Abstract
The past two decades have contributed a large body of preclinical work that has assisted in our understanding of the underlying pathophysiological mechanisms that cause chronic pain. In this context, it has been recognized that effective treatment of pain is a priority and that treatment often involves the use of one or a combination of agents with analgesic action. The current review presents an evidence-based approach to the pharmacotherapy of chronic pain. Medline searches were done for all agents used as conventional treatment in chronic pain. Published papers up to June 2005 were included. The search strategy included randomized, controlled trials, and where available, systematic reviews and meta-analyses. Further references were found in reference sections of papers located using the above search strategy. Agents for which there were no controlled trials supporting efficacy in treatment of chronic pain were not included in the present review, except in cases where preclinical science was compelling, or where initial human work has been positive and where it was thought the reader would be interested in the scientific evidence to date.
Keywords: Anticonvulsants, Antidepressants, Cannabinoids, Chronic pain, Opioids, Pharmacotherapy, Topical analgesics
Abstract
Les deux dernières décennies ont contribué à un vaste ensemble de travaux précliniques qui nous ont permis de mieux comprendre les mécanismes physiopathologiques sous-jacents responsables de la douleur chronique. Dans ce contexte, il est admis que le traitement efficace de la douleur est une priorité et qu’il exige souvent le recours à un agent ou à une association d’agents ayant une action analgésique. La présente analyse présente une démarche probante de la pharmacothérapie de la douleur chronique. Des recherches dans Medline ont été effectuées sur tous les agents utilisés pour le traitement classique de la douleur chronique. Les articles publiés jusqu’en juin 2005 ont été inclus. La stratégie de recherche incluait les essais aléatoires et contrôlés et, si elles étaient disponibles, les analyses systématiques et les méta-analyses. D’autres références ont été obtenues dans la partie des références des articles trouvés à l’aide de la stratégie de recherche précédente. Les agents dont aucun essai contrôlé n’étayait l’efficacité pour le traitement de la douleur chronique étaient exclus de la présente analyse, sauf si les données scientifiques précliniques étaient convaincantes, si les travaux humains initiaux étaient positifs ou si on pensait que les données scientifiques colligées jusque-là étaient intéressantes pour le lecteur.
Persistent pain is an escalating public health problem, currently affecting approximately 29% of Canadians (1). It is anticipated to affect one in three Canadians over the next two decades. Pain is the most common reason why Canadians seek help from health professionals; 21.5% of patients seen by primary care physicians suffer from persistent pain (2). At any one time, seven million Canadians are taking pain medication, yet many do not find relief. Intractable pain is a major cause of suffering and disability in our society. To live every day with severe pain is an extremely adverse experience that challenges every fibre of an individual’s being. People who have coped admirably well with major life adversity in the past can find themselves particularly challenged by the experience of relentless pain.
Traditionally, clinicians have conceptualized chronic pain as a symptom of disease or injury. Treatment was focused at addressing the underlying cause with the expectation that the pain would then resolve. It was thought that the pain itself could not kill. There is mounting evidence that ‘pain can kill’. It has been demonstrated that uncontrolled pain compromises immune function, promotes tumour growth, and can compromise healing with an increase in morbidity and mortality following surgery (3,4). Constant pain at moderate to severe levels, especially when associated with depression, can increase suicide risk. Often, chronic pain may cause more suffering and disability than the injury or illness that caused it in the first place. Alarming figures recently emphasized during the Global Day Against Pain (cosponsored by the World Health Organization, the International Association for the Study of Pain and the European Foundation of International Association for the Study of Pain Chapters) stated that more than 50% of patients still suffer severe and intolerable pain after surgery and trauma <http://www.painreliefhumanright.com>. Studies have demonstrated that 30% to 50% of patients suffer from chronic pain 1.5 to more than two years after such surgeries as open inguinal hernia repair (5) or thoracotomy (6), and that acute pain after surgery predicts long-term pain two years later (6).
We now recognize that the treatment of pain must be given high priority. The American Board for Hospital Accreditation has adopted pain as ‘the fifth vital sign’. This has resulted in mandated routine assessment and treatment of pain in hospital settings in all populations across the life cycle. The Canadian Pain Society has successfully advocated for a similar approach in Canada. Beginning in 2005, the Canadian Council on Health Services Accreditation now includes pain assessment and management in the Achieving Improved Measurement Standards (7).
Exponential growth in pain research in the past three decades has increased our understanding of underlying mechanisms of the pathophysiology of chronic pain. It is now known that peripheral and central events related to disease or injury can trigger long-lasting changes in the spinal cord and brain that lead to continued generation of afferent information through pain conducting systems (8–12). By such means, pain can persist beyond the point where normal healing takes place.
The system involved in pain transmission has ascending and descending branches at multiple levels. Complex interactions take place with feedback loops and multiple neurotransmitters involved. As described by Patrick Wall, “sensory systems are not dedicated and hard wired but are held in a steady state by elaborate dynamic control mechanisms” (12). Under normal or physiological conditions, it is nociceptors, sensory neurons (Table 1) and their projections that transmit patterns of impulses that are ultimately interpreted by the brain as pain. Following tissue damage, a number of changes take place within pain conducting systems. These changes can be broadly categorized as due to sensitization, structural reorganization and disinhibition (8). Neurons in this system can be pushed outside of their normal working range. Thus, nociceptors that are normally only responsive to noxious stimuli can begin to fire in response to stimuli that do not normally cause pain (eg, light touch), and receptors that normally respond to light touch can now evoke activity in the nociceptive system. This abnormal pattern of transmission assists in the understanding of clinical symptoms and signs such as allodynia, hyperalgesia, anesthesia dolorosa, phantom limb and phantom visceral pain (see Table 1 for definitions).
TABLE 1.
Definitions of pain terms
| Pain | An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage |
| Allodynia | Pain due to a stimulus which does not normally provoke pain |
| Dysesthesia | An unpleasant abnormal sensation, whether spontaneous or evoked |
| Hyperalgesia | An increased response to a stimulus which is normally painful |
| Anesthesia dolorosa | Pain in an area or region that is anesthetic |
| Nociceptor | A receptor preferentially sensitive to a noxious stimulus or to a stimulus that would become noxious if prolonged |
| Neuropathic pain | Pain initiated or caused by a primary lesion or dysfunction in the nervous system |
Data from reference 281
In the past, poorly understood chronic pain conditions, such as those noted above, have frequently been attributed to psychological pathology. However, chronic pain is no longer conceptualized according to a dichotomy where pain is thought to be due to either physical or psychological causes. This view is simplistic, ignores a huge body of research and results in ‘patient blaming’. Current research supports a holistic mind and body approach to the conceptualization and management of pain. It is in this context that appropriate pharmacotherapy for chronic pain should take place.
Chronic pain may result from a sustained sensory abnormality occurring as a result of ongoing peripheral pathology, such as chronic inflammation. It may also be autonomous and independent of the trigger that initiated it, as in neuropathic pain, or may contain elements of both. Thus, patients may present with nociceptive pain (pain due to tissue damage), neuropathic pain (pain due to nerve damage) or a combination of both. Pain may also be present in the absence of tissue damage. As considered above, because multiple levels of the nervous system are involved, numerous factors, such as overall health and conditioning, psychological issues, and metabolic, hormonal and circadian influences, can influence the experience of pain. It is important to consider the probable mechanism or mechanisms of pain to identify an overall management program and the agent or combination of agents most likely to benefit the patient (10,13). The present review focuses on pharmacotherapeutic options for patients with chronic, non-cancer pain, and aims to assist clinicians in choosing agents for an overall pain management program.
METHODS
MEDLINE searches were performed for all agents used as conventional treatment in chronic pain. Published papers up to June 2005 were included. Search strategy included randomized, controlled trials, and where available, systematic reviews and meta-analyses. Further references were found in reference sections of papers located using the above search strategy. Agents for which there were no controlled trials supporting efficacy in the treatment of chronic pain were not included in the review, except in cases where preclinical science was compelling or where initial human work has been positive and where it was thought that the reader would be interested in the scientific evidence to date (eg, certain anticonvulsants). Details are presented in the appropriate sections.
GENERAL PRINCIPLES OF PHARMACOTHERAPEUTICS IN CHRONIC PAIN
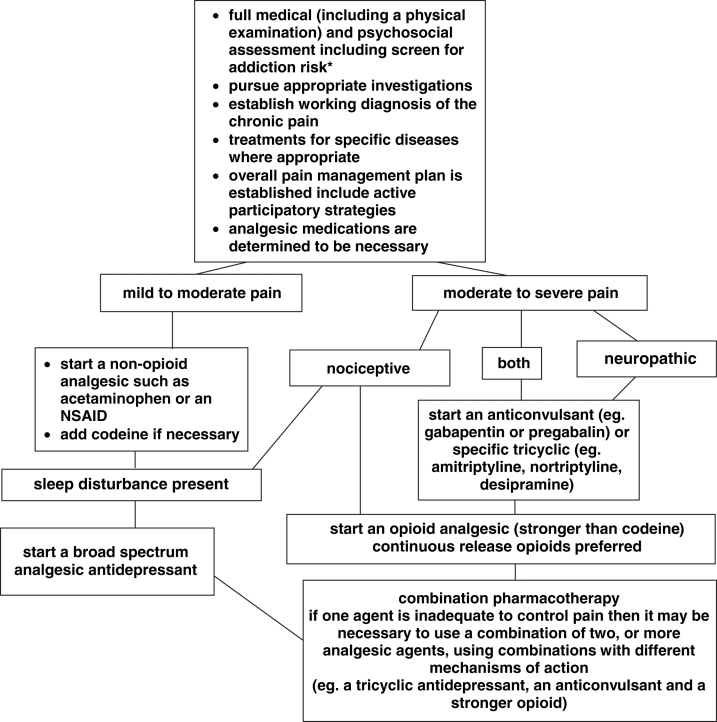
A treatment algorithm for chronic pain is presented in Figure 1. Once the physician has established the working diagnosis and has identified that medication is necessary, the usual approach is to start with a nonopioid analgesic such as a nonsteroidal anti-inflammatory drug (NSAID) or acetaminophen for mild to moderate pain (see specific section on each drug class). If this is inadequate and if there is an element of sleep loss, the next step may be to add an antidepressant with analgesic qualities. If there is a component of neuropathic pain, then a trial of one of the anticonvulsant analgesic agents is appropriate. If these steps are inadequate, then an opioid analgesic may be added. The use of opioids in chronic, noncancer pain is reviewed in more detail in the appropriate section (page 23). In an individual patient, one or several mechanisms may be at play in the etiology of the pain and more than one pharmacotherapeutic agent may be necessary for pain control; thus, it may be appropriate to use a combination of agents with different mechanisms of action in an effort to obtain adequate pain control. This combination approach has been supported by a recent randomized, double-blind, active-placebo-controlled trial (14), which found that gabapentin and morphine combined achieved better analgesia at lower doses than when the agents were used alone.
Figure 1).
Treatment algorithm for pharmacotherapy of chronic non-cancer pain. Note: In general, if one agent in a class of medications does not provide adequate analgesia or causes limiting side effects it is worth pursuing serial trials of one or two others from the class. *See Table 11. NSAID Nonsteroidal anti-inflammatory drug
NSAIDs
Mechanisms of action
For some time, it has been understood that the anti-inflammatory effects of the NSAIDs are due to the inhibition of enzymes that synthesize prostaglandins. Initially, it was thought that relief of pain was secondary to relief of inflammation, but subsequent research has indicated that there is poor correlation between anti-inflammatory activity and analgesic efficacy. In addition, there is research to indicate that NSAIDs exert their analgesic action not only through peripheral inhibition of prostaglandin synthesis, but also through a variety of other peripheral and central mechanisms (15–19).
The production of prostaglandins begins with membrane phospholipids, which are precursors to arachidonic acid. NSAIDs inhibit the cyclo-oxygenase (COX) step in this pathway. There are two structurally distinct forms of the COX enzyme, COX-1 and COX-2. COX-1 is a component of normal cells mediating production of prostaglandins involved in normal physiological functions such as cytoprotection of gastric mucosa and regulation of renal bloodflow, and COX-2 is the inducible form of the enzyme expressed in inflammatory cells (20,21).
Because COX-2 is the form of the enzyme present in inflammatory cells, it was initially thought that COX-2 inhibition was the probable mechanism of action for NSAID-mediated analgesia. However, the literature examining this question reveals essentially no differences in clinical efficacy between NSAIDs exhibiting preferential activity for COX-1 or COX-2 (22).
There is increasing evidence that NSAIDs also exhibit a central mechanism of action (15,18–20). Spinal and supraspinal NSAIDs are antinociceptive in animal models (19) and exhibit 10 to 100 times the potency when administered spinally compared with systemically (20). When co-administered, spinal ketorolac and morphine demonstrate a synergistic rather than a mere additive interaction (18). Intrathecal injection of ibuprofen or acetylsalicyclic acid (ASA) suppresses hyperalgesia induced by N-methyl-D-aspartate (NMDA), d,l-alpha-amino-3-hydroxy-5-methyl-4-isoxalone propionic acid and substance P (23). NSAIDs decrease thalamic activity evoked by electrostimulation of nociceptive primary afferents, systemic NSAIDs decrease release of spinal prostaglandin E2, and NSAIDs block centrally mediated hyperalgesia evoked by spinal NMDA and substance P (19). A number of mechanisms have been implicated in central actions of NSAIDs, including central prostaglandin synthesis and mechanisms involving opioid, serotonergic and NMDA or excitatory amino acids (16,19). Thus, the mechanism of action of NSAIDs in production of analgesia is probably multifactorial, with both peripheral and central effects, and prostaglandin inhibition is only one component.
Conventional NSAIDs versus COX-2 selective agents
The COX-2 selective inhibitors (also called COXIBs) were designed in an effort to reduce the gastrointestinal (GI) side effects associated with the conventional NSAIDs. Large, randomized, controlled trials demonstrated improved GI safety for rofecoxib (Vioxx, Merck Frosst Canada) (24) and lumiracoxib (another COX-2 selective agent not available in Canada) (25). A large trial (26) of celecoxib (Celebrex, Pfizer Canada Inc) demonstrated improved GI safety at six months, but the final analysis at 300 days did not find improved protection against ulcers for celecoxib compared with nonselective NSAIDs (27). (In this study, celecoxib was used at a dose of 400 mg twice daily, a higher dose than is recommended.) Recent literature has determined that COX-2 selective agents are associated with an increased risk of cardiovascular events such as stroke and myocardial infarction (28,29). The selective COX-2 agents inhibit production of prostacyclin, a potent inhibitor of platelet aggregation. This is believed to lead to a prothrombotic state secondary to unopposed activity of COX-1 mediated thromboxane A2 (which is proatherothrombotic), resulting in an increased risk of myocardial infarction and stroke (28). The COX-2 selectivity of rofecoxib is approximately 10 times greater than that of celecoxib, which may explain the observation that the risk of myocardial infarction is greater with rofecoxib than with celecoxib (30).
Subsequent to these studies, rofecoxib was withdrawn from the market by the manufacturer in the fall of 2004. The United States Food and Drug Administration (FDA) and Health Canada have reviewed the evidence and released statements on April 7, 2005. These statements can be found at <www.fda.gov/bbs/topics/news/2005/NEW01171.html> and <www.hc-sc.gc.ca/english/protection/warnings/2005/2005_17.html>, respectively. Health Canada has recommended usage restrictions on celecoxib, and the FDA has asked the manufacturer to include a boxed warning in the celecoxib label. Health Canada has indicated that patients who have had a heart attack or stroke, experienced serious chest pain related to heart disease or had serious disease of the heart such as congestive heart failure should not use celecoxib. The medication should be prescribed and used in the lowest possible dose, for the shortest necessary time, and should only be used to treat pain and inflammation of arthritis and certain types of acute pain. Health Canada further warns that patients who have serious risk factors for heart attack or stroke should be aware that using celecoxib may increase this risk. Health Canada and the FDA have also requested that the manufacturer of a third COX-2 selective agent, valdecoxib, withdraw it from the market on the basis of serious and possibly life-threatening skin reactions until safety issues have been resolved.
The only COX-2 selective agent available in Canada at the time of writing is celecoxib; however, others will likely become available as the safety issues are further clarified.
Clinical guidelines
The Third Canadian Consensus Conference regarding an evidence-based approach to prescribing NSAIDs was held in January 2005, at which time the literature, including the recent trials regarding the cardiovascular risks associated with the COX-2 selective agents, was reviewed and recommendations were made. These recommendations are summarized in Table 2 (27).
TABLE 2.
Summary of recommendations from the Third Canadian Consensus Conference regarding an evidence-based approach to prescribing nonsteroidal anti-inflammatory drugs (NSAIDs)
| Recommendation | |
|---|---|
| Patient-physician communication | Patients should be fully informed about evolving information regarding the benefits and risks of their treatment options. |
| Indications | NSAIDs, including COXIBs, are generally more effective and preferred by patients over acetaminophen, although a trial of acetaminophen is warranted in some patients. |
| Gastrointestinal toxicity | In patients with risk factors for perforations, ulcers and gastric bleeding, a COXIB is the NSAID of choice, depending on the patient’s cardiovascular risks. |
| If NSAIDs must be used in high-risk patients with a history of gastrointestinal bleeding, a proton pump inhibitor should be prescribed as well. | |
| NSAIDs can adversely affect the entire gastrointestinal tract; however, the prevalence of clinically relevant NSAID-associated lower gastrointestinal disease is unclear. | |
| Renal issues | Before starting an NSAID or COXIB, determine renal status and creatinine clearance in patients older than 65 years or in those with comorbid conditions that may affect renal function. |
| Advise patients that if they cannot eat or drink that day, they should withhold that day’s dose of NSAID/COXIB. | |
| Hypertension | In patients receiving antihypertensive drugs, remeasure blood pressure within a few weeks after initiating NSAID therapy and monitor appropriately; drug doses may need to be adjusted. |
| Cardiovascular events | Patients on rofecoxib have been shown to have an increased risk of cardiovascular events, and data suggest that this risk may be an effect of the NSAID/COXIB class. Physicians and patients should weigh the benefits and risks of therapy. |
| Geriatric considerations | NSAIDs/COXIBs should be used with caution in elderly patients, who are at greatest risk of serious gastrointestinal, renal and cardiovascular side effects. |
Data from reference 27. COXIBS Cyclo-oxygenase-2 selective inhibitors
General guidelines:
NSAIDs are most commonly administered orally, but certain agents are available for parenteral and rectal administration. Recently, topical agents have received increased attention (see section on topical analgesics, page 30). Some NSAIDs are equivalent to ASA in action, while others are more efficacious. There is no risk of physiological tolerance, but there is a ceiling effect (ie, increasing the dose above a certain level does not produce additional analgesia). Side effect profiles and pharmacokinetics vary among agents and from patient to patient as a result of differences in rate of absorption, metabolism, elimination and ratio of bound-to-unbound drug. All attempts to rank order the NSAIDs in terms of analgesic efficacy have been unsuccessful, and broad comparisons are the best available approach. As mentioned, there is poor correlation between the anti-inflammatory activity and analgesia; this is not surprising given the fact that these actions involve different mechanisms.
There are, however, principles that can guide the clinician in making an appropriate choice. Simplicity of dosing, tolerability, comparative toxicity, efficacy and cost are the main issues to take into consideration (31). Details regarding NSAID agents currently available in Canada appear in Table 3. In chronic pain conditions, the once-a-day or twice-a-day administration of a long half-life drug has a clear advantage. If a patient fails to respond to one agent, it is reasonable to select another, perhaps from a different class. For chronic use, the lowest dose that provides satisfactory results should be maintained. Keep in mind that elderly patients are at higher risk for adverse effects, particularly GI bleeding, which may be dose related, so lower doses should be used (at least to start), creatinine clearance should be checked and adverse events should be monitored closely.
TABLE 3.
Nonsteroidal anti-inflammatory drugs (NSAIDs)
| Drug class | Drug name | Common trade name | Usual dose po in mg | Maximum daily dose (mg) | Analgesic efficacy compared with ASA 650 mg po | Comments |
|---|---|---|---|---|---|---|
| Salicylates | ASA | Aspirin* | 325–650, q4-6h | 4000 | Irreversible antiplatelet effect | |
| Diflunisal | Dolobid† | 250–500, bid | 1500 | Superior | No antiplatelet effect at lower doses | |
| Propionic acids | Ibuprofen | Motrin‡, Advil§ | 200–800, tid | 3200 | Superior at both doses | Available as suppository, 50 mg/100 mg |
| Naproxen | Naprosyn¶ | 125–500, bid | 1250 | Available as suppository, 500 mg | ||
| Naproxen sodium | Anaprox¶ | 275–550, od/bid | 1375 | 275 mg is comparable in efficacy with ASA with slower onset and longer duration of action; 550 mg is superior | ||
| Oxaprozin | Daypro** | 600–1800, od | 1800 | |||
| Ketoprofen | – | 25–100, tid | 300 | 25 mg comparable; 50 mg superior | Available as suppository, 50 mg/100 mg | |
| Indole acetic acids | Indomethacin | Indocid†† | 25–50, tid | 200 | Comparable | High incidence of side effects, not recommended for routine use |
| Sulindac | – | 150–200, bid | 400 | Superior | ||
| Pyrolizine carboxylic acid | Ketorolac | Toradol¶ | 10, q6h
7 days maximum |
40 | Superior | IM formulation available, 10 mg/30 mg q4-6h (120 mg/day maximum) |
| Pyranocarboxylic acid | Etodolac | Ultradiol‡‡ | 200–600, bid | 1200 | Comparable | Relatively COX-2 selective; food markedly decreases absorption |
| Phenylacetic acids | Diclofenac sodium | Voltaren§§ | 25–50
25–75 |
150
150 |
Diclofenac potassium at 50 mg and 100 mg superior | IM diclofenac reported to be efficacious in renal colic; monitor liver chemistry |
| Diclofenac + misoprostol | Arthrotec-50** Arthrotec-75** |
50, 200 μg
75, 200 μg |
||||
| Anthranilic acids | Mefenamic acid | – | 500
250 |
1500 | Comparable | Use restricted to intervals of one week |
| Floctafenine | – | 200–400 | 1200 | Comparable | ||
| Oxicams | Piroxicam | – | 20 | 20 | Comparable in efficacy with ASA, with slower onset, longer duration | Oxicams exhibit long t1/2 (>50 h); mobicox is relatively COX-2 selective |
| Meloxicam | Mobicox*** | 7.5–15, od | 15 | |||
| Tenoxicam | Mobiflex¶ | 20–40, od | 40 | |||
| Napthylalkanones | Nabumetone | Relafen††† | 1000–2000, od | 2000 | Relatively COX-2 selective; gastric, renal and hematological safety long t1/2 (>24 h) | |
| COXIBs | Celecoxib | Celebrex** | 100 mg, bid (OA)
200 mg, bid (RA) |
400 | Comparable in efficacy | Highly COX-2 selective, improved GI toxicity, minimal platelet effects, similar renal toxicity, CV risk |
Data from reference 31. Note: Vioxx has been withdrawn from the market at the time of writing pending collection of additional safety information regarding cardiovascular risk.
