Abstract
Wnt proteins regulate the formation of central synapses by stimulating synaptic assembly, but their role at the vertebrate neuromuscular junction (NMJ) is unclear. Wnt3 is expressed by lateral motoneurons of the spinal cord during the period of motoneuron-muscle innervation. Using gain- and loss-of-function studies in the chick wing, we demonstrate that Wnt signaling is necessary for the formation of acetylcholine receptor (AChR) clusters without affecting muscle growth. Similarly, diaphragms from Dishevelled-1 mutant mice with deficiency in Wnt signaling exhibit defects in cluster distribution. In cultured myotubes, Wnt3 increases the number and size of AChR clusters induced by agrin, a nerve-derived signal critical for NMJ development. Wnt3 does not signal through the canonical Wnt pathway to induce cluster formation. Instead, Wnt3 induces the rapid formation of unstable AChR micro-clusters through activation of Rac1, which aggregate into large clusters only in the presence of agrin. Our data reveal a role for Wnts in post-synaptic assembly at the vertebrate NMJ by enhancing agrin function through Rac1 activation.
Keywords: acetylcholine receptor, clustering, Dvl1 mutant, neuromuscular junction, Rac
Wnt proteins regulate various aspects of neuronal connectivity, from axon guidance to dendritic development and synapse formation (1). At central synapses, Wnts act as retrograde signals that regulate terminal axon remodeling and presynaptic differentiation (2, 3). At peripheral synapses, a role for Wnt signaling was first identified in invertebrate systems. In Drosophila, the Wnt homologue Wingless (Wg) positively regulates the correct assembly of presynaptic active zones and clustering of post-synaptic components (4). In contrast, the Caenorhabditis elegans Wnt homologue lin44 inhibits the formation of synapses at specific areas along the axon (5). Therefore, in invertebrates, Wnt factors can promote or inhibit the formation of peripheral synapses. However, a role for Wnt signaling at vertebrate peripheral synapses is less understood.
At the vertebrate cholinergic neuromuscular junction (NMJ), agrin, a heparan sulfate proteoglycan secreted by motoneurons (6, 7), induces post-synaptic differentiation by aggregating acetylcholine receptors (AChR) and other proteins at the post-synaptic membrane (8–10). This effect is mediated through sequential activation of Rho GTPases; agrin induces a rapid and transient activation of Rac1 that is necessary for the initial phase of AChR micro-cluster formation, whereas the subsequent RhoA activation is crucial for the coalescence of the micro-clusters into full-sized AChR clusters (11, 12). Although initial evidence suggested that agrin was crucial for initiation of post-synaptic development (6, 7), agrin also plays a later maintenance role (13, 14). These various functions of agrin at different developmental stages might be achieved through other factors that influence agrin activity.
Here we report that Wnt3, which is expressed by motoneurons at the time when they invade muscle regions in the limb (3), induces the clustering of AChRs during early stages of NMJ assembly in chick wing muscles. Conversely, exposure to the Wnt antagonist Sfrp1 dramatically reduces the number of AChR aggregates in the chick limb, suggesting that endogenous Wnts are required for AChR clustering during neuromuscular innervation. Importantly, diaphragms from mice lacking Dishevelled-1 (Dvl1), a scaffold protein required in all Wnt pathways (15) (Fig. 1A), exhibit abnormal AChR cluster distribution, indicating a requirement for Wnt signaling in post-synaptic differentiation at the mouse NMJ. In myotubes, Wnt3 induces a rapid activation of Rac1 and the accumulation of AChR micro-clusters, which are converted into full-sized clusters in the presence of agrin. Our findings demonstrate a function for Wnts as modulators of post-synaptic differentiation at vertebrate peripheral synapses by collaborating with agrin.
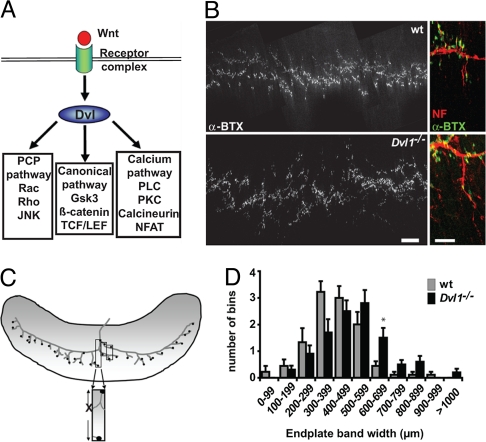
Fig. 1.
Deficiency in Wnt signaling affects AChR cluster distribution in mice. Loss of Dvl1 function results in defects in the distribution of clusters in the diaphragm. (A) Diagram shows that Wnt ligand binding to its receptor complex activates Dvl, which then activates Wnt signaling pathways. (B) Representative maximal projections from E18.5 WT and Dvl1−/− mutant diaphragms stained with α-BTX (Left). WT diaphragms display a narrow band of AChR clusters along the length of the diaphragm, whereas in Dvl1−/− diaphragms clusters are more dispersed. (Scale bar, 200 μm.) At higher magnification, apposition of AChR clusters (α-BTX) with nerve (neurofilament/βIII-tubulin) can be seen in both diaphragms (Right). (Scale bar, 50 μm.) (C) Diagram shows how clusters were measured in the diaphragm of WT and Dvl1 mutant mice. The x axis of the graph depicted in D corresponds to the widest distance of clusters. (D) The average cluster distribution is shifted to wider values in Dvl1−/− diaphragms (*P < 0.0001) compared with those of WT animals. Eleven measurements per diaphragm were obtained from nine (WT) or 10 (Dvl1−/−) mice.
