Abstract
Oxidative damage mediated by reactive oxygen species results in the generation of deleterious by-products. The oxidation process itself and the proteins modified by these molecules are important mediators of cell toxicity and disease pathogenesis. Aldehydic products, mainly the 4-hydroxy-2-alkenals, form adducts with proteins and make them highly immunogenic. Proteins modified in this manner have been shown to induce pathogenic antibodies in a variety of diseases including systemic lupus erythematosus (SLE), alcoholic liver disease, diabetes mellitus (DM) and rheumatoid arthritis (RA). 8-oxodeoxyguanine (oxidatively modified DNA) and low density lipoproteins (LDL) occur in SLE, a disease in which premature atherosclerosis is a serious problem. In addition, immunization with 4-hydroxy-2-nonenal (HNE) modified 60 kD Ro autoantigen induces an accelerated epitope spreading in an animal model of SLE. Advanced glycation end product (AGE) pentosidine and AGE modified IgG have been shown to correlate with RA disease activity. Oxidatively modified glutamic acid decarboxylase is important in type 1 DM, while autoantibodies against oxidized LDL are prevalent in Behcet’s disease. The fragmentation of scleroderma specific autoantigens occurs as a result of oxidative modification and is thought to be responsible for the production of autoantibodies through the release of cryptic epitopes. The administration of antioxidants is a viable untried alternative for preventing or ameliorating autoimmune disease, particularly on account of the overwhelming evidence for the involvement of oxidative damage in autoimmunity. However, this should be viewed in the light of disappointing results obtained with the use of antioxidants in cardiovascular disease.
Keywords: lupus, autoimmunity, epitopes, autoantibodies, antigens
1. Free radicals
Reactive oxygen species (ROS) are oxygen-based molecules possessing high chemical reactivity. These include free radicals (superoxide and hydroxyl radicals) and non-radical species (hydrogen peroxide) which can be produced even at basal conditions by a number of ways. Free radicals are active species containing atoms or molecules with one or more unpaired electrons occupying an outer orbital. They can arise either by the univalent pathway of oxygen reduction or as a consequence of enzymic/non-enzymic reactions. The superoxide anion radical O2 − is formed as a consequence of the one electron reduction of O2. The two electron reduction product of O2 in the fully protonated form is hydrogen peroxide (H2O2) while the three electron reduction product of O2 is the hydroxyl radical (OH.). A number of enzymic and non-enzymic reactions reduce oxygen to the more reactive superoxide radical. Superoxide is also released consequent to the in vitro oxidation of a number of compounds. H2O2 may be formed consequent to either the divalent reduction of oxygen by the enzymes urate-, D amino acid- and glycolate oxidases or by the univalent reduction of oxygen to superoxide and subsequent conversion of superoxide to hydrogen peroxide by superoxide dismutase. Though hydrogen peroxide is not a free radical by itself, it can lead to the formation of the more dangerous hydroxyl radical via the Fenton type reaction [1,2].
2. Antioxidant defense
Enzymatic (superoxide dismutase (SOD), catalase and the peroxidases) and non-enzymatic (ascorbic acid, reduced glutathione and vitamin E) antioxidant defense systems control ROS production by scavenging or decreasing ROS levels, thereby maintaining an appropriate cellular redox balance. Alterations of this normal balance resulting from elevated ROS production and/or decreased anti-oxidant levels leads to a state of oxidative stress and thus an enhanced susceptibility of membranes and biological molecules to react with free radicals.
SOD converts superoxide into H2O2 (which is further converted into water by catalase/glutathione peroxidase). Four types of SOD have been identified based on their tissue distribution. SOD1 (copper/zinc containing SOD) is found in the cytoplasm of virtually all eukaryotic cells. SOD2 (manganese containing SOD) is located in the matrix of the mitochondria of all aerobes. Ferrous SOD is mainly located in the cytosol of prokaryotes. SOD3 (extracellular Cu-Zn SOD) is present in mammals in extracellular fluids or is membrane associated. Except for Photobacterium leiognathi and Caulobacter crescentus, prokaryotes do not contain this enzyme [3].
3. Lipid peroxidation
Stress or any other factor that compromises the activity of antioxidant enzymes may trigger a potentially dangerous pathway of peroxidative damage. Peroxidative damage brought about by free radicals has been shown to be involved in the pathogenesis of several diseases. Increased oxidant stress has been associated with the observed increase in lipid peroxidation in these diseases. Lipid peroxidation has been defined as oxidative degeneration of polyunsaturated fatty acids, set into motion by free radicals [4].
