Abstract
In this study, we test the hypothesis that there are differential splicing patterns between the expressed oxytocin (OT) and vasopressin (VP) genes in the rat supraoptic nucleus (SON). We quantify the low abundance, intron-containing heteronuclear RNAs (hnRNAs) and the higher abundance mRNAs in the SON using two-step, quantitative SYBR Green real-time reverse transcription (RT)-PCR and external standard curves constructed using synthetic 90 nt sense-strand oligonucleotides. The levels of OT and VP mRNA in the SON were found to be similar, ∼108 copies/SON pair, whereas the copy numbers of VP hnRNAs containing intron 1 or 2 and the OT hnRNA containing intron 1 are much lower, i.e., ∼102–103 copies/rat SON pair. However, the estimated copy number of the intron 2-containing OT hnRNA is much larger, ∼106 copies/SON pair. The relative distributions of all the OT and VP RNA species were invariant and independent of the physiological status of the rats (e.g., osmotically stimulated or lactating rats). Using intron-specific riboprobes against hnRNAs, we demonstrate by fluorescence in situ hybridization strong signals of OT hnRNA containing intron 2 predominantly in the cytoplasm, in contrast to the localization of the VP hnRNA found only in the nuclei. Taken together, these data support the view that the splicing patterns between OT and VP gene transcripts are different and show that there is a selective cytoplasmic retention of OT intron 2.
Keywords: real-time polymerase chain reaction, intron retention, hypothalamus
vasopressin (VP) and oxytocin (OT) genes are expressed in specific neuronal populations in the central nervous system, such as the magnocellular neurons (MCNs) located in the supraoptic nucleus (SON) and paraventricular nucleus of the hypothalamo-neurophypophysial system (HNS). The OT and VP neuropeptides play fundamental roles in the regulation of body fluid and electrolyte homeostasis and reproduction (1, 7). OT and VP gene expression in the HNS has been extensively studied under many physiological conditions and by a variety of methods which measured mRNA levels, e.g., by Northern blots (53) and quantitative in situ hybridization histochemistry (qISHH) (30, 31, 60). One limitation of using OT and VP mRNA measurements to monitor gene expression changes in the HNS is that the steady-state levels of OT and VP mRNA in MCNs are so abundant that they mask the small changes of OT and VP mRNA that occur during short hyperosmotic stimuli (31, 36, 47). This situation typically found in neuroendocrine cells was addressed by the development and successful use of intron-specific probes in qISHH to measure heteronuclear RNA (hnRNA) levels of diverse gene transcripts in neurons (12, 19, 20, 24). Since the pool sizes for specific hnRNAs are usually small and the half-life of hnRNAs are very short (in minutes), compared with the larger pool sizes and much longer half-lives (in hours to days) of mRNAs (2, 13, 14, 56), measurements of hnRNAs, in principle, have a more relevant relationship to ongoing transcriptional activity. Several laboratories have successfully developed and used intron-specific riboprobes to measure OT and VP hnRNA levels by qISHH under different physiological conditions (23–25, 61, 62).
The need for a more quantitative and sensitive method to measure hnRNA levels than by qISSH became apparent to us when we unexpectedly found in a qISSH study of OT hnRNA and mRNA in the SON that a very short intron 2-specific riboprobe designed to detect OT hnRNA was much more effective and reliable than a longer intron 1-specific riboprobe in detecting the OT hnRNA (61). Structural analyses of the secondary structures of the two intronic sequences in the OT hnRNA as well as the secondary structures in the two intronic probes revealed no significant differences. We hypothesized that the most likely explanation for this difference is that intron 1 is excised from the primary OT transcript at a much more rapid rate than the splicing of intron 2, and therefore the amount of intron 1-containing OT hnRNA (OT hnRNA 1) should be much smaller than that of intron 2-containing OT hnRNA (OT hnRNA 2) in the SON. In this study, we test this hypothesis using two-step quantitative, real-time RT-PCR to measure the mRNA, hnRNA 1, and hnRNA 2 levels for both OT and VP in the SONs from adult male and acute osmotic stimulated rats, as well as virgin, pregnant, and lactating female rats.
Quantitative real-time RT-PCR together with external standard curves is a powerful method that allows for rapid and accurate quantification of gene transcripts (35, 52, 54, 63) and has been widely used in studies directed at problems in neuroscience (55), cancer research (10, 18, 22) and molecular diagnostics (17, 38, 57). Virtually all of these studies mainly focus on measuring the steady-state and abundant mRNA levels, and reports about detecting rare gene transcripts, such as hnRNAs, have been sparse. Here, we measure both high abundance transcripts, which are OT and VP mRNA, and low abundance transcripts, OT and VP hnRNAs, in the rat SON using a two-step quantitative, real-time RT-PCR with external standard curves that were generated using 90 nt sense-strand standard oligonucleotides corresponding to the specific transcripts' sequences (8, 9). This approach allowed us to obtain the amplification efficiencies of all the specific PCR primer pairs used in our studies from the corresponding external standard curves. We found that real-time RT-PCR with external standard curves constructed using synthetic 90 nt sense-strand oligonucleotides is a sensitive and reliable method to detect hnRNAs in the rat SON.
We describe in this paper the results of examining OT and VP gene expression under various physiological conditions, and in addition, we present data consistent with the aforementioned hypothesis that the levels of OT hnRNA 2 are greater than those of OT hnRNA 1 under both basal and stimulated conditions. The copy numbers of VP hnRNA 1 and hnRNA 2 are both closer to the level of OT hnRNA 1 than OT hnRNA 2 under the same physiological conditions. The finding that OT hnRNA 2 is orders of magnitude more abundant than the other three hnRNAs suggests that this is occurring by the process of intron retention in the SON. We further examined this hypothesis by doing fluorescence in situ hybridization (FISH) using tyramide signal amplification. In support of this hypothesis, we demonstrate that, in contrast to VP hnRNA, which is exclusively localized in the nucleus, the more intense signals of OT hnRNA 2 detected using the intron 2-specific riboprobe are predominantly found in the cytoplasm of the MCNs. These data further reinforce the hypothesis that there are different splicing patterns between the OT and VP primary transcripts and that there is a selective retention of OT intron 2 in the MCNs.
MATERIALS AND METHODS
Animals.
Adult male and female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used. Animals were individually housed in a temperature-controlled room (21–23°C) with lights on from 7:00 AM to 7:00 PM and given food and water ad libitum. All procedures were carried out in accordance with the guidelines set forth by the National Institutes of Health (NIH) Care and Animal Use Guidelines and were reviewed and approved by the NIH Institutional Animal Care and Use Committee.
Lactating females were killed on day 7 of lactation, and pregnant females were killed on day 19 of pregnancy. These animals and the control adult males and females were killed around 12 noon. For the acute osmotic stimulation experiments, male rats (n = 5 per group) received either an isotonic (0.9% normal saline) or hyperosmotic (1.5 M NaCl) intraperitoneal injection (1 ml solution/100 g body wt) and killed 2 h later.
For the real-time PCR analysis, the rats were decapitated under isoflurane anesthesia, their brains were quickly removed and immediately cooled in ice-cold Hanks' balanced salt solution, and the SONs were rapidly dissected (See below). Brains from the adult male, virgin female, and 7-day lactating rats that were used for the isotopic and fluorescence in situ hybridization studies were frozen on powdered dry ice and stored at −80°C until sectioning.
Tissue isolation and rat SON total RNA purification.
One millimeter coronal slices from each brain were made starting from the front of the optic chiasm using a Jacobowitz brain slicer (Zivic Laboratories, Pittsburg, PA), and the slice, located approximately −0.8 to −1.8 mm posterior to the bregma (40), was placed on a piece of parafilm on a hard black surface. Bilateral punches of SON nucleus were collected with a 14-gauge, stainless steel needle with a flattened end and then transferred to a 1.5 ml tube containing 350 μl lysis buffer for the total RNA extraction. RNA extraction and purification were performed according to the manufacturer's protocol with the RNeasy Micro Kit (Qiagen, Valencia, CA). The tissue was immediately homogenized with a pellet pestle motor (Kontes, Vineland, NJ) after being placed into the lysis buffer, followed by the addition of 70% ethanol to adjust the binding conditions. Then the sample was applied to the RNeasy MinElute Spin Column for adsorption of RNA to membrane. DNase I (Qiagen, Valencia, CA) was freshly prepared and added on the column membrane directly, and the DNase digestion was carried out at room temperature for 15 min. The sample was then washed several times and eluted using 14 μl RNase-free water. This kit combines RNA extraction and DNase treatment in one spin column system and removes the contaminating genomic DNA. This produces a high yield of total RNA, which is particularly important for the detection of low abundance hnRNA species in the total RNA, such as primary transcripts and transcription intermediates. In some experiments, bilateral SCNs were also punched out of the same 1 mm slice and the total RNA was similarly extracted. After the extraction, the concentration of total RNA was measured on a NanoDrop-1000 spectrophometer (NanoDrop Technologies, Wilmington, DE). The average total RNA obtained from a pair of SONs of one animal was 1,621 ng, and the A260/A280 ratios of total RNA from all the animals ranged between 1.9 and 2.1.