Results
Wnt Signaling Deficiency Affects NMJ Differentiation in the Mouse Diaphragm.
To examine the role of Wnt signaling at vertebrate peripheral synapses, we analyzed the Dvl1 mutant mouse, which exhibits a subtle behavioral phenotype (16) as well as defects in dendrite development and in central synaptogenesis (17, 18). To determine possible defects in NMJ formation, we analyzed the diaphragm. No significant differences were found in the number or distribution of AChR clusters at E14.5 (data not shown), when early NMJ differentiation begins independently of nerve-derived factors (13). At E18.5, quantification (Fig. 1) reveals a statistically significant (P < 0.0001) change in cluster distribution in the Dvl1 mutant compared with WT mice, manifested by an increase in the average endplate band width (Fig. 1 B–D). However, no changes in the size of synaptic boutons were observed [supporting information (SI) Fig. S1]. These findings suggest that Wnt-Dvl signaling is required for the proper distribution of AChR clusters at the vertebrate NMJ.
Wnt3 Enhances the Clustering of AChRs in the Chick Wing.
To analyze the role of Wnt signaling in NMJ formation in more detail, we examined AChR aggregation in the chick fore-wing, where muscle differentiation commences at Hamburger and Hamilton stage (HH) 25. We first characterized the appearance of AChR clusters during early wing development. By HH26, nerves have not yet reached the most distal region of the outgrowing wing (19) (data not shown), but they localize in close proximity to the earliest formed muscle fibers in each small muscle mass (Fig. S2). AChRs aggregate into very small puncta on the surface of nascent muscle fibers, which we refer to here as micro-clusters. By HH27/28, many more muscle fibers with AChR micro-clusters have formed in each muscle mass, but bigger and brighter AChR aggregates appear soon after (Fig. S2). Although nerves are found close to muscles, neural processes are not yet detected within the muscle mass. Thus, AChR cluster formation in the chick forelimb muscles follows nerve arrival at the muscle field, but precedes invasion. Our data in the wing confirm the events previously reported in the chick hind-limb (20).
The expression of Wnt3 by motoneurons at the time when they arrive at the muscle field raised the possibility of Wnt3 involvement in NMJ formation (3). To address the role of Wnt proteins during vertebrate NMJ formation, we implanted pellets of cells expressing Wnt3 into embryonic chick wings before the appearance of AChR clusters. Control experiments showed that transfected QT6 cells retain their ability to secrete recombinant proteins throughout the period of implantation (Fig. S3A). Cells expressing Wnt3, or control red fluorescent protein (RFP), were implanted into the wing (Fig. S3B) after formation of dorsal and ventral muscle masses at stage HH24/25, and were analyzed at stage HH27/28 when a significant number of AChR clusters have formed (Fig. S2). Implanted cells expressed Wnt3 and Sfrp1 proteins within the limb (Fig. S3B). As Wnts have previously been implicated in muscle differentiation (21–23), we first examined whether the relatively late Wnt3 exposure affects wing muscle development. The shape and size of the muscle masses in implanted wings were not different from control contralateral wings (Fig. 2 A and B), demonstrating that muscle development or growth is not affected by cell implantation in any of the experimental conditions used.
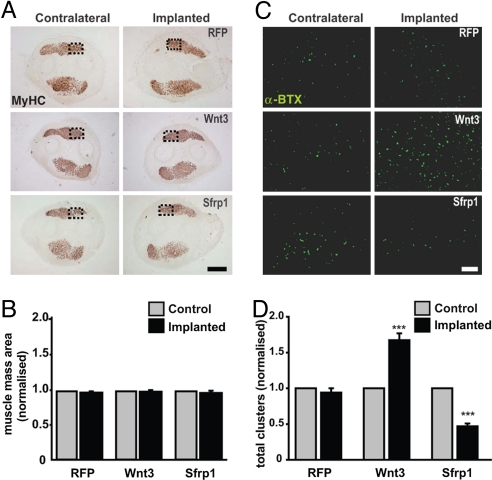
Fig. 2.
Wnt signaling regulates AChR clustering in vivo. Cell transplantation experiments in the chick wing show that gain of Wnt3 function increases clustering whereas Sfrp1 inhibits clustering. (A) Equivalent transversal cryo-sections from wings implanted with cells expressing RFP, Wnt3-HA, or Sfrp1-myc (Right), as well as their equivalent sections in the control contralateral wing (Left) were stained with an anti-muscle myosin antibody. The shape and size of muscle masses are not affected by the different treatments. (Scale bar, 500 μm.) (B) Quantification of the area of muscle mass reveals no changes. (C) Adjacent sections were labeled with fluorescent α-BTX to visualize AChR clusters. (Scale bar, 50 μm.) (D). Quantification reveals a significant increase in the number of AChR clusters in the presence of Wnt3. In contrast, a significant decrease in cluster number is observed in the presence of Sfrp1 compared with controls. Data in B and D were normalized to contralateral control wings (n = 5 animals per treatment; ***, P < 0.001 vs. RFP treatment, t test).