Bayer Inc, Canada;
Merck, USA;
McNeil Consumer Healthcare, Canada;
Wyeth Consumer Healthcare, Canada;
Hoffmann-La Roche Canada;
Pfizer Canada Inc;
Merck Frosst Canada;
Proctor & Gamble, Canada;
Novartis Pharmaceuticals Canada;
Boehringer Ingelheim, Canada;
GlaxoSmithKline Inc, Canada. ASA Acetylsalicylic acid; bid Twice daily; CV Cardiovascular; COX-2 Cyclo-oxygenase-2; GI Gastrointestinal; IM Intramuscular; OA Osteoarthritis; od Once daily; po By mouth; q Every; RA Rheumatoid arthritis; t1/2 Half life; tid Three times daily
Adverse effects:
There are a number of adverse effects that one must be aware of when using NSAIDs. These are reviewed below according to the system affected. Further details appear in Tables 2, 4, 5 and 6.
TABLE 4.
Nonsteroidal anti-inflammatory drug (NSAID) adverse effects
| Adverse effects of most concern | |
|---|---|
| Gastrointestinal | Gastrointestinal ulceration and intolerance |
| Renal | Inhibition of prostaglandin-mediated renal function |
| Hemostatic | Blockade of platelet function |
| Pregnancy | Inhibition of uterine motility may prolong gestation |
| Immune | Hypersensitivity reactions |
| Cardiovascular | Increased blood pressure |
| Interactions | Warfarin: NSAIDs bind to plasma proteins and can displace from binding site |
TABLE 5.
Risk factors for the development of nonsteroidal anti-inflammatory drug (NSAID)-associated gastroduodenal ulcers
| Established risk factors |
|
| Probable risk factors |
|
TABLE 6.
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) in relation to gastrointestinal (GI) safety
Patients at low risk for GI complications (ie, <65 years of age with no risk factors for upper GI complications)*
|
Patients >65 years or any patient with a suspected history of ulcer*
|
In elderly patients without cardiovascular risks*
|
Patients with GI risk factors on low-dose ASA who require a NSAID*
|
Ulcer‡
|
Prophylaxis after ulcer heals‡
|
Gastric:
Adverse effects of NSAIDs on the GI tract include dyspepsia and an increased risk of gastric or duodenal ulcer or upper GI bleed and death. The mechanism involves a decrease in prostaglandin I2 and prostaglandin E2, which normally inhibit acid secretion, enhance mucosal blood flow and promote cytoprotective mucus (32). The NSAIDs vary considerably with respect to gastric toxicity. A systematic review (33) of 43 trials, involving over 1.3 million patients who had taken a nonselective NSAID for two months or longer found that one in five patients developed endoscopically visible ulcers, one in 70 were symptomatic, one in 150 experienced a bleed or perforation, and one in 1200 died. Infection with Helicobacter pylori is a predisposing factor for ulcers even in the absence of NSAIDs, but NSAIDs can increase the risk of ulcers associated with H pylori; thus, both eradication of H pylori and the concomitant use of a proton pump inhibitor decreases the incidence of ulcers in those requiring NSAIDs (27).
As mentioned previously, the COX-2 selective class was developed in an effort to decrease GI side effects and is still the NSAID of choice in patients at risk of perforation, ulcer or bleed, depending on cardiovascular risk (27). Of the older nonselective NSAIDs, nabumetone and ibuprofen are agents that have been well studied. Both have been reported to have a favourable GI safety profile (22,34). Nabumetone exhibited a total incidence of gastric perforation, ulcer formation and GI bleeding of 0.03% in a meta-analysis of 4471 patients in eight controlled trials (35). Although ketorolac is relatively COX-2-specific, it is highly gastrotoxic and, as a result, carries a five-day dosing restriction (22).
In a review of GI toxicity, it was identified that 81% of patients who developed serious GI complications with NSAIDs reported no previous dyspepsia (32). Prevention is therefore a priority. A number of risk factors have been identified, and these are listed in Table 5. The management of NSAID-related GI risk is presented in Table 6.
Renal:
Elderly patients are at particular risk for renal toxicity. Because renal dysfunction can be present even in the presence of a normal serum creatinine value, the Consensus Conference Group has recommended that creatinine clearance should be checked both before and after initiating conventional NSAIDs and the COX-2 selective agents (27). A creatinine clearance slide rule was developed, allowing physicians to align the patient’s serum creatinine level against weight and read the calculated creatinine clearance according to the patient’s age and sex. It is available upon request by e-mail at creatinineclearance@aol.com. The COX-2 selective agents do not offer greater renal safety. The risk of NSAID-associated renal dysfunction is low in most people, and renal complications are usually reversible on timely withdrawal of the NSAID in individuals without previous renal disease. In situations of renal compromise or in concomitant therapy with drugs affecting renal function (eg, diuretics, antihypertensives and cyclosporine A), the risks may be much higher and caution should be used (27).
Hemostases:
The inhibition of COX-1 is associated with decreased platelet thromboxane A2 with subsequent increases in bleeding time. Individuals taking anticoagulants or clopidogrel (Plavix, Sanofi-Synthelabo Canada Inc), or those with bleeding disorders may have their risk of bleeding increased by the use of a conventional NSAID. COX-2 selective agents should not alter bleeding time. Specifically, nabumetone has no significant effect on bleeding time, sulindac has mixed effects and indomethacin demonstrates pronounced effects on bleeding time (22). When platelet function is of particular concern, acetaminophen or one of the nonacetylated salicylates should be considered.
Cardiovascular:
The increased risk of myocardial infarction and stroke seen with the COX-2 selective agents was reviewed above (see the section on conventional NSAIDs versus COX-2 selective agents, page 13). In addition, the NSAIDs can raise blood pressure in normotensive and hypertensive individuals (average systolic increase 3 mmHg to 7 mmHg and average diastolic increase 1 mmHg to 3 mmHg) in 7% to 16% of patients, respectively. Furthermore, NSAIDs, including the COX-2 selective agents, antagonize the antihypertensive effects of agents that act on the renin-angiotensin-aldosterone system. This includes beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Calcium channel blockers appear to be the least affected. Thus, blood pressure should be monitored regularly and the lowest effective dose of NSAID should be used for the shortest time possible (27).
On the one hand, the FDA has asked the manufacturers of NSAIDs to revise their labels to include a boxed warning highlighting the potential for increased risk of cardiovascular events and GI bleeding associated with their use. On the other hand, the nonselective NSAIDs have been associated with reduced odds of nonfatal myocardial infarction relative to nonusers (30), and low-dose ASA continues to be used for stroke prevention. As new evidence emerges, the details regarding the risk-benefit assessment in the use of NSAIDs, both selective and nonselective, will be further clarified. In the meantime, the recommendations presented in Table 2 provide the best evidence-based guidance to date.
ACETAMINOPHEN
Acetaminophen is an effective oral analgesic and antipyretic. It is equianalgesic and equipotent with ASA in most types of pain, excluding inflammatory arthritic pain. In head-to-head patient preference studies comparing acetaminophen with NSAIDs in the treatment of osteoarthritis, over twice as many patients preferred NSAIDs to acetaminophen. Given its safety profile, however, acetaminophen can still be considered the first-line drug for patients with osteoarthritis (27). Acetaminophen does not possess a significant anti-inflammatory effect (36). Until recently, the mechanism of action for this agent has been poorly understood. It is a weak inhibitor of prostaglandin synthesis in peripheral tissues, but has been postulated to exert analgesic effects by selective inhibition of prostaglandin formation in the brain (37). Growing evidence supports the involvement of a central serotonergic mechanism in analgesic actions of acetaminophen (38–40).
Acetaminophen is rapidly absorbed from the GI tract and reaches peak plasma levels in 30 min to 60 min. It is metabolized by the liver and excreted in urine. The plasma half-life is 2 h to 3 h, and plasma protein binding is negligible (36). In recommended doses, acetaminophen is well tolerated and side effects are mild. The GI profile of this agent is usually benign. On occasion, patients may experience abdominal pain or diarrhea that may improve with decrease or discontinuation of acetaminophen. There is no effect on platelet function. The main concern is that of hepatotoxicity in patients who are alcoholic or who have liver disease. In acute overdose, a potentially fatal adverse effect is hepatic necrosis. The conventional oral or rectal adult dose of acetaminophen is 500 mg to 1000 mg every 4 h to 6 h (see maximum doses below). There is a shallow dose-response curve, so increasing the dose further does not confer additional analgesia. The total daily dose should not exceed 4000 mg, and for chronic use, the dose should be limited to 2500 mg/day.
ANTIDEPRESSANTS
Tricyclic antidepressants
The first controlled trial of the analgesic effect of amitriptyline in patients who were not depressed was performed in patients with postherpetic neuralgia (41). Several reviews of randomized, controlled trials have concluded that tricyclic antidepressants (TCAs) exhibit clear analgesic efficacy in a number of chronic pain conditions (42–47). Specifically, TCAs have demonstrated analgesia in pain caused by diabetic neuropathy, postherpetic neuralgia (for which there is a solid body of evidence to support efficacy), tension headache, migraine, atypical facial pain, fibromyalgia and low back pain. However, TCAs do not appear to be efficacious in painful HIV sensory neuropathy (48), spinal cord injury (49) and cisplatin-induced neuropathy (50). In neuropathic pain, TCAs relieve brief lan-cinating pain, constant dysesthetic pain, allodynia and spontaneous pain. The pain relief from TCAs is generally moderate in degree, and is accompanied by side effects such as sedation, postural hypotension, and anticholinergic side effects such as dry mouth and constipation. The analgesic effect is independent of the effect on mood. TCAs with a balanced inhibition of serotonin (5-hydroxytryptamine [5-HT]) and noradrenaline (NA) reuptake, such as amitriptyline, imipramine and clomipramine, as well as agents with greater NA reuptake inhibition, such as desipramine and nortriptyline, appear to be effective analgesics. A meta-analytic review (47) of controlled trials examining antidepressants in the treatment of neuropathic pain provides some guidance in assisting with which of the TCAs to use first. This review included 21 eligible placebo-controlled trials, 15 of which involved tricyclics. Of these, 13 trials contained information allowing calculation of numbers needed to treat (NNTs) for individual TCAs (NNT: the number of patients that must be treated for one patient to obtain a defined reduction in their pain; in this case, a 50% reduction in the pain). Table 7 provides further detail regarding NNT for benefit, and minor and major harm. There is some support for doxepin in the treatment of chronic, non-cancer pain according to another good systematic review, but NNT data were not available (51).
TABLE 7.
Average numbers needed to treat (NNT) among placebo-controlled trials examining tricyclic antidepressants (TCAs), and serotonin and noradrenaline reuptake inhibitor antidepressants for neuropathic pain for benefit (50% reduction of pain), and minor and major harm
| Agent (references) | NNT ‘benefit’ | NNT ‘minor harm’ | NNT ‘major harm’* | Number of studies† |
|---|---|---|---|---|
| Amitriptyline (47,51) | 2.4 | 20.4 | 30.5 | 6 |
| Imipramine (47,51,77) | 2.1 | 1.4 | 13.7 | 4 |
| Desipramine (47,51) | 2.4 | 12.4 | 15.2 | 3 |
| Nortriptyline (47,51) | 2.6 | 1.4 | – | 3 |
| Clomipramine (47,51) | 2.1 | no dichotomous data available | 8.7 | 1 |
| Average TCAs | 2.3 | 8.9 | 17 | |
| Venlafaxine (51,76,77) | 4.0 | 2 | ||
| SSRIs (51,88) | 6.7 | 3 |
Major harm consists of withdrawal from the study due to adverse effects;
This column refers to the number of studies for which there was adequate information with which to calculate an average NNT. Please note that these figures derive from studies using different methodologies, different data analyses, with different numbers of patients. There are few comparative trials and the external validity may be poor because of selection that goes into trials. Thus the NNT data is a rough guide only. SSRIs Serotonin-specific reuptake inhibitors
Mechanisms of action:
Initially, it was thought that the primary mechanism of action for analgesia might be the reuptake blockade of NA and 5-HT, leading to enhanced synaptic activity of these amines in pathways modulating pain, particularly those originating in the brain stem and projecting to the spinal cord. Accumulating evidence supports other potential mechanisms of action as presented below.
Opioid action:
Key observations supporting an opioid connection are the ability of naloxone (an opioid antagonist) to inhibit antinociception, and the ability of chronic antidepressant administration to alter endogenous opioid levels (met-and leu-enkephalin) and opioid binding in the central nervous system (CNS) (52). Antidepressants have low affinity for opioid receptors, so the opioid link may be related to indirect mechanisms.
Sodium and calcium channel blockade:
Antidepressants can block sodium (53,54) and calcium channels (55), both of which are important in neuronal and nociceptive signalling. Thus, the possibility exists that these properties may contribute to antinociception. Indeed, local anesthetic actions have been documented following peripheral administration of antidepressants to adjacent nerves (56,57).
NMDA receptor antagonism:
Antidepressants block NMDA receptors that are known to be important in central sensitization, which contributes to inflammatory and neuropathic pain. Amitriptyline has been demonstrated to exhibit NMDA antagonist activity in the presence of inflammatory hyperalgesia (58).
Adenosine:
Adenosine receptor antagonists such as methylxanthines (caffeine, theophylline) have been demonstrated to inhibit antinociception by TCAs following acute (59–62) and chronic systemic administration (63) in animal models, supporting an adenosine link in their action.
Moderate doses of caffeine, equivalent to those used by the majority of adults in coffee-consuming populations, are capable of blocking the antinociceptive actions of amitriptyline in pre-clinical studies (63). Thus, it is possible that two cups of coffee per day or more could limit the analgesic effect of TCAs. We await studies in humans to answer this question definitively. Potassium channels: Antidepressants open certain potassium channels, thus stabilizing membranes and leading to an inhibitory effect on neuronal activity. This inhibitory effect may contribute to a central antinociceptive action (64,65).
Other actions:
TCAs block receptors for a number of other neurotransmitters, including histamine H1, muscarinic and nicotinic cholinergic, 5-HT2 and alpha-adrenergic receptors. These actions explain certain side effects, but may also contribute to the analgesic properties because each is involved in nociceptive signalling (66,67).
Clinical guidelines:
Because most patients with chronic pain experience poor sleep, and because a number of the antidepressants have sedative qualities that can benefit sleep, a TCA with some sedation (eg, amitriptyline) is generally chosen as a first-line therapy when insomnia is also present. If patients find these agents too sedating, a less sedating agent such as desipramine or nortriptyline is chosen. Table 8 presents details that should aid clinicians in choosing an appropriate agent.
TABLE 8.
Analgesic antidepressants
| Neurotransmitter profile
|
Most common side effects (%)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Common trade name | Therapeutic range for pain (mg/24 h) | Half-life (h) | NA | 5-HT | Sedation | Orthostatic hypotension | Weight gain | Dry mouth | Constipation | GI distress, nausea, diarrhea |
|
Tricyclics | |||||||||||
| Amitriptyline | Elavil† | 10–150* | 10–46 | +++ | +++ | >30 | >10 | >30 | >30 | >10 | >2 |
| Doxepin | Sinequan‡ | 10–150* | 8–36 | +++ | ++ | >30 | >10 | >10 | >30 | >10 | <2 |
| Trimipramine | Surmontil§ | 10–150* | 7–30 | ++ | + | >30 | >10 | >10 | >10 | >10 | <2 |
| Imipramine | Tofranil¶ | 10–150* | 4–34 | +++ | +++ | >10 | >30 | >10 | >30 | >10 | >10 |
| Clomipramine | Anafranil** | 10–150* | 17–37 | +++ | ++++ | >2 | >10 | >10 | >30 | >10 | >10 |
| Desipramine | Norpramin§ | 10–150* | 12–76 | +++++ | ++ | >2 | >2 | >2 | >10 | >2 | >2 |
| Nortriptyline | Aventyl†† | 10–100* | 13–88 | ++++ | ++ | >2 | >2 | >2 | >10 | >10 | <2 |
|
Serotonin/noradrenaline reuptake inhibitors | |||||||||||
| Venlafaxine | Effexor‡‡ | 37.5–225 | 3–7 (parent)
9–13 (metabolite) |
++ | ++++ | >10 | >10 | <2 | >10 | >10 | >30 |
| Duloxetine | Cymbalta§§ | 60–120 | 10 | ++++ | +++++ | >10 | <10 | <2 | >10 | >10 | >10 |
The therapeutic range for depression is up to 200 mg/24 h for nortriptyline and to 300 mg/24 h for the remaining tricyclic antidepressants; generally, these doses are not required for an analgesic effect and the usual dose will consist of 75 mg/24 h or less;
1560678 Ontario Inc, Canada;
ERFA Canada Inc;
Aventis Pharma Inc, Canada;
Novartis Pharmaceuticals, Canada;
Oryx Pharmaceuticals Inc, Canada;
Pharmel Inc, Canada;
Wyeth Canada;
Lilly, USA. 5-HT 5-Hydroxytryptamine; GI Gastrointestinal; NA Noradrenaline. Adapted from reference 286
Doses less than those used for depression generally have been used in analgesic regimens. Unfortunately, there is little data regarding dose-response relationships with the analgesic actions of antidepressants (43). Usual guidelines are to start patients at a dose of 10 mg to 25 mg given at bedtime (unless one of the more activating agents is chosen; then, the dose should be taken in the morning). The dose may then be titrated every three to five days by a further 10 mg to 25 mg (in elderly patients titrate every five to seven days) until a therapeutic response is achieved or persistent bothersome side effects occur. There is a broad dose range within which analgesic effects can occur, but for most TCAs, a therapeutic response occurs between 10 mg/day and 75 mg/day in most patients.
One study (68) examining imipramine for treatment of diabetic neuropathy found that most patients appeared to obtain optimum relief at or below 400 nM/L, a plasma concentration that required imipramine doses of 125 mg/day to 350 mg/day. These investigators caution that because of variability in pharmacokinetics and plasma TCA concentrations needed to obtain an optimum response, one should not necessarily discontinue treatment because of inadequate response at standard doses such as 100 mg/day. Thus, in selected patients it may be reasonable to increase the dose levels that are normally used for depression as long as limiting side effects do not occur; however, this will be the case in a small minority of patients. Blood levels for TCAs may be obtained to assure adequate dosing in a situation in which the physician finds that higher doses are necessary. Special caution should be used in elderly populations, who may require the lower range of dosing and conservative titration schedules. Pain relief approaches maximum values after four days of treatment at the therapeutic level. If a patient does not experience a therapeutic response, or if bothersome side effects occur, it is reasonable to try another agent.
The main drawback of the TCAs is their adverse side effect profile (Table 8). This is related to the fact that TCAs exhibit activity on a number of neurotransmitter receptors with resultant anticholinergic, sedating, autonomic and cardiovascular effects. For this reason, TCAs must be used with caution in patients with a history of cardiovascular disease, glaucoma, urinary retention and autonomic neuropathy, and with extreme caution in elderly patients (42). The main contraindications to the use of the antidepressants are significant cardiac arrythmias, prostatic hypertrophy and narrow angle glaucoma. A study of depressed patients with ischemic heart disease found that 20% of patients treated with nortriptyline after a myocardial infarction developed adverse cardiac events (69). The Fourth International Conference on the Mechanisms and Treatment of Neuropathic Pain (42) therefore recommended a screening electrocardiogram to check for cardiac conduction abnormalities before beginning treatment with TCAs, especially in patients older than 40 years. Caution is also recommended when there is a risk of suicide or accidental death by overdose.
There is some variation among the TCAs with regard to side effect profiles. Most are sedating, cause anticholinergic side effects such as dry mouth and constipation, and can cause postural hypotension and troublesome weight gain. In elderly patients, the risk of postural hypotension is increased. Nortriptyline and desipramine have fewer adverse effects and are generally the better tolerated of these agents (42).
Potential drug interactions of importance include interference with the antihypertensive effects of guanethidine, clonidine and similarly acting compounds; a risk of paralytic ileus when used in combination with anticholinergic drugs; enhanced response to alcohol, barbiturates and other CNS depressants; decreased insulin sensitivity with amitriptyline; and a possible serotonin syndrome when used with other serotonergic agents (eg, serotonin-specific reuptake inhibitors [SSRIs], sumatriptan and other triptans which are serotonin agonists). When used with opioids, TCAs may enhance the analgesic effect, but may also lead to additive sedation. When used with opioids, plasma levels of desipramine are increased; there is also marked inhibition of conversion of codeine to morphine with most of the TCAs (42). This is probably due to a decrease in activity of the cytochrome 2D6 isoenzyme. Except in special circumstances, one should avoid using the TCAs with irreversible monoamine oxidase inhibitors (phenelzine and tranylcypromine) due to a risk of serotonin syndrome.
Serotonin and NA reuptake inhibitors
Venlafaxine:
Venlafaxine is an effective antidepressant with strong inhibition of 5-HT and NA reuptake, and minimal muscarinic, histaminergic and adrenergic activity; it does not have the anticholinergic side effect profile of the TCAs. This agent is of interest because of its balanced neurotransmitter profile (making it similar in action to TCAs) and its similar structure to tramadol, an analgesic with both opioid agonist and monoaminergic activity (70). A number of uncontrolled reports indicate that venlafaxine is effective in postherpetic neuralgia, painful polyneuropathy, headache, neuropathic pain, atypical facial pain and radicular back pain (71–74). A recent randomized, controlled trial (n=244) (75) examining venlafaxine extended release in the treatment of painful diabetic neuropathy found that the NNT for 50% pain reduction was 4.5 at week 6, and noted that the NNT values for the higher dose of venlafaxine extended release (150 mg/day to 225 mg/day) were comparable with those of tricyclics and gabapentin. A randomized, controlled trial (n=29) (76) examined venlafaxine compared with imipramine and placebo in treating painful polyneuropathy and demonstrated that venlafaxine 225 mg/day was superior to placebo and was comparable with imipramine 150 mg/day in reducing constant, paroxysmal and pressure-evoked pain. In this study, the NNT to obtain one patient with moderate or better pain relief was 5.2 for venlafaxine and 2.7 for imipramine. Venlafaxine was not superior to imipramine with respect to tolerability, because a higher number of patients withdrew because of side effects with venlafaxine than with imipramine. There was a higher incidence of dry mouth and sweating with imipramine and tiredness with venlafaxine. A smaller, lower dose placebo-controlled trial (77) (n=13) using venlafaxine 37.5 mg/day to 75 mg/day found no difference from placebo on average daily pain intensity; however, average pain relief and maximum pain intensity were significantly lower with venlafaxine than with placebo in the treatment of neuropathic pain following treatment of breast cancer. Thus, there is initial evidence indicating that venlafaxine in a dose range of 150 mg/day to 225 mg/day may exhibit some analgesic effect; however, the NNT to obtain an analgesic effect is higher than with the TCA group. Further controlled trials are necessary. One potential advantage is the different and nonanticholinergic side effect profile compared with TCAs.