Reverse-transcription PCR identification of primary transcripts and intermediate RNA forms of OT and VP gene transcripts.
Three pairs of gene-specific primers for both the OT and VP predicted gene transcripts were designed by using the MacVector 7.0 program (Oxford Molecular, Oxford, UK), and the rat OT and VP gene sequences (GenBank accession no. X59496), respectively. Supplemental Table S1A shows the sequence of the primers with their estimated melting temperature (Tm) values and the predicted sizes of their PCR products.1 These primers were used to amplify the OT or VP cDNAs from primary transcripts (RNA species containing both intron 1 and intron 2) and intermediate RNA forms (e.g., hnRNA 1, or hnRNA 2). The terms hnRNA 1 or hnRNA 2 of OT or VP refer to partially spliced RNA transcripts containing only intron 1 or intron 2, respectively (see Fig. 1). BLAST results show that these primers are specific for the OT or VP genes. The reverse transcription primers for these experiments are shown in Supplemental Table S1B. The OT and VP intron-specific primers in Supplemental Table S1B that are located in intron 2 of the OT or VP transcripts would in principle reverse transcribe either the primary transcripts or hnRNA 2. The OT and VP gene specific primers located in the 3′-untranslated region (UTR) of the OT or VP transcripts would reverse transcribe the primary transcripts or hnRNA 1.
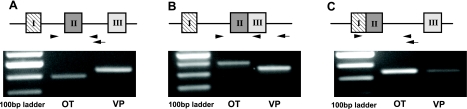
Fig. 1.
Gel analysis of reverse-transcription PCR products of oxytocin (OT) and vasopressin (VP) primary transcripts and transcription intermediates from the rat supraoptic nucleus. Reverse-transcription PCR products were loaded on to a 1.5% agarose gel containing ethidium bromide for gel electrophoresis. The arrows in the schematic diagrams in A–C show the binding sites of the specific primers for reverse transcription. The arrowheads in the panels show the binding sites of the PCR primer pairs to the predicted cDNAs derived from the OT and VP transcripts in the rat SON. A: RT-PCR products derived from the OT and VP primary transcripts. B: RT PCR products derived from transcription intermediates containing only intron 1 of the OT or VP gene. C: RT-PCR products derived from the transcription intermediates containing only intron 2 of the OT or VP gene. The strongest band in the 100 bp ladder marker is 500 bp. The primers used for the reverse transcription and the PCR, as well as the predicted amplicon lengths are shown in Supplemental Table S1. All the products have the predicted sizes of the RNA forms.
Reverse transcription was performed using 200 ng total RNA extracted and purified from the SON or SCN of each animal, 2 pmol of either OT or VP primers, and 200 units of SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) in a total volume of 20 μl. The reaction was incubated at 65°C for 5 min followed by incubation at 55°C for 60 min. cDNA synthesis was followed by RNase H treatment (Invitrogen). A reverse transcription negative control for each sample to detect the genomic DNA was performed simultaneously and underwent the same procedures but without SuperScript III Reverse Transcriptase.
PCR was performed using Platinum Taq DNA polymerase (Invitrogen) according to the company's recommended reaction conditions, except for the annealing temperature, which was determined by the MacVector 7.0 program. Six microliters of the PCR products were loaded and analyzed on 1.5% agarose gel. The bands of the PCR products with the right sizes were purified and sequenced (Macrogen, Rockville, MD).
Design of primers for real-time PCR and gene-specific reverse transcription.
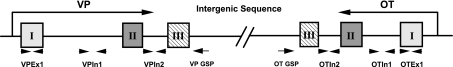
Based on the DNA sequences of the OT and VP genes, three pairs of primers for each gene were designed to amplify the specific cDNA representing the OT and VP transcripts containing exons and/or different introns derived from the SON total RNA, which are named OTex1, OTin1, OTin2, VPex1, VPin1, and VPin2 and illustrated diagrammatically in Fig. 2. The quantifications of these specific PCR products are related to the levels of OT mRNA, OT hnRNA 1, OT hnRNA 2, VP mRNA, VP hnRNA 1, and VP hnRNA 2 in the rat SON total RNA extracts. BLAST results show that these primers are specific for the OT or VP gene. The sequences of these six pairs of primers are shown in Supplemental Table S2, with their estimated Tm values and lengths of PCR products. The Tm values of the forward and reverse primers in each pair are similar, which is critical for qPCR (8).
Fig. 2.
Schematic diagram of the rat OT and VP genes illustrating the real-time PCR and reverse transcription primer binding sites. The OT and VP genes are located in the same chromosome in a tail-to-tail configuration and each has 3 exons (I, II, and III) separated by 2 introns. The arrows below the genes denote localizations of the gene-specific primers (GSP) for the reverse transcription of the different OT and VP RNA species, such as mRNA and hnRNA. The locations of the primers used for the real-time PCR of the various OT and VP RNA species are denoted by arrowheads. The sequences of forward and reverse primers for the real-time PCR are shown in Supplemental Table S2.
The OT and VP gene specific primers used for the reverse transcription in the real-time PCR experiments are shown in Supplemental Table S1B. The binding sites of both primers are located in the 3-UTR of the OT or VP gene but are upstream of their poly-A region and can be used to simultaneously reverse-transcribe both mRNAs and hnRNA (including primary and intermediate transcripts) of the OT and VP genes. The gene-specific primer can provide the greatest sensitivity for quantitative assays compared with random primer and oligo-dT primer (9) and would not reverse transcribe any free-standing excised introns.
Design of 90 nt single-strand sense oligonucleotides for standard curves.
In this study, synthesized single-strand sense oligonucleotides were used to generate standard curves specific for each kind of real-time PCR product. The general method is described elsewhere (8, 37) and was used here with minor variations. Briefly, the 90 nt single-strand oligonucleotides were designed according to the anticipated DNA sequences of the real-time PCR products derived from each primer pair. The sequences of the 90 nt oligonucleotides are shown in Supplemental Table S3, having the same primer sequences on the two ends and similar GC content compared with the sequences of the corresponding PCR products. All of these oligonucleotides were commercially synthesized and purified by polyacrylamide gel electrophoresis (Sigma, St. Louis, MO).
Real-time PCR.
The real-time PCR was performed using the QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA) on a Smart Cycler instrument (Cepheid, Sunnyvale, CA). The amplification mixture included 12.5 μl of 2× QuantiTect SYBR Green PCR Master Mix, 0.3 μM each primer for OT or 1.0 μM each primer for VP, 2 μl of cDNA preparation from SON RNA (the cDNA generated from 20 ng SON total RNA), or 2 μl of diluted 90 nt oligonucleotides, and an appropriate volume of DNase and RNase-free water to a final volume of 25 μl. The real-time PCR using the diluted 90 bp oligonucleotides as template were repeated at least four times for each dilution. The optimal PCR cycling conditions included one cycle of initial activation at 95°C for 15 min, followed by 45 cycles for OT and 50 cycles for VP of denaturing at 95°C for 15 s, annealing at 61°C for OT and 60°C for VP for 15 s, and extension at 72°C for 15 s. The melting curve and specific Tm for each PCR product was generated and was used to check product uniformity and specificity. In preliminary experiment, some PCR products were randomly selected and run on 1.2% agarose gel to verify the reliability of the melting curves and temperature.
Standard curve construction and quantification analysis of real-time PCR.
The standard curve method used in this study has been described elsewhere (37), with minor variations. Briefly, the synthesized oligonucleotides were diluted with DNase and RNase-free water in 10-fold serial dilutions over a range that spanned the sample concentrations, and the real-time PCR was performed to the get the cycle threshold (Ct) values for the diluted oligonucleotides (Supplemental Fig. S1). The standard curves for real-time PCR using OT- and VP-specific primers were constructed by plotting the Ct values from serially diluted oligonucleotide solutions against the logarithm of the initial oligonucleotide template amount using StatView 5.0 software (SAS Institute, Cary, NC). For each pair of primers, the linear standard curve was determined from five serial oligonucleotide dilutions to get a wide range of Ct values and to allow direct quantification of every sample of rat SON RNA (Supplemental Fig. S2). The dilution ranges of each oligonucleotides were determined by preliminary real-time PCR data of cDNA from rat SON total RNA and diluted oligonucleotides. Using the slope value in the equation, we calculated the efficiency by the formula E = 10−1/slope − 1 (58). If the slope value is in the range from −3.3 to −3.8, the efficiency will be close to 1, which indicates the PCR is efficient. Supplemental Figure S2 shows that the amplification efficiencies (E) for all primer pairs are ∼1. For the OT gene, the amplification efficiency of OT intron 1 is higher than that of OT exon 1, and the latter is higher than OT intron 2. Among the three pairs of primers for the VP gene, VP intron 2 has the highest amplification efficiency, which is ∼1.1, and the efficiencies for VP intron 1 and VP exon 1 are very similar, which are both ∼0.98. Most of the slope values of these six standard curves are in the range from −3.3 to −3.8, which means the real-time PCR for all these six pairs of primers were performed efficiently and can be used to detect the amounts of the OT and VP RNA species in the rat SON total RNA.