Quantification of AChR clusters near the implantation site on dorsal and ventral muscle masses of the wing showed that control RFP-expressing cells do not alter the number of AChR aggregates compared with the non-implanted contralateral wing (Fig. 2 C and D). In contrast, Wnt3-expressing cells induce a 70% increase in the number of AChR clusters (Fig. 2 C and D). To address whether endogenous Wnt activity regulates AChR clustering in vivo, we examined the effect of the secreted Wnt inhibitor Sfrp1 (24). Sfrp1 halves the number of AChR clusters formed on wing muscle compared with contralateral wings or those implanted with control RFP-expressing cells (Fig. 2 C and D). Together with the studies in the mouse diaphragm, these results provide strong evidence for an in vivo role of Wnt signaling at the vertebrate NMJ.
Wnt3 Enhances the Clustering Activity of Agrin on Myotubes.
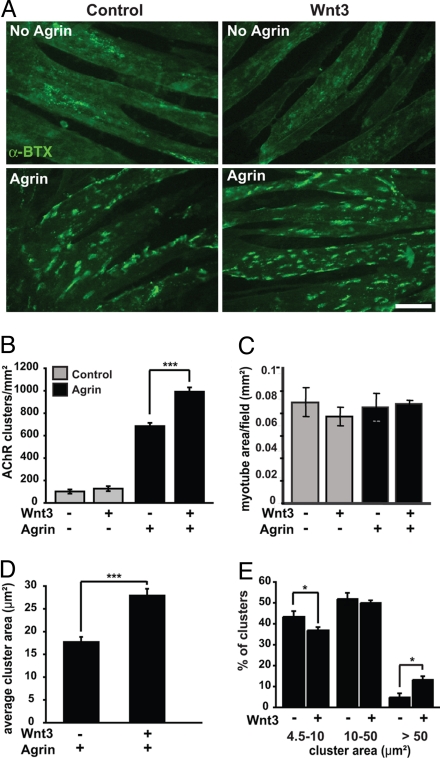
To address the mechanism by which Wnts regulate AChR clustering, we analyzed whether Wnt3 directly affects the clustering of AChRs in myotubes. As observed in vivo, no differences in myotube morphology or size are apparent following treatment with Wnt3 alone or in combination with agrin (Fig. 3 A and C). When myotubes were exposed overnight to Wnt3, no differences in the number of clusters were observed compared with controls (Fig. 3 A and B). We then examined whether Wnt3 could affect the ability of agrin to induce clustering. Addition of agrin causes a dramatic increase in the number of AChR clusters. Interestingly, when agrin and Wnt3 were applied together, a significant increase in the number and size of AChR clusters was observed compared with agrin alone (Fig. 3 A, B, and D). Consistently, analysis of the distribution of cluster sizes showed that Wnt3 decreases the percentage of small clusters (4.5–10 μm2) with a concomitant increase in the proportion of large clusters in the presence of agrin (>50 μm2), without affecting the number of medium-sized clusters (10–50 μm2; Fig. 3E). Interestingly, Wnt1, Wnt3a, and Wnt5a do not enhance agrin-mediated clustering (Fig. S4). Together, these findings indicate that, although Wnt3 alone is unable to induce AChR clustering, it can enhance agrin activity.
Fig. 3.
Wnt3 increases the number of agrin-induced AChR clusters in muscle cells in vitro. Myotubes derived from the C2C12 cell line were exposed to control or Wnt3 conditioned medium overnight in the absence or presence of agrin. (A) Wnt3 alone does not affect the number, size, or distribution of AChR clusters (Upper). However, Wnt3 significantly increases the number of agrin-induced AChR aggregates compared with agrin alone (Lower). (Scale bar, 40 μm.) (B) Quantification shows that Wnt3 enhances the activity of agrin by ≈50% (***, P < 0.001, t test). Data are expressed as the number of AChR clusters per mm2 myotube area. (C) Myotube differentiation is not affected, as no difference in the area of myotubes was observed in the various treatments. (D) Quantification shows that, in the presence of agrin, Wnt3 increases the average cluster area by ≈60% (***, P < 0.001, t test). (E) Analyses of size distribution reveals that Wnt3 decreases the percentage of small clusters (*, P < 0.05), does not alter the proportion of medium-sized clusters (P > 0.05), but does increase the proportion of large clusters compared with agrin alone (*, P < 0.05).
Wnts Expressed by Myotubes Do Not Influence AChR Clustering In Vitro.
