There are an estimated 1.5 million people with type 1 diabetes (T1D) and 20 million with type 2 diabetes (T2D) in the U.S. today including at least 150 000 younger than 20 years(1). Of concern, both T1D and T2D are increasing in youth and presenting at younger ages(2-4), implying a longer burden of disease and earlier onset of vascular complications(5).
Cardiovascular disease (CVD) is the leading cause of death in people with both T1D(6) and T2D(7) and the antecedents of adult CVD are present in children(8-10). Several studies demonstrate tracking of childhood CVD risk factors into adulthood(9-15). Furthermore, CVD risk factors in childhood correlate with abnormalities in surrogate markers of atherosclerosis (such as carotid intima thickness and arterial elasticity)(14;15) and atherosclerotic lesions in pathology evaluations(9;13). Although data indicate that progress has been made reducing microvascular complications in T1D(16;17) and that intensive management with lower HbA1c can reduce CVD events(18), evidence from the Pittsburgh Epidemiology of Diabetes Complications Study suggests a lack of similar progress in reduction of macrovascular as compared with microvascular complications(16). Furthermore, people with both T1D and T2D suffer macrovascular complications and death at earlier ages than non-diabetics(7;19). Importantly, dyslipidemia is a significant CVD risk factor in persons with diabetes(7;20-22) and target low-density lipoprotein cholesterol (LDL) levels continue to be lowered in adults with diabetes (DM)(7).
Observational data have emerged recently on prevalence of dyslipidemia in youth with DM(23-26). Yet, despite recent American Diabetes Association (ADA) and American Heart Association (AHA) clinical recommendations on treatment of dyslipidemia in youth with DM(27-30), no treatment data exist in dyslipidemic youth with DM on which to base clinical care. Instead current pediatric recommendations are generated by consensus expert opinion or are extrapolated either from adult data or treatment data on youth with familial hypercholesterolemia(27-31).
Given that dyslipidemia is an important and potentially modifiable CVD risk factor, data to inform clinical decision making regarding screening criteria and treatment of dyslipidemia in this high-risk population are of significant public health importance(32). Data from clinical trials in youth with DM are needed to determine the appropriate management strategy.
In this article, recent data and current recommendations on dyslipidemia in youth with DM will be reviewed. Evidence supporting the treatment of dyslipidemia in youth with DM will be discussed as well as current treatment options and recommended monitoring. Finally, the question of whether lipid abnormalities in youth with DM should be treated will be addressed.
DATA ON ATHEROSCLEROSIS IN YOUTH
Landmark studies such as the Bogalusa Heart Study(33), the Muscatine Study(34), the Young Finns Study(14), and the Pathobiologic Determinants of Atherosclerosis in Youth (PDAY)(35) demonstrate that the atherosclerotic process begins in childhood and the extent of atherosclerosis (based on postmortem examination or utilization of surrogate markers of atherosclerosis) relates to the presence and degree of CVD risk factors. Although hyperglycemia is an important CVD risk factor in these studies, no explicit differentiation of T1D versus T2D is made nor is there specific analysis of subjects with DM. The PDAY study developed a risk score based on CVD risk factors to predict atherosclerosis in people 15-34 years(36) that was validated in a number of studies(37-39) and NHANES data was used to establish sex and age specific cut-points standardized to National Cholesterol Education Program (NCEP) thresholds(40). These studies demonstrate tracking of CVD risk factors, especially for those in extreme categories of abnormal lipids and strongly suggest that efforts to reduce CVD risk factors in youth can reduce development of atherosclerosis and delay clinical CVD later in life(41).
DATA ON LIPIDS IN YOUTH WITH DIABETES
Recent data indicate that dyslipidemia is present in youth with DM. We reported that 18.6% of children with T1D had abnormal TC (>200 mg/dl) or high density lipoprotein cholesterol (HDL-c) (<35 mg/dl) levels in a retrospective cross-sectional analysis(23). Longitudinal analysis of data from the same clinic population revealed sustained abnormalities in a similar range(26). HbA1c was significantly related to TC and non-HDL-c (calculated as the TC minus HDL-c), and BMI z-score was inversely related to HDL-c.
Evidence that abnormal fasting lipid levels are present in youth with DM comes from the SEARCH for Diabetes in Youth (SEARCH) study in which 3% of subjects with T1D had LDL-c levels >160 mg/dl, 14% >130 mg/dl and almost half (48%) had LDL-c levels over the threshold for recommended LDL-c of 100 mg/dl (24). Reported prevalences were higher for youth with T2D at 9%, 24%, and 57% for the same cut-points, suggesting obesity has a negative impact on LDL-c, although we need to know much more about the mechanism of the increase in LDL-c sometimes seen with obesity. A recent review of complications in youth with T2D reports a wide range of dyslipidemia (15-62.5%)(42). In the SEARCH study, only 23 (1%) of T1D youth and 13 (5%) of T2D youth were on lipid lowering medications. Our data and SEARCH data(43) support optimizing glycemic control and lifestyle interventions aimed at obesity as essential components of managing lipid abnormalities in this population.
In a large (n=27,358) cross-sectional T1D cohort from Germany and Austria, Schwab documented the presence of dyslipidemia (defined as TC > 200 mg/dl, LDL-c > 130 mg/d or HDL-c < 35 mg/dl) in 29% of T1D subjects under 26 years, with a higher percentage (34%) in the 17-26 year old age group. Only 0.4% of this cohort were on lipid lowering medications including 0.8% of 17-26 year olds(25).
Findings from these studies suggest initiation of lipid-lowering medication in children with DM is lacking in light of newer, more aggressive lipid-lowering recommendations by the ADA or AHA. However, applying the 2003/2005 ADA or AHA 2006/2007 guidelines to these data may not reflect current practice. With obesity increasing in all youth(44) including youth with DM(45), more children will likely meet criteria for treatment. However, no outcome data exist to support pharmacologic treatment of CVD risk factors in youth with T1D or T2D(32). Retrospective data regarding pharmacologic treatment of dyslipidemia(26) suggest that more rigorous therapy as well as patient education on the importance of continued therapy may be needed to meet current ADA and AHA goals for lipids.