4. Phases of lipid peroxidation
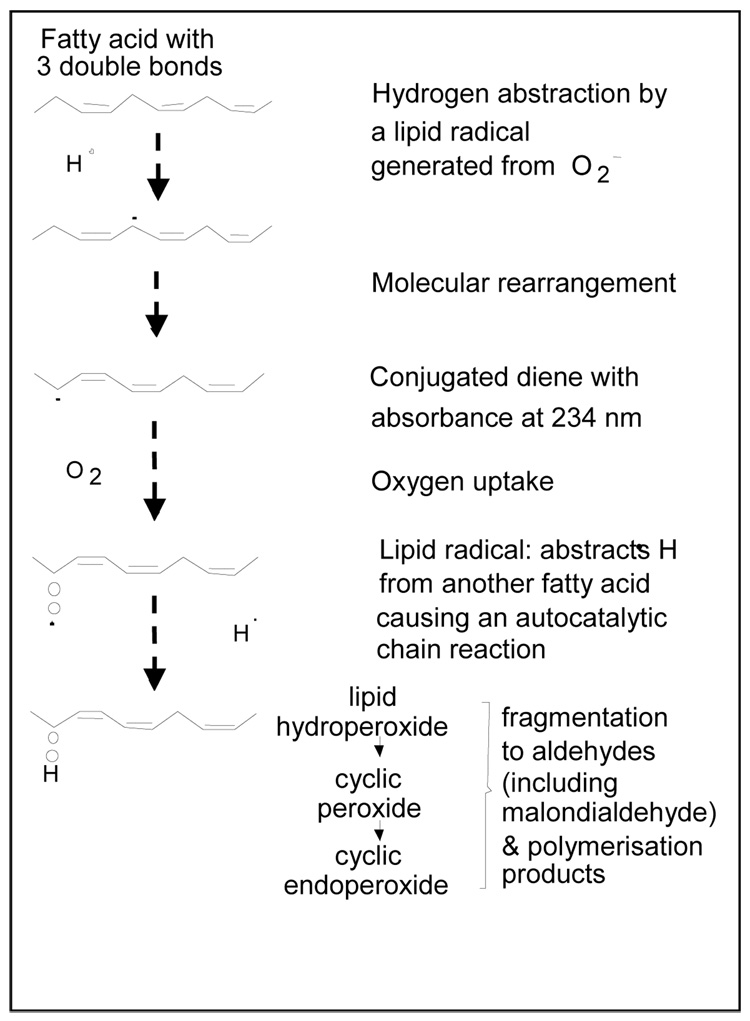
Oxidation of any polyunsaturated fatty acid is a chain reaction process and can be divided into three stages: initiation, propagation and termination (Figure 1). In the initiation phase a primary reactive radical (x.), abstracts a hydrogen atom from a methylene group of a polyunsaturated fatty acid to start the peroxidation. This leaves an unpaired electron on the carbon, resulting in the formation of a conjugated diene. The carbon-centered fatty acid radicals combine with molecular oxygen, in the propagation phase, yielding highly reactive peroxyl radicals that react with another lipid molecule to form hydroperoxides. Peroxyl radicals are capable of producing new fatty acid radicals, resulting in a radical chain reaction. In this reaction, the peroxyl radicals themselves are converted to stable termination phase products, lipid hydroperoxides. The lipid peroxidation process can result in a number of deleterious end products [3,4].
Figure 1.
Lipid peroxidation (Reference # 40)
Lipid peroxidation occurs as a consequence of increased oxidative stress resulting from the disruption of the pro-oxidant/antioxidant balance and is an important pathogenic process in oxygen toxicity. The effect is seen indirectly by the decrease in the levels of antioxidant enzymes or antioxidants like ascorbic acid, reduced glutathione or vitamin E. Or, more directly, increased amounts of conjugated dienes, isoprostanes, 4-hydroxynonenal, 4-hydroxy-2-nonenal-modified proteins, malondialdehyde release or malondialdehyde-modified proteins can be observed. In addition there are other markers of lipid peroxidation. These include free radical generated prostaglandin isomers (isoprostanes), 4-hydroxy-2-nonenal (membrane lipid peroxidation product) or 4-hydroxy 2-nonenal modified proteins, malondialdehyde (end-product of lipid peroxidation), malondialdehyde-modified proteins, protein-bound acrolein, free radical modified DNA, conjugate dienes (intermediate product of free radical damage) and protein carbonylation have all served as indices of peroxidative damage. As an alternative to these indirect methods, owing to the fact that the free radicals are paramagnetic, electron paramagnetic resonance spectroscopy combined with spin trapping has become an important tool to directly assess free radicals [3–5].
5. Reactive oxygen species and protein modification
The process of lipid peroxidation releases aldehydic products of lipid peroxidation (α, β-unsaturated aldehydes), mainly the 4-hydroxy-2-alkenals, that can form adducts with free amino groups of lysine and other amino acids. Aldehyde-modified proteins are highly immunogenic [3–7].