The recommended starting dose for venlafaxine is 37.5 mg tablets titrated every three to seven days to a maximum daily dose of 225 mg, given as two divided doses. The most common side effects are nausea, dyspepsia, sweating, somnolence and insomnia. In the larger trial reviewed above, seven patients had clinically important electrocardiogram changes (primary atrioventricular block, ventricular extrasystoles and atrial fibrillation) thought to be possibly treatment related, but overall, it was identified that the safety and tolerability of venlafaxine was not compromised at the higher dose level (75). In elderly patients, an increase in blood pressure is possible, so blood pressure should be monitored.
Duloxetine:
Like venlafaxine, duloxetine hydrochloride exhibits potent and relatively balanced 5-HT and NA reuptake inhibition. In addition it also lacks significant affinity for muscarinic, histamine H1, alpha-1-adrenergic, dopamine and opioid receptors. Preclinical work has supported an antinociceptive effect in models of persistent and inflammatory pain (78). To date, there are three randomized, controlled trials examining the efficacy of duloxetine in the treatment of pain. Two of the three trials examined duloxetine in treatment of diabetic neuropathy and the other in the treatment of fibromyalgia. In a multidose trial of 457 patients with diabetic neuropathy, duloxetine 60 mg/day and 120 mg/day significantly reduced pain severity beginning at week 1 and this continued throughout the 12-week study as compared with placebo. A dose of 20 mg/day did not differ significantly from placebo (79). A second 12-week trial comparing duloxetine 60 mg/day and 120 mg/day with placebo in 334 patients with diabetic neuropathic pain found that duloxetine was significantly more effective than placebo in reducing pain scores (80). In fibromyalgia, it was found that duloxetine exhibited efficacy for pain at weeks 1 to 4, but that by week 5, significance was lost and was not regained for the 12 weeks of the trial, with regard to the primary outcome measure for pain (Fibromyalgia Impact Questionnaire Pain Item) (78). The authors noted that there was significant improvement in the total score on the Fibromyalgia Impact Questionnaire at weeks 4 and 12 regardless of the patient’s depression status (38% of enrolled patients had a major depressive disorder); however, this measure includes items on fatigue, tiredness on awakening and stiffness as well as pain. A randomized, controlled trial examined duloxetine 60 mg once daily in the treatment of painful physical symptoms in patients with a major depressive disorder (81). According to the primary outcome measure for pain (Brief Pain Inventory Average Pain Score), results indicated that duloxetine was significantly better than placebo at early and intermediate visits (one, two and five weeks), but the difference was not significant at the end of the study (seven weeks). Thus, at present, there is preliminary evidence to suggest that duloxetine may be helpful in painful diabetic neuropathy, and there is no support for a sustained analgesic effect in fibromyalgia or for painful physical symptoms in patients with major depressive disorder. Further randomized, controlled trials in nondepressed patients with pain are required.
SSRIs
The SSRI antidepressants are generally used as the first-line treatment in depression due to equivalent efficacy and a better side effect profile (most common side effects include agitation, anxiety, sleep disturbance, tremor, sexual dysfunction and headache). SSRIs are also safer in cases of overdose. The literature regarding their potential as analgesics has been conflicting (43). Of 10 controlled trials examining SSRIs in the treatment of chronic headache, three found SSRIs to be no better than placebo and two found them to be marginally superior to placebo. In the remainder, there was some improvement but the analgesic effect was not superior to the comparison drug (82). There are three placebo-controlled trials using SSRIs in diabetic neuropathy; the larger study (n=46) found no difference between fluoxetine and placebo (83), while the two smaller studies found that paroxetine (84) and citalopram (85) exhibited some analgesic effect compared with placebo. In studies examining SSRIs compared with TCAs (83,84,86), analgesia with TCAs was superior in every case (43).
In a review of placebo-controlled trials involving painful polyneuropathy, the NNT value for 50% pain relief for TCAs was 2.6 and for SSRIs was 6.7; values for other agents were 2.5 for sodium channel-blocking anticonvulsants, 4.1 for calcium channel-blocking anticonvulsants like gabapentin and 3.4 for tramadol (87). A further systematic review of antidepressants for diabetic neuropathy and postherpetic neuralgia reported similar NNT values for TCAs (NNT 2.1 to 3.5); much less benefit was observed with SSRIs, which did not differ from placebo (88). Thus, the literature indicates that the SSRIs are less likely to exhibit efficacy as analgesics. In the case of comorbid depression when treatment of the depression is the priority, if TCAs are contraindicated, and venlafaxine has either failed or is too costly for the patient, then one may make the decision to use an SSRI as a first-line agent. When using SSRIs it is important to be aware of the metabolism in the liver by cytochrome P450 isoenzymes and potential interactions. Citalopram and escitalopram have the least impact on the cytochrome P450 isoenzymes (89). In elderly patients, fluoxetine should be avoided due to its extensive half-life (two to three days with active metabolite seven to nine days).
Other antidepressants
There is a single trial examining the dopamine and NA reuptake inhibitor bupropion in neuropathic pain, and this demonstrated an analgesic effect at a dose of 150 mg to 300 mg (90). However, the side effect profile (related to the dopaminergic system, delusions, hallucinations, seizure risk) argues against the use of this agent in elderly populations. The serotonin-2 antagonist/reuptake inhibitor trazodone is not an analgesic. Three of four placebo-controlled trials regarding trazodone were negative. There are no randomized, controlled trials examining the monoamine oxidase inhibitors in nondepressed patients with pain (43).
In conclusion, there is clear support that TCAs are analgesic and therefore a reasonable option to consider in the treatment of pain. The side effect profile obliges clinicians to use caution in elderly populations. In situations in which there are relative contraindications to the use of the TCAs (see above), analgesic agents other than the antidepressants are recommended as first-line agents. At present, there is inadequate evidence to support using venlafaxine or duloxetine as first-line agents.
Comorbid pain and depression
How does one choose the best antidepressant for patients suffering with comorbid pain and depression? No single antidepressant drug has proved to be more efficacious than any other for treatment of depression (91). However, recent evidence indicates that the dual-action antidepressants may exhibit increased efficacy in the treatment of depression alone. A recent meta-analysis of eight randomized, controlled trials comparing SSRIs with venlafaxine found that at high doses, 45% of patients achieved remission on venlafaxine, 35% on SSRIs and 25% on placebo (92). This observation, together with preliminary evidence suggesting that venlafaxine may be analgesic, provides support for using a serotonin and NA reuptake inhibitor such as venlafaxine as the first-line agent in treating comorbid pain and depression. However, it is important to be aware that existing evidence has not demonstrated a clear analgesic effect with venlafaxine (see venlafaxine section on page 19).
ANTICONVULSANTS
There is good evidence that certain anticonvulsants exhibit analgesic action in neuropathic pain. This is on the basis of their ability to reduce neuronal excitability (11). There are differences among agents with regard to the specific mechanisms. For example, gabapentin modulates neuronal calcium channels, and carbamazepine and lamotrigine act on sodium channels, while topiramate acts on both. It has been argued that the best name for this class of drugs would be neuromodulators (93). The most well-studied agents are gabapentin, pregabalin and carbamazepine; however, there is growing evidence for lamotrigine, topiramate and oxcarbazepine. Table 9 presents further detail regarding anticonvulsant neuromodulators exhibiting analgesic potential, proposed mechanisms of action and dosing.
TABLE 9.
Anticonvulsants with documented analgesic effects
| Agent | Dose range (mg/day) | Mechanism of action | Indications supported by at least one RCT* | Side effects | Comments |
|---|---|---|---|---|---|
| Gabapentin (Neurontin†) | 1200–3600 | N-type calcium channel blocker | PHN, DN, mixed neuropathic pain | Sedation, dizziness ataxia, confusion | Does not require metabolism in liver, so is a better choice in liver dysfunction; clearance will be diminished in renal dysfunction |
| Pregabalin (Lyrica†) | 150–600 | α2-δ protein of voltage- gated calcium channels | PHN, DN | Dizziness, somnolence | Analgesic effect is seen within first three days; does not require liver metabolism; renal excretion primary route of elimination |
| Carbamazepine (Tegretol‡) | 200–2000§ | Sodium channel blockade | TN | Sedation, dizziness, ataxia, diplopia, hepatitis, rash, hyponatremia | CBC, electrolyte and liver function studies pretreatment and every two weeks for three months, then less frequently§ (the hyponatremia may result in a confusional state); most serious potential side effects are aplastic anemia, hepatitis, serious dermatological reactions¶ |
| Lamotrigine (Lamictal**) | 200–400 | Sodum channel blockade | TN, DN, poststroke pain, spinal cord injury | Mild rash to serious dermatological reactions†† | CBC and liver function studies pretreatment and at four weeks |
| Oxcarbazepine (Trileptal‡) | 600–1200 | Sodium channel blockade | TN | Sedation, headache, dizziness, rash¶, vertigo, ataxia, nausea, diplopia, hyponatremia | CBC, electrolyte and liver function studies pretreatment and at four weeks
Serious dermatological reactions and multiorgan hypersensitivity reactions have been reported |
| Topiramate (Topamax‡‡) | 50–200 | Sodium channel blockade, ↑ GABA inhibition, ↓ glutamate excitation, modulates calcium channels | Migraine prophylaxis | Paresthesia, fatigue, nausea, anorexia, weight loss, changes in taste | Effect is modest; topiramate was associated with approximately one less migraine per month than placebo in three large RCTs |
For details see section relating to the specific agent in the text;
Pfizer Canada Inc;
Novartis Pharmaceuticals Canada Inc;
Doses of up to 2000 mg/day may be required in trigeminal neuralgia (TN) (116);
Life threatening dermatological reactions such as Stevens-Johnson syndrome, toxic epidermal necrolysis and lupus may be serious and require discontinuation of carbamazepine and oxcarbazepine;
GlaxoSmithKline Inc, Canada;
Rash ranging from simple morbilliform type to potentially serious rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported (283);
Janssen-Ortho Inc, Canada. CBC Complete blood count; DN Diabetic neuropathy; GABA Gamma-aminobutyric acid; PHN Postherpetic neuralgia; RCT Randomized, placebo-controlled trial; ↑ Increases; ↓ Decreases
Gabapentin
Gabapentin (Neurontin, Pfizer Canada Inc) has become widely used for the management of chronic neuropathic pain and epilepsy. This agent was originally developed as a structural analogue of gamma-aminobutyric acid (GABA) but does not actually bind to GABA or affect GABA reuptake or metabolism (11,94). Gabapentin binds with high affinity to the α2-δ subunit of voltage-dependent calcium channels; while it is thought that inhibition of high voltage activated calcium currents is a potential mechanism of action in analgesia (95), this remains to be determined (11).
Several large, randomized, controlled trials have provided evidence that gabapentin is significantly more analgesic than placebo in postherpetic neuralgia (96,97), diabetic neuropathy (98–100) and mixed diagnoses of neuropathic pain (101). There is also support for an analgesic effect in spinal cord injury (102) and trigeminal neuralgia (101), and in prophylaxis of chronic daily headache (103). Overall, gabapentin was well tolerated, and the most common adverse events were mild to moderate dizziness and somnolence, most of which occurred in the initiation phase (96,101). Additional side effects include ataxia and confusion (11). Dosing usually starts with 300 mg orally once daily; this can be increased by 300 mg every five days until adequate analgesia is accomplished or until limiting side effects are encountered. The large trials used doses of 1800 mg/day (96) and 2400 mg/day (96,101,104); in the trial using two doses, there did not appear to be a significant difference between the two doses with regard to efficacy compared with placebo, but the trial was not designed to look at dose response (96). Most authors recommend a dose range of 1800 mg/day to 3600 mg/day (93,96,97). There are only two comparative trials comparing gabapentin with other analgesics: one head-to-head trial with amitriptyline (98) that found that both agents exhibited similar efficacy but with different side effects and one placebo-controlled trial examining gabapentin alone and in combination with morphine (14), which demonstrated the combination to be superior than either agent alone.
Pregabalin
Pregabalin, like gabapentin, is a structural analogue of GABA but does not interact with GABA-A or -B receptors. The exact mechanism of action is unclear but it is known to selectively bind with high affinity to α2-δ protein, an auxiliary subunit of voltage-gated calcium channels, and thus may modulate presynaptic release of excitatory neurotransmitters (105). Large, randomized, controlled trials have indicated that pregabalin exhibits significant analgesic efficacy in postherpetic neuralgia (106,107) and painful diabetic peripheral neuropathy (108–110).
Pregabalin is generally well tolerated; the most common side effects are dizziness and somnolence. Renal excretion is the primary route of elimination; 98% of the administered drug is eliminated unchanged in the urine (105). Pregabalin has been studied in individuals with varying degrees of renal function and was effectively cleared in patients with end-stage renal failure undergoing dialysis (111). It has also been determined that the analgesic effect of pregabalin is evident within the first week of treatment (106,107,109,110). The effective dose range is from 150 mg/day to 600 mg/day orally and is given in two or three divided doses per day; 150 mg/day may be inadequate in some patients. In patients who received start doses of 300 mg/day, significant analgesia was identified within one to three days (106,112). An initial dose of 150 mg/day may be increased to 300 mg after three to seven days based on response and tolerability; if necessary, the dose may be increased to 600 mg/day after an additional seven days. In cases of renal impairment the lower dose range should be used.
The overall benefit of pregabalin is that it seems to offer approximately a 30% advantage over placebo, which is similar to that of gabapentin. To date, there are no head-to-head trials.
Carbamazepine
Carbamazepine’s (Tegretol, Novartis Pharmaceuticals Canada Inc) primary mechanism in stabilizing neuronal excitability is through sodium channel blockade (11,95). Controlled trials have demonstrated analgesic effects in trigeminal neuralgia, diabetic neuropathy and migraine prophylaxis (94,113–115). Survey data suggest that carbamazepine may also be of benefit in glossopharyngeal neuralgia, paroxysmal pain in multiple sclerosis (MS), postsympathectomy pain, lancinating pain in cancer and post-traumatic neuralgia (94,113,116). Carbamazepine remains the most successful first-line approach in treatment of trigeminal neuralgia (115). Effective doses range from 400 mg/day to 800 mg/day to as much as 2000 mg/day (115). It is recommended that dosing ‘start low and go slow’; start with a controlled release preparation of 100 mg to 200 mg every 8 h to 12 h orally, with as-needed rescue doses of the shorter acting preparation of 100 mg to 200 mg every 4 h. Dose escalation with the longer-acting preparation may then be determined based on therapeutic and side effects (115).
Oxcarbazepine
Oxcarbazepine (Trileptal, Novartis Pharmaceuticals Canada Inc) is a ketoanalogue of carbamazepine and is essentially 100% absorbed and converted almost immediately to the active 10-hydroxy metabolite, MHD (10,11-dihydro-10-hydroxy-5H-dibenzo(b,f)azepine-5-carboxamide). Both oxcarbazepine and its metabolite inhibit voltage-dependent sodium channels, while the metabolite also inhibits potassium channels (117). A recent review found three randomized, controlled trials demonstrating an analgesic effect in trigeminal neuralgia, and one controlled trial found comparable analgesia between amitriptyline and oxcarbazepine in cancer-related neuropathic pain, with fewer adverse events in the oxcarbazepine group. There were two case series reporting an analgesic effect in postherpetic neuralgia (117).
Lamotrigine
Lamotrigine (Lamictal, GlaxoSmithKline Inc, Canada) is a use-dependent inhibitor of neuronal sodium channels. Lamotrigine has been reported to exhibit analgesic effects in a case series of patients with painful diabetic neuropathy (118) and in open trials of trigeminal neuralgia, diabetic neuropathy, pain in MS and complex regional pain syndrome (119). Randomized, controlled trials have demonstrated a significant analgesic effect for lamotrigine compared with placebo in patients with trigeminal neuralgia (300 mg/day) (120), diabetic neuropathy (200 mg/day, 300 mg/day and 400 mg/day) (121), central poststroke pain (200 mg/day) (122) and incomplete spinal cord injury pain (400 mg/day) (123). In HIV neuropathy, there was initial evidence of greater reduction in pain scores for patients on lamotrigine (300 mg/day) than for patients taking placebo (124). A larger trial found no difference in average pain score among HIV patients on lamotrigine compared with placebo when looking at the whole sample, but when subgroups were examined, patients receiving antiretroviral therapy exhibited significantly reduced pain with lamotrigine (mean dose 377 mg/day to 402 mg/day) compared with those receiving placebo. A trial examining a dose of 200 mg/day of lamotrigine in a group of patients with mixed diagnoses of neuropathic pain did not demonstrate greater analgesic effect compared with placebo (125). In an open, prospective, dose-ranging trial in patients with trigeminal neuralgia (with or without MS), it was found that complete remission could occur at doses as low as 100 mg/day in one patient, with five of five MS-positive patients and eight of 15 MS-negative patients responding with complete remission at doses of 150 mg/day to 200 mg/day and the remainder experiencing partial or complete reduction of pain at the maximum dose of 400 mg/day (126).
Topiramate
Topiramate (Topamax, Janssen-Ortho Inc, Canada) has generated significant interest due to its multiple mechanisms of action which are of potential relevance to the management of chronic pain. These include modulation of voltage-gated sodium channels, potentiation of the inhibitory neurotransmitter GABA, blockade of the excitatory amino acid glutamate, modulation of voltage-gated calcium channels and inhibition of carbonic anhydrase (127). Topiramate has been found to decrease allodynia in preclinical models of neuropathic pain (127), and in case reports and open label studies, has demonstrated an analgesic effect in diabetic neuropathy, trigeminal neuralgia and cluster headache and other types of neuropathic pain that has not responded to standard agents (117,127,128). However, controlled trials of topiramate in treatment of chronic neuropathic pain have been disappointing to date. A randomized, placebo-controlled, multiple crossover pilot study of topiramate in trigeminal neuralgia found an analgesic effect in the main study, but not in the confirmatory study, in three subjects (129). In diabetic neuropathy, an initial double-blind controlled trial found that topiramate was more effective than placebo in 18 patients who received topiramate (130); subsequently, three large, double-blind, placebo-controlled trials reported by the Topiramate Diabetic Neuropathy Pain Study Group did not find topiramate to be significantly more effective than placebo in painful diabetic polyneuropathy (131). Further study is required to determine whether there is a role for topiramate in other types of neuropathic pain.
Several randomized, placebo-controlled trials examining topiramate in the treatment of migraine prophylaxis have demonstrated a statistically significant reduction in the frequency of migraine headaches with topiramate compared with placebo (132–138). Doses have ranged from 50 mg/day to 200 mg/day. The weight of evidence has indicated that a dose of 100 mg/day appears to lead to only a modest reduction in the mean frequency of migraines experienced per month. For example, in the three largest trials (133–135), topiramate 100 mg/day was associated with approximately one less migraine per month than placebo. The most frequently reported side effects were paresthesia, nausea, fatigue, anorexia, weight loss, cognitive difficulties such as memory trouble and altered taste.
Antidepressants versus anticonvulsants as analgesics
A systematic review of randomized, placebo-controlled trials using anticonvulsants and antidepressants for treatment of diabetic neuropathy and postherpetic neuralgia found that TCAs and anticonvulsants exhibited efficacy compared with placebo and that the NNT for one patient to experience 50% pain relief was approximately three for both (NNT=3.4 for antidepressants and NNT=2.7 for anticonvulsants for diabetic neuropathy, NNT=2.1 for antidepressants and NNT=3.2 for anticonvulsants for postherpetic neuralgia). There was no significant difference in minor adverse events; however, antidepressants were more likely to be associated with major adverse events leading to withdrawal from the study, with a number needed to harm of 17 (of 17 patients treated, one would withdraw due to adverse events). The majority of adverse events observed with the antidepressants were the classic antimuscarinic effects such as dry mouth, constipation and blurred vision. With the anticonvulsants, the most common adverse events were transient CNS effects such as dizziness, somnolence or disturbance of gait (139). There is only one head-to-head comparison of amitriptyline and gabapentin. This was a study in painful diabetic neuropathy in which it was found that both agents exhibited similar efficacy but different side effect profiles (98). Thus, evidence to date supports similar analgesic efficacy between the antidepressant and anticonvulsant agents, and the clinician may be guided primarily by the side effect profile and comorbidities an individual patient may present with. Table 10 presents further detail regarding NNT for antidepressants versus anticonvulsants. When one considers the fact that the NNT for ‘major harm’ (an adverse event that leads to withdrawal from the study) for the antidepressants is 17, while for the anticonvulsants it is the same as for placebo, the risk-benefit analysis favours using an anticonvulsant first in the absence of comorbid insomnia or depression.
TABLE 10.
Comparative numbers needed to treat (NNT) (for greater than 50% pain relief) and numbers needed to harm (NNH) (for withdrawal from the study) for tricyclic antidepressants, anticonvulsants and opioids in the treatment of neuropathic pain
| NNT | NNH | |
|---|---|---|
| Tricyclic antidepressants | ||
| Amitriptyline | 2.4 | |
| Clomipramine | 2.1 | |
| Desipramine | 2.4 | |
| Imipramine | 2.1 | |
| Nortriptyline | 2.6 | |
| Average combined tricyclic antidepressants | 2.3 | 14.7 |
| Anticonvulsants | ||
| Carbamazepine | 1.7* | 21.7 |
| Gabapentin | 3.8 | 26.1 |
| Lamotrigine | 4.0 | – |
| Pregabalin | 4.2 | 11.7 |
| Average combined anticonvulsants | 3.4 | 19.8 |
| Opioids | ||
| Morphine | 2.5 | NS |
| Oxycodone | 2.6 | NS |
| Tramadol | 3.9 | 9.0 |
| Average combined opioids | 3 | 3 |
In trigeminal neuralgia. NS Nonsignificant. Data from reference 51
Trigeminal neuralgia: A unique type of neuropathic pain
Trigeminal neuralgia is a unique neuropathic pain disorder. It does not respond in the same way to the conventional treatments used for other types of neuropathic pain (115).
A recent review provides specific recommendations as follows. The first line of treatment should be a trial of carbamazepine. This leads to pain relief in the majority of patients when used appropriately (dose titration is reviewed in the section on carbamazepine, page 22, and common side effects and monitoring bloodwork appear in Table 9). If there is inadequate response to carbamazepine, the next step is to add baclofen. Baclofen may also be used as monotherapy if carbamazepine has to be discontinued (page 29). Baclofen does not have the potential for life-threatening adverse events such as aplastic anemia, hepatitis, Stevens-Johnson syndrome or lupus. Recent controlled trials also support a role for oxcarbazepine in treatment of trigeminal neuralgia (117). Beyond this, one must rely on the guidance reviewed for other types of neuropathic pain, because there are no randomized, controlled trials supporting the use of other agents in the treatment of trigeminal neuralgia.
Clinical guidelines
As presented above, data from randomized trials have demonstrated efficacy for the anticonvulsant neuromodulators in the management of neuropathic pain. Due to a lack of head-to-head trials, it is difficult to draw firm conclusions for choosing one anticonvulsant agent over the other. However, the literature provides significant information to guide clinicians in pursuing a reasonable approach to choosing appropriate agents.
