The coding regions of ion channel and transporter genes are highly conserved in mammals, and the number of voltage-gated ion channel genes is unchanged within the mammalian lineage, with no known loss or gain of ion channel α-subunit genes (8, 23, 52). Comparisons of the deduced amino acid sequences of the channels and transporters that contribute to the electrophysiological phenotype of ventricular myocytes are shown in Table 1. For those genes that are functionally important in all mammals, there is no evidence for significant changes in the functional properties of their protein products. The function of the cardiac sodium channel current (INa) is generally assumed to be invariant across mammalian phylogeny (25), and the function of the inward rectifier (IK1) is unchanged from similar currents found in simple invertebrates (14). For the calcium current, as described in this report, there are minor changes in the biophysical function of the channel, but these changes do not contribute in any obvious way to the changes in action potential duration. The sequence of the calcium channel α-subunit is highly conserved for such a large protein containing several loosely structured regions (Table 1), and the minor changes in the biophysical function of this channel may reflect changes in the stoichiometry of the accessory subunits associated with the main subunit. For the Ca2+-ATPase, its key functional property, the specific activity of the pump, is unchanged across mammalian phylogeny (15, 19, 43, 45), whereas its expression levels change significantly.
As described in this report, there was no significant change in the function of the IKs and IKr channels, which control action potential repolarization in larger mammals. The increase in KCNQ1 and KCNH2 mRNA expression is consistent with the increased current expression in guinea pig myocytes (22), although it is likely that other channel subunits also regulate the expression of these currents. Of the channels that are important for action potential repolarization in small mammals, only the Kv1.5 gene is <94% identical in mouse-human sequence comparisons. The relevant comparison for these channels, however, is within the subset of small mammals where these channels are expressed, and in this case sequence identity is much higher (Table 1).
Taken together, the evidence suggests that regulatory evolution is predominant in the evolution of action potential duration in the ventricular myocytes of mammals. A similar conclusion has been made for the evolution of a nonmammalian electrophysiological system, the giant axon of squid (35). This conclusion does not exclude the possibility that one or more channels show some gene-encoded change in functional properties, but clearly the major thrust of evolutionarily mediated modifications is at the level of channel expression.
Whether the graded changes in expression seen for multiple genes, including KCNQ1, KCNH2, SCN5A, and SERCA2, primarily reflect cis-regulatory evolution, as seems most likely for the Kv2.1 and Kv4.2 genes, remains an open question. There are quantitative changes in transcription factor gene expression in the ventricular myocytes from different mammalian species (33), possibly reflecting evolution of the cardiac regulatory network within the mammalian lineage. Determining whether changes in the transcription factor network contribute to the graded changes in ion channel and transporter gene expression will require a more detailed understanding of the regulation of the relevant genes.
Conclusions.
The evolution of ventricular myocyte electrophysiology appears to conform, in broad outline, to the principles derived from the study of developmental system evolution (6, 7, 49). In particular, differences in the functional properties of ion channels and transporters appear to be relatively small compared with the multiple, relatively large changes in channel and transporter expression levels. As demonstrated in this report, action potential repolarization and calcium handling evolve in mammals primarily by changes in the level of expression of the relevant ion channels and transporters, in general agreement with the concept that regulatory evolution is the predominant mechanism underlying the evolution of complex multicellular organisms. Although this issue is confused in the physiological and modeling literature, because multiple and shifting combinations of gene products can contribute to single physiologically identified currents, future efforts in this area should benefit from a recognition of the relative unity of the molecular underpinnings of mammalian cardiac electrophysiology.
GRANTS
Supported by National Institutes of Health Grants HL-28958 (D. McKinnon), NS-29755 (D. McKinnon), HL-084539 (H.-S. Wang), and HL-65299 (M. Sanguinetti) and American Heart Association Grant 0235467T (B. Rosati).
Supplementary Material
Acknowledgments
This work benefited from discussions with John True and his laboratory. The project was facilitated by support from Drs. I. Cohen, C. Malbon, and H.-Y. Wang.