4-hydroxy-2-nonenal is the most studied molecule, among the 4-hydroxy-2-alkenals. 4-hydroxy-2-nonenal, and related compounds, possess two very reactive electrophilic sites: the alkene bond and the aldehyde group. The alkene bond targets the three nucleophilic amino acids cysteine, histidine and lysine via Michael-type addition. The free aldehyde in the open-chain form of the alkenal adduct can react with a second lysine, histidine or cysteine and act as heterobifunctional crosslinking reagents. 4-hydroxy-2-nonenal also reacts avidly with certain antioxidants and enzyme cofactors including glutathione and lipoic acid (the cofactor for α-ketoglutarate dehydrogenase) [8].
6. Oxidation and immune response
Rabbits and mice immunized with oxidized LDL particles (ox-LDL) develop autoantibodies directed against epitopes in malondialdehyde and 4-hydroxy-2-nonenal modified low-density lipoproteins (LDL). The presence of antibodies against ox-LDL or malondialdehyde-LDL in atherosclerotic plaques and oxidation-specific antigens on surface of apoptotic cells have been demonstrated by numerous investigators The presence of anti-oxidized LDL is associated with more rapid progression of atherosclerosis. Antigens modified by oxidative by-products induce immune responses in alcoholic liver disease [9,10].
The reaction of the adaptive response is enhanced by oxidative processes. Oxidation of carbohydrates increased the antibody response to co-administered co-antigens. The use of the Schiff base-forming agent tucaresol, in addition, during immunization with protein antigen increased T cell dependent immune response. Direct modification of protein antigen has been shown to be required for the enhancement of the immune response [6,9–12].
7. Oxidative modification of proteins in autoimmune disease
Several human diseases are autoimmune in nature resulting from the abrogation of self-tolerance. Autoimmune disease may be either organ-specific or tissue specific. Organ specific diseases include type 1 diabetes, thyroiditis, myasthenia gravis, primary biliary cirrhosis and Goodpasture’s syndrome while systemic diseases include rheumatoid arthritis, progressive systemic sclerosis and systemic lupus erythematosus. Nearly all these diseases are characterized by the presence of autoantibodies. Autoantibodies have been shown to be typically present [13] several years prior to diagnosis of SLE and type I diabetes and serve as markers for future disease [14]. Inflammation, infection, drugs, ROS, environmental factors induce formation of neo-antigens. Table 1 summarizes the antigens that are oxidatively modified in autoimmune diseases. Oxidative damage has been implicated in several autoimmune diseases, including systemic lupus erythematosus [6,15–18].
Table 1.
Oxidatively modified antigens associated with autoimmune diseases
| Oxidized antigen involved | Diseases |
|---|---|
| Oxidized LDL and 8-oxodeoxyguanine | SLE |
| HNE-modified 60 kD Ro | Animal model of SLE |
| Oxidized LDL | Atherosclerosis |
| IgG modified with advanced glycation end product; pentosidine | Rheumatoid arthritis |
| Oxidatively modified glutamic acid hydroxylase | Type I diabetes mellitus |
| Oxidized LDL | Behcet’s disease |
7.1 Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic, complex inflammatory autoimmune disorder of unknown aetiology, characterized by the presence of autoantibodies directed against a multitude of self-antigens. The targets of these antibodies reside in the nucleus, cytoplasm or cell membranes. SLE could be a result of interplay of genetic and environmental factors or molecular mimicry [19].
Although there may be no active tolerance to many intracellular self-antigens, immune tolerance to self is maintained by elimination of self reactive lymphocytes in the thymus during the development of the immune system and by rendering the T lymphocytes that bind self antigens anergic in the periphery. The disruption of self-tolerance, which results in the appearance of autoreactive lymphocytes, results in autoimmunity. This autoimmune response is generally divided into three kinds, namely B-cell dominant, T-cell dominant, and combinational types. Autoimmune hemolytic anemia and myasthenia gravis belong to the category of B-cell dominant autoimmune diseases while experimental autoimmune encephalomyelitis, insulin-dependent diabetes mellitus and the collagen-induced arthritis are T-cell dominant autoimmune diseases. SLE arises from the emergence of both autoreactive T and B cells with an aetiology [6, 20–22].