In summary, carbamazepine remains an established first-line option in the treatment of trigeminal neuralgia (93,115). There is good evidence supporting gabapentin and pregabalin for the treatment of postherpetic neuralgia and painful diabetic neuropathy, and growing evidence for lamotrigine and oxcarbazepine as additional neuromodulators in neuropathic pain. Carbamazepine may also be used in neuropathic pain if the pain is predominantly electric shock-like or if it is caused by MS (Watson CP, personal communication). Large trials have identified that topiramate is not analgesic in painful diabetic neuropathy, and the efficacy of topiramate in other types of pain remains to be established. Thus, it is reasonable to use gabapentin, pregabalin and carbamazepine first (in the case of liver disease, carbamazepine should be avoided), and then to move to lamotrigine or oxcarbazepine if there is no response or if the patient is unable to tolerate side effects. In the case of trigeminal neuralgia, baclofen is an additional option (page 29).
Generally, the same guidelines are used when prescribing anticonvulsants as analgesics as when one is using these drugs for epilepsy. Table 8 presents further detail regarding side effects, dosing for pain conditions and appropriate laboratory work when necessary.
CHRONIC OPIOIDS IN NONCANCER PAIN
Conventional opioids
There is a growing body of evidence that controlled-release opioid analgesics have a role to play in a subset of patients with chronic pain, including neuropathic pain (140–151). Guidelines for the use of opioid analgesics in chronic, non-cancer pain have been established. Table 11 summarizes the principles of practice for the use of opioid analgesics in chronic noncancer pain. The reader is referred to the full consensus statement of the Canadian Pain Society for further detail (152).
TABLE 11.
Summary of principles of practice for the use of opioid analgesics in chronic noncancer pain from the consensus statement of the Canadian Pain Society*
| Evaluate the patient |
|
|
| Establish diagnosis | Identify nociceptive versus neuropathic mechanisms underlying the pain | |
| Assess psychological aspects | Identify comorbid psychiatric diagnoses, note that pain leads to psychological suffering and address this aspect in treatment | |
| Assess risk of addiction | Identify patients who may need a more detailed assessment | |
| Ask: Has your use of alcohol or other drugs ever caused a problem for you or those close to you? | ||
|
Office screening tools |
||
|
SISAP† |
CAGE-AID‡ |
|
|
In the past have you ever:
|
|
| Patients with a history of addiction will require more careful prescribing and closer follow-up | ||
| Indications for trial of opioid therapy | Patients with moderate to severe pain that is nociceptive, neuropathic or both. Patients with mild to moderate pain that has failed to respond to other treatments (modality-based or pharmacological) (in situations where a definitive diagnosis cannot be established a trial of opioids requires careful monitoring and specific goals) | |
| Establish an overall management plan | Treatment with chronic opioids should take place within an overall pain management plan that includes consideration of all appropriate therapies for that individual patient | |
| Identify reasonable goals of treatment | Improved pain control is a reasonable and appropriate goal. It is also useful to develop functional goals; however, failure to attain all functional goals should not necessarily be construed as therapeutic failure | |
| Obtain full informed consent | Review: risks and benefits of opioid therapy including possible side effects, small risk of addiction in low-risk patients, tolerance, physical dependence and withdrawal risk if suddenly discontinued; risks of additive side effects with other potentially sedating agents; conditions under which opioids will be prescribed. If concerned about noncompliance consider a written contract | |
| Use time-contingent dosing | The goal is to try and keep breakthrough doses to a minimum once stabilization phase is accomplished | |
| Consult appropriate pain, addiction or psychological specialists where necessary | This will also depend on availability of the appropriate specialists | |
| Periodic review (‘5 As’) | Assess: Analgesia, Activities, Adverse effects, Abuse behaviours, Adequate documentation | |
| Manage adverse effects of opioids/lack of efficacy | Institute treatment of side effects, if there is a decrease in function or intolerable side effects, gradual reduction of opioid may be indicated | |
| Document, document, document | To document evaluation process, rationale for opioid therapy in context of overall management plan, follow-up and compliance with federal regulations | |
Reference 152;
When applying the Screening Instrument for Substance Abuse Potential (SISAP) tool (284), use caution in the following patients: men who exceed four drinks/day or 16 drinks/week; women who exeed 3 drinks/day or 12 drinks/week; recreational use of marihuana or hashish for euphoriant effects; a patient younger than 40 who smokes;
One positive answer to any of the CAGE-AID questions would suggest caution. Two or more positive responses should strongly suggest assessment by an addiction specialist before embarking on chronic opioid therapy
Guidelines for the use of chronic opioids in noncancer pain emphasize the need for a thorough history and physical examination with appropriate diagnostic workup, development of an overall pain management approach based on the individual needs of the patient and regular follow-up (eg, every three months or more depending on the clinical situation). Continued prescribing should be on the basis of documented pain relief, improved function or both. Pain relief is considered an acceptable goal of opioid therapy. One physician should do the prescribing and one pharmacist should do the dispensing. A contract, either oral or written, is suggested but is not mandatory.
Mechanisms:
The endogenous opioid system is a part of the body’s natural defense network allowing for the modulation of pain-related transmission. The endogenous opioid peptides (beta-endorphin, enkephalins, dynorphins) inhibit synaptic transmission and are released at several CNS sites in response to noxious stimuli. Opioid receptors fall into three classes, designated μ, κ and δ. Opioid receptors are found in several areas of the brain and the brain stem, throughout the spinal cord and in the peripheral nervous system. In the brain, opioids alter mood and reaction to pain. In the brain stem, opioids increase the activity of cells involved in descending inhibition of pain. At the spinal and peripheral level, opioids have an inhibitory effect on transmission in nociceptive systems. At the molecular level, opioid receptors are linked to G proteins and are able to affect ion channel gating. On the presynaptic nerve terminal, agonists of all three receptor types result in closure of voltage-gated calcium channels, leading to decreased calcium influx and a decrease in neurotransmitter release. μ-Opioid receptor agonists also hyperpolarize second-order primary afferents by increasing potassium conductance, resulting in decreased neuronal excitability and inhibition of postsynaptic neurons (11,153). Previously, it was thought that opioid analgesia was mediated via central effects only. It is now known that opioids exhibit a peripheral action as well (154).
Most available opioids are μ-opioid receptor agonists or drugs with direct affinity for μ-opioid receptors. When an opioid is administered exogenously, it is essentially augmenting the endogenous system that is in place. The pure agonists have no apparent ceiling effect for analgesia. As the dose is raised, the analgesic effect increases until analgesia is achieved or dose-limiting adverse effects occur. Antagonists such as naloxone interfere with the action of agonists blocking their effects. Clinically, the antagonists are used to treat overdoses of opioids. It is important to be aware that agents with mixed agonist-antagonist and partial agonist action are available (see below).
Clinical guidelines:
In Canada, the opioids most commonly used for chronic pain management are codeine, morphine, oxycodone, hydromorphone, fentanyl and methadone (Table 12). These agents are all primarily μ-opioid receptor agonists. Generally the methods of initiating therapy, titrating dosage and managing side effects are similar to those used in cancer pain (155). This includes the World Health Organization ‘analgesic ladder approach’, which involves starting with the weaker opioid agonists such as codeine or acetaminophen/codeine combinations for milder pain (keeping in mind the maximum daily dose of acetaminophen), then moving up to stronger opioids for moderate to severe pain. Any opioid may be used, but the long-acting, continuous-release options are preferred because they result in a more consistent blood level and better overall analgesia for patients with continuous pain. Practical tips for prescribing opioids appear in Table 13 and continuous-release forms of opioids currently available in Canada are listed in Table 14.
TABLE 12.
Use of opioids in chronic pain management
| Dose equivalent to morphine 10 mg IM | ||||||
|---|---|---|---|---|---|---|
| Drug | Common trade name | IM | PO | IM:PO potency ratio | Elimination half-life (h) | Duration of action (h) |
|
Agonists | ||||||
| Morphine | Morphine | 10 | 20–30 | 1:3 | 2–3.5 | 3–4 |
| Oxycodone | Percocet (with acetaminophen)* | |||||
| Percodan (with acetylsalicylic acid)* | – | 10–15† | 1:15 | 2–4 | 3–6 | |
| Supeudol (Sabex Inc, Canada) | ||||||
| Hydromorphone | Dilaudid (Abbott Laboratories, Canada) | 2 | 4–6‡ | 1:5 | 2–3 | 2–4 |
| Fentanyl (transdermal) | Duragesic (transdermal) (Janssen-Ortho Inc, Canada) | 0.1§ | – | 1–2 | 72 per patch | |
| Methadone | Methadone hydrochloride powder Metadol (Pharmascience, Canada) | 1.7–5¶ | 1:2 | 15–190 | 4–24 | |
| Codeine | Codeine | 120 | 200 | 2–3 | 4–6 | |
| Tramadol | Tramacet (Janssen-Ortho Inc, Canada) (with acetaminophen) | 200** | 1:1.2 | 3–4 | 4–6 | |
| Meperidine†† | Demerol (Sanofi-Synthelabo Inc, Canada) | 75 | 300 | 1:4 | 2–3 | 2–4 |
|
Mixed agonists/antagonists | ||||||
| Pentazocine†† | Talwin (Sanofi-Synthelabo Inc, Canada) | 60 | 180 | 1:3 | 2–3 | 3–4 |
| Butorphanol | Transnasal butorphanol | 2 | 2.5–3.5 | 3–4 | ||
Endopharmaceuticals Inc, USA;
Oxycodone is approximately twice the potency of morphine;
Hydromorphone is approximately five times the potency of morphine;
Empirically: transdermal fentanyl 100 μg/h = intramuscular (IM) Morphine 2–4 mg/h (available in patches to deliver 25, 50, 75, 100 μg/h, detailed conversion are available in the Compendium of Pharmaceuticals and Specialties, 2005);
The equipotent methadone dose varies and depends on the previous dose of opioid and individual pharmacokinetics. Methadone ranges from four to 12 or more times the potency of morphine, the relative potency of methadone is higher with higher doses of the previous conventional opioid. Care should be taken in opioid rotation especially if the patient is on high doses of a conventional opioid, lower relative doses of methadone will be required, for detailed conversion ratios see [178];
Difficult to define equipotent dose because tramadol is only available in Canada in combination with acetaminophen. Tramadol has weak opioid agonist effects in a range similar to codeine, Tramacet tablets contain 37.5 mg of tramadol and 325 mg of acetaminophen, tramadol also exhibits monoaminergic effects, it is important to be aware of this in patients using antidepressants or other agents with monoaminergic action;
Meperidine and pentazocine are not appropriate for chronic use.
Transnasal relative potency and duration of action equivalent to parenteral morphine
TABLE 13.
Practical tips for prescribing opioids
| Opioid-naïve | |
| Mild to moderate pain | Start codeine 30 mg to 60 mg tid (all doses refer to oral route administration) |
| If a dose of codeine 180 mg/day is inadequate and it is determined that the use of a stronger opioid is necessary, discontinue codeine and replace with a stronger opioid as described below for moderate to severe pain | |
| Moderate to severe pain | |
| Option 1 | Start continuous-release morphine 10 mg to 15 mg q12h with regular morphine 5 mg q4h prn for breakthrough pain control up to three doses per 24 h as a start |
| Option 2 | It is also reasonable to start with a short-acting form of a stronger opioid such as morphine sulphate 5 mg, oxycodone 2.5 mg to 5 mg or Percocet* first and then transfer to a continuous release form once the initial dose requirement is established |
| Titrate dose every three to five days according to analgesic requirements and limiting side effects | |
| Patient already on an opioid with moderate to severe pain and poor pain control | Initiate a trial of increased dose, titrating in increments appropriate to the agent and the dose until adequate pain relief or limiting side effects are encountered |
| Reasonable dose increments in mg (start with q12h dosing; occasionally, more frequent dosing may be required, eg, q8h or q6h); morphine continuous release: 15, 30, 60, 75, 90, 120,150,180, 200, 230, 260; oxycodone continuous release: 10, 20, 30, 40, 50, 60, 70, 80, 100, 120, 140; hydromorphone continuous release: 3, 6, 12, 18, 24, 30, 36, 42, 48, 54, 60 | |
| Duragesic (in μg/h) : 25, 50, 75, 100, 125, 150, 175, 200 | |
| If one agent fails, one may switch to an equivalent dose of another agent. If two different opioid agents provide inadequate relief or limiting side effects then one may consider a trial of methadone |
TABLE 14.
Long-acting opioids currently available in Canada
| Drug | Common trade name | Availability (mg)* (fentanyl μg/h) |
|---|---|---|
| Codeine | Codeine-Contin† | 50, 100, 150, 200 |
| Morphine | MS-Contin† | 15, 30, 60, 100, 200 |
| M-Eslon‡ | 10, 15, 30, 60, 100, 200 | |
| Morphine-SR (generic) | 15, 30, 60, 100, 200 | |
| Kadian§ | 20, 50, 100 | |
| Oxycodone | Oxy-Contin† | 10, 20, 40, 80 |
| Hydromorphone | Hydromorph-Contin† | 3, 6, 12, 24 |
| Fentanyl | Duragesic patch¶ | 25, 50, 75, 100 |
Dosing for all preparations listed is every 12 h, except for Kadian, which is released over 24 h;
Purdue Pharma, Canada;
Aventis Pharma Inc, Canada;
Mayne Pharma (Canada) Inc;
Janssen-Ortho Inc, Canada
It is important to note that codeine is a poor analgesic for stronger moderate to severe pain and depends on conversion to morphine for its analgesic effect. In addition, there is significant interindividual variation in the metabolism of codeine. This is related to the fact that O-demethylation of codeine to morphine is dependent on cytochrome P450 isoenzyme 2D6, which is known to exhibit genetic polymorphism. Thus, some individuals produce little or no morphine from codeine and others produce significant amounts, although the amount produced may show wide variation (156–158). Thus, for individuals with moderate to severe pain, a stronger opioid (such as morphine or oxycodone) should be chosen in the first instance, and codeine is not recommended.
As mentioned above, agents with mixed actions at different receptor subtypes are available. Pentazocine exhibits agonist effects at κ-receptors and weak antagonist action at μ-receptors. Thus, pentazocine can produce κ-mediated psychotomimetic side effects. When given together with a μ-agonist, the antagonist effect at the μ-receptor can generate an acute withdrawal syndrome. Pentazocine is generally not recommended for chronic pain. The only other mixed agonist-antagonist currently available for outpatient pain management is butorphanol, which in its transnasal form can be helpful for management of episodic migraine-type headache or other sudden onset types of severe recurrent pain (as long as the patient is not on another μ-opioid agonist). Partial agonists for opioid receptors are also available. However, there are no agents in this category that are useful for management of chronic, non-cancer pain at the present time.
Other opioids not recommended for treatment of chronic pain include meperidine (very short duration of action, excitatory long-acting metabolites) and propoxyphene (cardiotoxic metabolites) (Table 15). However, there is not an absolute contraindication to the use of these particular opioids, because there may be clinical situations when these products are appropriate, eg, if other opioids are not effective, if these agents are better tolerated or if one is attempting to avoid allergies to standard opioids.
TABLE 15.
Opioids generally not recommended for treatment of chronic pain
| Pentazocine (Talwin, Sanofi-Synthelabo Inc, Canada) |
| Meperidine hydrochloride (Demerol, Sanofi-Synthelabo Inc, Canada) |
| Propoxyphene hydrochloride (Darvon, AAIPharma Inc, USA) |
Common side effects from opioids include sedation, nausea, sweating, constipation and pruritis. As long as appropriate dose titration is used, respiratory depression in the presence of ongoing pain is uncommon. Sedation or other CNS side effects, if present, usually occur in the titration phase of therapy. Patients just beginning opioid therapy are advised not to drive or operate heavy machinery. Once sedation clears, confusion and other cognitive impairment almost always disappear. If not, the usual cause is concomitant administration of benzodiazepines or barbiturates. The concurrent use of sedatives should be avoided.
As with antidepressant agents, if a trial with one opioid does not result in analgesia or leads to unacceptable side effects, it is reasonable to switch to another opioid. Variation in genetic coding for the μ-opioid receptor has been demonstrated, and rotation from one opioid to another may transform a patient’s pain from opioid-resistant to opioid-responsive; one must therefore view the current tables of opioid equivalence as loose guidelines at best, used to identify an approximate dose in an effort to avoid under- or overdosing (159). Tables 12 and 13 present further detail regarding currently available opioids.
Dual or multimechanism opioids
Methadone:
Methadone has been available for approximately a half-century (160). It is traditionally known for its role in assisting heroin addicts to exit street drug use, and in this context, its long half-life and duration of action have been known for some time. Accumulating evidence has identified a number of potential advantages for methadone over other opioids, including agonist action at both μ and δ opioid receptors (161,162), NMDA antagonist activity (163–168) and the ability to inhibit the reuptake of monoamines (165). These properties, in addition to pharmacoeconomic issues related to the very low cost of the generic hydrochloride methadone powder that is generally available (169,170), have led to increased interest in the use of methadone for the treatment of cancer pain (169–174), neuropathic pain (169,170,175,176) and chronic, noncancer pain (177). Methadone exhibits significant inter-individual variation in pharmacokinetics as well as incomplete cross-tolerance with conventional opioids, and it is important for clinicians to be aware of this in some detail so that patients are not exposed to unnecessary risk when switching from conventional opioids to methadone. A review of the use of methadone in chronic noncancer pain containing details has recently been published in Pain Research & Management (178). Of note, methadone powder is used to prepare a liquid methadone in a concentration requested by the physician. In drug addiction settings, the liquid used is often a fruit juice made with orange juice or lemon juice powder in an effort to decrease the chances of it being injected. This may make the liquid attractive to young children and death may result. Thus, it is important to review this risk in detail and ensure that the medication is clearly labelled and stored in a way that children or others do not have access to it. Water can be requested; however, it is important to be aware of the risk of diversion and safety in storage in any case. Methadone is available in tablet form as well. For details, see the recent review in Pain (172).
Tramadol:
Tramadol hydrochloride has been available for 25 years in Europe and for a decade in the United States. Tramadol is only marketed in Canada as a tablet in combination with acetaminophen. Tramadol has been demonstrated to be an effective and safe analgesic for moderate to severe chronic pain related to osteoarthritis, low back pain, fibromyalgia and diabetic neuropathy (179–183). An extended-release preparation has been found to exhibit efficacy in controlled trials of osteoarthritis pain using chronic dosing (182,183). Tramadol in combination with acetaminophen has been demonstrated to exhibit significant efficacy in chronic low back pain (179,181) and osteoarthritis (179).
Tramadol exhibits a dual mechanism of action with both a weak μ-opioid agonist and a monoaminergic action (inhibits the reuptake of NA and 5HT). Research regarding the equianalgesic efficacy of tramadol has yielded variable results. Initial reports suggested an efficacy similar to meperidine (184), but subsequent reports have found tramadol to be less effective than meperidine, but more effective than codeine or propoxyphene (185). Tramadol exhibits an oral potency ratio of 1:10 compared with 1:10 for morphine sulphate, which is similar to codeine (186).
The most common adverse effects are nausea, drowsiness, dizziness and headache, dry mouth, pruritis, diarrhea and constipation (180,181,183). Unlike other opioid agonists, respiratory depression is seldom, if ever, reported using recommended doses orally (184,185). However, a case report revealed that tramadol may result in respiratory depression in patients with renal failure (187). Urinary retention and constipation are very infrequent (186). In addition, minimal tolerance has been reported and the addiction potential is low (184,185). Because tramadol inhibits the reuptake of monoamines, it should not be used with monoamine oxidase inhibiters. The manufacturer recommends 50 mg to 100 mg of tramadol every 4 h to 6 h up to a maximum of 400 mg/day (or 300 mg/day in older patients) as a single agent; however, in combination with acetaminophen, patients should not take more than six tablets per day. Tramadol’s dual mechanism of action, low respiratory depressant effect and low abuse potential make it a unique analgesic to consider.
Tolerance, physical dependence and addiction
The assessment of addiction in pain treatment settings has received increasing attention, and there are a number of excellent reviews to assist clinicians in the assessment and treatment of patients with comorbid chronic pain and addiction (188–191). The Liason Committee on Pain and Addiction (a joint committee with representatives from the American Pain Society, American Academy of Pain Medicine and American Society of Addiction Medicine) in the USA was formed in July 1999 to encourage collaboration between pain and addiction specialists on issues of common interest, including research, education, clinical care and policy development (189). The Liason Committee on Pain and Addiction has clarified the importance of development of clear and unambiguous terms related to addiction that are consistent with current scientific and clinical understanding of pain, addiction and opioid pharmacology. It has been identified that three fundamental concepts must inform terminology related to addiction to reflect current clinical and basic science relating to addictions:
Although some drugs produce pleasurable reward, critical determinants of addiction also rest with the user.
Addiction is a multidimensional disease with neurobiological and psychosocial dimensions.
Addiction is a phenomenon distinct from physical dependence and tolerance. Table 16 presents the currently recommended definitions of tolerance, physical dependence and addiction.
TABLE 16.
Definitions developed by the Liaison Committee on Pain and Addiction (LCPA)
| Addiction |
| A primary, chronic, neurobiological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. |
It is characterized by behaviours that include one or more of the following:
|
| Physical dependence |
| A state of adaptation manifested by a drug class-specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug and/or administration of an antagonist |
| Tolerance |
| A state of adaptation in which exposure to the drug results in changes that result in a diminution of one or more of the drugs effects over time |
From reference 189. The LCPA is made up of representatives from the American Pain Society, the Americal Academy of Pain Medicine and the American Society of Addiction Medicine
Table 11 presents a summary of principles recommended by the Canadian Pain Society in the use of chronic opioids for noncancer pain, which includes physician office screening tools (SISAP and CAGE-AID) that assist in identification of individuals at risk of addiction. If a risk of addiction is identified, or if there is comorbid addiction, this is not necessarily a contraindication to the use of a chronic opioid. In such cases, the clinician must address treatment of both the pain and the addictive disorder, and assure that there is clear structure in place for doing so. This should involve a complete assessment of addiction potential, consultation with an addiction specialist when appropriate, clear limits around prescribing of medications, limited dispensing including daily dispensing in some cases, signed contracts and urine testing in appropriate circumstances.
Although one may speculate that because methadone maintenance programs have been successful in assisting street drug addicts, methadone may be a better choice in patients with comorbid pain and addiction, this has not been determined scientifically and awaits appropriate study. To date, there are no controlled trials examining the use of methadone in this population, nor are there any head-to-head trials comparing methadone with other opioids in this population. However, until further information is available, it is reasonable to consider methadone as a first-line option in this population (179).