The Ct value of the real-time PCR for cDNA from each rat SON RNA sample was put in the corresponding equation drawn from the corresponding standard curve, and the amount of oligonucleotides at the same Ct value was calculated and expressed as copy numbers, which are theoretically equivalent to the copy numbers of the starting cDNA templates from 20 ng of rat SON total RNA, and this was corrected by using the amount of total RNA present in the SON pair of the rat. The copy numbers of the genomic DNA were calculated using the Ct values from every rat SON sample and were subtracted from the copy numbers of the starting cDNA template to get the relative starting copy numbers of the transcript RNAs (mRNA or hnRNA) in the SON pair of each rat.
Isotopic in situ hybridization histochemistry and FISH using tyramide signal amplification.
The isotopic in situ hybridization histochemistry (ISHH) protocol used here, including the tissue sectioning, labeling of riboprobes, hybridization conditions, and phosphoimaging procedures to detect VP heteronuclear RNA and mRNAs in hypothalamic brain slices, is the same as we have described elsewhere (61).
For the FISH, four different riboprobes were used. These include an OT intron 2-specific probe, an OT exon-specific probe, and the VP intron-specific and VP exon-specific probes, which we have previously used and described elsewhere (61). Digoxigenin (DIG) labeling of these probes was done per the manufacturer's instructions (Roche Diagnostics, Indianapolis, IN) using PCR amplified VP (30 ng) and OT (100 ng) sequences as a template. Overnight hybridization at 55°C was done using 2 μl out of the 100 μl purified DIG-labeled riboprobe per slide in a total of 80 μl hybridization solution (same as the hybridization solution used in the ISHH hybridization). The slides were then washed as previously described (61), the endogenous peroxidase was inhibited by incubation in DAKO peroxidase blocker (Dako North America, Carpinteria, CA) at room temperature for 10 min, processed for the DIG labeling probe by incubating in 1:500 of anti-DIG-HRP (Roche Diagnostics) made in 1× TBS (Tris-buffered saline: 0.1 M Tris·Cl pH 7.5, 150 mM NaCl) containing 5% normal goat serum (Vector, Burlingame, CA) at 4°C overnight, and amplified in the 1:20,000 FITC Tyramide [kindly provided by Dr. Eva Mezey, National Institute of Dental and Craniofacial Research, National Institutes of Health (NIH)] in 10 ml 0.1 M Tris·HCl (pH 8.0) with one tablet of hydrogen peroxide-urea (Sigma-Aldrich) at room temperature for 10 min for the OT and VP exon-specific probes and the OT intron-specific probe. For the VP intron-specific probe, the slides were developed in the 1:500 fluorophore-labeled tyramide in 1xplus amplification diluent from the TSA Plus fluorescence kit (PerkinElmer, Waltham, MA). After being rinsed in 1× TBS buffer, only the slides hybridized with the OT intronic probe were incubated in 1:500 of antifluorescence-horseradish peroxidase (Roche Diagnostics) in the 1× TBS with 5% normal goat serum at 4°C overnight and amplified in the 1:20,000 FITC Tyramide for the second time, because the OT intronic probe is very short, and signals generated from this probe are either very weak or not visible after a one-time amplification. Finally, the slides with OT intronic probe were stained with 1:50,000 DAPI at room temperature for 1 min, and the same was done for the other OT and VP intronic and exonic probes. The sections were viewed in a Leica DMR6000 fluorescence microscope, and pictures were taken with a Hamamatsu camera and Volocity software (Improvision, Lexington, MA). High-resolution images were analyzed using NIH Image software, version 1.35.
Statistical analyses.
The statistically significant differences of OT and VP hnRNA or mRNA relative copy numbers in the rat SON among groups were calculated either by ANOVA followed by Fisher's PLSD test, using the Statview 5.0 (SAS Institute, Cary, NC) program, or by a two-tailed Mann-Whitney/Wilcoxon U-test. Differences between groups were considered statistically significant when P < 0.05. Results are expressed as copy number per animal or percentage of control (means ± SE).
RESULTS
Identification of OT and VP primary transcripts and transcription intermediates by reverse-transcription-PCR.
To obtain evidence that the primary transcripts and specific transcription intermediates of OT and VP gene expression are in fact present in the total RNA extracted from the rat SONs, we identified the OT and VP primary transcripts and hnRNA forms by qualitative RT-PCR. Figure 1 shows the predicted RT-PCR products (i.e., cDNAs derived from putative OT and VP primary transcripts and hnRNAs) on ethidium bromide-stained agarose gels after electrophoresis. Figure 1A shows the PCR products derived from the OT or VP primary transcripts. Figure 1B shows the PCR products derived from OT or VP hnRNA 1, i.e., the transcription intermediates containing only intron 1. Figure 1C shows the PCR products derived from the OT or VP hnRNA 2, i.e., the intermediate RNA forms containing intron 2, but no intron 1. All the DNA sequencing results for the OT and VP primary transcripts, hnRNA 1, and hnRNA 2 PCR products that validate their identities are shown in Supplemental Figs. S3–S8. BLAST results of the sequenced PCR products confirm that they are the predicted sequences.
Evaluation of changes of OT and VP mRNAs and hnRNAs in the SON after acute hyperosmotic stimulation in vivo using real-time RT-PCR.
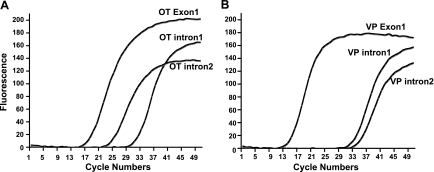
In a previous study, using quantitative ISHH (qISHH), we reported that there was an increase in VP hnRNA but not OT hnRNA in the SON, following an acute hyperosmotic stimulus produced by intraperitoneal salt injection (61). This is in contrast to the situation of chronic salt loading during which both the OT and VP hnRNA are greatly increased (62). In both of these qISHH studies, we were only able to use a riboprobe directed against intron 2 of the OT gene, since all our efforts to construct an effective riboprobe against OT intron 1 for qISHH were unsuccessful. It is not clear why the very short intron 2 sequence provides a better riboprobe for detecting OT hnRNA in the MCNs by qISHH than the longer riboprobes that targeted intron 1. However, we wanted to determine if these observations of a refractory response of OT gene expression to acute hyperosmotic stimuli found for OT intron 2 also held true for OT intron 1, and hence, we tried to quantitatively measure the VP and OT hnRNAs using a real-time RT PCR assay. In the present study, we used the quantitative real-time RT-PCR method described here in which effective primers (Supplemental Table S2) and standards (Supplemental Table S3) could be synthesized and used for all four introns. The same OT or VP PCR primers used to amplify the synthesized 90 nt oligonucleotides described above for the construction of the standard curves (Supplemental Table S3, Fig. S1) were also used to amplify the OT and VP gene-specific reverse-transcribed cDNAs from the rat SON total RNAs. Figure 3 shows representative fluorescence growth curves of OT (Fig. 3A) and VP (Fig. 3B) real-time PCR products using a SON total RNA sample from the same rat. From these growth curves, we can see that OT exon 1-bearing RNA has a much lower Ct value compared with that of OT intron 2, and that the Ct value of OT intron 2 is much lower than OT intron 1 in the same rat (Fig. 3A). The Ct value of VP exon 1 is also much lower than those of both VP intron 1 and intron 2, but in contrast to OT, the Ct values between VP intron 1 and VP intron 2 (Fig. 3B) are close to each other. This difference in the presence of intron 2 vs. intron 1-bearing hnRNA levels between the OT and VP genes was found under all the physiological conditions that we studied (see Table 1). For most of the samples described in this paper, no genomic DNA contamination of the RNAs was detected up to 50 cycles, and when DNA was detected, the Ct values for the genomic DNA contaminants were at least eight cycles greater than those of the corresponding RNAs in these samples. Therefore, the Ct values from genomic DNA are sufficiently out of the range of the RNA analyses, and would not influence the RNA quantifications derived from the standard curves.
Fig. 3.
Illustrations of real-time PCR of OT and VP RNAs using cDNA derived from the total RNA of the same rat SON. Representative real-time PCR growth curves for OT (A) and VP (B) specific PCR primers using cDNA template from total RNA of the same rat SON are shown. A: from the growth curves, we can see that the real-time PCR product from OT exon 1 has a lower cycle threshold (Ct) value compared with those of OT intron 1 and 2. Note that the Ct value of OT intron 2 is significantly lower than OT intron 1, even though the cDNA template used here from the same rat total RNA. B: in the same rat SON total RNA sample, the Ct value of VP exon 1 is much lower than those of both VP intron 1 and intron 2, but the Ct values between VP intron 1 and intron 2 are similar to one another. These differences in OT and VP hnRNA patterns were found in all the SON total RNA samples that we studied (See also Figs. 5 and 6).