DATA ON LIPIDS IN ADULTS WITH DM
Although lipid levels in patients with T1D have been found to be comparable to or better than in non-diabetic adults (lower TC, LDL-c, and TG and higher HDL-c)(46), adults with T1D still commonly have dyslipidemia and are known to be at higher risk for atherosclerotic disease compared with the general population. Dyslipidemia is clearly a major risk factor for atherosclerosis and CVD in adults with both T1D and T2D(7). The NCEP considers the presence of DM to be the risk equivalent of a history of coronary disease with similar goals for lipid lowering(47). There is consideration that lipids in those with DM may be more atherogenic. Possible mechanisms include differences in lipoprotein particle size, LDL-c oxidation, and increased transvascular LDL-c transport in patients with T1D (47-49).
In contrast, dyslipidemia in T2D is characterized by decreased HDL-c and elevated triglycerides with variable TC and LDL-c levels, although LDL-c particles are smaller, denser, and more atherogenic(7). Studies using statins to reduce LDL-c 30-40% in adults with T2D have shown a 30-40% relative reduction in coronary heart disease risk(21;22).
In summary, adults with T1D have been reported to have a better lipoprotein profile than non-diabetic adults(46), however abnormal lipid levels within T1D subjects predict worse CVD outcomes(20). Although the effectiveness of statin treatment of elevated LDL-c in adults with T2D is well-established(7), no clinical trials exist in persons with T1D demonstrating LDL-c reduction results in improved CVD outcomes. Most of the pediatric lipid data is in T1D, whereas in adults more data exist in T2D. In addition to CVD, dyslipidemia may also be an important risk factor for microvascular complications in patients with DM(50) and the relationship of dyslipidemia to micro- and macrovascular complications and whether this differs by DM type requires further study.
CURRENT RECOMMENDATIONS & GUIDELINES
Screening and Treatment
The ADA in 2003 and 2005 recommended screening for dyslipidemia in patients with T1D ≥2 years of age in the presence of a positive or unknown family history, otherwise at ≥12 years of age, (once glycemic control has been obtained in the newly diagnosed patient), and then every 5 years if normal and at diagnosis and every 2 years in patients with T2D(27;28) (Table I; available at www.jpeds.com). Ideally, the ADA recommends that screening samples should be obtained in the fasting state.
Table I.
ADA Recommendations on Lipid Screening and Management in Youth with Diabetes
|
Diabetes Care 26:2194, '03 Diabetes Care 28:186, '05 |
Type 1 Diabetes | Type 2 Diabetes |
|---|---|---|
| Initial Screening Age (once glycemic control obtained) |
>2 yrs at diagnosis if unknown or +family history; otherwise at 12 years (puberty) |
At diagnosis |
| Re-screening if lipids normal | 5 years | 2 years |
| Optimal concentration | LDL-c <100 mg/dl HDL-c >35 mg/dl Triglyceride <150 mg/dl |
|
| Management of elevated LDL-c | Goal LDL-c <100 mg/dl | |
| Initial Therapy | Glycemic control, MNT, physical activity, weight control, tobacco cessation |
|
| After 3-6 months | LDL-c >160 mg/dl: begin medication LDL-c 130-159 mg/dl: “recommended” after MNT failure based on other CVD risk factors Counsel on pregnancy if statin started |
|
However, given the difficulties of obtaining fasting samples (which include safety issues in DM patients taking insulin), the Adult Treatment Panel III suggests screening with non-fasting TC and HDL-c, followed by a complete fasting lipoprotein panel if screening results are abnormal(47). Non-HDL-c is used as a secondary target in adults per the NCEP guidelines, particularly among patients with elevated triglycerides(47) and has better predicted CVD deaths in adults with T1D than other lipoproteins(51). Data in adults indicate that TC, HDL-c, and non-HDL-c are minimally affected by fasting status(52). Although data are needed in youth with DM, the Bogalusa study has shown that adverse non-HDL-c level as compared with LDL-c level better predicts adult dyslipidemia and is related to CVD risk factors in adulthood(53).
The AHA recently published a scientific statement on CVD risk reduction in high risk pediatric patients including youth with T1D and T2D(29) (Figure 1; available at www.jpeds.com). Five steps are outlined: 1) risk is stratified by disease process, 2) CVD risk factors are assessed, 3) tier-specific cut-points and treatment goals are specified, 4) lifestyle change is advocated, and 5) if goals are not met then disease specific management is recommended. Youth with T1D are categorized as Tier I or high risk patients (based on data for clinical evidence of CAD <30 years of age); T2D youth are considered Tier II or moderate risk (based on pathophysiologic evidence of accelerated atherosclerosis). Step 2 consists of assessment of all CV risk factors (fasting lipids, smoking history, family history of early CAD [males ≤55 years, females ≤65 years] in an expanded 1st degree pedigree, blood pressure [on 3 separate occasions, interpreted for age, sex, and height], BMI, and a physical activity history.) (Of note, glycemic goals are given, but for youth with DM they are subsumed under the recommendation for endocrinologist-directed treatment.) Youth with T2D and two or more additional risk factors advance to the higher risk tier I. Tier I goals are slightly more aggressive than tier II (BMI ≤85% v. 90% for age/sex, BP≤90% v. 95% for age/sex, and LDL-c ≤100 mg/dl v ≤130 mg/dl.) For tier II patients, therapeutic lifestyle change is recommended for 6 months and medications are to be considered if goals are not met. For tier I patients, recommendations are to: 1) intensify glucose management per their endocrinologist, 2) assess BMI, fasting lipids, and management of weight and lipids for 6 months including nutritionist evaluation and dietary education with goals of total fat <30% of calories, saturated fat <10%, cholesterol <300 mg/dl, avoiding trans fats, and adequate calories for growth. Additional goals are ≥1 hour of active play and ≤2 hours of “screen time” daily. If goals are not met after 6 months a more intensive weight loss and exercise program is recommended prior to beginning a statin at the lowest dose to achieve the LDL-c goal of <100 mg/dl. Of note, although the recent ADA/AHA statement on primary prevention of CVD in people with DM stated that the recommendations for T2D appear appropriate for T1D(7), the AHA guidelines for youth differentiate the distinct pathophysiologic processes in youth(29).