OTHER AGENTS USED FOR CHRONIC PAIN
Oral local anesthetics
Voltage-gated sodium ion channels are the key transducers converting depolarization from peripheral receptors to action potentials that are communicated from the periphery to the spinal cord (11). The introduction of oral forms of local anesthetics (such as lidocaine, which block sodium channels) has spurred interest in the analgesic potential of this class of drugs, particularly for neuropathic pain. In well-controlled clinical trials, tocainide was effective for trigeminal neuralgia (192); however, this agent is too toxic for clinical use. Mexiletine, a class IB antiarrythmic agent, is a structural analogue of lidocaine; it has been used with mixed success to treat various neuropathies (193). There are four randomized, controlled trials examining the analgesic potential of mexiletine in diabetic neuropathy, three with positive results (194–196) and one demonstrating no difference between mexiletine and placebo (197). Evidence indicates that patients with stabbing or burning pain, heat sensations or tingly, crawling sensations benefit the most (195).
One controlled trial (198) demonstrated a significant effect of mexiletine on prominent allodynia associated with neuropathic pain, but no significant effect on other pain measures. There is one randomized, controlled trial (199) that demonstrated an analgesic effect in peripheral nerve injury. With regard to other types of pain, studies to date have found no significant analgesic effect for mexiletine in HIV neuropathy (48,200), spinal cord injury (201) or prevention of the onset of chronic pain subsequent to breast surgery for cancer (202,203).
A review (193) of mexiletine in the treatment of diabetic neuropathy concluded that mexiletine is a reasonable alternative in patients who have not had a satisfactory response to, or who cannot tolerate, TCAs or other agents available for neuropathic pain.
Two studies have examined the association between response to intravenous lidocaine and oral mexiletine. One prospective two-dose lidocaine study (2 mg/kg and 5 mg/kg) demonstrated that subsequent response to oral mexiletine was significantly correlated with the average response to the two intravenous lidocaine infusions (204). A second study determined that the effects of intravenous lidocaine and oral mexiletine on mechanical allodynia in patients with peripheral nerve injury were highly correlated, but there was a weaker correlation for spontaneous pain (205). In summary, the evidence to date supports a role for mexiletine as a third-line agent in the treatment of diabetic neuropathy, but not for other types of neuropathic pain.
The initial dose of mexiletine should be low (100 mg/day to 150 mg/day), and dose escalation should proceed gradually until analgesic effects occur or side effects become problematic (116). Dose escalation at weekly intervals is reasonable (193). Most patients experience a therapeutic response at a medium dose regimen of 450 mg/day (195), but doses of 675 mg/day may be necessary (196). The maximum daily dose used for this indication is 10 mg/kg (194), up to 900 mg/day (198). Mexiletine has been reported to aggravate or induce arrythmias in some patients, so an electrocardiogram should be obtained during dose escalation. A cardiologist should be consulted before considering mexiletine for treatment of neuropathic pain in an individual who has been treated for an arrythmia. Mexiletine is contraindicated in patients with second- or third-degree heart block. Patients with hepatic dysfunction have reduced capacity to eliminate mexiletine and are at risk of developing toxicity, so lower doses and slower titration schedules are recommended (193). The most common side effect is nausea (prevalence 10% to 30%); other reported side effects include fatigue, dry mouth, vomiting, gastric pain, headache shakiness, dizziness, tinnitus, palpitations, pruritis and allergies (193).
Overall, due to poor relative efficacy when compared with other analgesics and the potential for serious side effects, mexiletine is not a very useful analgesic.
Neuroleptics
As a general rule, neuroleptics are not analgesic and should be avoided for the treatment of pain. Methotrimeprazine (Nozinan, Aventis Pharma Inc) has been demonstrated to exhibit analgesic properties in cancer pain (116), and pimozide was found to demonstrate better efficacy than carbamazepine in one randomized, controlled trial for trigeminal neuralgia (206). However, these neuroleptics are associated with unpleasant extrapyramidal side effects and irreversible tardive dyskinesia, so caution is advised. Furthermore, in a well-controlled trial, it was found that the addition of fluphenazine to amitriptyline did not confer any additional analgesia in the treatment of postherpetic neuralgia (207).
Clonidine
Like opioid receptors, the alpha-2-adrenergic receptor is a transmembrane G protein-coupled receptor. Activation of this receptor opens postsynaptic potassium channels, inhibits presynaptic voltage-gated calcium channels and inhibits adenyl cyclase. Alpha-2-adrenergic receptors are present both pre- and postsynaptically and, like opioids, when activated, they reduce neurotransmitter release and decrease postsynaptic transmission, resulting in an overall inhibitory effect (11).
The alpha-2-adrenergic agonist clonidine (Catapres, Boehringer Ingelheim Canada Ltd) has been found to produce antinociception in a variety of nociceptive tests, and both spinal and supraspinal mechanisms have been implicated in such activity (208). Preclinical work indicates that systemic and spinal administration of clonidine and other adrenergic agonists inhibit pain behaviors in nerve injury models. Clonidine exerts a prominent effect on allodynia as well as spontaneous pain. In humans, a number of controlled trials support an analgesic effect for clonidine administered epidurally in the treatment of arachnoiditis (209), reflex sympathetic dystrophy (now complex regional pain syndrome type 1) (210) and cancer pain (211). A double-blind, randomized comparison of the effects of epidural clonidine, lignocaine and the combination demonstrated equivalent analgesia between clonidine and the local anesthetic in patients with low back and leg pain and neuropathic pain (212). An earlier controlled trial had identified no statistical difference between the analgesia experienced with epidural morphine and epidural clonidine, and noted that the analgesia with clonidine could last up to one month, while that with morphine lasted up to 24 h (213). In a single-dose, randomized, double-blind, double-dummy, crossover comparison of extradural and intravenous clonidine in 10 patients with chronic back pain, it was demonstrated that intravenous and extradural clonidine were both efficacious and that clonidine by either route produced a high incidence of adverse events such as sedation and decreases in arterial pressure and heart rate (214). Controlled trials have demonstrated an analgesic effect for transdermal clonidine in diabetic neuropathy (patch dose of 0.1 mg/day to 0.3 mg/day) (215,216).
In summary, the evidence indicates that both epidural and systemic clonidine (given intravenously or transdermally) are analgesic, but use is limited by side effects such as sedation and postural hypotension. At present, transdermal clonidine is not available in Canada. Controlled trials of oral clonidine in pain treatment are lacking. The above trials identifying a systemic analgesic effect suggest that it may be reasonable to consider a trial of oral clonidine when patients have not responded to previous agents, and when there is thought to be a sympathetically maintained component. Whether clonidine is efficacious in such situations, however, awaits further study. One should begin with a low dose and gradually increase the dose until limiting side effects occur. Withdrawal should be done gradually to avoid rebound hypertension.
Baclofen
Baclofen (Lioresal, Novartis Pharmaceuticals Canada Inc), known primarily for its antispasticity action (217,218), has also been shown to have an antinociceptive action in a variety of experimental models, including nerve injury models (219). Baclofen selectively activates GABA-B receptors pre- and postsynaptically, leading to a decrease in excitatory transmission and an increase in inhibition, resulting in marked segmental inhibition. In human studies, open (220) and controlled trials (221) have found baclofen to exhibit an analgesic effect in trigeminal neuralgia. Uncontrolled reports have supported an effect in glossopharyngeal and vagoglossopharyngeal neuralgia, and episodic and allodynic pain in some patients with opthalmic postherpetic neuralgia (220). Intrathecal administration of baclofen has also been reported to suppress spontaneous and allodynic dysesthetic pain in open trials of patients with spinal lesions (222,223) and with central poststroke pain (223).
Baclofen is an additional option for the treatment of trigeminal neuralgia (115). The usual starting dose is 5 mg to 10 mg three times a day. This dose is increased every second day until the patient is pain free or side effects occur. The usual maintenance dose is 50 mg to 60 mg daily in divided doses. Patients with severe trigeminal neuralgia may need to take baclofen at 3 h to 4 h intervals due to its short half-life (approximately 3 h to 4 h in most patients). The dose of baclofen may be gradually tapered after the patient has been pain-free for several weeks. If pain does not recur, the patient may remain without medication until the next exacerbation (220). When discontinuing baclofen, it is important to remember that one must gradually taper the dose, because abrupt withdrawal can result in seizures.
The most common side effects are drowsiness, dizziness and GI distress. Approximately 10% of patients cannot tolerate baclofen because of side effects. A rare complication of baclofen is an acute confusional state that appears shortly after starting or stabilizing treatment. This alteration in mental status resolves after discontinuing the drug. Occasionally, patients who have been using baclofen successfully over a period of time may appear somnolent, retarded or depressed for no apparent reason. In this case, decrease or discontinuation of baclofen may be followed by improvement. If patients exhibit a disturbance of consciousness, seizures, respiratory depression, muscular hypotonia, hyporeflexia, hallucinations, impaired memory, catatonia or mania, this is an indication of acute or chronic toxicity and must be actively managed. Baclofen should be avoided in patients with renal disease, because this increases the chances of intoxication. Baclofen must be tapered and discontinued slowly after prolonged use, because hallucinations, manic psychosis or seizures can occur if it is abruptly discontinued (220). If baclofen or carbamazepine alone are inadequate to control trigeminal neuralgia, a combination of the two can be used (115,220).
Guidelines for treatment of glossopharyngeal or vagoglossopharyngeal neuralgia and the lancinating and allodynic pains of opthalmic postherpetic neuralgia are the same as for the treatment of trigeminal neuralgia.
THE TRIPTANS FOR MIGRAINE
The treatment of migraine-related pain involves features distinct from the treatment of other types of pain. In the case of migraine, a significant subgroup of patients benefit from treatment with the triptan class of serotonin receptor agonists (224). The best studied and most well-known agent in this group is sumatriptan, which is available as sumatriptan hemisulfate (Imitrex nasal spray, GlaxoSmithKline, Canada) or sumatriptan succinate (Imitrex tablets or autoinjector). Others include almotriptan malate (Axert, Janssen-Ortho Inc, Canada), eletriptan (Relpax, Pfizer Canada Inc), naratriptan hydrochloride (Amerge, GlaxoSmithKline, Canada), rizatriptan benzoate (Maxalt, Merck Frosst Canada Ltd) and zolmatriptan (Zomig, AstraZeneca Canada Inc), which is also available as a nasal spray. A meta-analysis of 53 trials identified that at marketed doses, all oral triptans are effective and well tolerated. The differences among them were relatively small; however, rizatriptan 10 mg, eletriptan 80 mg and almotriptan 12.5 mg provide the highest likelihood of success, while sumatriptan has had the longest clinical experience and along with zolmatriptan is available in a wider range of formulations (224). In addition, naratriptan (Amerge) exhibits a slower onset and longer duration of action, which may be an advantage in individuals with protracted migraine. Rizatriptan benzoate (Maxalt) is also supplied in a rapidly disintegrating oral wafer. Table 17 presents further detail regarding the triptans that are currently available. The bottom line is, that with any agent there will be individual differences and it is worthwhile trying different drugs in a class.
TABLE 17.
Triptans for the management of migraine
| Agent | Trade name | Usual dose (mg) | Tmax (h) | T1/2 (h) | Formulations | Advantages |
|---|---|---|---|---|---|---|
| Sumatriptan | Imitrex* | 50–100 | 0.5–5 | 1.9–2.2 | Tablets† | |
| 6 | 0.25 | 1.7–2.3 | Autoinjector | |||
| 5–20 | 1–1.5 | 1.3–5.4 | Nasal spray† | Nasal spray is useful if vomiting present | ||
| Almotriptan malate | Axert‡ | 6.25–12.5 | 1–3 | 3–4 | Tablets | Longer action but slower onset, useful for patients with longer duration headache |
| Eletriptan | Relpax§ | 20–40 | 2 | 4 | Tablets | Works in patients who are refractory to sumatriptan |
| Naratriptan hydrochloride | Amerge* | 1–2.5 | 2–5 | 5–8 | Tablets | |
| Rizatriptan benzoate | Maxalt¶ | 5–10 | 1–1.5 | 1.7–3 | Tablets
Wafer |
The wafer is an advantage if access to water is difficult, it may be placed under the tongue and is still well absorbed |
| Zolmitriptan | Zomig** | 2.5–5 | 2 | 2.5–3 | Tablets | Nasal spray is useful if vomiting is present, rapimelt tablet similar advantage to the wafer, easy administration |
| 2.5–5 | 3 | 3 | Nasal spray | |||
| 2.5–5 | 3 | 3 | Rapimelt tablet |
GlaxoSmithKline Inc, Canada;
Sumatriptan exhibits low oral and intranasal bioavailability related to incomplete absorption and hepatic and presystemic metabolism;
Janssen-Ortho Inc, Canada;
Pfizer Canada Inc;
Merck Frosst Canada Inc;
AstraZeneca Canada Inc. T1/2 Half-life; Tmax Time to maximum concentration
The triptans are selective for the 5-HT1B and 5-HT1D serotonin receptor subtypes, where they act as agonists. The mechanism of action involves the reduction of sensory activation in the periphery and nociceptive transmission in the brainstem trigeminal nucleus where they diminish central sensitization (11,225). They also result in vasoconstriction, opposing the vasodilation, thought to be involved in the pathophysiology of migraine, although the role of vasoconstriction in the antimigraine action remains unclear. The triptans have replaced the vasoconstrictive agent ergotamine tartrate. These agents should not be used in patients with a history, symptoms or signs of ischemic cardiac, cerebrovascular or peripheral vascular disease, valvular heart disease, cardiac arrythmias or other significant cardiovascular disease. In addition, triptans should be avoided in persons with hemiplegic and basilar migraine (226).
This section has focused on the triptans. However, it is important to remember that the first line of treatment in migraine is to counsel patients about diet, particularly in terms of food triggers such as alcohol, red wine, nitrates in preserved meat and monosodium glutamate, the importance of regular sleep, not sleeping in, regular exercise and generally healthful approaches to living. If it is decided that pharmacotherapy is needed, the next step is to add a standard over-the-counter analgesic such as acetaminophen, ASA, ibuprofen or an ASA/acetaminophen/caffeine combination (227,228). These measures assist many patients in control of migraine.
For migraine prophylaxis, pharmacotherapeutic options include propranalol (229), sodium valproic acid (and possibly topiramate) (230), calcium antagonists (the most useful are flunarizine, verapamil and cardizem) (231,232) and TCA analgesics (233). None of these agents have been demonstrated to exhibit high efficacy and there is no evidence on which to base the choice of one over the other (11); however, prophylactic agents are useful and it is worth pursuing serial trials of the agents listed. In addition, the value of nonpharmacological approaches should be emphasized, because a meta-analytic review found substantial support for the effectiveness of both biofeedback approaches and pharmacological approaches, with no preference for one over the other (234). Thus, it appears that a combined approach is the way to go in migraine prophylaxis.
TOPICAL ANALGESICS
The involvement of peripheral mechanisms in the generation of chronic pain suggests the use of topical agents in management. There is considerable interest in this area, with the probability that new agents will become available (66). Controlled trials have demonstrated efficacy for topical NSAIDs (235–237), capsaicin (238), local anesthetics (239–241) and doxepin (242–244). There is preliminary evidence that a topical combination of amitriptyline and ketamine is analgesic in neuropathic pain (details are presented below) (245,246).
Topical NSAIDs
Systematic reviews of randomized, controlled trials have identified that topical NSAIDs are effective in relieving pain in acute (soft tissue trauma and sprains) and chronic pain (247–249). In acute strains and sprains, topical NSAIDs were significantly better than placebo over one week, with an NNT of 3.9. In chronic conditions, such as arthritis and rheumatism, topical NSAIDs administered over two weeks demonstrated an NNT of 3.1 (247). For drugs with at least three placebo-controlled trials, ketoprofen (NNT=2.6), felbinac (NNT=3.0), ibuprofen (NNT=3.5) and piroxicam (NNT=4.2) exhibited significant efficacy (247). In other words, one in three patients using a topical NSAID for treatment of chronic pain in arthritis and rheumatism achieves a successful outcome who would not have achieved this with a placebo.
Topical NSAIDs exhibited few adverse events; these were primarily cutaneous in nature (rash or pruritis at the site of application) and GI adverse events were rare compared with oral use (248). The evidence supports local enhanced delivery with NSAID concentrations in subadjacent synovium comparable with those after oral administration, and subcutaneous concentrations far exceeding those after oral administration (248). In addition, it has been determined that this was not due to the effects of rubbing; placebo preparations were also rubbed into the affected parts (247).
More recently, data from three randomized, controlled trials regarding a new topical agent available in Canada (diclofenac 1.5% in dimethlysulfoxide [Pennsaid, Diemthaid Healthcare Ltd, Canada]) indicates that this agent is significantly better than placebo in the treatment of osteoarthritis of the knee (235–237). The topical agent was as effective as the oral agent and exhibited fewer adverse events (237). Currently, topical diclofenac is the only topical NSAID available in Canada by prescription.
There are a number of over-the-counter salicylate-containing preparations available in Canada (most contain trolamine salicylate or methyl salicylate). These agents generally fall into the category of rubefacients (or agents that act by counterirritation). A systematic review (250) of topical rubefacients containing salicylates notes that salicylates are difficult to categorize, because they do not seem to work in the same way as other NSAIDs. This review indicated that trials of rubefacients are limited by number, size, quality and validity; the best assessment of limited information suggests that rubefacients containing salicylates may be efficacious in acute pain and moderately to poorly efficacious in chronic arthritic and rheumatic pain. In acute conditions the NNT was 2.1; in chronic conditions the NNT was 5.3. It was concluded that topical salicylates may have efficacy in acute pain at seven days, but poor to moderate efficacy for chronic pain at 14 days (250). Table 18 presents the topical NSAIDs and some of the rubefacients available in Canada.
TABLE 18.
| Product name | Active agent | |
|---|---|---|
| Topical NSAID | Pennsaid‡ | Diclofenac |
| Topical rubefacients | Myoflex 10%§ | Trolamine salicylate |
| Myoflex extra strength 15%§ | Trolamine salicylate | |
| Aspercreme 10%¶ | Trolamine salicylate | |
| Extra strength aspercreme 15%¶ | Trolamine salicylate | |
| Bengay muscle pain** | Trolamine salicylate | |
| Arthritis cream | Trolamine salicylate | |
| Actiflex†† | Trolamine salicylate | |
| Crème analgesique extra puissant sans odeur | Trolamine salicylate | |
| Hot stuff‡‡ | Methyl salicylate | |
| Oil of wintergreen | Methyl salicylate | |
| Physio rub | Methyl salicylate | |
| Medicated analgesic cream | Methyl salicylate | |
| Ultra strength heat rub | Methyl salicylate | |
| Antiphlogistine rub | Methyl salicylate | |
| A-535 heat§§ |
Prescription required;
Available without a prescription;
Dimethaid Health Care Ltd, Canada;
Bayer Inc, Canada;
Chattem, Canada;
Pfizer Canada Inc;
Pangeo Pharma (Canada) Inc;
Mueller Sports Medicine Inc, USA;
Church & Dwight Canada Corp
Capsaicin
Capsaicin is the active ingredient of chili peppers and similar plants in the capsicum family. Capsaicin is available as a topical cream in two strengths: 0.025% and 0.075% (Zostrix, Medicis Canada Ltd). The mechanism of action is thought to be due to a reduction in pain-related neuropeptides such as substance P, with blockade of afferent input (238). Hypoalgesia is also associated with degeneration of epidermal nerve fibres (251).
A recent systematic review revealed six double-blind, placebo-controlled trials (656 patients) pooled for neuropathic pain conditions, with a NNT of 5.7, and three controlled trials examining capsaicin in musculoskeletal pain (368 patients) with a NNT of 8.1 (251). An earlier meta-analysis identified that capsaicin cream was better than placebo in the treatment of diabetic neuropathy, osteoarthritis and psoriasis (252). Thus, there is some evidence that topical capsaicin is better than placebo for treatment of pain from diabetic neuropathy, osteoarthritis and possibly psoriasis (28,251).
Treatment with capsaicin causes a burning sensation, which compromises the blinding of clinical trials and decreases the clinical utility of this agent. Of interest is an apparent persisting benefit in some patients who respond, remain improved and do not continue to require capsaicin. The fact that the pain in these patients was present for prolonged periods before capsaicin makes it unlikely that this effect was just the natural resolution of the pain condition (238).
Capsaicin may be beneficial to some patients with neuropathic or arthritic pain as an adjuvant analgesic, but is unlikely to be adequate as the sole analgesic agent. The frequency of application of either the 0.025% or the 0.075% cream should be four to five times daily. Treatment should persist for four to six weeks, because the onset and best response may take this long to occur. Patients should be instructed to wash their hands after each application or to use rubber gloves to apply the cream, and to avoid eye contact. The adverse effect is a burning sensation. This will occur in 80% of patients. The severity of burning appears to be worse in conditions such as postherpetic neuralgia or psoriasis where the skin is permanently scarred, than in pain conditions where the skin is normal. The burning may decrease with repeated applications. One can also decrease burning by using another analgesic or applying a topical local anesthetic to improve tolerance. Coughing and sneezing can occur. To date, there has been no evidence of toxic effects on nerves with the low doses used in topical application, although this is a concern (238).
Topical TCAs and ketamine
There are two randomized, controlled trials demonstrating that topical doxepin 3% to 5% is analgesic in a mixed group of patients with neuropathic pain (243,244); one demonstrated an earlier onset of analgesic effect when doxepin was used in combination with capsaicin (243). Doxepin 5% topical cream (Zonalon, Medicis Canada Ltd) is available in Canada for the treatment of pruritis. Topical amitriptyline 2% with ketamine 1% given over six to 12 months in an open-label trial was associated with long-term perceived analgesic effect and good to excellent patient satisfaction, and was well tolerated in a group of patients with mixed diagnoses of neuropathic pain (253). In a three-week randomized, placebo-controlled trial, the concentration of amitriptyline 2% and ketamine 1% was not significantly better than placebo (254); however, a higher concentration of amitriptyline 4% and ketamine 2% was significantly better than placebo in postherpetic neuralgia (245). Thus, there is preliminary support that a combination of amitriptyline and ketamine is analgesic in postherpetic neuralgia in a 4% amitriptyline and 2% ketamine concentration. Topical amitriptyline and ketamine cream is not yet available in Canada.
Topical lidocaine patch
Subsequent to initial data suggesting that a topical gel containing 5% lidocaine led to a significant decrease in the pain of postherpetic neuralgia over the torso or limbs (255), a topical 5% lidocaine patch has been developed. There are three randomized, controlled trials examining the lidocaine patch in postherpetic neuralgia (239–241) and one in patients with a variety of peripheral neuropathic pain syndromes (256); all have found that the lidocaine 5% patch provides significantly better analgesia than a vehicle placebo patch. In a recent review of these studies, the author concludes that the topical 5% lidocaine patch holds promise for the treatment of postherpetic neuralgia and other neuropathic conditions (257).
CANNABINOIDS
Cannabinoids available by prescription
The potent antinociceptive and antihyperalgesic effects of cannabinoid agonists in animal models of acute and chronic pain, the presence of cannabinoid receptors in pain-processing areas of the brain, spinal cord and periphery, and evidence supporting endogenous modulation of pain systems by cannabinoids provide support that cannabinoids exhibit significant potential as analgesics (258–261).