Address for reprint requests and other correspondence: D. McKinnon, Dept. of Physiology and Biophysics, BST Rm. 124, Level 6, Stony Brook Univ., Stony Brook, NY 11794-8661 (e-mail: dmckinnon@notes.cc.sunysb.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol 13: 298–307, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol 476: 279–293, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bezzina CR, Shimizu W, Yang P, Koopmann TT, Tanck MW, Miyamoto Y, Kamakura S, Roden DM, Wilde AA. Common sodium channel promoter haplotype in Asian subjects underlies variability in cardiac conduction. Circulation 113: 338–344, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bru-Mercier G, Deroubaix E, Capuano V, Ruchon Y, Rucker-Martin C, Coulombe A, Renaud JF. Expression of heart K+ channels in adrenalectomized and catecholamine-depleted reserpine-treated rats. J Mol Cell Cardiol 35: 153–163, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Carroll SB Evolution at two levels: on genes and form. PLoS Biol 3: 1159–1166, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll SB, Grenier JK, Weatherbee SD. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Malden, MA: Blackwell Science, 2004.
- 8.Demuth JP, De Bie T, Stajich JE, Cristianini N, Hahn MW. The evolution of mammalian gene families. PLoS ONE 1: e85, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon JE, Shi W, Wang HS, MacDonald C, Yu H, Wymore R, Cohen IS, McKinnon D. Role of the Kv4.3 potassium channel in ventricular muscle: a molecular correlate for the transient outward current. Circ Res 79: 659–668, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Dong M, Sun X, Prinz AA, Wang HS. Effect of simulated Ito on guinea pig and canine ventricular action potential morphology. Am J Physiol Heart Circ Physiol 291: H631–H637, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Elzinga G, Westerhof N. Matching between ventricle and arterial load. An evolutionary process. Circ Res 68: 1495–1500, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res 32: W273–W279, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Targeted deletion of Kv4.2 eliminates Ito,f and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res 97: 1342–13, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Hagiwara S, Miyazaki S, Rosenthal NP. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol 67: 621–638, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton N, Ianuzzo CD. Contractile and calcium regulating capacities of myocardia of different sized mammals scale with resting heart rate. Mol Cell Biochem 106: 133–141, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Hoekstra HE, Coyne JA. The locus of evolution: evo devo and the genetics of adaptation. Evolution 61: 995–1016, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Holt JP, Rhode EA, Kines H. Ventricular volumes and body weight in mammals. Am J Physiol 215: 704–715, 1968. [DOI] [PubMed] [Google Scholar]
- 18.Hoppe UC, Marban E, Johns DC. Molecular dissection of cardiac repolarization by in vivo Kv4.3 gene transfer. J Clin Invest 105: 1077–84, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hove-Madsen L, Bers DM. Sarcoplasmic reticulum Ca2+ uptake and thapsigargin sensitivity in permeabilized rabbit and rat ventricular myocytes. Circ Res 73: 820–828, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Hume JR, Giles W, Robinson K, Shibata EF, Nathan RD, Kanai K, Rasmusson R. A time- and voltage-dependent K+ current in single cardiac cells from bullfrog atrium. J Gen Physiol 88: 777–778, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loughrey CM, Smith GL, MacEachern KE. Comparison of Ca2+ release and uptake characteristics of the sarcoplasmic reticulum in isolated horse and rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol 287: H1149–H1159, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Kamiya K, Opthof T, Yasui K, Kodama I. Density and kinetics of IKr and IKs in guinea pig and rabbit ventricular myocytes explain different efficacy of IKs blockade at high heart rate in guinea pig and rabbit: implications for arrhythmogenesis in humans. Circulation 104: 951–956, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Moulton G, Attwood TK, Parry-Smith DJ, Packer JC. Phylogenomic analysis and evolution of the potassium channel gene family. Receptors Channels 9: 363–377, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Pandit SV, Clark RB, Giles WR, Demir SS. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys J 81: 3029–3051, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA 99: 6210–6215, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto RD, Elson RC, Szucs A, Rabinovich MI, Selverston AI, Abarbanel HD. Extended dynamic clamp: controlling up to four neurons using a single desktop computer and interface. J Neurosci Methods 108: 39–48, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Pond AL, Nerbonne JM. ERG proteins and functional cardiac IKr channels in rat, mouse, and human heart. Trends Cardiovasc Med 11: 286–294, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Popovic ZB, Richards KE, Greenberg NL, Rovner A, Drinko J, Cheng Y, Penn MS, Fukamachi K, Mal N, Levine BD, Garcia MJ, Thomas JD. Scaling of diastolic intraventricular pressure gradients is related to filling time duration. Am J Physiol Heart Circ Physiol 291: H762–H769, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Prothero J Heart weight as a function of body weight in mammals. Growth 43: 139–510, 1979. [PubMed] [Google Scholar]
- 31.Rosati B, Dun W, Hirose M, Boyden PA, McKinnon D. Molecular basis of the T- and L-type Ca2+ currents in canine Purkinje fibres. J Physiol 579: 465–471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosati B, Grau F, Kuehler A, Rodriguez S, McKinnon D. Comparison of different probe-level analysis techniques for oligonucleotide microarrays. Biotechniques 36: 316–322, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Rosati B, Grau F, McKinnon D. Regional variation in mRNA transcript abundance within the ventricular wall. J Mol Cell Cardiol 40: 295–302, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Rosati B, Grau F, Rodriguez S, Li H, Nerbonne JM, McKinnon D. Concordant expression of KChIP2 mRNA, protein and transient outward current throughout the canine ventricle. J Physiol 548: 815–822, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal JJ, Bezanilla F. A comparison of propagated action potentials from tropical and temperate squid axons: different durations and conduction velocities correlate with ionic conductance levels. J Exp Biol 205: 1819–1830, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature 384: 80–83, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt-Nielsen K Scaling: Why Is Animal Size so Important? Cambridge, UK: Cambridge Univ. Press, 1984.
- 39.Sham JS, Hatem SN, Morad M. Species differences in the activity of the Na+-Ca2+ exchanger in mammalian cardiac myocytes. J Physiol 488: 623–631, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi W, Wymore RS, Wang HS, Pan Z, Cohen IS, McKinnon D, Dixon JE. Identification of two nervous system specific members of the erg potassium channel gene family. J Neurosci 17: 9423–9432, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl WR Scaling of respiratory variables in mammals. J Appl Physiol 22: 453–460, 1967. [DOI] [PubMed] [Google Scholar]
- 42.Striessnig J Pharmacology, structure and function of cardiac L-type Ca2+ channels. Cell Physiol Biochem 9: 242–269, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Su Z, Li F, Spitzer KW, Yao A, Ritter M, Barry WH. Comparison of sarcoplasmic reticulum Ca2+-ATPase function in human, dog, rabbit, and mouse ventricular myocytes. J Mol Cell Cardiol 35: 761–767, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Sun X, Wang HS. Role of the transient outward current Ito in shaping canine ventricular action potential—a dynamic clamp study. J Physiol 564: 411–419, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J 389: 151–159, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vornanen M, Ryokkynen A, Nurmi A. Temperature-dependent expression of sarcolemmal K+ currents in rainbow trout atrial and ventricular myocytes. Am J Physiol Regul Integr Comp Physiol 282: R1191–R1199, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Feng ZP, Duff HJ. Glucocorticoid regulation of cardiac K+ currents and L-type Ca2+ current in neonatal mice. Circ Res 85: 168–173, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Westerhof N, Elzinga G. Normalized input impedance and arterial decay time over heart period are independent of animal size. Am J Physiol Regul Integr Comp Physiol 261: R126–R133, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Wray GA The evolutionary significance of cis-regulatory mutations. Nat Rev Genet 8: 206–216, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Wymore R, Gintant GA, Wymore RT, Dixon JE, McKinnon D, Cohen IS. Tissue and species distribution of mRNA for the IKr-like K+ channel, erg. Circ Res 80: 261–268, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Yin L, Bien H, Entcheva E. Scaffold topography alters intracellular calcium dynamics in cultured cardiomyocyte networks. Am J Physiol Heart Circ Physiol 287: H1276–H1285, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev 57: 387–395, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Zobel C, Kassiri Z, Nguyen TT, Meng Y, Backx PH. Prevention of hypertrophy by overexpression of Kv4.2 in cultured neonatal cardiomyocytes. Circulation 106: 2385–2391, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.