Figure 1. AHA Guidelines for Risk Stratification and Treatment in Youth with Diabetes.
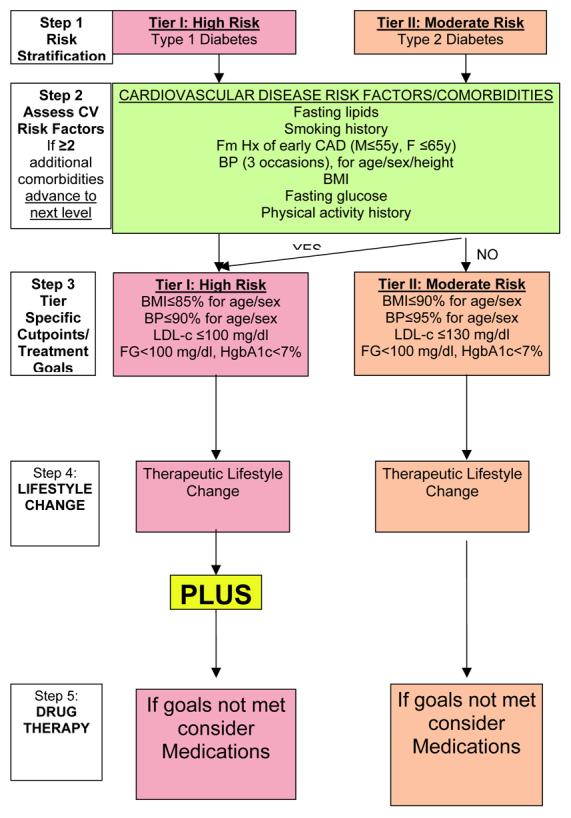
[adapted from Kavey(29)]
In contrast, the earlier ADA guidelines from 2003 and 2005 recommend treatment with medication for LDL-c ≥160 mg/dl in addition to emphasizing blood glucose control and dietary and exercise counseling. In children with LDL-c levels between 130-159 mg/dl, glucose control and dietary and exercise counseling are recommended for 6 months with medication to be considered if the LDL-c remains >130 mg/dl. The treatment goals are LDL-c < 100 mg/dl, HDL-c > 35 mg/dl and TG < 150 mg/dl(27;28). In adults with DM and overt CVD, the ADA goal LDL-c has been lowered to 70 mg/dl (54). Finally, the International Society for Pediatric and Adolescent Diabetes has recently published Clinical Practice Consensus Guidelines very similar to the ADA guidelines(55).
Medical Nutritional Therapy
Medical nutritional therapy (MNT) is a key element in managing existing DM and can prevent or slow the rate of development of diabetic complications (56;57). Specific MNT goals per the ADA for individuals with DM are a lipoprotein profile that reduces the risk for CVD and to prevent or delay the rate of development of the chronic complications of DM by modifying nutrient intake and lifestyle(56;57). Additionally, the recommendations note that in order to address individual nutrition needs, personal and cultural preferences need to be taken into account as well as willingness to change (56). The recommendations are to limit saturated fat to < 7% of total calories, to limit intake of trans fat, limit dietary cholesterol to <200mg/day, and to consume two or more servings of fish per week (commercially fried fish excluded) to provide n-3 polyunsaturated fatty acids (56).
The goal of MNT for youth with T1D is to provide adequate energy to meet the nutritional needs and ensure normal growth and development during this specific time in the life cycle (56;57). In contrast, dietary goals for youth with T2D are generally directed at weight reduction or maintenance. Numerous adult studies demonstrate that diets low in total fat, saturated fat, and cholesterol can lower LDL-c (58). The Dietary Intervention Study in Children (DISC) examined children's adaptations to a fat-reduced diet and found that dietary changes over a three year period are safe and effective in modestly lowering LDL-c (although less than a 5mg/dl greater reduction in LDL-c in the intervention compared with the usual care group) while maintaining adequate growth, iron stores, and nutritional adequacy (59). The DISC study further demonstrated that dietary modifications can be achieved without adverse side effects in pubertal children at up to 7.4 years of follow-up(60). NHANES data from 1999-2002 reported that the ADA clinical practice recommendations for adults with DM at that time were far from being achieved (61). The SEARCH study reported that in a large cohort of children with DM aged 10-22 years only 15% met the ADA and AHA recommendations for total and saturated fat intake(62).
Pharmacologic Treatment
Clinical trials of both lifestyle modification and pharmacologic interventions are needed to address efficacy and long-term safety in youth with DM. Data on the safety of lipid-lowering medications in youth with DM require prospective study in carefully controlled clinical trials, including the long-term use and potential teratogenic issues for adolescent females. Current AHA recommendations to initiate pharmacologic treatment with a statin at the lowest dose and (Table II; available at www.jpeds.com) stress appropriate patient selection criteria: age (>10 years, and ≥Tanner Stage 2, preferably after menarche), other CVD risk factors in addition to levels of LDL-c, preference of patient and family, and screening for contraindications (especially hepatic disease)(30). Measuring creatinine kinase and liver transaminases are recommended prior to starting the lowest initial statin dose. Monitoring liver tests and musculoskeletal symptoms for rhabdomyolysis, a reversible but potentially life-threatening adverse event are recommended(27).
Table II.
Medication Initiation, Titration, and Monitoring Recommendations (adapted from McCrindle(30))
Patient Selection:
|
Initiation and Titration of Medication:
|
On-going Monitoring:
|
Lipid-lowering medications in youth have been reviewed recently(63;64). Currently approved medications include bile-acid sequestrants (which are poorly tolerated) and statins. Fibrates are the first line treatment of hypertriglyceridemia in adults and although also used for this indication in youth, they are neither approved nor do data exist on their use in youth with DM. Niacin, or nicotinic acid, can both lower LDL-c and raise HDL-c but is rarely used in children as flushing is a frequent side effect, although timed-release formulations may reduce this. Ezetimibe is a more recent pharmacologic option that inhibits intestinal cholesterol absorption and is approved for use in youth ≥10 years with familial hypercholesterolemia. Ezetimibe has been combined with a statin and data in adults demonstrate additive effects in lowering LDL-c(65). Whether ezetimibe has a role in pediatrics is under investigation, however the ENHANCE trial recently reported no additional benefit in carotid IMT with ezetimibe added to simvastatin compared with simvastatin alone in hypercholesterolemic adults (mean LDL-c>300mg/dl at baseline), although it did significantly decrease hsCRP (66-68). The range of expected effects of lipid-lowering depends both on the dosage of lipid-lowering medication, the degree of dyslipidemia, and concomitant therapies such as MNT, physical activity, and glycemic control and has been catalogued recently(30).