At present, there are two oral cannabinoids available in Canada. These are nabilone (Cesamet, Valeant Canada Ltd) and Marinol (Solvay Pharma Inc, Canada) (a synthetic preparation of delta-9-tetrahydrocannabinol [THC], which is the main psychoactive ingredient in cannabis). The listed indication for both of these agents is nausea and vomiting following chemotherapy (as second- or third-line options). Any use in pain applications would be considered ‘off-label’ use. There is some support that oral THC preparations exhibit a mild to moderate analgesic effect equivalent to codeine 60 mg to 120 mg daily and that higher doses are associated with central effects such as sedation (262). There is one randomized, controlled trial that has demonstrated a modest effect for synthetic THC (Marinol) in the treatment of central pain in MS using a dose of 10 mg (263). There are no randomized, controlled trials examining nabilone in the treatment of chronic pain, but there are two clinical reports showing modest benefit in some patients (264,265).
A multicentre, randomized, controlled trial examining an oral cannabis extract demonstrated an improvement in objective mobility and pain in MS, but no significant effect on the Ashworth scale for spasticity after 15 weeks of treatment (266); however, in the 80% of patients followed for 12 months (double-blinded) there was a statistically significant small treatment effect on muscle spasticity on the Ashworth scale (267).
In April 2005, a novel buccal spray extract containing THC and cannabidiol (a nonpsychoactive cannabinoid found in cannabis), Sativex (GW Pharma Ltd, United Kingdom) was approved by Health Canada and received a conditional notice of compliance. A randomized, controlled trial of 48 patients demonstrated a statistically significant decrease in pain of brachial plexus avulsion, but not only by the full two-point reduction on the 11-point numeric rating scale required for clinical significance (268), a further randomized, controlled trial has demonstrated a significant reduction in pain and improved sleep in 64 patients with central pain due to MS (269). The indication for Sativex is as adjunctive treatment for symptomatic relief of neuropathic pain in MS. Further trials are ongoing.
In making a decision regarding whether to use a cannabinoid, one may apply all of the same guidelines as reviewed for opioids. In other words, a comprehensive assessment of the cause of the pain and a psychosocial history, including screening for past and current risks for addiction, must be completed. Conventional therapies should be tried or considered before initiating a trial of a cannabinoid. Cannabinoids should generally be considered as a third line of treatment. A recent symposium of articles published in this Journal provide further information regarding the use of cannabinoids in pain (259,270–276).
Side effects include euphoria, anxiety, panic, paranoia, psychosis, sedation, dizziness, somnolence, depression, ataxia, cognitive effects, tachycardia, postural hypotension and palpitations. There are effects on reaction time and motor control, so patients should be advised that driving can be affected and to use discerning judgment, as you would advise patients when using any potentially sedating agent. Table 19 presents the available agents, dose preparations and dose guidelines.
TABLE 19.
Cannabinoids available by prescription in Canada
| Agent | Trade name | Strengths available (mg) | Start dose (range) | Route of administration | Listed indications |
|---|---|---|---|---|---|
| Nabilone | Cesamet* | 0.5, 1 | 0.5 mg/hs (1–2 mg bid) | Oral | Antiemetic in cancer chemotherapy |
| Synthetic Δ-9-THC | Marinol† | 2.5, 5, 10 | 5 mg (5–10 mg bid) | Oral | Antiemetic in cancer chemotherapy |
| Extract of naturally occurring Δ-9-THC and CBD | Sativex‡§ | 2.7 Δ-9-THC/2.5 CBD** | –¶ | Transbuccal | Adjunctive treatment for pain in multiple sclerosis |
Valeant Canada Ltd;
Solvay Pharma Inc, Canada;
GW Pharma Ltd, United Kingdom;
Approved under a conditional notice of compliance by Health Canada as of April 2005;
Insufficient published information to make recommendations regarding dose at the time of writing;
Each spray contains 2.7 mg delta-9-tetrahydro-cannabinol (Δ-9-THC) and 2.5 mg cannabidiol (CBD). bid Twice a day; hs At bedtime
Marihuana Medical Access Regulations
Since July 2001, the Marihuana Medical Access Regulations have been established, allowing Canadians to apply to possess cannabis (marihuana) for medicinal purposes. This program involves an application process initiated by the patient. The patient requires the support of a physician who also completes a form. The patient sends the forms, along with two passport photos, to the Office of Cannabis Medical Access, Health Canada, where the application is reviewed. If all requirements are met, a license to possess is granted. Patients may also apply for a license to produce or may purchase cannabis from Health Canada. Further information regarding this program is available at <www.hc-sc.gc.ca/hecs-sesc/ocma/index.htm>. As of March 4, 2005, there are 813 Canadians authorized to possess marihuana for medical purposes and there are 352 physicians in Canada who have supported these applications. Updated statistics are available at the Web site quoted above.
There is a need for further study regarding marihuana as a therapeutic agent with regard to both efficacy and safety, neither of which have been established in controlled trials. There are studies underway. In the meantime, it is important for physicians in Canada to be aware that 10% to 16% of patients presenting to pain and MS clinics are using cannabis for symptom control, and that pain is one of the top symptoms for which people in the general population are using cannabis for medical purposes (277–280). Physicians should always ask about the use of cannabis and any other herbal or complementary therapies as a routine part of history-taking.
The Special Access Programme available through Health Canada
The Special Access Programme is available to physicians who would like to obtain nonmarketed drugs for patients with serious or life-threatening conditions when conventional therapies have failed, or are unsuitable or unavailable. Chronic pain that has been unresponsive to agents marketed in Canada can be a serious condition for consideration under this program. Thus, if a physician knows of an agent that may benefit their patient and if that agent is not yet available in Canada, it may be possible to obtain the drug through the Special Access Programme. The Web site for the Special Access Programme, with all of the forms and documentation, is <http://www.hc-sc.gc.ca/dhp-mps/acces/drugs-drogues/index_e.html>. The contact phone number is 613-941-2108.
THE FUTURE
There are many new agents in development. New models for persistent pain have allowed researchers to define the pharmacology of analgesia in more detail. It is known that numerous mechanisms at multiple levels may be involved in an individual presenting with persistent pain. We have already learned that more than one systemic agent is often necessary to target relevant mechanisms (11), and that when two agents are used, it is sometimes possible to obtain better analgesia at lower doses and with fewer side effects (14). In the future, it is probable that treatment protocols will include both systemic and topical agents targeting both central and peripheral mechanisms. Novel neuromodulators targeting sodium and calcium channels, NMDA receptor antagonists, purinergic agents, and agents targeting the endocannabinoid system and others are all in development, as well as agents for topical use, and these will provide additional options as analgesics in the future.
CONCLUSIONS
Chronic pain involves multiple pathophysiological mechanisms with peripheral and central components. The management of chronic pain requires an interdisciplinary approach. Pharmacotherapy for pain must take place within an overall management plan that maximizes the patient’s involvement in the pursuit of health, even in the face of pain. This review has provided information regarding the major classes of medication used to assist in the management of chronic pain, including the NSAIDs, acetaminophen, antidepressants and anticonvulsants. The use of chronic opioids has been reviewed, along with an approach to comorbid pain and addiction. Emerging areas, including topical approaches and an introduction to the field of cannabinoids, have also been presented.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of Drs Harold Merskey, John Clark and Jana Sawynok for review of the entire manuscript, and Ms Lisa Grandy Allen and Ms Theresa Hurley of the Drug Information Centre, Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia for assistance in research of portions of the manuscript
Footnotes
FUNDING: Jointly supported by the Department of Psychiatry, Dalhousie University, Halifax, Nova Scotia, and the College of Physicians and Surgeons of Nova Scotia.
REFERENCES
- 1.Moulin DE, Clark AJ, Speechley M, Morley-Forster PK. Chronic pain in Canada – prevalence, treatment, impact and the role of opioid analgesia. Pain Res Manag. 2002;7:179–84. doi: 10.1155/2002/323085. [DOI] [PubMed] [Google Scholar]
- 2.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: A World Health Organization Study in Primary Care. JAMA. 1998;280:147–51. doi: 10.1001/jama.280.2.147. (Erratum in 1998;280:1142) [DOI] [PubMed] [Google Scholar]
- 3.Liebeskind JC. Pain can kill. Pain. 1991;44:3–4. doi: 10.1016/0304-3959(91)90141-J. [DOI] [PubMed] [Google Scholar]
- 4.Page GG. Acute pain and immune impairment. Pain Clin Updates. 2005;XIII:1–4. [Google Scholar]
- 5.Poobalan AS, Bruce J, King PM, Chambers WA, Krukowski ZH, Smith WC. Chronic pain and quality of life following open inguinal hernia repair. Br J Surg. 2001;88:1122–6. doi: 10.1046/j.0007-1323.2001.01828.x. [DOI] [PubMed] [Google Scholar]
- 6.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Council on Health Services Accreditation Standards Document. 2005, Canadian Council on Health Services Accreditation. p. Standard 13.11.
- 8.Woolf CJ, Bennett GJ, Doherty M, et al. Towards a mechanism-based classification of pain? Pain. 1998;77:227–9. doi: 10.1016/S0304-3959(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 9.Woolf CJ, Decosterd I. Implications of recent advances in the understanding of pain pathophysiology for the assessment of pain in patients. Pain. 1999;(Suppl 6):S141–7. doi: 10.1016/S0304-3959(99)00148-7. [DOI] [PubMed] [Google Scholar]
- 10.Woolf CJ, Max MB. Mechanism based pain diagnosis: Issues for analgesic drug development. Anesthesiology. 2001;95:241–9. doi: 10.1097/00000542-200107000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Griffin RS, Woolf CJ. Pharmacology of analgesia. In: Golan DE, editor. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Philadelphia: Lippincott, Williams and Wilkins; 2005. pp. 229–43. [Google Scholar]
- 12.Wall PD. Introduction to the edition after this one. In: Wall PD, Melzack R, editors. The Textbook of Pain. 3. London: Churchill Livingstone; 1994. pp. 1–7. [Google Scholar]
- 13.Dworkin RH. An overview of neuropathic pain: Syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–9. doi: 10.1097/00002508-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–34. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 15.McCormack K. Non-steroidal anti-inflammatory drugs and spinal nociceptive processing. Pain. 1994;59:9–43. doi: 10.1016/0304-3959(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 16.Cashman JN. The mechanisms of action of NSAIDS in analgesia. Drugs. 1996;52:13–23. doi: 10.2165/00003495-199600525-00004. [DOI] [PubMed] [Google Scholar]
- 17.Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136–46. [PubMed] [Google Scholar]
- 18.Malmberg AB, Yaksh TL. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiology. 1993;79:270–81. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Yaksh TL, Dirig DM, Malmberg AB. Mechanism of action of nonsteroidal anti-inflammatory drugs. Cancer Invest. 1998;16:509–27. doi: 10.3109/07357909809011705. [DOI] [PubMed] [Google Scholar]
- 20.Wallace JL, Cirino G. The development of gastrointestinal-sparing nonsteroidal anti-inflammatory drugs. Trends Pharmacol Sci. 1994;15:405–6. doi: 10.1016/0165-6147(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433–42. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 22.Spangler RS. Cyclooxygenase 1 and 2 in rheumatic disease: Implications for nonsteroidal anti-inflammatory drug therapy. Semin Arthritis Rheum. 1996;26:435–46. doi: 10.1016/s0049-0172(96)80024-2. [DOI] [PubMed] [Google Scholar]
- 23.Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cycloocygenase inhibition. Science. 1992;257:1278–9. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 24.Bombardier C, Laine L, Reicin A, et al. VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–8. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 25.Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: Randomised controlled trial. Lancet. 2004;364:665–74. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- 26.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 27.Tannenbaum H, Bombardier C, Davis P, Russell AS. An evidence-based approach to prescribing nonsteroidal antiinflammatory drugs. Third Canadian Consensus Conference. J Rheumatol. 2006;33:140–57. [PubMed] [Google Scholar]
- 28.Levesque LE, Brophy JM, Zhang B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: A population study in elderly adults. Ann Intern Med. 2005;142:481–9. doi: 10.7326/0003-4819-142-7-200504050-00113. [DOI] [PubMed] [Google Scholar]
- 29.Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: Nested case-control study. Lancet. 2005;365:475–81. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 30.Kimmel SE, Berlin JA, Reilly M, et al. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med. 2005;142:157–64. doi: 10.7326/0003-4819-142-3-200502010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Baumann TJ. Pain management. In: Piro JT, Talbert RL, Hayes PE, Yee GC, Matzke GR, Posey LM, editors. Pharmacotherapy. A Pathophysiologic Approach. 5. New York: McGraw Hill; 2002. p. 1107. [Google Scholar]
- 32.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 33.Tramer MR, Moore RA, Reynolds DJ, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: A new model applied to chronic NSAID use. Pain. 2000;85:169–82. doi: 10.1016/s0304-3959(99)00267-5. [DOI] [PubMed] [Google Scholar]
- 34.Singh G, Ramey DR, Morfeld D, Fries JF. Comparative toxicity of non-steroidal anti-inflammatory agents. Pharmacol Ther. 1994;62:175–91. doi: 10.1016/0163-7258(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 35.Lipani J, Poland M. Clinical update of the relative safety of nabumetone in long term trials. Inflammopharmacology. 1995;3:351–6. [Google Scholar]
- 36.Sunshine A, Olsen NZ. Nonnarcotic analgesics. In: Wall PD, Melzack R, editors. Textbook of Pain. 3. United Kingdom: Churchill Livingstone; 1994. pp. 923–42. [Google Scholar]
- 37.Chandrasekharan NV, Dai H, Roos KL, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–31. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelissier T, Alloui A, Caussade F, et al. Paracetamol exerts a spinal antinociceptive effect involving an indirect interaction with 5-hydroxytryptamine3 receptors: In vivo and in vitro evidence. J Pharmacol Exp Ther. 1996;278:8–14. [PubMed] [Google Scholar]
- 39.Bonnefont J, Alloui A, Chapuy E, Clottes E, Eschalier A. Orally administered paracetamol does not act locally in the rat formalin test: Evidence for a supraspinal, serotonin-dependent antinociceptive mechanism. Anesthesiology. 2003;99:976–81. doi: 10.1097/00000542-200310000-00034. [DOI] [PubMed] [Google Scholar]
- 40.Bonnefont J, Courade JP, Alloui A, Eschalier A. [Antinociceptive mechanism of action of paracetamol] Drugs. 2003;63:1–4. [PubMed] [Google Scholar]
- 41.Watson CP, Evans RJ, Reed K, Merskey H, Goldsmith L, Warsh J. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32:671–3. doi: 10.1212/wnl.32.6.671. [DOI] [PubMed] [Google Scholar]
- 42.Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 43.Lynch ME. Antidepressants as analgesics. A review of random controlled trials examining analgesic effects of antidepressant agents. J Psychiatry Neurosci. 2001;26:30–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Max MB. Antidepressants as analgesics. In: Fields HL, Liebeskind JC, editors. Progress in Pain Research Management. Seattle: IASP Press; 1994. pp. 229–46. [Google Scholar]
- 45.Max MB. Thirteen consecutive well-designed randomized trials show that anti-depressants reduce pain in diabetic neuropathy and postherpetic neuralgia. Pain Forum. 1995;4:248–53. [Google Scholar]
- 46.McQuay HJ, Moore RA. Antidepressants and chronic pain. BMJ. 1997;314:763–4. doi: 10.1136/bmj.314.7083.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McQuay HJ, Tramer M, Nye BA, Carroll D, Wiffen PJ, Moore RA. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68:217–27. doi: 10.1016/s0304-3959(96)03140-5. [DOI] [PubMed] [Google Scholar]
- 48.Kieburtz K, Simpson D, Yiannoutsos C, et al. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. AIDS Clinical Trial Group 242 Protocol Team. Neurology. 1998;51:1682–8. doi: 10.1212/wnl.51.6.1682. [DOI] [PubMed] [Google Scholar]
- 49.Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: Results of a randomized controlled trial. Pain. 2002;96:365–73. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 50.Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98:195–203. doi: 10.1016/s0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 51.Onghena P, Van Houdenhov B. Antidepressant-induced analgesia in chronic nonmalignant pain: A meta-analysis of 39 placebo controlled studies. Pain. 1992;49:205–19. doi: 10.1016/0304-3959(92)90144-Z. [DOI] [PubMed] [Google Scholar]
- 52.Eschalier A, Ardid D, Dubray C. Tricyclic and other antidepressants as analgesics. In: Sawnyok J, Cowan A, editors. Novel Aspects of Pain Management: Opioids and Beyond. New York: Wiley; 1999. pp. 303–20. [Google Scholar]
- 53.Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C., III Inhibition of neuronal Na+ channels by antidepressant drugs. J Pharmacol Exp Ther. 1998;284:208–14. [PubMed] [Google Scholar]
- 54.Song JH, Ham SS, Shin YK, Lee CS. Amitriptyline modulation of Na(+) channels in rat dorsal root ganglion neurons. Eur J Pharmacol. 2000;401:297–305. doi: 10.1016/s0014-2999(00)00460-x. [DOI] [PubMed] [Google Scholar]
- 55.Lavoie PA, Beauchamp G, Elie R. Tricyclic antidepressants inhibit voltage-dependent calcium channels and Na+-Ca2+ exchange in rat brain cortex synaptosomes. Can J Physiol Pharmacol. 1994;68:1414–8. doi: 10.1139/y90-215. [DOI] [PubMed] [Google Scholar]
- 56.Gerner P, Mujtaba M, Sinnott CJ, Wang GK. Amitriptyline versus bupivacaine in rat sciatic nerve blockade. Anesthesiology. 2001;94:661–7. doi: 10.1097/00000542-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 57.Khan MA, Gerner P, Want GK. Amitriptyline for prolonged cutaneous analgesia in the rat. Anesthesiology. 2002;96:109–16. doi: 10.1097/00000542-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 58.Eisenach JC, Gebhart GF. Intrathecal amitriptyline acts as an N-methyl-D-aspartate receptor antagonist in the presence of inflammatory hyperalgesia in rats. Anesthesiology. 1995;83:1046–53. doi: 10.1097/00000542-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 59.Esser MJ, Sawynok J. Caffeine blockade of the thermal anti-hyperalgesic effect of acute amitriptyline in a rat model of neuropathic pain. Eur J Pharmacol. 2000;399:131–9. doi: 10.1016/s0014-2999(00)00336-8. [DOI] [PubMed] [Google Scholar]
- 60.Sierralta F, Pinardi G, Mendez M, Miranda HF. Interaction of opioids with antidepressant-induced antinociception. Psychopharmacology (Berl) 1995;122:374–8. doi: 10.1007/BF02246269. [DOI] [PubMed] [Google Scholar]
- 61.Ulugol A, Karadag HC, Tamer M, Firat Z, Aslantas A, Dokmeci I. Involvement of adenosine in the anti-allodynic effect of amitriptyline in streptozotocin-induced diabetic rats. Neurosci Lett. 2002;328:129–32. doi: 10.1016/s0304-3940(02)00491-3. [DOI] [PubMed] [Google Scholar]
- 62.Esser MJ, Sawnyok J. Caffeine blockade of the thermal anti-hyperalgesic effect of acute amitriptyline in a rat model of neuropathic pain. Eur J Pharmacol. 2000;399:131–9. doi: 10.1016/s0014-2999(00)00336-8. [DOI] [PubMed] [Google Scholar]
- 63.Esser MJ, Chase T, Allen GV, Sawynok J. Chronic administration of amitriptyline and caffeine in a rat model of neuropathic pain: Multiple interactions. Eur J Pharmacol. 2001;430:211–8. doi: 10.1016/s0014-2999(01)01276-6. [DOI] [PubMed] [Google Scholar]
- 64.Galeotti N, Ghelardini C, Capaccioli S, Quattrone A, Nicolin A, Bartolini A. Blockade of clomipramine and amitriptyline analgesia by an antisense oligonucleotide to mKv1.1, a mouse Shaker-like K+ channel. Eur J Pharmacol. 1997;330:15–25. doi: 10.1016/s0014-2999(97)10134-0. [DOI] [PubMed] [Google Scholar]
- 65.Galeotti N, Ghelardini C, Bartolini A. Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology. 2001;40:75–84. doi: 10.1016/s0028-3908(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 66.Sawynok J. Topical and periphally acting analgesics. Pharm Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- 67.Sawynok J, Esser MJ, Reid AR. Antidepressants as analgesics: An overview of central and peripheral mechanisms of action. J Psychiatry Neurosci. 2001;26:21–9. [PMC free article] [PubMed] [Google Scholar]
- 68.Sindrup SH, et al. Concentration response relationship in imipramine treatment of diabetic symptoms. Clin Pharmacol Ther. 1990;47:509–15. doi: 10.1038/clpt.1990.65. [DOI] [PubMed] [Google Scholar]
- 69.Roose SP, Laghrissi-Thode F, Kennedy JS, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA. 1998;279:287–91. doi: 10.1001/jama.279.4.287. [DOI] [PubMed] [Google Scholar]
- 70.Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses. 1998;51:167–8. doi: 10.1016/s0306-9877(98)90112-8. [DOI] [PubMed] [Google Scholar]