Table 1.
Comparisons of ratios of OT and VP mRNA and hnRNA values between male, virgin, pregnant, and lactating female rats
| Conditions | n | OT mRNA/OT mRNA* | OT hnRNA1/OT hnRNA1† | OT hnRNA2/OT hnRNA2‡ | OT hnRNA2/OT hnRNA1§ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. Ratios of OT mRNA and hnRNA contents under various physiological conditions | ||||||||||
| Female control | 5 | 1.0 | 1.0 | 1.0 | 831 | |||||
| Pregnant, 19 days | 5 | 1.12 | 0.54 | 0.88 | 1370 | |||||
| Lactating, 7 days | 4 | 3.56 | 4.64 | 1.7 | 305 | |||||
| Male control | 5 | 1.24 | 1.06 | 0.55 | 431 | |||||
| Conditions | n | VP mRNA/VP mRNA* | VP hnRNA1/VP hnRNA1† | VP hnRNA2/VP hnRNA2‡ | VP hnRNA1/VP hnRNA2§ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. Ratios of VP mRNA and hnRNA content under various physiological conditions | ||||||||||
| Female control | 5 | 1.0 | 1.0 | 1.0 | 8.86 | |||||
| Pregnant, 19 days | 5 | 1.10 | 1.14 | 1.25 | 8.04 | |||||
| Lactating, 7 days | 4 | 2.37 | 3.60 | 3.62 | 8.81 | |||||
| Male control | 5 | 1.30 | 1.59 | 1.28 | 11.0 | |||||
Ratios compared with oxytocin (OT) mRNA (in A) or vasopressin (VP) mRNA (in B) in the female controls (whose ratio equals one).
Ratios compared with OT heteronuclear (hn) RNA1 (in A) or VP hnRNA1 (in B) from the female control (whose ratio equals one).
Ratios compared with OT hnRNA2 (in A) or VP hnRNA2 (in B) from the female control (whose ratio equals one).
Ratios of ratios of OT hnRNA2 compared with OT hnRNA1(in A) or VP hnRNA2 compared VP hnRNA1 (in B) in the group of animals under the same physiological condition.
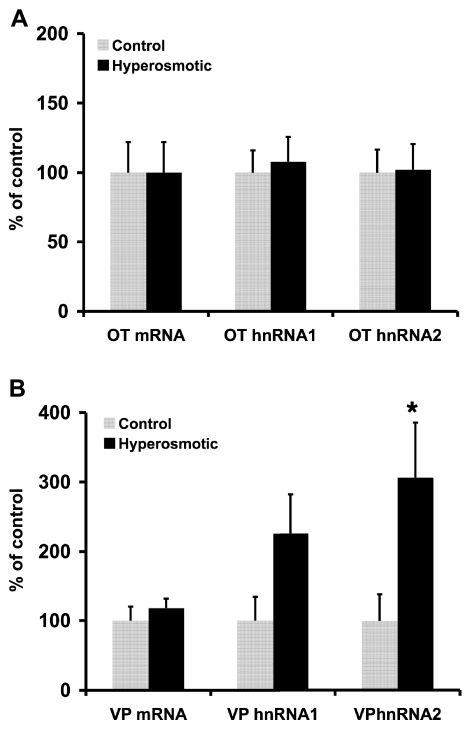
Given that we could use these real-time RT-PCR primers and standard curves to measure the amounts of OT and VP mRNA and different hnRNAs in the rat SON under various physiological conditions, we first used this method to study the changes in OT and VP hnRNAs and mRNAs in the SON after acute hyperosmotic stimulation. These data are shown in Fig. 4. Compared with the RNAs in the control rat SONs, both intron-specific assays indicate an increase in VP hnRNA following an acute salt load; however, only the VP hnRNA 2 level increase was statistically significant (P < 0.05). It is notable that under the same conditions, the levels of OT hnRNA 1, OT hnRNA 2, OT mRNA, and VP mRNA were unchanged (Fig. 4). Therefore, acute hyperosmotic stimulation significantly increases only VP hnRNA in the SON, indicating that VP but not OT gene transcription is increased under this physiological condition. Therefore, these data both support the conclusions previously reported using qISHH (61) and extend those conclusions by providing a more complete quantitative analysis of the hnRNA response of the MCNs in the SON by evaluating all four hnRNAs in response to the acute hyperosmotic stimuli. This further suggests that there are different signal-transcription coupling mechanisms influencing OT and VP gene expression responses to hyperosmotic stimuli (62).
Fig. 4.
Comparisons of OT and VP hnRNA and mRNA levels in supraoptic nuclei (SONs) of acutely hyperosmotically stimulated male rats vs. SONs in isotonic controls. Real-time PCR analysis of the various OT and VP RNA species in SON total RNA extracted from male rats that had received an acute hyperosmotic injection of 1.5 M NaCl (1% body wt) (n = 5), were compared with real-time PCR values of SONs from control males, which had received an isotonic saline injection of equal volume (n = 5). Changes in the OT hnRNAs and mRNA are shown in A, and in VP hnRNAs and mRNA in B. The acute osmotic stimulus produced significant increases only in VP hnRNA 2 (P < 0.05) and increases in VPhnRNA 1 but did not effect the expression of either OT hnRNA 1, OT hnRNA 2, OT mRNA, or VP mRNA. Data are presented as percentages of isotonic control. *Significantly different from male isotonic control, P < 0.05.
Steady-state OT and VP hnRNA and mRNA levels in SONs of normal male, and normal, lactating, and pregnant female rats as determined by real-time PCR.
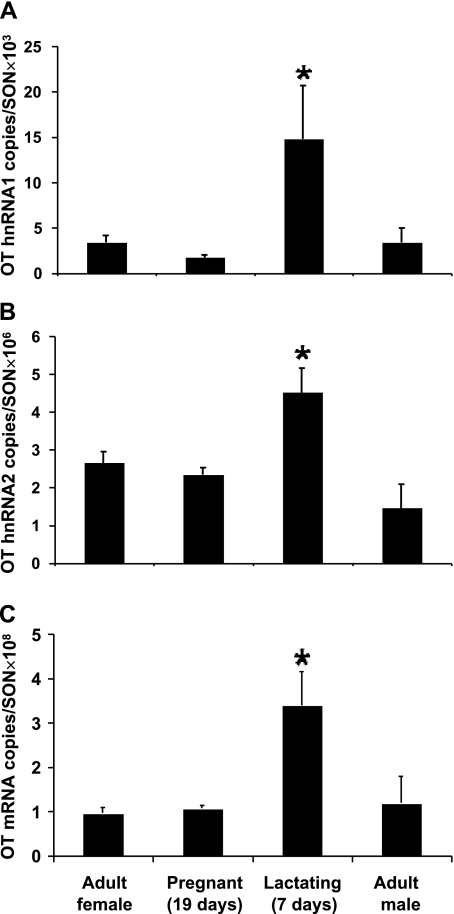
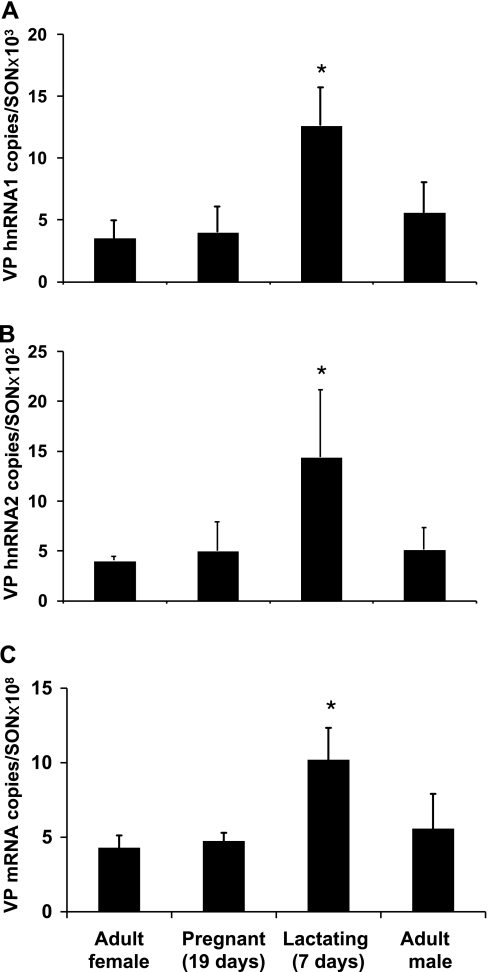
Quantitative real-time RT-PCR studies were made using SON total RNA from adult male and female rats, 19-day pregnant rats, and 7-day lactating rats, and the results of these studies are illustrated in Figs. 5 and 6. As described earlier, the relative copy numbers of OT and VP transcripts in the rat SON were calculated using the Ct values from the real-time PCR and the equations drawn from the standard curves (see methods). For the OT gene in the adult male or female rat, the relative copy number of OT mRNA is ∼108/SON pair (Fig. 5C) is much larger than that of OT hnRNA 2, which is ∼106/SON pair (Fig. 5B). Interestingly, the copy number of OT hnRNA 2 is much greater than OT hnRNA 1, which is ∼103/SON pair (Fig. 5A). Compared with the adult female, lactation caused a significant increase in OT hnRNA 1 (P < 0.05), OT hnRNA 2 (P < 0.05) and OT mRNA (P < 0.05). However, none of these three transcripts changed significantly in the 19-day pregnant rats (P > 0.05 in each case). The copy number of VP mRNA in the adult male and female rat SON is ∼108/SON pair (Fig. 6C), which is very similar to that of the OT mRNA levels, but much bigger than those of VP hnRNA 1 (Fig. 6A) and VP hnRNA 2 (Fig. 6B). Unlike the situation for the OT RNAs, the copy numbers between VP hnRNA 1 and hnRNA 2 are similar, which is ∼103/SON pair. Lactation increased VP hnRNA 1, VP hnRNA 2, and VP mRNA expression compared with the adult females. As was the case for the OT gene, the expression of VP mRNA and hnRNAs did not change significantly in the 19-day pregnant rats compared with the adult females.