For lowering of triglycerides, diet and glycemic control are recommended by the ADA unless triglycerides are over 1,000 mg/dl, in which case, the child is at increased risk for pancreatitis and fibric acid derivatives should be considered(27) whereas the AHA has a lower threshold of 700 mg/dl(29). Even though the most recent dietary guidelines from the AHA mention the benefits of fish oils in the diet and the use of plant stanols/sterols (29), there are no current guidelines for youth with DM from the ADA regarding these options, nor are there guidelines specific for lowering CVD risk.
Contrasts in current ADA/AHA guidelines
The recent AHA guidelines for dyslipidemia screening and treatment in youth with DM have lower LDL-c cut-points for treatment (<100 mg/dl) than previous AHA and ADA guidelines as well as a lower age limit of 10 years. Therapeutic life-style change is recommended as a first step prior to pharmacologic treatment by both Associations. Also, although the AHA guidelines stress that most T2D patients will have 2 or more additional CVD risk factors and therefore be classified as tier I or high risk, some pediatric endocrinologists would consider youth who have presented with T2D to be at higher risk of future CVD than youth with T1D. Additionally, some pediatric endocrinologists might object to youth with well-controlled T1D categorized in the high risk tier I (with youth with homozygous familial hypercholesterolemia, chronic kidney disease/end-stage kidney disease, post orthotopic heart transplant, and Kawasaki disease with current coronary artery aneurysms), although the AHA guidelines explicitly state that individualization of the recommendations are needed(29).
CLINICAL TRIALS TO TREAT DYSLIPIDEMIA IN YOUTH WITH DIABETES
Treatment goals for dyslipidemia in adults with DM have become more aggressive(54), awareness of increased CVD risk in DM has increased(6;7;18), and the first treatment recommendations for dyslipidemia in youth with DM have been published(27-30). However, no clinical trials of pharmacologic agents in youth with DM have been performed and the risk exists that these medications may start to be used routinely in youth with DM in the absence of safety or efficacy data from clinical trials. While these drugs have a relatively benign safety profile in adults and the few studies in hypercholesterolemic youth(30), appropriately designed and powered clinical trials to evaluate safety and efficacy are needed. However, prior to their wide-scale implementation per practice guidelines pediatric endocrinologists must become comfortable prescribing dyslipidemia medications---less likely to occur in the absence of safety, efficacy, and cost-effectiveness data in dyslipidemic youth with DM. Therefore, clinical trials must be adequately powered and designed to determine safety as well as efficacy. Of note, a multi-center, international clinical trial to pharmacologically treat dyslipidemia and hypertension in youth with T1D has been registered http://www.controlled-trials.com/ISRCTN91419926/ and the TODAY study in youth with T2D has practice guideline based algorithms to treat dyslipidemia and hypertension, although these are not the study's primary end-points(69). Ultimately, data on the relationship of clinical intervention to health care outcomes are needed in addition to surrogate markers such as lipid levels or measures of vascular health, as outlined below. However, these data will require long-term study and likely multi-center study collaboration. The cost of collecting such data makes it unlikely that it will ever be performed. Therefore refinement and validation of current surrogate markers of CVD are of importance.
SURROGATE NON-INVASTIVE MEASURES OF SUBCLINICAL CVD
Multiple non-invasive techniques for assessing cardiovascular risk have been recently reviewed(70;71). Electron beam CT to evaluate coronary artery calcification (CAC) has been used in adults(72-74), but no CAC was detected in an adolescent T1D population(75). B-mode ultrasound to evaluate carotid intima media thickness (IMT) has demonstrated increased IMT thickness in youth with T1D(76-78). In youth, CVD risk from T1D has been compared with that of familial hypercholesterolemia, as measured by carotid IMT(76).
Arterial stiffness measures including pulse wave velocity and pulse wave analysis are non-invasive techniques for evaluating sub-clinical cardiovascular disease(79-83) including studies in children, to assess atherosclerotic vascular disease. Studies in children (aged 7-18 years) have demonstrated the usefulness of arterial stiffness measures in detecting sub-clinical aortic changes in otherwise healthy children and adolescents(84) and in children with T1D(85).
Brachial artery distensibility is another method that has been used to measure vascular disease in a young adult population. The technique provides a measure of vascular function proven to be associated with atherosclerosis at a different site than pulse wave analysis. In the Bogalusa Heart study, decreased distensibility of the brachial artery was negatively correlated with several established cardiovascular risk factors including age, blood pressure and adiposity as well as LDL-c and VLDL-c levels in over 900 asymptomatic young adults(86).
Haller et al have demonstrated endothelial dysfunction in youth with T1D as compared with non-diabetic controls using reactive hyperemia-peripheral artery tonometry, a newer non-invasive technique to assess endothelial function(87). In the absence of long-term follow-up data tracking youth into adulthood to monitor CVD events and mortality, reliable surrogate markers of CVD to reduce time of follow-up and to increase power will be necessary. However, limitations to surrogate markers have been reviewed recently and must be considered when interpreting study results(88).
DISCUSSION
We must emphasize that no prospective data on safety, cost, or outcomes on dyslipidemia medications in adolescents with DM exist and therefore the question of how aggressive treatment of CVD risk factors should be in this population is indeed uncertain. Due to a lack of clinical trial data, current controversy over treatment of dyslipidemia in youth with DM (Table III; available at www.jpeds.com) could be considered analogous to pre-DCCT debates on the wisdom of tight glycemic control. Arguments against aggressive treatment of dyslipidemia in youth with DM are numerous and include: a lack of data on safety, efficacy, outcome, or even surrogate marker data; intra- and interindividual variability; medication cost; potential life-time treatment; and data that early vascular lesions can regress with treatment as adults. Conversely, arguments for aggressive treatment include: safety in adults with DM and in youth with familial hypercholesterolemia; data from landmark studies on the presence of early atherosclerosis in youth; an increased risk of coronary heart disease in young adults with DM as compared with non-diabetics; a preponderance of data on coronary heart disease risk reduction with statin treatment in adults; tracking of lipids from youth to adulthood; concern of negative “vasculo-metabolic memory” analogous to that of “metabolic memory” suggested by the DCCT/EDIC for the persistent effect of elevated HbA1c on CVD(18).