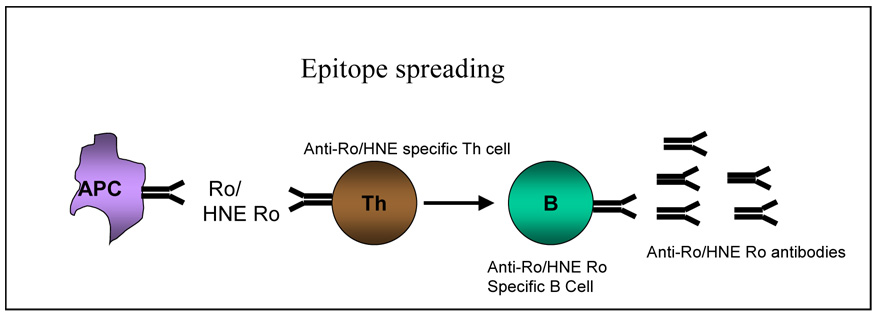
Molecular mimicry of viral or bacterial antigens with self-determinants has been touted as one of the pathogenic mechanisms for the appearance of autoreactive cells [22]. The diversification and amplification of autoimmunity in an individual could be explained by epitope spreading. The concept of epitope spreading has been extended to other autoimmune diseases since it was first described in experimental autoimmune encephalomyelitis [23]. Epitope spreading may occur intramolecularly (within a single antigen) or intermolecularly (within different antigens) and is defined as the progression of an autoimmune response from initial activation to a chronic state involving increased targeting of autoantigens by T cells and antibodies. Once immune tolerance to one component is abrogated, B- and T-cell responses can diversify to other components of the macromolecule with the recognition of other epitopes in the intact particle (Figure 2 and Figure 3). Several investigations based on immunization of non-autoimmune mice with self-peptides support the view that the highly diverse B-and T-cell autoimmune responses in SLE might originate from a single protein or even a single cryptic self epitope without the need for foreign pathogens or molecular mimics [23–26].
Figure 2.
Model showing mechanisms of B and T-cell epitope spreading. Figure 2A: Antigen presenting cells (APCs) (macrophages or dendritic cells) present novel self peptides from 60 kD Ro or the 60 kD HNE-Ro neoantigen to T cells, which in turn provide help to autoreactive B cells. Clonal expansion of B cells capable of binding to 60 kD Ro or 60 kD HNE Ro occurs
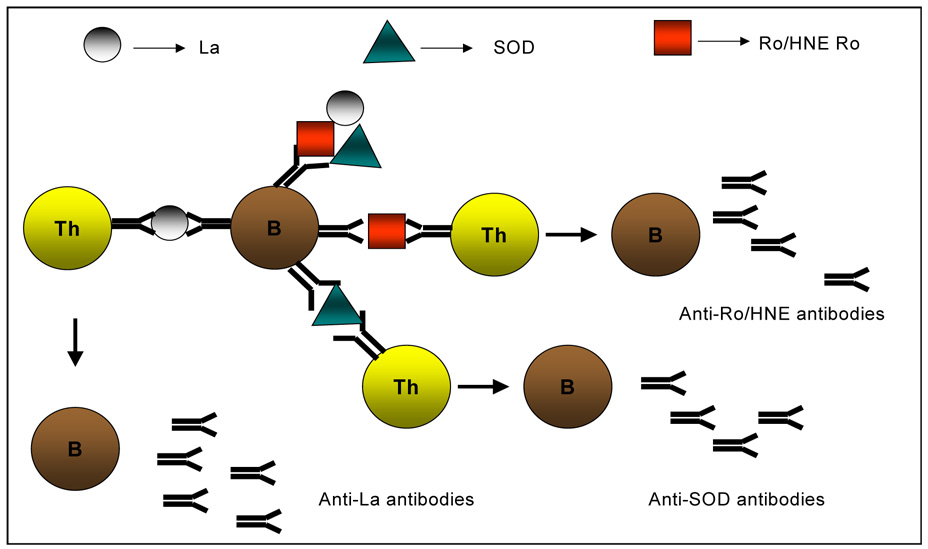
Figure 3.
B cells internalize multiple proteins such as 60 kD Ro, HNE-modified 60 kD or SOD, present epitopes from each protein to naïve T cells resulting in diversification of autoreactive T cells. Finally, T cells assist a diversified B-cell response. The cascade continues, with T cells activating additional autoreactive B cells and B cells presenting additional self epitopes, until there is autoreactivity to numerous autoantigens.