As various Wnts are expressed in muscle tissue (22, 25, 26) and inhibition of endogenous Wnt-like activity in vivo reduces post-synaptic differentiation at the NMJ (Fig. 2D), we tested whether Wnts secreted by muscle cells might contribute to agrin-mediated AChR clustering. We used the secreted Wnt antagonist Sfrp1 (24), which blocks both canonical and non-canonical Wnt signaling, and Dickkopf-1 (Dkk1), which specifically blocks canonical signaling through its interaction with the LRP5/6 co-receptor (27). Neither Sfrp1 nor Dkk1 significantly affect the number of endogenous or agrin-induced AChR clusters in cultured myotubes (Fig. 4A). These results suggest that Wnts expressed by myotubes do not significantly contribute to the clustering activity of agrin.
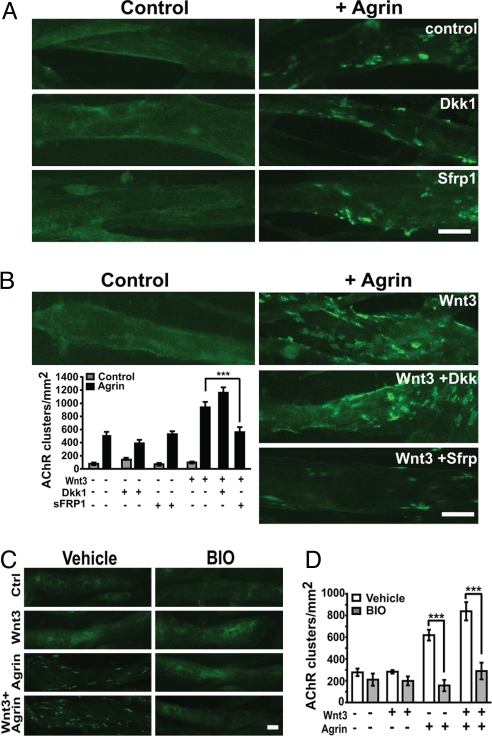
Fig. 4.
The canonical Wnt pathway does not enhance agrin-mediated clustering. Myotubes were treated with Dkk1 or Sfrp1 in the absence or presence of agrin and Wnt3. (A) Neither Dkk1 nor Sfrp1 significantly reduces the number of AChR clusters induced by agrin. (B) Dkk1 does not affect the clustering induced by Wnt3 and agrin together. However, Sfrp1 reduces the clustering effect of Wnt3 plus agrin to the level of agrin alone (Lower, ***, P < 0.001). (C and D) The Gsk3 inhibitor BIO significantly reduces the clustering induced by agrin (***, P < 0.001) or both agrin and Wnt3 (***, P < 0.001). (Scale bar, 20 μm.)
Wnt3 Activates a Non-Canonical Pathway to Cluster AChRs.
To analyze the mechanism by which Wnt3 enhances AChR clustering, we examined the role of the canonical Wnt pathway. We found that Dkk1 has no significant effect on the clustering activity of Wnt3 (Fig. 4 A and B). In contrast, Sfrp1, reduces the number of clusters induced by Wnt3 and agrin by ≈50%, down to the levels induced by agrin alone (Fig. 4B). We then examined the contribution of Gsk3β, a serine/threonine kinase that is inhibited by Wnts specifically in the canonical pathway (reviewed in ref. 28). BIO, a specific Gsk3 inhibitor (29–31), does not enhance the clustering activity of agrin (Fig. 4 C and D). On the contrary, inhibition of Gsk3 reduces the level of clustering to control levels in both conditions, agrin-treated and agrin and Wnt3-treated myotubes (Fig. 4D). Thus, inhibition of Gsk3 partially blocks the clustering activity of agrin, which is consistent with the finding that lithium, a Gsk3 inhibitor, reduces agrin-induced AChR aggregation (32). Taken together, our results indicate that the canonical pathway is not involved in Wnt3-mediated AChR clustering.
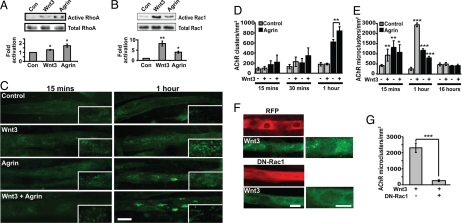
One of the non-canonical Wnt signaling pathways activates the small GTPases Rac1 and RhoA (18, 33–35). Importantly, Rac1 and RhoA have previously been shown to be essential for agrin-induced clustering of AChRs (11, 12). We therefore analyzed the activity of endogenous RhoA, Rac1, and Cdc42. Agrin rapidly increases RhoA activity (twofold increase in 15 min; Fig. 5A), whereas Wnt3 alone induces a subtle increase (1.5 fold; Fig. 5A). Inhibition of the RhoA/ROCK pathway with the ROCK inhibitor Y27632 (36) does not affect the basal number of AChR clusters (Fig. S5), but reduces the number of AChR clusters in the presence of agrin alone or agrin and Wnt3 together by 29% and 38%, respectively (Fig. S5). However, in the presence of the inhibitor, Wnt3 can still enhance the clustering activity of agrin, suggesting that ROCK is not required for Wnt3-mediated activity.
Fig. 5.