Table III.
Pros and Cons of Pharmacologic Treatment
| PROS | CONS |
|---|---|
| Lipids track into adulthood | Wait until adults
|
| Lipids associated with atherosclerosis in childhood |
No data that treatment in Youth will reduce long-term CVD complications |
| Lipids important micro- and macrovascular risk factor |
Primum non nocere
|
| DM considered a CVD risk factor equivalent in adults |
Cost: 1) number needed to treat to prevent CVD event unable to be calculated, but undoubtedly high; 2) many years of treatment required with potential for life- time treatment |
| Earlier DM onset→longer DM disease burden, potential adverse “vasculo- metabolic memory” and increased “area under the curve” for CVD risk factors |
Variability, regression to mean of lipids |
| Long-term elevated risk of CVD (PDAY, Young Finns, Bogalusa) |
No outcome data, no safety data in youth with diabetes |
| Preponderance of data on lowering CHD risk in adults, why wait? |
Although determination of risk in adult individuals has been traditionally calculated as the risk for an event within 10 years, another perspective in pediatric diabetes is that the age at which an individual will be at risk for an event should also be considered when determining initiation of medication. In adolescents with DM, the risk for an event prior to the age of 35 years is thought to be significant in children who also have elevated LDL-c levels. The risk of CVD events in T1D patients in their twenties is dramatically increased from the non-diabetic population(19;51). A recent study suggests that moderate reductions in LDL-c sustained over a life-time could markedly reduce CVD---a two-fold larger reduction was reported in heart disease in subjects with genetically lower LDL-c as would have been expected from similar LDL-c reductions in statin studies(89). Persistent decreases in CVD deaths in the treatment as compared with the placebo group have been reported in lipid-lowering trials(90;91), suggesting long-term risk reduction from short-term lipid-lowering. In the high CVD risk setting of DM, modest LDL-c (and other CVD risk factors) reductions over time could lower long-term CVD complications (Figure 2; available at www.jpeds.com). The hypothesis that early treatment of CVD risk factors in youth with DM will reduce future CVD events/mortality remains to be tested. The intensity of treatment must be balanced with safety, cost, additional issues and the hypothesized future risk reduction.
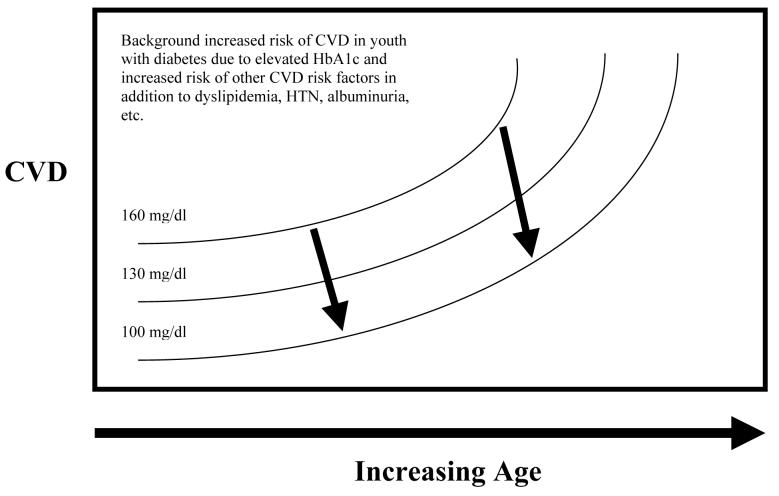
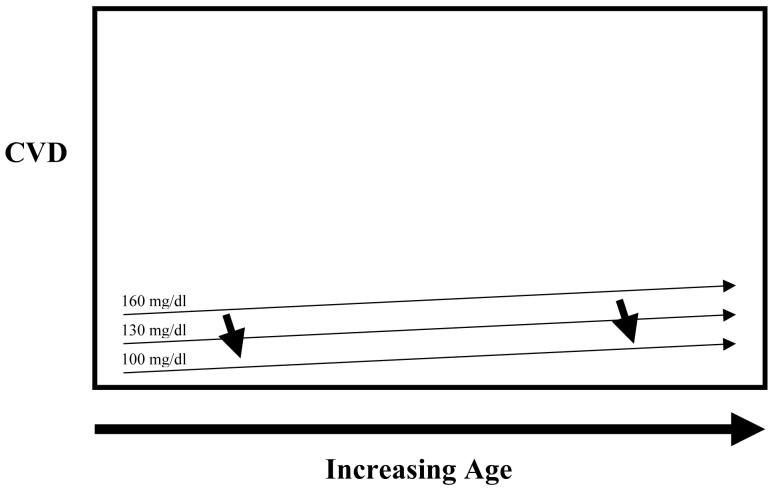
Figure 2A and 2B. Hypothetical Relationship of LDL-c, LDL-c-lowering and Future CVD in Youth with Diabetes.
Youth with diabetes are at increased risk of future cardiovascular disease. The question remains as to the timing of pharmacologic intervention. Numerous factors influence this decision including background risk of CVD for youth with diabetes, safety of pharmacologic agents, degree of long-term benefit to be gained from intervention, etc. Currently, data are insufficient to determine the degree of benefit to be obtained from early intervention on CVD risk factors in youth with diabetes in reducing this cumulative area under the curve of CVD risk factors. Figure 2A represents possible long-term risk reduction assuming high background CVD risk and benefit from LDL-c lowering, and Figure 2B represents lower background CVD risk and less benefit from LDL-c lowering.