- 71.Galer BS. Neuropathic pain of peripheral origin: Advances in pharmacologic treatment. Neurology. 1995;45(Suppl 9):S17–25. doi: 10.1212/wnl.45.12_suppl_9.s17. [DOI] [PubMed] [Google Scholar]
- 72.Songer DA, Schule H. Venlafaxine for the treatment of chronic pain. Am J Psychiatry. 1996;153:737. doi: 10.1176/ajp.153.5.737a. [DOI] [PubMed] [Google Scholar]
- 73.Sumptom JE, Moulin DE. Treatment of neuropathic pain with venalfaxine. Ann Pharmacother. 2001;35:557–9. doi: 10.1345/aph.10206. [DOI] [PubMed] [Google Scholar]
- 74.Taylor K, Rowbotham MC. Venlafaxine hydrochloride and chronic pain. West J Med. 1996;165:147–8. [PMC free article] [PubMed] [Google Scholar]
- 75.Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: A double-blind, placebo-controlled study. Pain. 2004;110:697–706. doi: 10.1016/j.pain.2004.05.010. (Erratum in 2005;113:248) [DOI] [PubMed] [Google Scholar]
- 76.Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: A randomized, controlled trial. Neurology. 2003;60:1284–9. doi: 10.1212/01.wnl.0000058749.49264.bd. [DOI] [PubMed] [Google Scholar]
- 77.Tasmuth T, Hartel B, Kalso E. Venlafaxine in neuropathic pain following treatment of breast cancer. Eur J Pain. 2002;6:17–24. doi: 10.1053/eujp.2001.0266. [DOI] [PubMed] [Google Scholar]
- 78.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–84. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 79.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–18. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 80.Wernicke JF. Duloxetine in treatment of diabetic neuropathic pain. Pharmacotherapy. 2004;24:1422. (Abst) [Google Scholar]
- 81.Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res. 2005;39:43–53. doi: 10.1016/j.jpsychires.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 82.Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12:384–9. doi: 10.1046/j.1525-1497.1997.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250–6. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 84.Sindrup SH, Gram LF, Brosen K, Eshoj O, Mogensen EF. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990;42:135–44. doi: 10.1016/0304-3959(90)91157-E. [DOI] [PubMed] [Google Scholar]
- 85.Sindrup SH, Bjerre U, Dejgaard A, Brosen K, Aaes-Jorgensen T, Gram LF. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther. 1992;52:547–52. doi: 10.1038/clpt.1992.183. [DOI] [PubMed] [Google Scholar]
- 86.Bendtsen L, Jensen R, Olesen J. A non-selective (amitriptyline), but not a selective (citalopram), serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension type headache. Neurol Neurosurg Psychiatry. 1996;61:285–90. doi: 10.1136/jnnp.61.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sindrup SH, Jensen TS. Pharmacologic treatment of pain in polyneuropathy. Neurology. 2000;55:915–20. doi: 10.1212/wnl.55.7.915. [DOI] [PubMed] [Google Scholar]
- 88.Collins SL, Moore RA, McQuay HJ, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: A quantitative systematic review. J Pain Symptom Manag. 2000;20:449–559. doi: 10.1016/s0885-3924(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 89.Gallagher RM, Verma S. Mood and anxiety disorders in chronic pain. In: Dworkin RH, Breitbart WS, editors. Psychosocial Aspects of Pain: A Handbook for Healthcare Providers, Progress in Pain Research and Management. Vol. 27. Seattle: IASP Press; 2004. pp. 139–78. [Google Scholar]
- 90.Semenchuk MR, Sherman S, Davis B. Double blind randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology. 2001;57:1583–8. doi: 10.1212/wnl.57.9.1583. [DOI] [PubMed] [Google Scholar]
- 91.Mulrow CD, Williams JW, Jr, Chiquette E, et al. Efficacy of newer medications for treating depression in primary care practices. Am J Med. 2000;108:54–64. doi: 10.1016/s0002-9343(99)00316-2. [DOI] [PubMed] [Google Scholar]
- 92.Thase ME. Evaluating antidepressants therapies: Remission as the optimum outcome. J Clin Psychiatry. 2003;64:18–25. [PubMed] [Google Scholar]
- 93.Backonja M. Neuromodulating drugs for the symptomatic treatment of neuropathic pain. Curr Pain Headache Rep. 2004;8:212–6. doi: 10.1007/s11916-004-0054-4. [DOI] [PubMed] [Google Scholar]
- 94.Berde CB. New and old anticonvulsants for management of pain. IASP Newsletter Technical Corner January/February. 1997. pp. 3–5. [Google Scholar]
- 95.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10:685–92. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- 96.Rice ASC, Maton S NPS Group. Gabapentin in postherpetic neuralgia: A randomised, double blind, placebo controlled study. Pain. 2001;94:215–24. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 97.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: A randomized controlled trial. JAMA. 1998;280:1837–42. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 98.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double blind study comparing efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med. 1999;159:1931–7. doi: 10.1001/archinte.159.16.1931. [DOI] [PubMed] [Google Scholar]
- 99.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: A randomized controlled trial. JAMA. 1998;280:1831–6. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 100.Gorson KC, Schott C, Herman R, Ropper AH, Rand WM. Gabapentin in the treatment of painful diabetic neuropathy: A placebo controlled, double blind, crossover trial. J Neurol Neurosurg Psychiatry. 1999;66:251–2. doi: 10.1136/jnnp.66.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serpell MG NPS Group. Gabapentin in neuropathic pain syndromes: A randomised, double blind, placebo-controlled trial. Pain. 2002;99:557–66. doi: 10.1016/S0304-3959(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 102.Levendoglu F, Ogun CO, Ozerbil O, Ogun TC, Ugurlu H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29:743–51. doi: 10.1097/01.brs.0000112068.16108.3a. [DOI] [PubMed] [Google Scholar]
- 103.Spira PJ, Beran RG Australian Gabapentin Chronic Daily Headache Group. Gabapentin in the prophylaxis of chronic daily headache: A randomized, placebo-controlled study. Neurology. 2003;61:1753–9. doi: 10.1212/01.wnl.0000100121.58594.11. [DOI] [PubMed] [Google Scholar]
- 104.Bridges D, Thompson SWN, Rice ASC. Mechanisms of neuropathic pain. Br J Aneasth. 2001;87:12–26. doi: 10.1093/bja/87.1.12. [DOI] [PubMed] [Google Scholar]
- 105.Frampton JE, Foster RH. Pregabalin in the treatment of postherpetic neuralgia. Drugs. 2005;65:111–8. doi: 10.2165/00003495-200565010-00011. [DOI] [PubMed] [Google Scholar]
- 106.Dworkin RH, Corbin AE, Young JP, Jr, et al. Pregabalin for treatment of postherpetic neuralgia: A randomized, placebo-controlled trial. Neurology. 2003;60:1274–83. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- 107.Sabatowski R, Galvez R, Cherry DA, et al. 1008-045 Study Group. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: Results of a randomized, placebo-controlled clinical trial. Pain. 2004;109:26–35. doi: 10.1016/j.pain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 108.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: A randomized, placebo-controlled trial. J Pain. 2005;6:253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 109.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: A randomized controlled trial. Neurology. 2004;63:2104–10. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 110.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: A double-blind, placebo-controlled trial. Pain. 2004;110:628–38. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 111.Randinitis EJ, Posvar EL, Alvey CW. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol. 2003;43:277–83. doi: 10.1177/0091270003251119. [DOI] [PubMed] [Google Scholar]
- 112.Rowbotham M, Young J, Sharma U, et al. Pregabalin shows reduction in pain by day three of treatment. J Pain. 2003;4(Suppl 1):63. [Google Scholar]
- 113.McQuay H, Carroll D, Jadad AR, Wiffen P, Moore A. Anticonvulsant drugs for management of pain: A systematic review. BMJ. 1995;311:1047–52. doi: 10.1136/bmj.311.7012.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Backonja M, Serra J. Pharmacologic management part 1: Better-studied neuropathic pain diseases. Pain Med. 2004;5:S28–S47. doi: 10.1111/j.1526-4637.2004.04020.x. [DOI] [PubMed] [Google Scholar]
- 115.Watson CPN. Management issues of neuropathic trigeminal pain from a medical perspective. J Orofac Pain. 2004;18:366–73. [PubMed] [Google Scholar]
- 116.Portenoy RK. Drug therapy for neuropathic pain. Drug Therapy. 1993;23:41–53. [Google Scholar]
- 117.Guay DP. Oxcarbazepine, topiramate, zonisamide, and levetiracetam: Potential use in neuropathic pain. Am J Geriatr Pharmacother. 2003;1:18–37. doi: 10.1016/s1543-5946(03)80013-2. [DOI] [PubMed] [Google Scholar]
- 118.Eisenberg E, Alon N, et al. Abstracts 8th World Congress on Pain. Seattle: IASP Press; 1996. Lamotrigine in the treatment of painful diabetic neuropathy; p. 372. [Google Scholar]
- 119.McCleane G. Lamotrigine in the management of neuropathic pain: A review of the literature. Clin J Pain. 2000;16:321–6. doi: 10.1097/00002508-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 120.Zakrzewska JM, Chaudhry Z, Nurmikko TJ, Patton DW, Mullens EL. Lamotrigine (lamictal) in refractory trigeminal neuralgia: Results from a double-blind placebo controlled crossover trial. Pain. 1997;73:223–30. doi: 10.1016/S0304-3959(97)00104-8. [DOI] [PubMed] [Google Scholar]
- 121.Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotrigine reduces painful diabetic neuropathy: A randomized, controlled study. Neurology. 2001;57:505–9. doi: 10.1212/wnl.57.3.505. [DOI] [PubMed] [Google Scholar]
- 122.Vestergaard K, Andersen G, Gottrup H, Kristensen BT, Jensen TS. Lamotrigine for central poststroke pain: A randomized controlled trial. Neurology. 2001;56:184–90. doi: 10.1212/wnl.56.2.184. [DOI] [PubMed] [Google Scholar]
- 123.Finnerup NB, Sindrup SH, Bach FW, Johannesen IL, Jensen TS. Lamotrigine in spinal cord injury pain: A randomized controlled trial. Pain. 2002;96:375–83. doi: 10.1016/S0304-3959(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 124.Simpson DM, Olney R, McArthur JC, Khan A, Godbold J, Ebel-Frommer K. A placebo-controlled trial of lamotrigine for painful HIV-associated neuropathy. Neurology. 2000;54:2115–9. doi: 10.1212/wnl.54.11.2115. [DOI] [PubMed] [Google Scholar]
- 125.McCleane G. 200 mg daily of lamotrigine has no analgesic effect in neuropathic pain: A randomized, double-blind, placebo controlled trial. Pain. 1999;83:105–7. doi: 10.1016/s0304-3959(99)00095-0. [DOI] [PubMed] [Google Scholar]
- 126.Lunardi G, Leandri M, Albano C, et al. Clinical effectiveness of lamotrigine and plasma levels in essential and symptomatic trigeminal neuralgia. Neurology. 1997;48:1714–7. doi: 10.1212/wnl.48.6.1714. [DOI] [PubMed] [Google Scholar]
- 127.Chong MS, Libretto SE. The rationale and use of topiramate for treating neuropathic pain. Clin J Pain. 2003;19:59–68. doi: 10.1097/00002508-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 128.McGeeney BE. Topiramate in the treatment of cluster headache. Curr Pain Headache Rep. 2003;7:135–8. doi: 10.1007/s11916-003-0023-3. [DOI] [PubMed] [Google Scholar]
- 129.Gilron I, Booher SL, Rowan JS, Max MB. Topiramate in trigeminal neuralgia: A randomized, placebo-controlled multiple crossover pilot study. Clin Neuropharmacol. 2001;24:109–12. doi: 10.1097/00002826-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 130.Edwards KR, Glanz MJ. Efficacy and safety of topiramate in the treatment of painful diabetic neuropathy: A double-blind, placebo-controlled study. Neurology. 2000;54:A81. [Google Scholar]
- 131.Thienel U, Neto W, Schwabe SK, Vijapurkar U Topiramate Diabetic Neuropathic Pain Study Group. Topiramate in painful diabetic polyneuropathy: Findings from three double-blind placebo controlled trials. Acta Anaesthesiol Scand. 2004;110:221–31. doi: 10.1111/j.1600-0404.2004.00338.x. [DOI] [PubMed] [Google Scholar]
- 132.Mei D, Capuano A, Vollono C, et al. Topiramate in migraine prophylaxis: A randomised double-blind versus placebo study. Neurol Sci. 2004;25:245–50. doi: 10.1007/s10072-004-0350-0. [DOI] [PubMed] [Google Scholar]
- 133.Brandes JL, Saper JR, Diamond M, et al. MIGR-002 Study Group. Topiramate for migraine prevention: A randomized controlled trial. JAMA. 2004;291:965–73. doi: 10.1001/jama.291.8.965. [DOI] [PubMed] [Google Scholar]
- 134.Silberstein SD, Neto W, Schmitt J, Jacobs D MIGR-001 Study Group. Topiramate in migraine prevention: Results of a large controlled trial. Arch Neurol. 2004;61:490–5. doi: 10.1001/archneur.61.4.490. [DOI] [PubMed] [Google Scholar]
- 135.Diener HC, Tfelt-Hansen P, Dahlof C, et al. MIGR-003 Study Group. Topiramate in migraine prophylaxis – results from a placebo-controlled trial with proplanalol as an active control. J Neurol. 2004;251:943–50. doi: 10.1007/s00415-004-0464-6. [DOI] [PubMed] [Google Scholar]
- 136.Silvestrini M, Bartolini M, Coccia M, Baruffaldi R, Taffi R, Provinciali L. Topiramate in the treatment of chronic migraine. Cephalalgia. 2003;23:820–4. doi: 10.1046/j.1468-2982.2003.00592.x. [DOI] [PubMed] [Google Scholar]
- 137.Storey JR, Calder CS, Hart DE, Potter DL. Topiramate in migraine prevention: A double-blind placebo-controlled study. Headache. 2001;41:968–75. doi: 10.1046/j.1526-4610.2001.01190.x. [DOI] [PubMed] [Google Scholar]
- 138.Edwards KR, Potter DL, Wu SC, Kamin M, Hulihan J. Topiramate in the preventive treatment of episodic migraine: A combined analysis from pilot, double-blind, placebo-controllled trials. CNS Spectr. 2003;8:428–32. doi: 10.1017/s1092852900018733. [DOI] [PubMed] [Google Scholar]
- 139.McQuay HJ. Neuropathic pain: Evidence matters. Eur J Pain. 2002;6:11–8. doi: 10.1053/eujp.2001.0316. [DOI] [PubMed] [Google Scholar]
- 140.Arkinstall W, Sandler A, Goughnour B, Babul N, Harsanyi Z, Darke AC. Efficacy of controlled release codeine in chronic non-malignant pain: A randomized, placebo-controlled clinical trial. Pain. 1995;62:169–78. doi: 10.1016/0304-3959(94)00262-D. [DOI] [PubMed] [Google Scholar]
- 141.Moulin DE, Iezzi A, Amireh R, Sharpe WK, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996;347:143–7. doi: 10.1016/s0140-6736(96)90339-6. [DOI] [PubMed] [Google Scholar]
- 142.Harati Y, Gooch C, Swenson M, et al. Double blind randomized trial of tramadol for the treatment of pain of diabetic neuropathy. Neurology. 1998;50:1842–6. doi: 10.1212/wnl.50.6.1842. [DOI] [PubMed] [Google Scholar]
- 143.Watson CPN, Babul N. Efficacy of oxycodone in neuropathic pain, a randomized trial in postherpetic neuralgia. Neurology. 1998;50:1837–41. doi: 10.1212/wnl.50.6.1837. [DOI] [PubMed] [Google Scholar]
- 144.Caldwell JR, Hale ME, Boyd RE, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs: A double blind, randomized, multicenter, placebo controlled trial. J Rheumatol. 1999;26:862–9. [PubMed] [Google Scholar]
- 145.Peloso PM, Bellamy N, Bensen W, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip and knee. J Rheumatol. 2000;27:764–71. [PubMed] [Google Scholar]
- 146.Roth SH, Fleischmann RM, Burch FX, et al. Around-the-clock controlled-release oxycodone therapy for osteoarthritis-related pain: Placebo-controlled trial and long-term evaluation. Arch Intern Med. 2000;160:853–60. doi: 10.1001/archinte.160.6.853. [DOI] [PubMed] [Google Scholar]
- 147.Huse E, Larbig W, Flor H, Birbaumer N. The effect of opioids on phantom limb pain and cortical reorgnization. Pain. 2001;90:47–55. doi: 10.1016/s0304-3959(00)00385-7. [DOI] [PubMed] [Google Scholar]
- 148.Caldwell JR, Rapoport RJ, Davis JC, et al. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: Results from a randomized, placebo-controlled, double-blind trial and open label extension trial. J Pain Symptom Manage. 2002;23:278–91. doi: 10.1016/s0885-3924(02)00383-4. [DOI] [PubMed] [Google Scholar]
- 149.Maier C, Hildebrandt J, Klinger R, Henrich-Eberl C, Lindena G MONTAS Study Group. Morphine responsiveness, efficacy and tolerability in patients with chronic non-tumor associated pain – results of a double-blind, placebo-controlled trial (MONTAS) Pain. 2002;97:223–33. doi: 10.1016/S0304-3959(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 150.Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59:1015–21. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- 151.Watson CP, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J. Controlled-release oxycodone relieves neuropathic pain: A randomized controlled trial in painful diabetic neuropathy. Pain. 2003;105:71–8. doi: 10.1016/s0304-3959(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 152.Jovey RD, Ennis J, Gardner-Nix J, et al. Canadian Pain Society. Use of opioid analgesics for the treatment of chronic noncancer pain – A consensus statement and guidelines from the Canadian Pain Society, 2002. Pain Res Manage. 2003;8(Suppl A):3A–28A. doi: 10.1155/2003/436716. [DOI] [PubMed] [Google Scholar]
- 153.Schumacher HE, Basbaum AI, Way WL. Opioid analgesics and antagonists. In: Katzung BG, editor. Basic and Clinical Pharmacology. 9. New York: Lange Medical Books, McGraw-Hill; 2004. pp. 497–518. [Google Scholar]
- 154.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–90. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 155.Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med. 1996;335:1124–32. doi: 10.1056/NEJM199610103351507. [DOI] [PubMed] [Google Scholar]
- 156.Williams DG, Hatch DJ, Howard RF. Codeine phosphate in paediatric medicine. Br J Anaesth. 2001;86:413–21. doi: 10.1093/bja/86.3.413. [DOI] [PubMed] [Google Scholar]
- 157.Chen ZR, Somogyi AA, Bochner F. Polymorphic O-demethylation of codeine. Lancet. 1988;2:914–5. doi: 10.1016/s0140-6736(88)92529-9. [DOI] [PubMed] [Google Scholar]
- 158.Chen ZR, Somogyi AA, Reynolds G, Bochner F. Disposition and metabolism of codeine after single and chronic doses in one poor and weven extensive metabolizers. Br J Clin Pharmacol. 1991;31:381–90. doi: 10.1111/j.1365-2125.1991.tb05550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lipkowski AW, Carr DB. Rethinking opioid equivalence. Pain Clinical Updates. 2002;X:1–4. [Google Scholar]
- 160.Wolff K, Rostami-Hodjegan A, Shires S, et al. The pharmacokinetics of methadone in healthy subjects and opiate users. Br J Clin Pharmacol. 1997;44:325–34. doi: 10.1046/j.1365-2125.1997.t01-1-00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pasternak G. Multiple morphine and enkephalin receptors and the relief of pain. JAMA. 1988;259:1362–7. [PubMed] [Google Scholar]
- 162.Pasternak G. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci. 2001;22:67–70. doi: 10.1016/s0165-6147(00)01616-3. [DOI] [PubMed] [Google Scholar]
- 163.Ebert B, Andersen S, Krogsgaard-Larsen P. Ketobemidone, methadone and pethidine are non-competitive N-methyl-D-aspartate (NMDA) antagonists in the rat cortex and spinal cord. Neurosci Lett. 1995;187:165–8. doi: 10.1016/0304-3940(95)11364-3. [DOI] [PubMed] [Google Scholar]
- 164.Davis A, Inturrisi C. d-methadone blocks morphine tolerance and N-methyl-D-aspartate-induced hyperalgesia. J Pharm Exp Ther. 1999;289:1048–53. [PubMed] [Google Scholar]
- 165.Codd EE, Shank RP, Schupsky JJ, Raffa RB. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: Structural determinants and role in antinociception. J Pharm Exp Ther. 1995;274:1263–9. [PubMed] [Google Scholar]
- 166.Callahan RJ, Au JD, Paul M, Liu C, Yost CS. Functional inhibition by methadone of N-methyl-D-aspartate receptors expressed in Xenopus oocytes; stereospecific and subunit effects. Anesth Analg. 2004;98:653–9. doi: 10.1213/01.ane.0000099723.75548.df. [DOI] [PubMed] [Google Scholar]
- 167.Ebert B, Thorkildsen C, Andersen S, Christrup LL, Hjeds H. Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochem Pharmacol. 1998;56:553–9. doi: 10.1016/s0006-2952(98)00088-4. [DOI] [PubMed] [Google Scholar]
- 168.Gorman A, Elliott K, Inturrisi C. The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat brain and spinal cord. Neurosci Lett. 1997;223:5–8. doi: 10.1016/s0304-3940(97)13391-2. [DOI] [PubMed] [Google Scholar]
- 169.Gagnon B, Almahrezi A. Methadone in the treatment of neuropathic pain. Pain Res Manage. 2003;8:149–54. doi: 10.1155/2003/236718. [DOI] [PubMed] [Google Scholar]
- 170.Moulin D. Use of methadone for neuropathic pain. Pain Res Manage. 2003;8:131–2. doi: 10.1155/2003/749572. [DOI] [PubMed] [Google Scholar]
- 171.Bruera E, Sweeney C. Methadone use in cancer patients with pain. J Palliative Med. 2002;5:127–37. doi: 10.1089/10966210252785097. [DOI] [PubMed] [Google Scholar]
- 172.Ripamonti C, Zecca E, Bruera E. An update on the clinical use of methodone for cancer pain. Pain. 1997;70:109–15. doi: 10.1016/s0304-3959(96)03286-1. [DOI] [PubMed] [Google Scholar]
- 173.Fainsinger R, Schoeller T, Bruera E. Methadone in the management of cancer pain: A review. Pain. 1993;52:137–47. doi: 10.1016/0304-3959(93)90125-9. [DOI] [PubMed] [Google Scholar]
- 174.Davis M, Walsh D. Methadone for the relief of cancer pain: A review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9:73–83. doi: 10.1007/s005200000180. [DOI] [PubMed] [Google Scholar]
- 175.Altier N, Dion D, Boulanger A, Choiniere M. Successful use of methadone in the treatment of chronic neuropathic pain arising from burn injuries: A case study. Burns. 2001;27:771–5. doi: 10.1016/s0305-4179(01)00032-8. [DOI] [PubMed] [Google Scholar]
- 176.Gagnon B, Bruera E. Differences in ratios of morphine to methadone in patients with neuropathic pain versus non-neuropathic pain. J Pain Symptom Manag. 1999;18:120–4. doi: 10.1016/s0885-3924(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 177.Hays H, Woodroffe M. Use of methadone in treating chronic noncancer pain. Pain Res Manage. 1998;4:23–7. [Google Scholar]
- 178.Lynch ME. A review of the use of methadone for treatment of chronic non-cancer pain. Pain Res Manage. 2005;10:133–44. doi: 10.1155/2005/286713. [DOI] [PubMed] [Google Scholar]
- 179.Mullican WS, Lacy JR ftT-A-S Group. Tramadol/acetaminophen combination tablets and codeine/acetaminophen combination capsules for the management of chronic pain: A comparative trial. Clin Ther. 2001;23:1429–45. doi: 10.1016/s0149-2918(01)80118-1. [DOI] [PubMed] [Google Scholar]