Wnt3 induces a rapid increase in the formation of micro-clusters. (A) Pull-down assays from myotubes treated with Wnt3 or agrin show that Wnt3 induces a slight increase in endogenous activated Rho (1.5 fold), whereas agrin increases GTP-Rho levels to a greater extent (twofold; normalized to control, Left). (B) Rac1 assays from lysates reveal that both Wnt3 (eightfold) and agrin (fourfold) activate Rac1 within 15 min, compared with untreated controls (Left, normalized to 1). Total levels of Rac1 and RhoA were used as loading controls. The blots are representative of three independent experiments. (C) Time course of cluster formation. Myotubes were exposed to control, Wnt3, agrin, or both for 15 min or 1 h. After 15 min (Left), agrin, Wnt3, or both increase the number of micro-clusters. However, after 1 hour, Wnt3 alone induces a stronger effect (second panel, Right, inset). In contrast, agrin alone or both agrin and Wnt3 significantly increase the formation of large clusters (Lower, Right). (D) Quantification shows that Wnt3, agrin, or both do not affect the formation of large clusters after 15 or 30 min. However, agrin induces the formation of large clusters after 1 h (55% increase), with a further increase (26%) in the presence of Wnt3 (**P < 0.01). (E) Quantification reveals an increase in micro-cluster formation after 15 min in the presence of Wnt3 alone (**, P < 0.01), agrin alone (**, P < 0.01), or both (*, P < 0.05). After 1 h, the number of micro-clusters formed in response to Wnt3 alone is significantly increased (100% increase) compared with agrin treatment. In contrast, after 16 h treatment, the number of micro-clusters in all conditions is the same as in controls. (F) Wnt3 induces microcluster formation in RFP-transfected control myotubes after 1 h (Upper), whereas DN-Rac1 expression abolishes the effect of Wnt3 (Lower). (Scale bar, 20 μm.) (G) Quantification shows that DN-Rac1 reduces the formation of micro-clusters by ≈90% after 1 h exposure to Wnt3 compared with control RFP-expressing myotubes (***, P < 0.001).
We next examined whether Wnt3 affects Rac1 activity. Within 15 min, Wnt3 activates Rac1 (eightfold) to a much greater extent than agrin (fourfold; Fig. 5B). Both Wnt3 and agrin can also activate Cdc42 but to a lesser degree (data not shown). Thus, Wnt3 on its own can activate Rac1, which is implicated in AChR clustering, and yet Wnt3 is unable to induce AChR clustering without agrin.
Wnt3 Induces the Formation of AChR Micro-clusters.
The activation of small Rho GTPases within 15 min of Wnt exposure prompted us to examine the effects of Wnt3 and agrin on cluster formation over shorter periods of time. We found that Wnt3, agrin, or Wnt3 and agrin together do not significantly affect cluster formation at 15 or 30 min (Fig. 5 C and D). In contrast, a 1-hour exposure to agrin alone or Wnt3 plus agrin significantly induces cluster formation, as observed after overnight treatment (Fig. 5 C and D). Thus, Wnt3, in combination with agrin, induces clustering after 1 h, whereas alone it is unable to induce full-sized AChR clusters.
A distinct role for Rac1 and RhoA in AChR clustering has been postulated. Rac1 activation leads to the formation of AChR micro-clusters (12), whereas RhoA activation is required for further aggregation of micro-clusters into typical large AChR clusters (11). Therefore, we tested whether Wnt3 could regulate the formation of micro-clusters, defined as clusters with an area <4.5 μm2 (12). Wnt3 alone induces a 124% increase in the formation of AChR micro-clusters after 15 min compared with controls (Fig. 5 C and E). During this period, agrin alone or in combination with Wnt3 increases the number of micro-clusters equally well, which consolidate into large clusters after 1 h (Fig. 5 C, D, and E). However, after 1 h of Wnt3 treatment, the number of micro-clusters exceeds those induced by agrin by twofold (Fig. 5 C and E). Interestingly, treatment with both agrin and Wnt3 induces fewer micro-clusters than agrin or Wnt3 alone (Fig. 5 C and E; ***, P < 0.001), with a concomitant increase in the appearance of large clusters (Fig. 5 C and D). At 16 h, few micro-clusters are present in any condition (Fig. 5E), whereas the number of large clusters is higher in the presence of agrin or agrin and Wnt3 (Fig. 3 A and B). These results strongly suggest that Wnt3 can induce microcluster formation, which become full-sized clusters only in the presence of agrin.
To determine the function of Rac1 in Wnt3-mediated microcluster formation, myotubes were transfected with dominant-negative (DN) Rac1 and micro-cluster formation was analyzed after 1 h of Wnt3 treatment, the time at which the response is maximal. Consistent with a crucial role for Rac1 in the initiation of AChR clustering, DN-Rac1 effectively blocks the formation of micro-clusters induced by Wnt3 (Fig. 5 F and G).