Fig. 5.
OT hnRNA and mRNA levels in the SONs of adult male, and adult (virgin), pregnant, and lactating female rats. Real-time PCR was performed using primers for the detection of transcripts containing OT intron 1, OT intron 2 and OT exon 1 to measure the levels of OT heteronuclear RNA (hnRNA 1) (A), OT hnRNA 2 (B), and OT mRNA (C), respectively, in the SON total RNA from adult female (n = 5), 19-day pregnant (n = 5), 7-day lactating female (n = 4), and adult male (n = 5) rats. The data are expressed as copy numbers of RNA in SON pair per rat. As expected, the OT mRNA levels is much greater than the hnRNAs levels, and the OT hnRNA 2 are orders of magnitude greater than OT hnRNA 1. Compared with the adult female controls, only the lactating rats showed significant increases in OT hnRNA 1 (P < 0.05), OT hnRNA 2 (P < 0.05), and OT mRNA (P < 0.001) levels. *Significantly different from adult female control, P < 0.05.
Fig. 6.
VP hnRNA and mRNA levels in the SONs of adult male, and adult (virgin), pregnant, and lactating female rats. Real-time PCR was performed using primers for the detection of transcripts containing VP intron 1, VP intron 2, and VP exon 1 to measure the levels of VP hnRNA 1 (A), VP hnRNA 2 (B), and VP mRNA (C), respectively, in the SON total RNA from adult female (n = 5), 19-day pregnant (n = 5), 7-day lactating (n = 4), and adult male (n = 5) rats. The data are expressed as copy numbers of RNA in SON pair per rat. As expected, the VP mRNA level is much greater than the VP hnRNAs levels, but the VP hnRNA 1 and hnRNA 2 levels are much closer compared with the OT transcription pattern. Compared with the adult female control, only lactation significantly increased both VP hnRNA 1 (P < 0.05) and VP mRNA (P < 0.05) expression, and VP hnRNA 2 (P = 0.053) also increased and was close to but did not reach statistical significance. *Significantly different from adult female, P < 0.05.
The ratios of OT and VP mRNA and hnRNA contents are compared in Table 1. In the rat SON, the copy number of OT mRNA is ∼100-fold greater than the OT hnRNA 2, and OT hnRNA 2 is 100 to 1,000-fold higher than OT hnRNA 1. The VP mRNA copy number is five orders of magnitude greater than that of VP hnRNA 1, and the VP hnRNA 1 is only eightfold greater than VP hnRNA 2. The relatively large amount of steady-state OT hnRNA 2 in the SON compared with all the other hnRNA forms, under all the different physiological conditions studied, suggests that incompletely spliced transcripts still containing intron 2 are present and that a process of OT intron 2 retention may be occurring in the OT MCNs (see discussion).
Locations of OT and VP hnRNA and mRNA in the magnocellular neurons of rat SON.
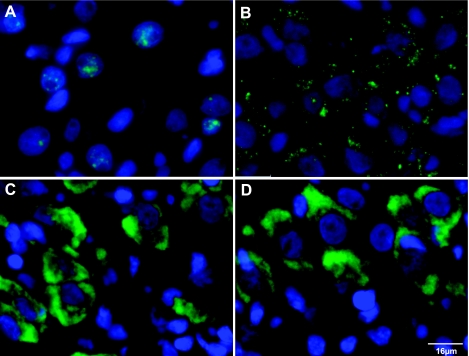
Using an OT intron 2-specific riboprobe, a VP intron 1-specific riboprobe, and OT and VP exon-specific riboprobes, we were able to detect and topographically localize the OT and VP hnRNAs and mRNAs in the MCNs in the rat SON. These data are illustrated in Fig. 7. The sections hybridized with the VP intron-specific riboprobe shows the green signals from VP hnRNA are exclusively located in the nuclei, which are stained blue by DAPI (Fig. 7A). In contrast, the OT hnRNA 2 is predominantly localized to the cytoplasm (Fig. 7B). These data are consistent with the hypothesis that there is selective retention of OT intron 2 in the MCNs and shows that this partially spliced OT hnRNA is largely present in the cytoplasm of the MCNs. For comparison, the cytoplasmic locations of the VP and OT mRNAs are shown in Fig. 7, C and D, respectively.
Fig. 7.
Localization of OT and VP mRNA and hnRNA in the rat supraoptic nucleus by fluorescence in situ hybridization (FISH). A: representative localizations of VP hnRNA as visualized by FISH using VP intron-specific riboprobe. The blue signals show the nuclei stained by DAPI. The green signal reflecting the VP hnRNA is localized only to the nuclei of the magnocellular neurons. B: illustration of OT hnRNA localization using an OT intron 2-specific riboprobe targeted to OT intron 2. The intense green FISH signals are predominantly located in the cytoplasm. C: VP mRNA localizations using a VP exon-specific probe show intense FISH signals in the cytoplasm. D: OT mRNA localizations using OT exon-specific probe show intense FISH signals in the cytoplasm. The scale bar is equal to 16 μm.
DISCUSSION
In the present study, we used two-step quantitative real-time RT-PCR to measure OT and VP mRNAs and hnRNAs in the SONs of rats under a variety of physiological conditions. We found that by using external standard curves together with two-step real-time RT-PCR we could reliably measure low abundance hnRNA transcripts in the rat SON. We used gene-specific reverse transcription in these procedures to measure and compare the copy numbers of OT and VP mRNA and hnRNAs from the same total RNA SON sample. The use of gene-specific primers for the reverse transcription provides the greatest sensitivity and specificity for the quantitative assays compared with random primers and oligo-dT primers in the two-step real-time PCR method (9). The cDNAs derived from the gene-specific primer upstream of the poly-A sequence should reflect all the different RNA components, such as cDNAs from primary transcripts, splicing intermediates (hnRNA 1 and hnRNA 2), and mRNAs. There are two ways to prepare external standard curves to determine copy numbers for each transcript in a given cell or tissue (8). The first involves in vitro-transcribed sense RNA from a plasmid with real-time PCR targeting sequence, and the second is use of single-stranded sense-strand oligonucleotides. Here, we used the single-stranded sense-strand oligonucleotide method to construct the standard curves. Earlier comparisons of the amplification efficiencies derived from standard curves prepared from T7-transcribed RNA vs. sense oligonucleotides standards found these methods to yield virtually identical results and therefore the sense oligonucleotides standard approach has been accepted as a legitimate alternative to the in vitro-transcribed standard RNA approach (8). For example, the oligonucleotide standard curve approach has been successfully used in quantifying HHV-6B genes (37).Therefore, in this paper we estimated copy numbers of the various transcript forms studied here by using oligonucleotide standard curves.
Comparisons between qISHH and quantitative real-time RT-PCR in studies of the rat SON.
Re-evaluation of the OT and VP gene expression responses to acute hyperosmotic stimulation initially determined by qISHH (61) was done by the real-time RT-PCR methods described in this paper. In the previous ISHH study (61), it was reported that there was a selective increase in VP hnRNA but not OT hnRNA after acute osmotic stimuli, although both neuropeptides are known to be robustly secreted in response to this stimulus. Since this finding was unexpected, and in those studies only an intron specific probe to OT intron 2 was adequate to measure the changes in OT hnRNA levels, we decided to re-examine these data using both intron 1- and intron-2 specific primers for the OT and VP genes in quantitative real-time RT-PCR assays. The data in Fig. 4 show that the conclusions based on the qISHH data are confirmed by the real-time RT-PCR experiments and also are supported by the results of the latter experiments when both the intron 1- and intron 2-specific primers were used. Therefore, both the qISHH data (61) and the present quantitative RT-PCR data show that VP gene transcription is increased during acute hyperosmotic perturbation, whereas OT transcription is not. This is in contrast to the situation obtained after 2 days of hyperosmotic stimulation (62).