- 180.Schnitzer TJ, Gray WL, Paster RZ, Kamin M. Efficacy of tramadol in treatment of chronic low back pain. J Rheumatol. 2000;27:772–8. [PubMed] [Google Scholar]
- 181.Ruoff GE, Rosenthal N, Jordan D, Karim R, Kamin M Protocol CAPSS-112 Study Group. Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: A multicenter, randomized, double blind, placebo-controlled outpatient study. Clin Ther. 2003;23:1123–40. doi: 10.1016/s0149-2918(03)80071-1. [DOI] [PubMed] [Google Scholar]
- 182.Wilder-Smith CH, Hill L, Spargo K, Kalla A. Treatment of severe pain from osteoarthritis with slow-release tramadol or dihydrocodeine in combination with NSAIDs: A randomised study comparing analgesia, antinociception and gastrointestinal effects. Pain. 2001;91:23–31. doi: 10.1016/s0304-3959(00)00414-0. [DOI] [PubMed] [Google Scholar]
- 183.Babul N, Noveck R, Chipman H, Roth SH, Gana T, Albert K. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: A randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Manag. 2004;28:59–71. doi: 10.1016/j.jpainsymman.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 184.Budd K. Chronic pain-challenge and response. Drugs. 1994;47(Suppl 1):33–8. doi: 10.2165/00003495-199400471-00006. [DOI] [PubMed] [Google Scholar]
- 185.Abramowicz M. Tramadol – A new oral analgesic. In: Abamowicz M, editor. The Medical Letter. Vol. 37. 1995. pp. 59–60. [PubMed] [Google Scholar]
- 186.Twycross RG. Opioids. In: Melzack R, Wall PD, editors. Textbook of Pain. London: Churchill Livingstone; 1994. pp. 943–62. [Google Scholar]
- 187.Barnung SK, Treschow M, Borgbjerg FM. Respiratory depression following oral tramadol in a patient with impaired renal function. Pain. 1997;71:111–2. doi: 10.1016/s0304-3959(97)03350-2. [DOI] [PubMed] [Google Scholar]
- 188.Savage SR. Assessment for addiction in pain treatment settings. Clin J Pain. 2002;18:S28–S38. doi: 10.1097/00002508-200207001-00004. [DOI] [PubMed] [Google Scholar]
- 189.Savage SR, Joranson DE, Covington EC, Schnoll SH, Heit HA, Gilson AM. Definitions related to the medical use of opioids: Evolution towards universal agreement. J Pain Symptom Manag. 2003;26:655–67. doi: 10.1016/s0885-3924(03)00219-7. [DOI] [PubMed] [Google Scholar]
- 190.Kirsh KL, Whitcomb LA, Donaghy K, Passik SD. Abuse and addiction issues in medically ill patients with pain; Attempts at clarification of terms and empirical study. Clin J Pain. 2002;18:S52–S60. doi: 10.1097/00002508-200207001-00006. [DOI] [PubMed] [Google Scholar]
- 191.Weaver M, Schnoll S. Abuse liability in opioid therapy for pain treatment in patients with an addiction history. Clin J Pain. 2002;18:S61–S69. doi: 10.1097/00002508-200207001-00007. [DOI] [PubMed] [Google Scholar]
- 192.Lindstrom P, Lindbolm U. The analgesic effect of tocainide in trigeminal neuralgia. Pain. 1987;28:45–50. doi: 10.1016/0304-3959(87)91058-X. [DOI] [PubMed] [Google Scholar]
- 193.Jarvis B, Coukell AJ. Mexiletine: A review of it's therapeutic use in painful diabetic neuropathy. Drugs. 1998;56:691–707. doi: 10.2165/00003495-199856040-00016. [DOI] [PubMed] [Google Scholar]
- 194.Dejgard A, Peterson P, Kastrup J. Mexiletine for treatment of chronic painful diabetic neuropathy. Lancet. 1988;1:9–11. doi: 10.1016/s0140-6736(88)90999-3. [DOI] [PubMed] [Google Scholar]
- 195.Stracke H, Meyer UE, Schumacher HE, Federlin K. Mexiletine in the treatment of diabetic neuropathy. Diabetes Care. 1992;15:1550–5. doi: 10.2337/diacare.15.11.1550. [DOI] [PubMed] [Google Scholar]
- 196.Oskarsson P, Ljunggren JG, Lins PE. Efficacy and safety of mexiletine in the treatment of painful diabetic neuropathy. The Mexiletine Study Group. Diabetes Care. 1997;20:1594–7. doi: 10.2337/diacare.20.10.1594. [DOI] [PubMed] [Google Scholar]
- 197.Wright J, Oki JM, Graves L. Mexiletine in the symptomatic treatment of diabetic peripheral neuropathy. Ann Pharmacother. 1997;31:29–34. doi: 10.1177/106002809703100103. [DOI] [PubMed] [Google Scholar]
- 198.Wallace MS, Magnuson S, Ridgeway B. Efficacy of oral mexiletine for neuropathic pain with allodynia: A double-blind, placebo-controlled, crossover study. Reg Anesth Pain Med. 2000;25:459–67. doi: 10.1053/rapm.2000.8583. [DOI] [PubMed] [Google Scholar]
- 199.Chabal C, Jacobson L, Mariano A, Chaney E, Britell CW. The use of oral mexiletine for the treatment of pain after peripheral nerve injury. Anesthesiology. 1992;76:513–7. doi: 10.1097/00000542-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 200.Kemper CA, Kent G, Burton S, Deresinski SC. Mexiletine for HIV-infected patients with painful peripheral neuropathy: A double-blind, placebo-controlled, cross over trial. J Acquir Immune Defic Syndr Human Retrovirol. 1998;19:367–72. doi: 10.1097/00042560-199812010-00007. [DOI] [PubMed] [Google Scholar]
- 201.Chiou-Tan FY, Tuel SM, Johnson JC, Priebe MM, Hirsh DD, Strayer JR. Effect of mexiletine on spinal cord injury dysesthetic pain. Am J Phys Med Rehabil. 1996;75:84–7. doi: 10.1097/00002060-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 202.Fassoulaki A, Patris K, Sarantopoulos C, Hogan Q. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesth Analg. 2002;95:985–91. doi: 10.1097/00000539-200210000-00036. [DOI] [PubMed] [Google Scholar]
- 203.Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. Regional block and mexiletine: The effect on pain after cancer breast surgery. Reg Anesth Pain Med. 2001;26:223–8. doi: 10.1053/rapm.2001.23205. [DOI] [PubMed] [Google Scholar]
- 204.Galer BS, Harle J, Rowbotham MC. Response to intravenous lidocaine infusion predicts subsequent response to oral mexiletine. J Pain Symptom Manage. 1996;12:161–7. doi: 10.1016/0885-3924(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 205.Attal N, Rouaud J, Brasseur L, Chauvin M, Bouhassira D. Systemic lidocaine in pain due to peripheral nerve injury and predictors of response. Neurology. 2004;62:218–25. doi: 10.1212/01.wnl.0000103237.62009.77. [DOI] [PubMed] [Google Scholar]
- 206.Lechin F, van der Dijs B, Lechin M. Pimozide therapy for trigeminal neuralgia. Arch Neurol. 1989;46:960–3. doi: 10.1001/archneur.1989.00520450030015. [DOI] [PubMed] [Google Scholar]
- 207.Graff-Radford SB, Shaw LR, Naliboff BN. Amitriptyline and fluphenazine in the treatment of postherpetic neuralgia. Clin J Pain. 2000;16:188–92. doi: 10.1097/00002508-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 208.Eisenach JC, De Kock M, Klimscha W. Alpha 2-adrenergic agonists for regional anesthesia: A clinical review of clonidine (1984–1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 209.Glynn C, O’Sullivan K. A double blind randomized comparison of the effects of epidural clonidine, lignocaine and the combination of clonidine and lignocaine in patients with chronic pain. Pain. 1995;64:337–43. doi: 10.1016/0304-3959(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 210.Rauck RL, Eisenach JC, Jackson K, Young LD, Southern J. Epidural clonidine treatment for refractory RSD. Anesthesiology. 1993;79:1163–9. [PubMed] [Google Scholar]
- 211.Eisenach JC, Rauck RL, Buzzanell C. Epidural clonidine analgesia for intractable cancer pain: Phase 1. Anesthesiology. 1989;71:647–52. doi: 10.1097/00000542-198911000-00004. [DOI] [PubMed] [Google Scholar]
- 212.Glynn C, O’Sullivan K. A double blind randomised comparison of the effects of epidural clonidine, lignocaine and a combination of clonidine and lignocaine in patients with chronic pain. Pain. 1996;64:337–43. doi: 10.1016/0304-3959(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 213.Glynn C, Dawson D, Sanders R. A double-blind comparison between epidural morphine and epidural clonidine in patients with chronic non-cancer pain. Pain. 1988;34:123–8. doi: 10.1016/0304-3959(88)90157-1. [DOI] [PubMed] [Google Scholar]
- 214.Carroll D, Jadad A, King V, Wiffen P, Glynn C, McQuay H. Single-dose, randomized, double-blind, double-dummy cross-over comparison of extradural and I.v. clonidine in chronic pain. Br J Anesth. 1993;71:665–9. doi: 10.1093/bja/71.5.665. [DOI] [PubMed] [Google Scholar]
- 215.Zeigler D, Lynch SA, Muir J, Benjamin J, Max MB. Transdermal clonidine versus placebo in painful diabetic neuropathy. Pain. 1992;48:403–8. doi: 10.1016/0304-3959(92)90092-P. [DOI] [PubMed] [Google Scholar]
- 216.Byas-Smith MG, Max MB, Muir J, Kingman A. Transdermal clonidine compared to placebo in painful diabetic neuropathy using a two-stage ‘enriched enrollment’ design. Pain. 1995;60:267–74. doi: 10.1016/0304-3959(94)00121-t. [DOI] [PubMed] [Google Scholar]
- 217.Krames E. Implantable devices for pain control: Spinal cord stimulation and intrathecal therapies. Best Pract Res Clin Anaesthesiol. 2002;16:619–45. doi: 10.1053/bean.2002.0263. [DOI] [PubMed] [Google Scholar]
- 218.Slonimski M, Abram SE, Zuniga RE. Intrathecal baclofen in pain management. Reg Anesth Pain Med. 2004;29:269–76. doi: 10.1016/j.rapm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 219.Bowery NG, Malcangio M. a-Aminobutyric acid and pain. In: Sawynok JA, editor. Novel Aspects of Pain Management: Opioids and Beyond. Mississauga: Wiley-Liss Inc; 1999. pp. 249–64. [Google Scholar]
- 220.Fromm GH. Baclofen as an adjuvant analgesic. J Pain Symptom Manag. 1994;9:500–9. doi: 10.1016/0885-3924(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 221.Fromm GH, Terrence CF, Chattha AS. Baclofen in the treatment of trigeminal neuralgia: A double blind study and long-term follow-up. Ann Neurol. 1984;15:240–4. doi: 10.1002/ana.410150306. [DOI] [PubMed] [Google Scholar]
- 222.Herman RM, Luzansky SC, Ippolito R. Intrathecal baclofen suppresses centralpain in patients with spinal lesions, a pilot study. Clin J Pain. 1992;8:338–45. [PubMed] [Google Scholar]
- 223.Tiara T, Kanamura H, Tanikawa T. A new approach to control central deafferentation pain: Spinal intrathecal baclofen. Stereotactic and Functional Neurosurgery. 1995;65:101–5. doi: 10.1159/000098905. [DOI] [PubMed] [Google Scholar]
- 224.Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: Detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–58. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 225.Ahn AH, Basbaum AI. Where do triptans act in the treatment of migraine? Pain. 2005;115:1–4. doi: 10.1016/j.pain.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tietjen GE. The risk of stroke in patients with migraine and implications for migraine management. CNS Drugs. 2005;19:683–92. doi: 10.2165/00023210-200519080-00004. [DOI] [PubMed] [Google Scholar]
- 227.Wenzel RG, Sarvis CA, Krause ML. Over-the -counter drugs for acute migraine attacks: Literature review and recommendations. Pharmacotherapy. 2003;23:494–505. doi: 10.1592/phco.23.4.494.32124. [DOI] [PubMed] [Google Scholar]
- 228.Lipton RB, Goldstein J, Baggish JS, Yataco AR, Sorrentino JV, Quiring JN. Aspirin is efficacious for treatment of acute migraine. Headache. 2005;45:283–92. doi: 10.1111/j.1526-4610.2005.05065.x. [DOI] [PubMed] [Google Scholar]
- 229.Holroyd KA, Penzien DB, Cordingley GE. Propranalol in the management of recurrent migraine: A meta-analytic review. Headache. 1991;31:333–40. doi: 10.1111/j.1526-4610.1991.hed3105333.x. [DOI] [PubMed] [Google Scholar]
- 230.Chronicle E, Mulleners CE. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database Syst Rev. 2004;(3):CD003226. doi: 10.1002/14651858.CD003226.pub2. [DOI] [PubMed] [Google Scholar]
- 231.Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis:the clinical experience. J Cardiovasc Pharmacol. 1991;18:S21–6. [PubMed] [Google Scholar]
- 232.Leone M, Grazzi L, La Mantia L, Bussone G. Flunarizine in migraine: A minireview. Headache. 1991;31:388–91. doi: 10.1111/j.1526-4610.1991.hed3106388.x. [DOI] [PubMed] [Google Scholar]
- 233.Tomkins GE, Jackson JL, O'Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: A meta-analysis. Am J Med. 2001;111:54–63. doi: 10.1016/s0002-9343(01)00762-8. [DOI] [PubMed] [Google Scholar]
- 234.Holroyd KA, Penzien DB. Pharmacological versus non-pharmacological prophylaxis of recurrent migraine headache: A meta-analytic review of clinical trials. Pain. 1990;42:1–13. doi: 10.1016/0304-3959(90)91085-W. [DOI] [PubMed] [Google Scholar]
- 235.Bookman AA, Williams KS, Shainhouse JZ. Effect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: A randomized controlled trial. CMAJ. 2004;171:333–8. doi: 10.1503/cmaj.1031793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (Pennsaid) in the treatment of primary osteoarthritis of the knee: A randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164:2017–23. doi: 10.1001/archinte.164.18.2017. [DOI] [PubMed] [Google Scholar]
- 237.Tugwell PS, WG A, Shainhouse JZ. Equivalence study of a topical diclofenac solution (Pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: A randomized controlled trial. J Rheumatol. 2004;31:2002–12. [PubMed] [Google Scholar]
- 238.Watson CPN. Topical capsaicin as an adjuvant analgesic. J Pain Symptom Manag. 1994;9:425–33. doi: 10.1016/0885-3924(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 239.Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: Double-blind placebo controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996;65:39–44. doi: 10.1016/0304-3959(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 240.Galer BS, Jensen MP, Ma T, Davies PS, Rowbotham MC. The lidocaine patch 5% effectively treats all neuropathic pain qualities: Results of a randomized, double blind, vehicle controlled, 3 week efficacy study with use of the neuropathic pain scale. Clin J Pain. 2002;18:297–301. doi: 10.1097/00002508-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 241.Galer BS, Rowbotham MC, Perander J, Friedman E. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: Results of an enriched enrollment study. Pain. 1999;80:533–8. doi: 10.1016/S0304-3959(98)00244-9. [DOI] [PubMed] [Google Scholar]
- 242.McCleane G. Topical application of doxepin hydrochloride can reduce the symptoms of complex regional pain syndrome: A case report. Injury Int J Care Injured. 2002;33:88–9. doi: 10.1016/s0020-1383(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 243.McCleane GJ. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: A randomized, double blind, placebo-controlled study. Br J Clin Pharmacol. 2000;49:574–9. doi: 10.1046/j.1365-2125.2000.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.McCleane GJ. Topical doxepin hydrochloride reduces neuropathic pain: A randomized, double-blind placebo controlled study. The Pain Clinic. 2000;12:47–50. doi: 10.1046/j.1365-2125.2000.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lockhart E. Topical combination of amitriptyline and ketamine for post herpetic neuralgia. J Pain. 2004;5(S1):82. [Google Scholar]
- 246.Lynch ME, Clark AJ, Sawnyok J. Topical amitriptyline, ketamine and a combination of both in the treatment of neuropathic pain. Clin J Pain. 2003;19:323–7. doi: 10.1097/00002508-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 247.Moore RA, Tramer MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ. 1998;316:333–8. doi: 10.1136/bmj.316.7128.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Heyneman CA, Lawless-Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases. Drugs. 2000;2000:555–74. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 249.Vaile JH, Davis P. Topical NSAIDs for musculoskeletal conditions. Drugs. 1998;56:783–99. doi: 10.2165/00003495-199856050-00004. [DOI] [PubMed] [Google Scholar]
- 250.Mason L, Moore RA, Edwards JE, McQuay HJ, Derry S, Wiffen PJ. Systematic review of efficacy of topical rubefacients containing salicylates for the treatment of acute and chronic pain. BMJ. 2004;328:995. doi: 10.1136/bmj.38040.607141.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang WY, Li Wan Po A. The effectiveness of topically applied capsaicin. A meta-analysis. Eur J Clin Pharmacol. 1994;46:517–22. doi: 10.1007/BF00196108. [DOI] [PubMed] [Google Scholar]
- 253.Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical amitriptyline and ketamine in neuropathic pain syndromes: an open-label study. J Pain. 2005;6:644–9. doi: 10.1016/j.jpain.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 254.Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical amitriptyline 2% and ketamine 1% in neuropathic pain syndromes: A randomized double blind placebo controlled trial. Anesthesiology. 2005;103:140–6. doi: 10.1097/00000542-200507000-00021. [DOI] [PubMed] [Google Scholar]
- 255.Rowbotham MC, Davies PS, Fields HL. Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol. 1995;37:246–53. doi: 10.1002/ana.410370216. [DOI] [PubMed] [Google Scholar]
- 256.Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: A randomized, double-blind, placebo-controlled study. Pain. 2003;106:151–8. doi: 10.1016/s0304-3959(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 257.Watson CP. Topical agents for neuropathic pain: A systematic review. In: Merskey J, Loeser JD, Dubner R, editors. The Paths of Pain 1975–2005. Seattle: IASP Press; 2005. pp. 483–501. [Google Scholar]
- 258.Rice ASC. Cannabinoids and pain. Curr Opin Invest Drugs. 2001;2:399–413. [PubMed] [Google Scholar]
- 259.Lynch ME. Preclinical science regarding cannabinoids as analgesics: An overview. Pain Res Manag. 2005;10(Suppl A):7A–14A. doi: 10.1155/2005/169093. [DOI] [PubMed] [Google Scholar]
- 260.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobio. 2001;63:569–609. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 261.Walker JM, Strangman NM, Huang SM. Cannabinoids and pain. Pain Res Manage. 2001;6:74–9. doi: 10.1155/2001/413641. [DOI] [PubMed] [Google Scholar]
- 262.Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2002;323:1–6. doi: 10.1136/bmj.323.7303.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004 doi: 10.1136/bmj.38149.566979.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Notcutt WG, Price M, Chapman G. Clinical experience with nabilone for chronic pain. Pharm Sci. 1997;3:551–5. [Google Scholar]
- 265.Martyn CN, Illis LS, Thom J. Nabilone in the treatment of multiple sclerosis. Lancet. 1995;345:579. doi: 10.1016/s0140-6736(95)90485-9. [DOI] [PubMed] [Google Scholar]
- 266.Zajicek J, Fox P, Sanders H, et al. UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre rendomised placebo-controlled trial. Lancet. 2003;362:1517–26. doi: 10.1016/S0140-6736(03)14738-1. [DOI] [PubMed] [Google Scholar]
- 267.Zajicek JP, Sanders HP, Wright DE, et al. Cannabinoids in multiple sclerosis (CAMS) study: Safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry. 2005;76:1664–9. doi: 10.1136/jnnp.2005.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: Results of a randomised controlled trial. Pain. 2004;112:299–306. doi: 10.1016/j.pain.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 269.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–9. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 270.Clark AJ, Lynch ME, Ware M, Beaulieu P, McGilveray IJ, Gourlay D. Guidelines for the use of cannabinoid compounds in chronic pain. Pain Res Manag. 2005;10(Suppl A):44A–6A. doi: 10.1155/2005/894781. [DOI] [PubMed] [Google Scholar]
- 271.Clark AJ, Lynch ME. Cannabinoids for pain management – what is their role? Pain Res Manage. 2005;10(Suppl A):5A–6A. doi: 10.1155/2005/978489. [DOI] [PubMed] [Google Scholar]
- 272.Ware M, Beaulieu P. Cannabinoids for the treatment of pain: An update on recent clinical trials. Pain Res Manage. 2005;10(Suppl A):27A–30A. doi: 10.1155/2005/847562. [DOI] [PubMed] [Google Scholar]
- 273.Ware M, Tawfik VL. Safety issues concerning the medical use of cannabis and cannabinoids. Pain Res Manage. 2005;10(Suppl A):31A–7A. doi: 10.1155/2005/312357. [DOI] [PubMed] [Google Scholar]
- 274.Beaulieu P. Toxic effects of cannabis and cannabinoids: Animal data. Pain Res Manage. 2005;10(Suppl A):23A–6A. doi: 10.1155/2005/763623. [DOI] [PubMed] [Google Scholar]
- 275.McGilveray IJ. Pharmacokinetics of cannabinoids. Pain Res Manage. 2005;10(Suppl A):15A–22A. doi: 10.1155/2005/242516. [DOI] [PubMed] [Google Scholar]
- 276.Gourlay D. Addiction and pain medicine. Pain Res Manage. 2005;10(Suppl A):38A–43A. doi: 10.1155/2005/512653. [DOI] [PubMed] [Google Scholar]
- 277.Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology. 2004;62:2098–100. doi: 10.1212/01.wnl.0000127707.07621.72. [DOI] [PubMed] [Google Scholar]
- 278.Ware MA, Doyle CR, Woods R, Lynch ME, Clark AJ. Cannabis use for chronic non-cancer pain: Results of a prospective survey. Pain. 2003;102:211–6. doi: 10.1016/s0304-3959(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 279.Ogborne AC, Smart AC. Cannabis users in the general Canadian population. Subst Use Misuse. 2000;35:301–11. doi: 10.3109/10826080009147698. [DOI] [PubMed] [Google Scholar]
- 280.Page SA, Verhoef MJ, Stebbins RA, Metz LM, Levy JC. Cannabis use as described by people with multiple sclerosis. Can J Neurol Sci. 2003;30:201–5. doi: 10.1017/s0317167100002584. [DOI] [PubMed] [Google Scholar]
- 281.Merskey H, Bogduk N, editors. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Terms. Seattle: IASP Press; 1994. [Google Scholar]
- 282.Simpson DM, McArthur JC, Olney R, et al. Lamotrigine HIV Neuropathy Study Team. Lamotrigine for HIV- associated painful sensory neuropathies: A placebo controlled trial. Neurology. 2003;60:1508–14. doi: 10.1212/01.wnl.0000063304.88470.d9. [DOI] [PubMed] [Google Scholar]
- 283.Namaka M, Gramlich CR, Ruhlen D, Melanson M, Sutton I, Major J. A treatment algorithm for neuropathic pain. Clin Ther. 2004;26:951–79. doi: 10.1016/s0149-2918(04)90171-3. (Erratum in 2004;26:2163) [DOI] [PubMed] [Google Scholar]
- 284.Coambs RB, Jarry JL, Santhiapillai ac, Abrahamsohn RV, Atance CM. The SISAP: A new screening instrument for identifying potential opioid abusers in the management of chronic nonmalignant pain in general medical practice. Pain Res Manage. 1996;1:155–62. [Google Scholar]
- 285.Canadian Pharmacists Association. Compendium of Pharmaceuticals and Specialties: The Canadian Drug Reference for Health Professionals. Ottawa: Canadian Pharmacists Association; 2005. pp. 1471–3. [Google Scholar]
- 286.Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 15. Cambridge: Hogrefe and Huber; 2005. pp. 44–5. [Google Scholar]