Although short-term treatment data for statins in youth with familial hypercholesterolemia report minimal adverse effects(30;92;93), data on the cost and risk/benefit of pharmacologic treatment of dyslipidemia in youth with DM are needed. Life-long attention to lifestyle modification in addition to diligent pharmacologic treatment may be required to meet and sustain recommended lipid goals. Additional[h1] data are needed as current guidelines could be cited to treat a 10 year old with an LDL-c of 101 mg/dl or to not treat an 18 year old with an LDL-c of 159 mg/dl. Even though guidelines must always be adapted by each physician to individual patients, data are needed to do so rationally. In our practice youth with DM who are dyslipidemic generally are offered participation in research treatment protocols consistent with ADA guidelines. Non-fasting TC, HDL-c, and non-HDL-c in youth also might serve as a more efficient screening tool much as a spot urine is used in lieu of overnight urine collections to improve microalbuminuria screening compliance in adolescents and a consensus panel has recently concluded that non-HDL-c constitutes a better screening index than LDL-c to identify high-risk patients(94). Also, as measurement variability of lipoproteins in youth with DM is unknown, repeat measurement to confirm abnormalities may be prudent prior to diagnosis of dyslipidemia.
Future research in youth should include prospective longitudinal studies on the natural history of dyslipidemia, clinical trials to examine safety and efficacy of lipid-lowering medications, and ultimately the long-term relationship of dyslipidemia and its treatment to future health outcomes in youth with DM.
Acknowledgments
Drs. Maahs, Wadwa, Klingensmith, and Rewers have received a grant (but no salary support) for a clinical trial evaluating the safety and efficacy of lipid-lowering medications in youth with type 1 diabetes and elevated LDL-c. Dr. Maahs is supported by a National Institute of Diabetes and Digestive and Kidney Diseases grant (K23 DK075360).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Liese AD, D'Agostino RB, Jr., Hamman RF, Kilgo PD, Lawrence JM, Liu LL, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Peds. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 2.EURODIAB ACE Study Group Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355:873–876. [PubMed] [Google Scholar]
- 3.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith G, Bloch C, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diab Care. 2007;30:503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 5.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296:421–426. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, et al. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489–3493. doi: 10.1161/CIRCULATIONAHA.104.529651. [DOI] [PubMed] [Google Scholar]
- 7.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diab Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 8.Lauer RM, Lee J, Clarke WR. Factors affecting the relationship between childhood and adult cholesterol levels: the Muscatine Study. Peds. 1988;82:309–318. [PubMed] [Google Scholar]
- 9.McGill HC, Jr., McMahan CA, Malcom GT, Oalmann MC, Strong JP. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol. 1997;17:95–106. doi: 10.1161/01.atv.17.1.95. [DOI] [PubMed] [Google Scholar]
- 10.McGill HC, Jr., McMahan CA, Malcom GT, Oalmann MC, Strong JP. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol. 1995;15:431–440. doi: 10.1161/01.atv.15.4.431. [DOI] [PubMed] [Google Scholar]
- 11.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to Syndrome X from childehood to young adulthood. Arch Intern Med. 1994;154:1842–1847. [PubMed] [Google Scholar]
- 12.Bao W, Srinivasan SR, Valdez R, Greenlund KJ, Wattigney WA, et al. Longitudinal changes in cardiovascular risk from childhood to young adulthood in offspring of parents with coronary artery disease: the Bogalusa Heart Study [see comments] JAMA. 1997;278:1749–1754. [PubMed] [Google Scholar]
- 13.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study [see comments] N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 14.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 15.Juonala M, Jarvisalo MJ, Maki-Torkko N, Kahonen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–1493. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- 16.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diab. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 17.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus--progress made, more to be done. J Clin Endocrinol Metab. 2006;91:3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- 18.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetol. 2003;46:760–765. doi: 10.1007/s00125-003-1116-6. [DOI] [PubMed] [Google Scholar]
- 20.Soedamah-Muthu SS, Chaturvedi N, Toeller M, Ferriss B, Reboldi P, Michel G, et al. Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diab Care. 2004;27:530–537. doi: 10.2337/diacare.27.2.530. [DOI] [PubMed] [Google Scholar]
- 21.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 22.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 23.Maahs DM, Maniatis AK, Nadeau K, Wadwa RP, McFann K, Klingensmith GJ. Total cholesterol and high-density lipoprotein levels in pediatric subjects with type 1 diabetes mellitus. J Pediatr. 2005;147:544–546. doi: 10.1016/j.jpeds.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 24.Kershnar AK, Daniels SR, Imperatore G, Palla SL, Petitti DB, Pettitt DJ, et al. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: The search for diabetes in youth study. J Pediatr. 2006;149:314–319. doi: 10.1016/j.jpeds.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 25.Schwab KO, Doerfer J, Hecker W, Grulich-Henn J, Wiemann D, Kordonouri O, et al. Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: cross-sectional data from the German diabetes documentation and quality management system (DPV) Diab Care. 2006;29:218–225. doi: 10.2337/diacare.29.02.06.dc05-0724. [DOI] [PubMed] [Google Scholar]
- 26.Maahs DM, Wadwa RP, McFann K, Nadeau K, Williams MR, Eckel RH, et al. Longitudinal lipid screening and use of lipid-lowering medications in pediatric type 1 diabetes. J Pediatr. 2007;150:146–50. doi: 10.1016/j.jpeds.2006.10.054. 150. [DOI] [PubMed] [Google Scholar]
- 27.Management of dyslipidemia in children and adolescents with diabetes. Diab Care. 2003;26:2194–2197. doi: 10.2337/diacare.26.7.2194. [DOI] [PubMed] [Google Scholar]