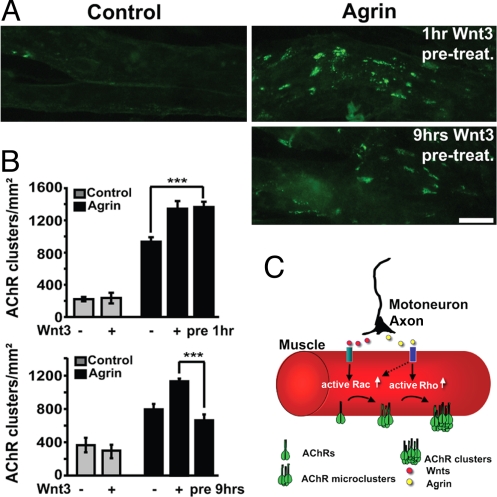
These experiments suggest that Wnt3-induced micro-clusters are unstable but may contribute to the enhanced agrin activity observed. To test this notion, myotubes were exposed to Wnt3, which was then removed before the addition of agrin. Preexposure to Wnt3 for 1 h significantly increases the number of large clusters compared with agrin alone, and the effect is similar when myotubes are treated with both Wnt3 and agrin (Fig. 6 A and B). In contrast, long-term preexposure (9 h) to Wnt3 does not enhance agrin activity (Fig. 6 A and B). These results strongly suggest that Wnt3 enhances agrin activity through the rapid and transient formation of AChR micro-clusters that can be aggregated and stabilized into larger clusters by agrin.
Fig. 6.
Short-term exposure to Wnt3 enhances agrin activity. Myotubes were incubated for 1 h or 9 h with control or Wnt3 conditioned medium. Myotubes were then washed several times to remove the conditioned media before the application of agrin (pretreatment). (A) One hour pretreatment with Wnt3 followed by agrin induces a similar enhancement of clustering as observed when myotubes were exposed to both proteins as the same time (Right, Upper). However, 9 h pretreatment with Wnt3 followed by agrin does not increase the number of AChR clusters compared with agrin alone (Right, Lower). (B) Quantification reveals that 1 h preexposure to Wnt3 results in a significant increase in the number of agrin-induced AChR clusters (***, P < 0.001, t test, Upper), whereas application of agrin to myotubes pretreated with Wnt3 for 9 h does not significantly change the number of clusters compared with agrin alone (Lower). (C) Diagram summarizing the coordinated activities of Wnts and agrin in clustering AChRs at the NMJ. Both agrin and Wnt3 induce the formation of AChR micro-clusters through Rac1 activation. Wnt3 is more effective in activating Rac1 than agrin. These unstable micro-clusters function as nucleating centers for the formation of stable large clusters upon RhoA activation by agrin. (Scale bar, 20 μm.)
Discussion
Here we provide evidence that Wnt signaling plays a positive role in post-synaptic differentiation at the vertebrate NMJ. Gain- and loss-of-function studies demonstrate that Wnt signaling is required in vivo for the proper clustering of AChRs, a hallmark of post-synaptic differentiation at the NMJ. In cultured myotubes, Wnt3 alone induces the formation of AChR micro-clusters through Rac1 activation, which fail to aggregate into large clusters. In the presence of agrin, however, Wnt3 promotes the formation of large clusters, thus enhancing agrin activity. We propose that Wnt factors collaborate with agrin by increasing the number of micro-clusters, which are subsequently converted into large AChR clusters by agrin.
Implantation of cells expressing Wnt3 in the chick wing increases the formation of AChR clusters in myofibers at the time when motoneuron axons reach the muscle field. In contrast, blockade of endogenous Wnts by implantation of Sfrp1-expressing cells results in a significant decrease in AChR clusters. This effect occurs in the absence of any significant change in muscle fiber differentiation or in the size of muscle masses, suggesting a direct effect of Wnt in the formation of AChR clusters in vivo. Although the endogenous source of Wnt is currently unknown, Wnt3 is a candidate as it is expressed in motoneurons at the time of limb muscle innervation (3). However, Wnt3 is expressed in only a subset of motoneurons, the LMC (3), suggesting that other Wnts together with Wnt3 might regulate post-synaptic differentiation at the vertebrate NMJ.
Recent studies have provided in vitro evidence suggesting that downstream effectors of the Wnt pathway participate in AChR clustering at the vertebrate NMJ. Dvl forms a ternary complex with the agrin receptor MuSK and its downstream kinase PAK1 (37), whereas APC, a protein involved in the canonical Wnt pathway, interacts with AChRs in an agrin-dependent fashion (38). Importantly, disruption of Dvl/MuSK or APC/AChR interaction strongly impairs agrin-dependent AChR clustering on the surface of muscle cells (37, 38). However, no evidence for their role in vivo has been presented to our knowledge. Here we show that diaphragms from Dvl1-null mutants exhibit a more dispersed distribution of AChR clusters. A stronger but similar phenotype has been observed in mutants that affect NMJ development including the agrin, HB9, ChAT, and cdk5 mutants (13, 39–41). Like agrin mutants, Dvl1 mutants exhibit a more detectable phenotype at E18 than at earlier stages (41). The mild Dvl1 phenotype could be caused by a possible compensation by the other Dvl genes, such as Dvl2 and Dvl3, which are broadly expressed. These results, together with our implantation experiments, demonstrate that Wnt-Dvl signaling plays a role in AChR clustering at the NMJ in both birds and mammals.