Free radical or ROS mediated damage occurs in SLE and other diseases. Significantly higher 4-hydroxy-2-nonenal-modified protein levels occur in children with lupus. SOD1 activity was decreased in lupus [3,4]. Malondialdehyde and conjugated dienes were significantly elevated in lupus patients compared to controls. Antibodies to SOD1 were significantly increased in SLE patients and are potentially responsible for the increased oxidative damage seen [3]. Oxidatively modified LDL's have been shown to elicit autoantibodies and oxidant stress has been attributed to the development of anti-phospholipid antibodies. Elevated levels of anti-oxLDL autoantibodies occur in SLE patients [27] and studies show that anti-oxLDL positively correlate with antiphospholipid antibodies and anti- β-2-glycoprotein. Antibodies to oxLDL that are cross-reactive with phosopholipids are thought to be due to binding to oxidized phospholipids (75). Circulating oxLDL/β-2-glycoprotein complexes and IgG immune complexes containing oxLDL/β-2-glycoprotein occur in SLE and/or phospholipid syndrome [28].
Increased levels of 8-oxo-deoxyguanine (8-oxodG) have been found in lymphocytes from patients with SLE. An investigation of blood monocytes from patients with SLE showed an impairment in the removal of 8-oxodG as a result of a deficient repair system [29].
Work from our laboratory showed increased oxidative damage and 4-hydroxy-2-nonenal-modification of proteins in SLE compared to normal controls (unpublished data). Therefore, we immunized rabbits with 4-hydroxy-2-nonenal-modified Ro 60 and unmodified Ro 60 to test our hypothesis that immunization of animals with 4-hydroxy-2-nonenal-modified Ro would result in accelerated epitope spreading. In both SLE and Sjogren’s syndrome, a common target of autoantibodies is the 60 kD Ro ribonucleoprotein. This structure is made up of a 60 kD protein non-covalently associated with at least one of four short uridine rich RNAs (the hY RNAs). These hY RNAs are also associated with the 48,000 MW La (or SSB) autoantigen. Anti-Ro is found in 25–40% of patients with SLE, while anti-La is found in substantially fewer patients. As hypothesized we found a rapid autoimmune response and development of lupus-like disease in the 4-hydroxy-2-nonenal-Ro immunized group. Thus, immunization with an oxidatively modified autoantigen accelerates the disease process in this animal model of SLE [30,31].
Oxidative modification of 60 kD Ro might result in the formation of chemical adducts which could serve as neo-antigens to which the immune system has probably not been exposed. The 60 kD Ro modified in this fashion might be more readily internalized, on account of its neo-conformation, than the unmodified Ro, by antigen presenting cells, such as dendritic cells or macrophages. These in turn present novel self-peptides to T cells, which can provide help to autoreactive B cells to elicit intramolecular spreading. B cells specific for either the modified or unmodified Ro could internalize the antigen, along with associated antigens, by means of its cell surface Ig receptor. Epitopes from each of these proteins could be then presented to naïve T cells, in the context of major histocompatibility complex Class II, resulting in a diversification of autoreactive T cells, which assist a diversified B cell response that can recognize separate B-cell antigenic determinants from the different antigens resulting in autoreactivity to numerous antigens.
7.2 Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by synovitis, chronic inflammation of the joints, erosion of the cartilage and bone. The exact pathogenesis in still unknown and treatment is non-curative. The presence of shared epitope QKRAA on the HLA-DRβ chain and the presence of rheumatoid factor (RF) have served as long-term outcome predictors of RA [6,32].
The damage to the cartilage and bones has been associated with the action of free radicals, proinflammatory cytokines and matrix metalloproteinases (MMPs). Inflammatory cells like macrophages, T and B cells and neutrophils infiltrate the inflamed synovial membrane. Reactive oxygen species such as superoxide, hydroxyl radicals, hypochlorous acid and nitric acid (involved in acute and chronic inflammation) are generated when these cells consume increased amounts of oxygen [33].
The rapid reaction between between superoxide and nitric oxide results in the formation of peroxynitrite which inhibits the activity of tissue inhibitor of metalloproteinases 1 (90), leading to elevated activities of MMP-1 (91) and MMP-3 (92). Oxidative stress, in addition, is associated with sequential oxidation processes that generate advanced glycation end (AGE) products that are damaging to proteins (93). Other potential contributing factors of RA, are the presence of circulating IgM anti-IgG advanced glycation end (AGE) products [34,35].
Advanced glycation results from non-enzymatic glycation of proteins. Oxidative stress has been shown to cause IgG to be modified by AGE products. AGE products had been primarily investigated in diabetes where they are implicated in tissue damage. Subsequent studies in patients with diabetes or rheumatic diseases revealed that antibodies directed against glycated IgG were mainly associated with RF-positive RA. Ligier et al and Lucey et al have shown IgM antibodies against glycated IgG. Earlier studies have shown that pentosidine (an AGE modification product which cross-links lysine and arginine) was elevated in 50% of patients with RA and that these increased pentosidine levels correlated with clinical disease activity. Increased oxidant stress in uncontrolled RA was reported to be responsible for the elevated levels of serum and urinary pentosidine. Free radicals have been reported to bring about AGE products. Oxygen free radicals have been identified in synovial fluid of 90% of patients with RA. These species have been found to correlate with tumor necrosis factor alpha in the blood [6,36,37].