- 28.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diab Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 29.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 30.McCrindle BW, Urbina EM, Dennison BA, Jacobson MS, Steinberger J, Rocchini AP, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948–1967. doi: 10.1161/CIRCULATIONAHA.107.181946. [DOI] [PubMed] [Google Scholar]
- 31.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr. 2003;142:368–372. doi: 10.1067/mpd.2003.205. [DOI] [PubMed] [Google Scholar]
- 32.Dahl-Jorgensen K, Larsen JR, Hanssen KF. Atherosclerosis in childhood and adolescent type 1 diabetes: early disease, early treatment? Diabetol. 2005;48:1445–1453. doi: 10.1007/s00125-005-1832-1. [DOI] [PubMed] [Google Scholar]
- 33.Newman WP, III, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med. 1986;314:138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 34.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–2819. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 35.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 36.A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. JAMA. 1990;264:3018–3024. doi: 10.1001/jama.1990.03450230054029. [DOI] [PubMed] [Google Scholar]
- 37.McMahan CA, Gidding SS, Malcom GT, Tracy RE, Strong JP, McGill HC., Jr. Pathobiological determinants of atherosclerosis in youth risk scores are associated with early and advanced atherosclerosis. Peds. 2006;118:1447–1455. doi: 10.1542/peds.2006-0970. [DOI] [PubMed] [Google Scholar]
- 38.Gidding SS, McMahan CA, McGill HC, Colangelo LA, Schreiner PJ, Williams OD, et al. Prediction of coronary artery calcium in young adults using the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score: the CARDIA study. Arch Intern Med. 2006;166:2341–2347. doi: 10.1001/archinte.166.21.2341. [DOI] [PubMed] [Google Scholar]
- 39.McMahan CA, McGill HC, Gidding SS, Malcom GT, Newman WP, Tracy RE, et al. PDAY risk score predicts advanced coronary artery atherosclerosis in middle-aged persons as well as youth. Atherosclerosis. 2007;190:370–377. doi: 10.1016/j.atherosclerosis.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Jolliffe CJ, Janssen I. Distribution of lipoproteins by age and gender in adolescents. Circulation. 2006;114:1056–1062. doi: 10.1161/CIRCULATIONAHA.106.620864. [DOI] [PubMed] [Google Scholar]
- 41.McGill HC, Jr., McMahan CA. Starting earlier to prevent heart disease. JAMA. 2003;290:2320–2322. doi: 10.1001/jama.290.17.2320. [DOI] [PubMed] [Google Scholar]
- 42.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–1831. doi: 10.1016/S0140-6736(07)60821-6. [DOI] [PubMed] [Google Scholar]
- 43.Petitti DB, Imperatore G, Palla SL, Daniels SR, Dolan LM, Kershnar AK, et al. Serum lipids and glucose control: the SEARCH for Diabetes in Youth study. Arch Pediatr Adolesc Med. 2007;161:159–165. doi: 10.1001/archpedi.161.2.159. [DOI] [PubMed] [Google Scholar]
- 44.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 45.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diab Care. 2003;26:2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 46.Wadwa RP, Kinney GL, Maahs DM, Snell-Bergeon J, Hokanson JE, Garg SK, et al. Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diab Care. 2005;28:1051–1056. doi: 10.2337/diacare.28.5.1051. [DOI] [PubMed] [Google Scholar]
- 47.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 48.Orchard TJ, Virella G, Forrest KY, Evans RW, Becker DJ, Lopes-Virella MF. Antibodies to oxidized LDL predict coronary artery disease in type 1 diabetes: a nested case-control study from the Pittsburgh Epidemiology of Diabetes Complications Study. Diab. 1999;48:1454–1458. doi: 10.2337/diabetes.48.7.1454. [DOI] [PubMed] [Google Scholar]
- 49.Colhoun HM, Otvos JD, Rubens MB, Taskinen MR, Underwood SR, Fuller JH. Lipoprotein subclasses and particle sizes and their relationship with coronary artery calcification in men and women with and without type 1 diabetes. Diab. 2002;51:1949–1956. doi: 10.2337/diabetes.51.6.1949. [DOI] [PubMed] [Google Scholar]
- 50.Leiter LA. The prevention of diabetic microvascular complications of diabetes: is there a role for lipid lowering? Diabetes Res Clin Pract. 2005;68(Suppl 2):S3–14. doi: 10.1016/j.diabres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline KL, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diab Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 52.Craig SR, Amin RV, Russell DW, Paradise NF. Blood cholesterol screening influence of fasting state on cholesterol results and management decisions. J Gen Intern Med. 2000;15:395–399. doi: 10.1046/j.1525-1497.2000.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srinivasan SR, Frontini MG, Xu J, Berenson GS. Utility of childhood non-high-density lipoprotein cholesterol levels in predicting adult dyslipidemia and other cardiovascular risks: the Bogalusa Heart Study. Peds. 2006;118:201–206. doi: 10.1542/peds.2005-1856. [DOI] [PubMed] [Google Scholar]
- 54.Standards of medical care in diabetes--2006. Diab Care. 2006;29(Suppl 1):S4–42. [PubMed] [Google Scholar]
- 55.Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. ISPAD clinical practice consensus guidelines 2006-2007. Microvascular and macrovascular complications. Pediatr Diabetes. 2007;8:163–170. doi: 10.1111/j.1399-5448.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 56.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes--2006: a position statement of the American Diabetes Association. Diab Care. 2006;29:2140–2157. doi: 10.2337/dc06-9914. [DOI] [PubMed] [Google Scholar]
- 57.Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diab Care. 2003;26(Suppl 1):S51–S61. doi: 10.2337/diacare.26.2007.s51. [DOI] [PubMed] [Google Scholar]
- 58.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12:911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 59.Lauer RM, Obarzanek E, Hunsberger SA, Van HL, Hartmuller VW, Barton BA, et al. Efficacy and safety of lowering dietary intake of total fat, saturated fat, and cholesterol in children with elevated LDL cholesterol: the Dietary Intervention Study in Children. Am J Clin Nutr. 2000;72:1332S–1342S. doi: 10.1093/ajcn/72.5.1332s. [DOI] [PubMed] [Google Scholar]
- 60.Obarzanek E, Kimm SY, Barton BA, Van Horn LL, Kwiterovich PO, Jr., Simons-Morton DG, et al. Long-term safety and efficacy of a cholesterol-lowering diet in children with elevated low-density lipoprotein cholesterol: seven-year results of the Dietary Intervention Study in Children (DISC) Peds. 2001;107:256–264. doi: 10.1542/peds.107.2.256. [DOI] [PubMed] [Google Scholar]