Another comparison between qISHH and quantitative real-time RT-PCR data that we evaluated relates to the observation is that there is an apparent discrepancy between VP hnRNA and VP mRNA levels in the SON and suprachiasmatic nucleus (SCN). It is well known that the VP MCNs in the SON synthesize large quantities of VP peptide, which they transport to, store in, and secrete from the posterior pituitary into the blood. In contrast, the smaller VP-synthesizing neurons in the SCN synthesize, store, and secrete much less VP neuropeptide within the nervous system in a circadian pattern (7). Consistent with these observations, other studies show that the VP mRNA levels in the SON are substantially higher than in the SCN (60). This is easily visualized by the differences in ISHH signals seen in these two nuclei when an exon-specific probe is used to detect VP mRNA (Supplemental Fig. S9, top right). Interestingly, when ISHH is done using a VP intron 1-specific riboprobe in the same brain at the circadian peak time of VP transcription in the SCN, the VP hnRNA levels in the SON and SCN appear to be similar (Supplemental Fig. S9, bottom right). To quantify these observations, we punched out the bilateral SON and SCN and determined the copy numbers of the VP mRNAs and hnRNAs in these nuclei using quantitative real-time RT-PCR technique (see methods). The results of these measurements are presented in Supplemental Table S4 and show that the SON contains 16-fold more VP mRNA than the SCN (P = 0.03). In contrast, the SON hnRNA/SCN hnRNA ratio is 2.8-fold, but this difference is not significantly different between the two nuclei (P = 0.41).
Thus, the quantitative real-time RT-PCR data in Supplemental Table S4 are consistent with the qualitative ISHH data presented in Supplemental Fig. S9. These data support the view that at the circadian peak time of VP gene expression in the SCN, the hnRNA levels in the SCN and SON are similar, in contrast to their mRNA levels, which are dramatically different. This apparent discrepancy reinforces the view that the hnRNA level primarily reflects transcription rates, whereas the mRNA reflects both transcriptional and mRNA degradative processes, which are more dynamically regulated in the SCN than in the SON.
No rigorous quantitative comparison has ever been made between these two nuclei with respect to their VP hnRNA levels, but consistent with our findings, a previous study using nuclear run methods showed equivalent levels of VP gene transcription in the light phase SON and SCN (11). Another group that used a novel standardization approach in ISHH measured copy number/μm3 of mRNA measured in the SON and SCN and showed that the ratio of the SON/SCN mRNAs was equal to 14.7 (60). This ISHH determined value is in excellent agreement with the SON/SCN ratio of 16 obtained from the studies in this paper using real-time RT-PCR (Supplemental Table S4). No standardized qISHH data are available to compare to the SON/SCN VP hnRNA ratio of 2.8 that we found by quantitative real-time RT-PCR (Supplemental Table S4). However, the latter difference on VP hnRNA between the two nuclei was not statistically significant, and this finding is consistent with the interpretation of comparable levels of VP hnRNA drawn from the visual inspections of the ISHH data in Supplemental Fig. S9 (bottom right). Thus, comparisons of copy number estimates between the same transcript species (e.g., mRNA or hnRNA) appear to be comparable in qISHH and RT-PCR methods.
Comparing copy numbers between different transcript species obtained by using different methods may be problematic. From the qISHH studies the ratio of VP mRNA/VP hnRNA 1 copy numbers in the rat SON was reported to be 40 (60). In a recent, single-step real-time PCR study from our laboratory, it was reported that this ratio was 439 (41), and in the present study, we find that this ratio is 1.29 × 105. Hence, we conclude that while these estimates of relative copy numbers can be legitimately compared between measurements of levels of the same RNA species (e.g., hnRNAs or mRNAs), comparisons between different RNA species and derived by different methods do not appear to be comparable. This may be due to differences in efficiencies of the probe hybridization and RT steps in ISHH and RT-PCR, respectively, for the low abundance RNA species (e.g., hnRNAs) and higher abundance RNA species (e.g., mRNAs). However, general changes in either hnRNA or mRNA evaluated by the same probes and methods can be compared using these calculated copy numbers.
OT and VP gene expression and reproductive status.
An unresolved biological issue that we addressed in this study is the question whether OT gene expression is increased in the MCNs in the SON during pregnancy. In a comprehensive review about the OT MCN system and pregnancy, it was noted that there was no consensus in the literature as to whether OT mRNA or OT hnRNA expression in the SON is altered during pregnancy (see pp. 41–43 in Ref. 44). A variety of assays to assess OT mRNA levels have been used to try and answer this question including liquid-phase hybridizations (64); slot blot (50) and Northern blot (26) hybridizations; and qISHH (33, 45). Compared with diestrus or virgin female rats, pregnant female rats (from 16–21 days of pregnancy) were both reported to have from 30 to 300% OT mRNA increases in pregnancy in some reports (26, 32, 33, 45, 53, 64) to no changes at all in other reports (6, 16, 50). In only one study in the literature was a probe to OT hnRNA used in ISHH, and this led to a report of no increase in pregnancy, but the OT hnRNA did appear to increase in the SON 2 days after birth (16).
Given the above lack of agreement between these studies about OT gene expression during pregnancy, we re-evaluated this question using the quantitative real-time RT-PCR methods described here, by examining both mRNA and hnRNA levels. To be comprehensive in this analysis we included assays for both OT and VP mRNAs and hnRNAs, and compared these values in male and virgin female rats, lactating female (7 days postpartum), and 19-day pregnant female rats. These data are shown in Figs. 5 and 6, and the actual copy numbers are shown in Table 1. From these data it can be seen that OT and VP gene expression did not differ between the adult male and female rats, nor was there any significant difference between the OT mRNAs and OT hnRNAs (as well as between the VP mRNAs and VP hnRNAs) between virgin females and day 19 pregnant females. There were, however, large differences between OT mRNAs and OT hnRNAs as well as between VP mRNAs and VP hnRNAs between the adult virgin female rats and day 7 lactating females. We conclude that OT gene expression is not altered during pregnancy but is greatly increased during lactation.
Splicing patterns of the OT and VP pre-mRNAs in the SON.
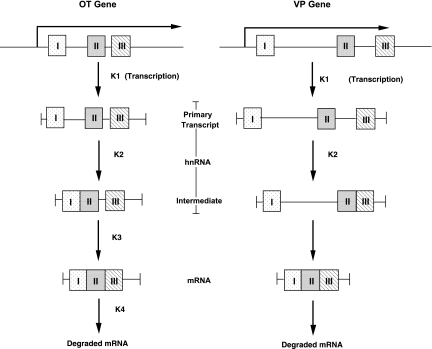
In the present study, we provide RT PCR evidence for the presence of the primary transcripts and transcription intermediates of the OT and VP genes in the rat SON (Fig. 1). The PCR products representing the OT and VP primary transcripts are shown to contain part of intron 1, all of exon 2 and part of intron 2 (Fig. 1A). The intron 1-bearing intermediates without intron 2 (hnRNA1) are shown in Fig. 1B, and the intermediates containing intron 2 without intron 1 are shown in Fig. 1C. The identities of all of these PCR products were validated by DNA sequence analyses (Supplemental Figs. S3–S8). From the qualitative PCR data shown in Fig. 1, we can conclude that the primary transcripts and intermediate RNA forms that we quantified here by real-time PCR are in fact present in the MCNs of rat SON. Based on the quantitative data for OT and VP hnRNAs and mRNAs shown in Table 1, we deduce a hypothetical model of the order of splicing of the introns of the OT and VP pre-mRNAs. This model is illustrated in Fig. 8. Given that these genes have only two introns, the underlying assumption of this analysis is that in each physiological condition studied, the lowest hnRNA copy number reflects the amount of primary transcript, and the higher copy number hnRNA represents the spliced intermediate. For example for the adult female SON data in Fig. 5, OT hnRNA 1, which is 3.19 × 103 copies/SON, is much lower than the OT hnRNA 2 copy number of 2.65 × 106, the latter being 831-fold higher (Table 1). Hence, we interpret these data as indicating that OT intron 1 is spliced first in the OT primary transcript, thereby leaving behind the remaining intermediate OT hnRNA 2 (Fig. 8). The reverse order then would be the case for the VP-pre-mRNA, where the adult female SON contains 3.95 × 102 copies/SON of VP hnRNA 2, which is one-eighth of the 3.5 × 103 intron 1-containing RNA species. We therefore interpret this as intron 2 being spliced first in the VP primary transcript, followed by the excision of intron 1 (Fig. 8).
Fig. 8.