In rheumatoid arthritis patients, the antioxidant defense system has been shown to be compromised. Studies have shown elevated blood malondialdehyde levels RA patients and significantly lower levels of blood concentrations of total thiols, glutathione and vitamin C compared to controls. These studies have found a shift in the oxidant/antioxidant balance in favor of lipid peroxidation, which could lead to the tissue damage observed in the disease. A statistically significant increase in the concentrations of antioxidants, along with a decrease in the concentrations of malondialdehyde was found after treatment of the disease. These results suggest the importance of therapeutic co-administration of antioxidants along with conventional drugs to such patients [38].
7.3 Type 1 diabetes mellitus
Type 1 diabetes mellitus is an autoimmune disease that is organ-specific with T cell mediated destruction of β cells of the pancreatic islet cell and ROS involvement. Studies have demonstrated that protein glycation, oxidation and nitration are elevated in cellular and extracellular proteins in diabetes. Glycation of proteins, oxidation of proteins and nitration is thought to contribute to vascular cell dysfunction and the development of retinopathy, nephropathy and neuropathy (microvascular diabetic complications). The quality and functional integrity of proteins are maintained by the cellular machinery by the degradation and replacement of damaged proteins (oxidation and glycation are the eain types of physiological damage). The glycated, oxidized and nitrated amino acid residues are liberated by cellular proteolysis as free adducts and released into plasma for excretion into the urine. Thus, the changes in plasma concentrations and excretion of glycation, oxidation and nitration adducts may reflect damage to tissues in diabetes, yielding new markers of the damaging effects of hyperglycemia. In a study of 21 type 1 diabetes mellitus patients and 12 control subjects, the concentrations of protein glycation, oxidation and nitration adduct residues were found to be increased in type 1 diabetes mellitus patients patients compared to normal controls (up to 3-fold in plasma protein and up to 1-fold in hemoglobin; except for decrease in pentosidine and 3-nitrotyrosine residues in hemoglobin). However, the same study found that the concentrations of protein glycation and oxidation free adducts increased up to 10-fold in plasma while urinary excretion was found to increase up to 15-fold in diabetic patients.
Type 1 diabetes mellitus is also distinguished by the presence of a number of autoantigens. Glutamic acid decarboxylase is one of the major, and most well characterized autoantigens. Treatment of β cell lysates with copper sulphate and iron sulphate produces high molecular weight complexes of glutamic acid decarboxylase independent of disulphide double bonds. Sera from patients with type 1 diabetes mellitus bind these complexes much more strongly than they bind the glutamic acid decarboxylase monomer. Thus, oxidative modification of glutamic acid decarboxylase may be important in type 1 diabetes mellitus patients pathogenesis [6,39].
7.4 Scleroderma or systemic sclerosis
Scleroderma or systemic sclerosis is a systemic autoimmune disease that affects several organs including skin, lung and kidneys leading to widespread tissue fibrosis as well as vasculopathy. Patients affected with systemic sclerosis have autoantibodies that bind several autoantigens. Addition of ferrous sulphate to HeLa cell extracts fragment specific scleroderma autoantigens in a unique way. RNA polymerase II, topoisomerase1, upstream binding factor and the 70 kD protein of U1 RNA are fragmented in this manner and this fragmentation was inhibited by metal ion chelators. Some of these fragments were also generated by copper mediated oxidation. The authors also investigated intact keratinocytes exposed to supraphysiological concentrations of copper in which oxidation was started by hydrogen peroxide addition. Topoisomerase was shown this way to be cleaved into the 95 kD fragment that was previously observed with in vitro studies. The authors propose that perfusion-reperfusion injury found in scleroderma in the presence of metal ions may produce these oxidatively modified autoantigens. Such modified antigens might initiate the autoimmune process through cryptic epitopes. Such a scenario, however, assumes the fact that autoantibodies arise consequent to the pathologic process. It is now well established that autoantibodies precede disease manifestations in many autoimmune diseases [6].
7.5 Behcet’s disease
Hulusi Behcet, a Turkish physician, first described this immunoinflammatory disease of unknown aetiology. This systemic disease is characterized by the presence of ocular occlusive vasculitis and thrombosis, and anterior or posterior uveitis in conjunction with oral aphthae, genital ulceration and cutaneous lesions.