In the diaphragm, it has been shown that AChR micro-clusters aggregate into large clusters in an agrin-dependent manner (42), but the mechanism of this conversion is not well understood. Here, we show that Wnt3 collaborates with agrin by inducing the formation of AChR micro-clusters. In cultured myotubes, Wnt3 alone induces the formation of micro-clusters within 15 min, with a greater effect at 1 h. However, these micro-clusters do not persist, as they disappear by 16 h. In contrast, agrin alone induces the formation of micro-clusters, which subsequently aggregate to form large clusters. Short-term, but not long-term, pretreatment with Wnt3 enhances the activity of agrin to form large clusters. These results strongly suggest that Wnt3 induces the formation of unstable micro-clusters that are converted into large clusters only in the presence of agrin.
How does Wnt3 enhance agrin activity? Although Wnt3 can activate canonical signaling (3, 43–46), in myotubes, Wnt3 regulates AChR clustering through a pathway that requires Rac1. Activation of Rac1 and RhoA correlates with the formation of micro-clusters and full-sized clusters, respectively (11, 12, 47). Compared with agrin, Wnt3 activates Rac1 more strongly than RhoA, and it induces the formation of only unstable AChR micro-clusters. Importantly, Wnt3 requires Rac1 to induce micro-clusters, as DN Rac1 blocks Wnt3 function. Agrin, in contrast, induces Rac1 less well than Wnt3, but still significantly, and also promotes microcluster formation. Agrin, however, activates Rho more highly than Wnt3, correlating with agrin's ability to aggregate micro-clusters into large clusters. Our data are consistent with a model proposed by Sanes and colleagues whereby agrin could stabilize AChR micro-clusters, which function as nucleating centres for the subsequent formation of large clusters (14) (Fig. 6C). Indeed, micro-clusters form at early stages of muscle innervation (42). Taken together, our findings demonstrate a role for Wnts at the vertebrate NMJ through a mechanism that involves agrin and the formation of micro-clusters through a Rac1-mediated pathway.
A recent study shows that Wnt3a, which activates the β-catenin signaling pathway, induces the dispersal of AChR clusters in the presence of agrin (26). Together with our findings, these results suggest that a balance between pro-assembly and pro-disassembly Wnts may contribute to the correct apposition between the nerve terminal and post-synaptic AChR clusters.
Materials and Methods
Manipulation of Chick Wing Buds.
For implantation assays, quail-derived QT6 cells were transiently transfected with RFP, Wnt3-HA, or Sfrp1-myc cDNAs using Lipofectamine (Invitrogen). Sixteen hours later, cells were trypsinized, pelleted, and embedded in collagen gels (Cellagen; ICN) before implantation into Rhode Island Red chicken embryo right wing buds at stage HH24/25 as previously described (48) and analyzed at stage HH27/28. Embryos were staged according to Hamburger and Hamilton (see SI Materials and Methods). Serial cryo-sections were examined for changes in muscle myosin accumulation and AChR clustering using fluorescent α-bungarotoxin (α-BTX, Molecular Probes), as well as with antibodies against the neural marker SNAP-25 (Synaptic Systems) to detect the nerve track and anti-HA (Roche Molecular Biochemicals) or anti-Myc (Sigma) antibodies to follow the implanted cells.
Analysis of Embryonic Mouse Diaphragms.
Diaphragms were dissected from E14.5 or E18.5 WT or Dvl1−/− embryos, and processed as previously described (49) with some modifications. See SI Materials and Methods for full information.
Myotube Culture and Staining.
C2C12 cells were grown on glass coverslips in DMEM/F12 medium containing 20% FCS, 2 mM L-glutamine, and penicillin/streptomycin for 2 days. Myoblast differentiation was triggered by addition of DMEM/F12 medium containing 2% horse serum. After 3 days, myotubes were treated with control EGFP or Wnt3-HA conditioned medium obtained from transfected QT6 cells with or without 200 pM neural agrin (R&D Systems) for the indicated times at 37 °C. AChR clusters were stained with Alexa488-conjugated α-BTX (1 μg/ml) for 1 h at 37 °C. Cells were subsequently washed and fixed in 4% paraformaldehyde for 10 min at 4 °C and cold methanol for 5 min and mounted in Fluoromount G. When recombinant Wnt proteins were used (Wnt3a and Wnt5a from R&D Systems), they were applied at a final concentration of 20 ng/ml. For inhibition studies, recombinant Dkk1 or Sfrp1 (R&D Systems) were applied to cultures overnight at final concentrations of 20 ng/ml and 2.5 μg/ml, respectively. BIO (6-bromoindirubin-3′-oxime; Calbiochem) was added to cultures at a final concentration of 1 μM overnight at 37 °C. The ROCK inhibitor Y27632 (Calbiochem) was used at a concentration of 100 μM for 2 hours before addition of Wnt3 or agrin.
Supplementary Material
Acknowledgments.
We thank Drs. Daniel Sussman and Tony Wynshaw-Boris for generously providing the Dvl1 mutant mice, Jeremy Nathans and Kate Nobes for constructs, Eleanna Stamatakou for helping with the breeding and genotyping of mice, and Daniel Ciantar for confocal assistance and analysis. We also thank members of our laboratory for useful discussion and comments on the manuscript. The UK Biotechnology and Biological Sciences Research Council, UK Medical Research Council (MRC), and the Wellcome Trust supported this work. S.M.H. is an MRC scientist with Program Grant support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806300105/DCSupplemental.