Differential splicing of the OT and VP pre-RNAs. From the relative steady-state levels of the mRNAs and hnRNAs found in the SON for the oxytocin (Fig. 5) and vasopressin (Fig. 6) genes, we deduced a speculative kinetic pattern of splicing. The K symbols represent theoretical rate constants, the actual values of which are unknown, and their associated numbers indicate the presumed order of splicing (see discussion). The OT primary transcript first has intron 1 excised out, thereby producing an intermediate, which only contains OT intron 2. OT intron 2 is subsequently excised, leading to the OT mRNA. In contrast, in the VP gene, intron 2 is first excised, followed by the removal of intron 1, thereby producing the VP mRNA. The most important distinction in splicing patterns between the OT and VP pre-mRNAs is the apparent accumulation of intron 2-containing hnRNA intermediate in the OT splicing process.
Evidence for retention of an intron 2-containing spliced intermediate.
The most notable difference between the splicing patterns of the OT and VP pre-mRNAs that we have found is that the amount of OT hnRNA 2 in the SON (∼106 copies/SON) is 100–1,000-fold greater than the amount of OT hnRNA 1 and both VP hnRNAs in the SON (Table 1). This suggests that OT intron 2 may be undergoing intron retention (i.e., a failure to undergo excision of a specific intron). That intron 2 appears to be accumulating in the OT MCN at a much higher level than in intron 1 had been indicated by our previous ISHH data, since OT intron 2-specific oligonucleotide probes were much more effective in detecting OT hnRNA than OT intron 1-specific probes (61). This was the case even though the conditions for ISHH were much more favorable for detecting the longer intron 1 than the very short 84 bp intron 2. It is important to note here that the oligonucleotide and riboprobes used in the ISHH studies (61) could have been measuring excised but not degraded intronic RNA in the nuclei of the MCNs. This cannot be the case for the real-time RT-PCR measurements in the current paper since in the two-step method used here the RT step used a downstream gene-specific primer prior to the PCR step (which used the intron-specific primers). Therefore, free, excised introns could have not been detected by this method.
The conclusion deduced from the PCR data that there is retention of OT intron 2 in the SON is supported by the results of our FISH analysis and the distributions of OT and VP hnRNAs and mRNAs, which are shown in Fig. 7. The localization of VP hnRNA detected by the intron 1-specific riboprobe is in the nucleus (Fig. 7A), while the OT hnRNA detected by the intron 2-specific riboprobe is mainly in the cytoplasm (Fig. 7B). As expected, the VP (Fig. 7C) and OT (Fig. 7D) mRNA are localized in the cytoplasm. These data are consistent with the hypothesis that there is a selective OT intron 2 retention in the SON and further indicate that the partially spliced OT hnRNA in the MCNs is being transported to the cytoplasm.
While the above suggestion that OT intron 2 retention is occurring in the SON is novel for OT and VP gene expression, this type of alternative splicing is known to occur in vertebrates (3, 4, 21, 27, 29). In a recent large-scale study of 21,106 human genes, it was found that ∼15% showed evidence of intron retention and of these 22% were conserved in the mouse genome (21). One correlation found between the probability of an intron being retained and structure is that retained introns are significantly shorter than nonretained introns. This is particularly relevant here since the OT intron 2 is very short, i.e., only 84 bp. Many well-known gene transcripts are known to undergo intron retention. These include proinsulin (34, 56), cyclo-oxygenase 1 and 3 (15, 43), apolipoprotein E (59), urocortin 1 (5), GnRH (46, 48, 49), and PNMT (51). In many of these cases, the intron-retention mechanism plays an important role in the regulation of gene expression. For the proinsulin mRNA variant, intron 1 containing the 5′-UTR is retained in developing chicks (34) in a tissue-specific manner and influences heart development. In the mouse β-cells in the islets of Langerhans, the splicing rate of intron 1 is orders of magnitude faster than the excision of intron 2 in proinsulin (half-time of intron 2 splicing is ∼7 min), and the presence of intron 2 in the hnRNA inhibits translation (56). In both systems, the intron 2-containing proinsulin hnRNA is transported to or leaks into the cytoplasm where it undergoes degradation at the same rate as mature mRNA (half-time >48 h). Another interesting example is the processing of the GnRH pre-mRNA where this excision of intron 1 is also developmentally regulated. The retention of intron 1 produces a longer 5′-UTR, which decreases the efficiency of translation, even though the intron 1-containing hnRNA can enter the cytoplasm. It is conceived that the role of this intron-retention is to allow for rapid control of translation by having a pool of untranslatable but splice-ready hnRNA in the cell (46, 48, 49). While the above-discussed partially spliced RNAs have access to the cytoplasm of the cell, in other systems the retained intron remains in the nucleus. The latter is the case for the APOE4 variant that retains intron 3 (i.e., APOEI3, in Ref. 59) in neurons. These authors point out that this variant provides a potential for a rapid response of the cell to stress or injury. In this way, there are two regulated sources of APOE mRNA that are available, conventional gene expression and splicing, the latter due to a reserve pool of APOEI-3 that can be rapidly induced to splice out intron 3 (59). A variant of this phenomenon is found in mouse liver, where there is an 8 kb nuclear-retained poly (A+) RNA (CTN-RNA) that is transcribed from the cationic amino acid transporter (mCAT2) gene and is retained in the nucleus due to the presence of its 3′-UTR. Stress stimuli cause posttranscriptional cleavage of this 3′-UTR and subsequent transport of the CTN-mRNA into the cytoplasm for translation of the CTN protein (42).
Little is known about the mechanism responsible for intron-retention. Some suggestions are: 1) that there is repression of splicing by polypyrimidine tract proteins in some cells and 2) that there is nonconsensus splicing sites in either the 5′ or 3′ end of the introns or in branch points of the intron (3, 4). These have been proposed for the retention of intron 1 in GnRH (46, 49). In this regard it is interesting that in addition to the “major spliceosome” mechanisms found in the nucleus (3, 4), there are also so-called “minor spliceosome” mechanisms located in the cytoplasm (28, 39). These differ not only in their locations of splicing, but also in their preferred consensus sites and specific small nuclear riboprotein particles (SnRNPs). The “minor class introns” spliced by this mechanism represent only 0.15–0.34% of all introns in vertebrates, tend to be shorter in length, and have considerably slower rates of processing (28). Hence, it is possible that OT intron 2 is in the “minor intron class” and whether the OT hnRNA 2 is spliced in the MCN's cytoplasm remains to be determined.
GRANTS
This research was supported by the Intramural research program of the NIH, National Institute of Neurological Disorders and Stroke.
Supplementary Material
Acknowledgments
We thank Shirley B. House for assistance with the dissections of the SCN and SON, Dr. Eva Mezey and Sharon Key for advice and help with the FISH study, and Dr. W. S. Young III for critical reading of the paper.