Excessive production of ROS is present in Behcet’s disease, with associated significant increase in malondialdehyde production and decreased glutathione peroxidase activity. Another study showed significantly elevated levels of autoantibodies against oxidized LDL and lipid hydroperoxides in a group of patients with Behcet’s disease compared to healthy controls. In addition this study found that erythrocyte SOD, catalase and plasma glutathione peroxidase activities were significantly lower in Behcet’s disease patients compared to controls. The decrease in these antioxidant enzymes would be responsible for the increased oxidative stress occurring in Behcet’s disease, the susceptibility of LDL to oxidation and thus may predispose these patients to atherothrombotic events [6].
8. Conclusion
The role of free radicals in the pathogenesis and development of diseases is well documented. Generation of ROS and enzymatic and non-enzymatic control of these harmful molecules is an ongoing process. Antibodies to antioxidant enzymes could result in the disruption in this balance resulting in oxidative stress, which is turn leads to pathological changes. This could lead to oxidatively modified autoantigens that serve as neo-antigens in promoting loss of tolerance to self. Immunization with modified autoantigens has shown accelerated epitope spreading and induction of disease. Administration of antioxidants or other dietary modulations is not studied in autoimmune disease, but could be helpful in preventing or ameliorating disease although results in cardiovascular disease are disappointing [40].
Acknowledgements
Supported by NIH grant ARO1844 to RHS and Oklahoma Center for the Advancement of Science and Technology to BTK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. Review. [DOI] [PubMed] [Google Scholar]
- 2.Fridovich I. In: Molecular Mechanism of oxygen activation. Hayaishi O, editor. New York: Acad. Press; 1974. p. 453. [Google Scholar]
- 3.Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci. 2003;73:1655–1666. doi: 10.1016/s0024-3205(03)00475-2. [DOI] [PubMed] [Google Scholar]
- 4.Kurien BT, Scofield RH. Curcumin/turmeric solubilized in sodium hydroxide inhibits HNE protein modification--an in vitro study. J Ethnopharmacol. 2007;110:368–373. doi: 10.1016/j.jep.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Reznick AZ, Cross CE, Hu ML, Suzuki YJ, Khwaja S, Safadi A, Motchnik PA, Packer L, Halliwell B. Modification of plasma proteins by cigarette smoke as measured by protein carbonyl formation. Biochem J. 1992;286(Pt 2):607–611. doi: 10.1042/bj2860607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurien BT, Hensley K, Bachmann M, Scofield RH. Oxidatively modified autoantigens in autoimmune diseases. Free Radic Biol Med. 2006;41:549–556. doi: 10.1016/j.freeradbiomed.2006.05.020. Review. [DOI] [PubMed] [Google Scholar]
- 7.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 8.Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 9.Maggi E, Chiesa R, Melisano G, CR, Astore G, Grossi A, et al. LDL oxidation in patients with atherosclerosis: a study of in vitro and in vivo oxidation markers. Arterioscler Thromb Vasc Biol. 1995;14:1892–1899. doi: 10.1161/01.atv.14.12.1892. [DOI] [PubMed] [Google Scholar]
- 10.Lehtimaki T, Lehtinen S, Solakivi T, Nikkila M, Jaakkola O, Jokela H, Yla-Herttuala S, Luoma JS, Koivula T, Nikkari T. Autoantibodies against oxidized low density lipoprotein in patients with angiographically verified coronary artery disease. Arterioscler Thromb Vasc Biol. 1999;19:23–27. doi: 10.1161/01.atv.19.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Willis MS, Klassen LW, Tuma DJ, Sorrell MF, Thiele GM. Adduction of soluble proteins with malondialdehyde-acetaldehyde (MAA) induces antibody production and enhances T-cell proliferation. Alcohol Clin Exp Res. 2002;26:94–106. [PubMed] [Google Scholar]
- 12.Allison ME, Fearon DT. Enhanced immunogenicity of aldehyde-bearing antigens: a possible link between innate and adaptive immunity. Eur J Immunol. 2000;30:2881–2887. doi: 10.1002/1521-4141(200010)30:10<2881::AID-IMMU2881>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 14.Scofield RH. Autoantibodies as predictors of disease. Lancet. 2004;363:1544–1546. doi: 10.1016/S0140-6736(04)16154-0. [DOI] [PubMed] [Google Scholar]
- 15.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 16.Kurien BT, Scofield RH. In vitro modification of solid phase multiple antigenic peptides/autoantigens with 4-hydroxy-2-nonenal (HNE) provide ideal substrates for detection of anti-HNE antibodies and peptide antioxidants. J Immunol Methods. 2005;303:66–75. doi: 10.1016/j.jim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol. 2007;5:567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]