References
- 1.Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Ann Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- 2.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 3.Krylova O, et al. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 4.Packard M, et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 7.Bowe MA, Fallon JR. The role of agrin in synapse formation. Ann Rev Neurosci. 1995;18:443–462. doi: 10.1146/annurev.ne.18.030195.002303. [DOI] [PubMed] [Google Scholar]
- 8.Steinbach JH. Developmental changes in acetylcholine receptor aggregates at rat skeletal neuromuscular junctions. Dev Biol. 1981;84:267–276. doi: 10.1016/0012-1606(81)90394-8. [DOI] [PubMed] [Google Scholar]
- 9.Englander LL, Rubin LL. Acetylcholine receptor clustering and nuclear movement in muscle fibers in culture. J Cell Biol. 1987;104:87–95. doi: 10.1083/jcb.104.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace BG. Regulation of agrin-induced acetylcholine receptor aggregation by Ca++ and phorbol ester. J Cell Biol. 1988;107:267–278. doi: 10.1083/jcb.107.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weston C, et al. Cooperative regulation by Rac and Rho of agrin-induced acetylcholine receptor clustering in muscle cells. J Biol Chem. 2003;278:6450–6455. doi: 10.1074/jbc.M210249200. [DOI] [PubMed] [Google Scholar]
- 12.Weston C, Yee B, Hod E, Prives J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J Cell Biol. 2000;150:205–212. doi: 10.1083/jcb.150.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 14.Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc Natl Acad Sci USA. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wharton KA., Jr. Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- 16.Lijam N, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad-Annuar A, et al. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 19.Hollyday M. Development of motor innervation of chick limbs. Prog Clin Biol Res. 1983;110(part A):183–193. [PubMed] [Google Scholar]
- 20.Dahm L. M., Landmesser LT. The regulation of synaptogenesis during normal development and following activity blockade. J Neurosci. 1991;11:238–255. doi: 10.1523/JNEUROSCI.11-01-00238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433:317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- 22.Anakwe K, et al. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development. 2003;130:3503–3514. doi: 10.1242/dev.00538. [DOI] [PubMed] [Google Scholar]
- 23.Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- 24.Rattner A, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Church VL, Francis-West P . Wnt signalling during limb development. Int J Dev Biol. 2002;46:927–936. [PubMed] [Google Scholar]
- 26.Wang J, et al. Wnt/beta -catenin signaling suppresses rapsyn expression and inhibits acetylcholine receptor clustering at the neuromuscular junction. J Biol Chem. 2008;283:21668–21675. doi: 10.1074/jbc.M709939200. [DOI] [PubMed] [Google Scholar]
- 27.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 28.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 29.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 30.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 31.Meijer L, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Sharma SK, Wallace BG. Lithium inhibits a late step in agrin-induced AChR aggregation. J Neurobiol. 2003;54:346–357. doi: 10.1002/neu.10134. [DOI] [PubMed] [Google Scholar]
- 33.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 34.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter CG, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 36.Kuwahara K, et al. The effects of the selective ROCK inhibitor, Y27632, on ET-1-induced hypertrophic response in neonatal rat cardiac myocytes–possible involvement of Rho/ROCK pathway in cardiac muscle cell hypertrophy. FEBS Lett. 1999;452:314–318. doi: 10.1016/s0014-5793(99)00680-8. [DOI] [PubMed] [Google Scholar]
- 37.Luo ZG, et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, et al. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neurosci. 2003;6:1017–1018. doi: 10.1038/nn1128. [DOI] [PubMed] [Google Scholar]
- 39.Fu AK, et al. Aberrant motor axon projection, acetylcholine receptor clustering, and neurotransmission in cyclin-dependent kinase 5 null mice. Proc Natl Acad Sci USA. 2005;102:15224–15229. doi: 10.1073/pnas.0507678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misgeld T, et al. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 41.Gautam M, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 42.Lin S, Landmann L, Ruegg MA, Brenner HR. The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation. J Neurosci. 2008;28:3333–3340. doi: 10.1523/JNEUROSCI.5590-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lan Y, et al. Expression of Wnt9b and activation of canonical Wnt signaling during midfacial morphogenesis in mice. Dev Dyn. 2006;235:1448–1454. doi: 10.1002/dvdy.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billiard J, et al. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol. 2005;19:90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- 45.Barrow JR, et al. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morkel M, et al. Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- 47.Weston CA, Teressa G, Weeks BS, Prives J. Agrin and laminin induce acetylcholine receptor clustering by convergent, Rho GTPase-dependent signaling pathways. J Cell Sci. 2007;120:868–875. doi: 10.1242/jcs.03367. [DOI] [PubMed] [Google Scholar]
- 48.Li X, et al. Hedgehog can drive terminal differentiation of amniote slow skeletal muscle. BMC Dev Biol. 2004;4:9. doi: 10.1186/1471-213X-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin W, et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.