Address for reprint requests and other correspondence: H. Gainer, Molecular Neuroscience Sect., Laboratory of Neurochemistry, National Inst. of Neurological Disorders and Stroke, National Institutes of Health, Bldg. 49, Rm. 5A78, Bethesda, MD 20892 (e-mail: gainerh@ninds.nih.gov).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev 84: 169–208, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Audibert A, Weil D, Dautry F. In vivo kinetics of mRNA splicing and transport in mammalian cells. Mol Cell Biol 22: 6706–6718, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black DL Mechanisms of alternative pre-messenger RNA splicing. Ann Rev Biochem 72: 291–336, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Black DL, Grabowski PJ. Alternative pre-mRNA splicing and neuronal function. Prog Mol Subcell Biol 31: 187–216, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Blanco E, Rojas R, Haeger P, Cuevas R, Perez C, Munita R, Quiroz G, Andres ME, Forray MI, Gysling K. Intron retention as an alternative splice variant of the rat urocortin 1 gene. Neuroscience 140: 1245–1252, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Brooks PJ The regulation of oxytocin mRNA levels in the medial preoptic area. Relationship to maternal behavior in the rat. Ann NY Acad Sci 652: 271–285, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev 81: 1197–1267, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bustin SA Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25: 169–193, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR–a perspective. J Mol Endocrinol 34: 597–601, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Bustin SA, Gyselman VG, Williams NS, Dorudi S. Detection of cytokeratins 19/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. Br J Cancer 79: 1813–1820, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter DA, Murphy D. Nuclear mechanisms mediate rhythmic changes in vasopressin mRNA expression in the rat suprachiasmatic nucleus. Brain Res 12: 315–321, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Chang MS, Hahn MK, Sved AF, Zigmond MJ, Austin MC, Sherman TG. Analysis of tyrosine hydroxylase gene transcription using an intron specific probe. J Neurosci Meth 94: 177–185, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Clement JQ, Maiti S, Wilkinson MF. Localization and stability of introns spliced from the Pem homeobox gene. J Biol Chem 276: 16919–16930, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Clement JQ, Qian L, Kaplinsky N, Wilkinson MF. The stability and fate of a spliced intron from vertebrate cells. RNA 5: 206–220, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui JG, Kuroda H, Chandrasekharan NV, Pelaez RP, Simmons DL, Bazan NG, Lukiw WJ. Cyclooxygenase-3 gene expression in Alzheimer hippocampus and in stressed human neural cells. Neurochem Res 29: 1731–1737, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Douglas AJ, Meeren HK, Johnstone LE, Pfaff DW, Russell JA, Brooks PJ. Stimulation of expression of the oxytocin gene in rat supraoptic neurons at parturition. Brain Res 782: 167–174, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin LL, Gibler TM, Van Gelder RN. Real-time quantitative polymerase chain reaction diagnosis of infectious posterior uveitis. Arch Ophthalmol 120: 1534–1539, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Favy DA, Lafarge S, Rio P, Vissac C, Bignon YJ, Bernard-Gallon D. Real-time PCR quantification of full-length and exon 11 spliced BRCA1 transcripts in human breast cancer cell lines. Biochem Biophys Res Commun 274: 73–78, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Fremeau RT Jr, Autelitano DJ, Blum M, Wilcox J, Roberts JL. Intervening sequence-specific in situ hybridization: detection of the pro-opiomelanocortin gene primary transcript in individual neurons. Brain Res 6: 197–201, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Fremeau RT Jr, Lundblad JR, Pritchett DB, Wilcox JN, Roberts JL. Regulation of pro-opiomelanocortin gene transcription in individual cell nuclei. Science 234: 1265–1269, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Galante PA, Sakabe NJ, Kirschbaum-Slager N, de Souza SJ. Detection and evaluation of intron retention events in the human transcriptome. RNA 10: 757–765, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerard CJ, Olsson K, Ramanathan R, Reading C, Hanania EG. Improved quantitation of minimal residual disease in multiple myeloma using real-time polymerase chain reaction and plasmid-DNA complementarity determining region III standards. Cancer Res 58: 3957–3964, 1998. [PubMed] [Google Scholar]
- 23.Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol 6: 1061–1069, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Herman JP, Schafer MK, Watson SJ, Sherman TG. In situ hybridization analysis of arginine vasopressin gene transcription using intron-specific probes. Mol Endocrinol 5: 1447–1456, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Herman JP, Sherman TG. Acute stress upregulates vasopressin gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. Ann NY Acad Sci 689: 546–549, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz MJ, Bloch KD, Kim NB, Amico JA. Expression of the endothelin-1 and oxytocin genes in the hypothalamus of the pregnant rat. Brain Res 648: 59–64, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Kan Z, States D, Gish W. Selecting for functional alternative splices in ESTs. Genome Res 12: 1837–1845, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konig H, Matter N, Bader R, Thiele W, Muller F. Splicing segregation: the minor spliceosome acts outside the nucleus and controls cell proliferation. Cell 131: 718–729, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nature Rev 8: 819–831, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Lightman SL, Young WS, 3rd. Corticotrophin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. J Physiol 403: 511–523, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lightman SL, Young WS, 3rd. Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol 394: 23–39, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipschitz DL, Crowley WR, Armstrong WE, Bealer SL. Neurochemical bases of plasticity in the magnocellular oxytocin system during gestation. Exp Neurol 196: 210–223, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Luckman SM, Larsen PJ. Evidence for the involvement of histaminergic neurones in the regulation of the rat oxytocinergic system during pregnancy and parturition. J Physiol 501: 649–655, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansilla A, Lopez-Sanchez C, de la Rosa EJ, Garcia-Martinez V, Martinez-Salas E, de Pablo F, Hernandez-Sanchez C. Developmental regulation of a proinsulin messenger RNA generated by intron retention. EMBO Rep 6: 1182–1187, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marino JH, Cook P, Miller KS. Accurate and statistically verified quantification of relative mRNA abundances using SYBR Green I and real-time RT-PCR. J Immunol Methods 283: 291–306, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Murphy D, Carter D. Vasopressin gene expression in the rodent hypothalamus: transcriptional and posttranscriptional responses to physiological stimulation. Mol Endocrinol 4: 1051–1059, 1990. [DOI] [PubMed] [Google Scholar]
- 37.Øster B, Höllsberg P. A sensitive quantification of HHV-6B by real-time PCR. Biol Proced Online 4: 88–93, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11: 305–312, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Patel AA, Steitz JA. Splicing double: insights from the second spliceosome. Nat Rev Mol Cell Biol 4: 960–970, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (Ed. 2). Academic, 1986. [DOI] [PubMed]
- 41.Ponzio TA, Yue C, Gainer H. An intron-based real-time PCR method for measuring vasopressin gene transcription. J Neurosci Meth 164: 149–154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, Spector DL. Regulating gene expression through RNA nuclear retention. Cell 123: 249–263, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Qin N, Zhang SP, Reitz TL, Mei JM, Flores CM. Cloning, expression, and functional characterization of human cyclooxygenase-1 splicing variants: evidence for intron 1 retention. J Pharmacol Exp Ther 315: 1298–1305, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Russell JA, Leng G, Douglas AJ. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol 24: 27–61, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Schriefer JA Diethylstilbesterol- and pregnancy-induced changes in rat neurointermediate lobe oxytocin, arginine vasopressin, methionine enkephalin and dynorphin. Neuroendocrinology 54: 185–191, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Seong JY, Park S, Kim K. Enhanced splicing of the first intron from the gonadotropin-releasing hormone (GnRH) primary transcript is a prerequisite for mature GnRH messenger RNA: presence of GnRH neuron-specific splicing factors. Mol Endocrinol 13: 1882–1895, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Shoji M, Kimura T, Kawarabayasi Y, Ota K, Inoue M, Yamamoto T, Sato K, Ohta M, Funyu T, Sonoyama T, Abe K. Effects of acute salt loading on vasopressin mRNA level in the rat brain. Am J Physiol Regul Integr Comp Physiol 266: R1591–R1595, 1994. [DOI] [PubMed] [Google Scholar]
- 48.Son GH, Jung H, Seong JY, Choe Y, Geum D, Kim K. Excision of the first intron from the gonadotropin-releasing hormone (GnRH) transcript serves as a key regulatory step for GnRH biosynthesis. J Biol Chem 278: 18037–18044, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Son GH, Park E, Jung H, Han J, Lee KH, Seong JY, Kim K. GnRH pre-mRNA splicing: solving the mystery of a nature's knockout, hpg mouse. Biochem Biophys Res Commun 326: 261–267, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Spinolo LH, Raghow R, Crowley WR. Oxytocin messenger RNA levels in hypothalamic, paraventricular, and supraoptic nuclei during pregnancy and lactation in rats. Evidence for regulation by afferent stimuli from the offspring. Ann NY Acad Sci 652: 425–428, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Unsworth BR, Hayman GT, Carroll A, Lelkes PI. Tissue-specific alternative mRNA splicing of phenylethanolamine N-methyltransferase (PNMT) during development by intron retention. Int J Dev Neurosci 17: 45–55, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Valasek MA, Repa JJ. The power of real-time PCR. Adv Physiol Educ 29: 151–159, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Van Tol HH, Bolwerk EL, Liu B, Burbach JP. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology 122: 945–951, 1988. [DOI] [PubMed] [Google Scholar]
- 54.Vandenbroucke II, Vandesompele J, Paepe AD, Messiaen L. Quantification of splice variants using real-time PCR. Nucleic Acids Res 29: E68–E68, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagatsuma A, Sadamoto H, Kitahashi T, Lukowiak K, Urano A, Ito E. Determination of the exact copy numbers of particular mRNAs in a single cell by quantitative real-time RT-PCR. J Exp Biol 208: 2389–2398, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Shen L, Najafi H, Kolberg J, Matschinsky FM, Urdea M, German M. Regulation of insulin preRNA splicing by glucose. Proc Natl Acad Sci USA 94: 4360–4365, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270: 41–49, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques 39: 75–85, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Xu Q, Walker D, Bernardo A, Brodbeck J, Balestra ME, Huang Y. Intron-3 retention/splicing controls neuronal expression of apolipoprotein E in the CNS. J Neurosci 28: 1452–1459, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young WS 3rd, Mezey E, Siegel RE. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridization histochemistry. Brain Res 387: 231–241, 1986. [DOI] [PubMed] [Google Scholar]
- 61.Yue C, Mutsuga N, Scordalakes EM, Gainer H. Studies of oxytocin and vasopressin gene expression in the rat hypothalamus using exon- and intron-specific probes. Am J Physiol Regul Integr Comp Physiol 290: R1233–R1241, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Yue C, Mutsuga N, Sugimura Y, Verbalis J, Gainer H. Differential kinetics of oxytocin and vasopressin heteronuclear RNA expression in the rat supraoptic nucleus in response to chronic salt loading in vivo. J Neuroendocrinol 20: 227–232, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Yun JJ, Heisler LE, Hwang II, Wilkins O, Lau SK, Hyrcza M, Jayabalasingham B, Jin J, McLaurin J, Tsao MS, Der SD. Genomic DNA functions as a universal external standard in quantitative real-time PCR. Nucleic Acids Res 34: e85, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zingg HH, Lefebvre DL. Oxytocin and vasopressin gene expression during gestation and lactation. Brain Res 464: 1–6, 1988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.