- 61.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diab Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 62.Mayer-Davis EJ, Nichols M, Liese AD, Bell RA, Dabelea DM, Johansen JM, et al. Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc. 2006;106:689–697. doi: 10.1016/j.jada.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Rodenburg J, Vissers MN, Daniels SR, Wiegman A, Kastelein JJ. Lipid-lowering medications. Pediatr Endocrinol Rev. 2004;2(Suppl 1):171–180. [PubMed] [Google Scholar]
- 64.Belay B, Belamarich PF, Tom-Revzon C. The use of statins in pediatrics: knowledge base, limitations, and future directions. Peds. 2007;119:370–380. doi: 10.1542/peds.2006-0787. [DOI] [PubMed] [Google Scholar]
- 65.Davidson MH, McGarry T, Bettis R, Melani L, Lipka LJ, LeBeaut AP, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 66.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, et al. Simvastatin with or without Ezetimibe in Familial Hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 67.Brown BG, Taylor AJ. Does ENHANCE Diminish Confidence in Lowering LDL or in Ezetimibe? N Engl J Med. 2008;358:1504–07. doi: 10.1056/NEJMe0801608. [DOI] [PubMed] [Google Scholar]
- 68.Drazen JM, Jarcho JA, Morrissey S, Curfman GD. Cholesterol Lowering and Ezetimibe. N Engl J Med. 2008;358:1507–08. doi: 10.1056/NEJMe0801842. [DOI] [PubMed] [Google Scholar]
- 69.Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Groner JA, Joshi M, Bauer JA. Pediatric precursors of adult cardiovascular disease: noninvasive assessment of early vascular changes in children and adolescents. Peds. 2006;118:1683–1691. doi: 10.1542/peds.2005-2992. [DOI] [PubMed] [Google Scholar]
- 71.Wadwa R, Rewers M. Update on noninvasive detection of cardiovascular disease in diabetes. Current Opinion in Endocrinology and Diabetes. 2005;12:267–272. [Google Scholar]
- 72.Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, et al. Effect of Type 1 Diabetes on the Gender Difference in Coronary Artery Calcification: a Role for Insulin Resistance?: The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diab. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 73.Snell-Bergeon JK, Hokanson JE, Jensen L, Mackenzie T, Kinney G, Dabelea D, et al. Progression of Coronary Artery Calcification in Type 1 Diabetes: The importance of glycemic control. Diab Care. 2003;26:2923–2928. doi: 10.2337/diacare.26.10.2923. [DOI] [PubMed] [Google Scholar]
- 74.Budoff MJ, Lane KL, Bakhsheshi H, Mao S, Grassmann BO, Friedman BC, et al. Rates of progression of coronary calcium by electron beam tomography. Am J Cardiol. 2000;86:8–11. doi: 10.1016/s0002-9149(00)00820-1. [DOI] [PubMed] [Google Scholar]
- 75.Gunczler P, Lanes R, Soros A, Verdu L, Ramon Y, Guevara B, et al. Coronary artery calcification, serum lipids, lipoproteins, and peripheral inflammatory markers in adolescents and young adults with type 1 diabetes. J Pediatr. 2006;149:320–323. doi: 10.1016/j.jpeds.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 76.Jarvisalo MJ, Jartti L, Nanto-Salonen K, Irjala K, Ronnemaa T, Hartiala JJ, et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–2947. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]
- 77.Krantz JS, Mack WJ, Hodis HN, Liu CR, Liu CH, Kaufman FR. Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. J Pediatr. 2004;145:452–457. doi: 10.1016/j.jpeds.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 78.Jarvisalo MJ, Putto-Laurila A, Jartti L, Lehtimaki T, Solakivi T, Ronnemaa T, et al. Carotid Artery Intima-Media Thickness in Children With Type 1 Diabetes. Diab. 2002;51:493–498. doi: 10.2337/diabetes.51.2.493. [DOI] [PubMed] [Google Scholar]
- 79.Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab. 2003;88:5375–5380. doi: 10.1210/jc.2003-030722. [DOI] [PubMed] [Google Scholar]
- 80.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance - An integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 81.Brooks BA, Molyneaux LM, Yue DK. Augmentation of central arterial pressure in Type 2 diabetes. Diabet Med. 2001;18:374–380. doi: 10.1046/j.1464-5491.2001.00479.x. [DOI] [PubMed] [Google Scholar]
- 82.Brooks B, Molyneaux L, Yue DK. Augmentation of central arterial pressure in type 1 diabetes. Diab Care. 1999;22:1722–1727. doi: 10.2337/diacare.22.10.1722. [DOI] [PubMed] [Google Scholar]
- 83.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 84.Lurbe E, Torro MI, Carvajal E, Alvarez V, Redon J. Birth weight impacts on wave reflections in children and adolescents. Hypertension. 2003;41:646–650. doi: 10.1161/01.HYP.0000048341.52293.7C. [DOI] [PubMed] [Google Scholar]
- 85.Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C, Schwartz RF, et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diab Care. 2004;27:2911–2917. doi: 10.2337/diacare.27.12.2911. [DOI] [PubMed] [Google Scholar]
- 86.Urbina EM, Srinivasan SR, Tang R, Bond MG, Kieltyka L, Berenson GS. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study) Am J Cardiol. 2002;90:953–958. doi: 10.1016/s0002-9149(02)02660-7. [DOI] [PubMed] [Google Scholar]
- 87.Haller MJ, Stein J, Shuster J, Theriaque D, Silverstein J, Schatz DA, et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes. 2007:8193–198. doi: 10.1111/j.1399-5448.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 88.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 89.Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 90.Tenkanen L, Manttari M, Kovanen PT, Virkkunen H, Manninen V. Gemfibrozil in the treatment of dyslipidemia: an 18-year mortality follow-up of the Helsinki Heart Study. Arch Intern Med. 2006;166:743–748. doi: 10.1001/archinte.166.7.743. [DOI] [PubMed] [Google Scholar]
- 91.Strandberg TE, Pyorala K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364:771–777. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 92.Arambepola C, Farmer AJ, Perera R, Neil HA. Statin treatment for children and adolescents with heterozygous familial hypercholesterolaemia: A systematic review and meta-analysis. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 93.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Buller HR, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 2004;292:331–337. doi: 10.1001/jama.292.3.331. [DOI] [PubMed] [Google Scholar]
- 94.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diab Care. 2008;31:811–822. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]