- 18.Naparstek Y, Plotz PH. The role of autoantibodies in autoimmune disease. Annu Rev Immunol. 1993;11:79–104. doi: 10.1146/annurev.iy.11.040193.000455. [DOI] [PubMed] [Google Scholar]
- 19.Boyd GW. An evolution-based hypothesis on the origin and mechanisms of autoimmune disease. Immunol Cell Biol. 1997;75:503–507. doi: 10.1038/icb.1997.78. [DOI] [PubMed] [Google Scholar]
- 20.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–153. doi: 10.1038/35100573. Review. [DOI] [PubMed] [Google Scholar]
- 21.Billingham RE, Brent L, Medawar PB. Quantitative studies on tissue transplantation immunity. III. Actively acquired tolerance. Proc. Natl. Acad. Sci. USA. 1956;238:357. [Google Scholar]
- 22.Rose NR, Mackay IR. Molecular mimicry: a critical look at exemplary instances in human diseases. Cell Mol Life Sci. 2000;57:542–551. doi: 10.1007/PL00000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 24.Scofield RH, Henry WE, Kurien BT, James JA, Harley JB. Immunization with short peptides from the sequence of the systemic lupus erythematosus-associated 60-kDa Ro autoantigen results in anti-Ro ribonucleoprotein autoimmunity. J Immunol. 1996;156:4059–4066. [PubMed] [Google Scholar]
- 25.Scofield RH, Kaufman KM, Baber U, James JA, Harley JB, Kurien BT. Immunization of mice with human 60-kd Ro peptides results in epitope spreading if the peptides are highly homologous between human and mouse. Arthritis Rheum. 1999;42:1017–1024. doi: 10.1002/1529-0131(199905)42:5<1017::AID-ANR22>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B'-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–461. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig WY. Autoantibodies against oxidized low density lipoprotein: a review of clinical findings and assay methodology. J Clin Lab Anal. 1995;9:70–74. doi: 10.1002/jcla.1860090113. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura E, Lopez LR. Are oxidized LDL/beta2-glycoprotein I complexes pathogenic antigens in autoimmune-mediated atherosclerosis? Clin Dev Immunol. 2004;11:103–111. doi: 10.1080/10446670410001722186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurien BT, Scofield RH. Lipid peroxidation in systemic lupus erythematosus. Indian J Exp Biol. 2006;44:349–356. Review. [PubMed] [Google Scholar]
- 30.Farris AD, Gross JK, Hanas JS, Harley JB. Genes for murine Y1 and Y3 Ro RNAs have class 3 RNA polymerase III promoter structures and are unlinked on mouse chromosome 6. Gene. 1996;174:35–42. doi: 10.1016/0378-1119(96)00279-x. [DOI] [PubMed] [Google Scholar]
- 31.Scofield RH, Kurien BT, Ganick S, McClain MT, Pye Q, James JA, Schneider RI, Broyles RH, Bachmann M, Hensley K. Modification of lupus-associated 60 kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitope spreading. Free Radic Biol Med. 2005;38:719–728. doi: 10.1016/j.freeradbiomed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Weyand CM, Hicok KC, Conn DL, Goronzy JJ. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992;117:801–806. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- 33.Gilston V, Blake DR, Winyard PG. Inflammatory mediators, free radicals and gene transcription. In: Winyard PG, Blake DR, Evans CH, editors. Free radicals and inflammation. Boston: Birkhauser; 2000. pp. 83–98. [Google Scholar]
- 34.Yoshihara Y, Obata K, Fujimoto N, Yamashita K, Hayakawa T, Shimmei M. Increased levels of stromelysin-1 and tissue inhibitor of metalloproteinases-1 in sera from patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:969–975. doi: 10.1002/art.1780380713. [DOI] [PubMed] [Google Scholar]
- 35.Scofield RH, Tardibono G, Ogden SB, Harley JB, Reichlin M, Kurien BT. Rheumatoid hyperviscosity: analysis of a patient with intermediate complexes that block other autoantibodies and a review of the literature. Semin Arthritis Rheum. 1998;27:382–391. doi: 10.1016/s0049-0172(98)80018-8. [DOI] [PubMed] [Google Scholar]
- 36.Bucala R, Cerami A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- 37.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 38.Jaswal S, Mehta HC, Sood AK, Kaur J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta. 2000;338:123–129. doi: 10.1016/j.cccn.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed N, Babaei-Jadidi R, Howell SK, Beisswenger PJ, Thornalley PJ. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia. 2005;48:1590–1603. doi: 10.1007/s00125-005-1810-7. [DOI] [PubMed] [Google Scholar]
- 40.Gutteridge JMC, Westermarck T, Halliwell B. Oxygen radical damage in biological systems. In: Johnson JE, Walford R, Harman D, Miquel J, editors. Free Radicals, Aging, and Degenerative Diseases. New York: Alan R. Liss; 1985. p. 99. [Google Scholar]