Abstract
Competition among different axons to reach the somatodendritic region of the target neuron is an important event during development to achieve the final architecture typical of the mature brain. Trasmitter-receptor matching is a critical step for the signaling between neurons. In the cerebellar cortex, there is a persistent competition between the two glutamatergic inputs, the parallel fibers and the climbing fibers, for the innervation of the Purkinje cells. The activity of the latter input is necessary to maintain its own synaptic contacts on the proximal dendritic domain and to confine the parallel fibers in the distal one. Here, we show that climbing fiber activity also limits the distribution of the GABAergic input in the proximal domain. In addition, blocking the activity by tetrodotoxin infusion in Wistar rat cerebellum, a synapse made by GABAergic terminals onto the recently formed Purkinje cell spines appear in the proximal dendrites. The density of GABAergic terminals is increased, and unexpected double symmetric/asymmetric postsynaptic densities add to the typical symmetric phenotype of the GABAergic shaft synapses. Moreover, glutamate receptors appear in these ectopic synapses even in the absence of glutamate transmitter inside the presynaptic terminal and close to GABA receptors. These results suggest that the Purkinje cell has an intrinsic tendency to develop postsynaptic assemblies of excitatory types, including glutamate receptors, over the entire dendritic territory. GABA receptors are induced in these assemblies when contacted by GABAergic terminals, thus leading to the formation of hybrid synapses.
Keywords: cerebellum, GABA receptor, spinogenesis, synaptic plasticity
The function of the nervous system critically relies on the establishment of precise synaptic connections between one neuron and its target cells. Electrical activity exerts a key role in controlling the specificity of neurotransmitter/receptor matching, both during the developmental period and in the mature brain (1, 2). Vertebrate skeletal muscle expresses five classes of neurotransmitter receptors at early developmental stages, four of which are eliminated during the achievement of the mature cholinergic phenotype. In this model, NMDA- and AMPA-receptor expression increases when activity is suppressed and decreases when activity is enhanced (3). Interestingly, under experimental glutamatergic innervation, the rat neuromuscular junction switches from cholinergic- to glutamatergic-type synapse (4). A similar activity-dependent control has been shown in the mature hippocampus. Developing hippocampal granule cells express a dual glutamatergic/GABAergic phenotype, but when development is completed, the GABAergic phenotype shuts off. However, different treatments, such as sustained synaptic activity or direct activation of glutamate receptors or BDNF administration, can rescue the developmental phenotype. This form of plasticity is not limited to the developmental critical period (5). These findings demonstrate an activity-dependent regulation of the matching of transmitters and their receptors in defining the identity of recently formed synapses.
Following a prolonged block of electrical activity of all neuronal populations by tetrodotoxin (TTX) or of the ionotropic glutamate receptors by AMPA receptor antagonist infusion, the Purkinje cell (PC) proximal dendritic compartment undergoes a profound restructuring of its architecture. A large number of new spines appear and they are mainly innervated by parallel fibers (PFs), an input normally restricted to the distal dendritic domain. In contrast, climbing fibers (CFs) undergo a morphological modification consisting of a reduced size of their varicosities and a loss of synaptic contacts with the PCs. This finding led to the hypothesis that in the absence of activity, the PC is intrinsically endowed with cues to be innervated by the PFs. The CFs, to achieve and maintain their dendritic territory, have to be active to displace the cues of the excitatory competitor afferents (6–8).
In addition to the two excitatory inputs, the PC also receives a strong inhibitory GABAergic input, mainly from both basket and stellate neurons, which is distributed along the PC somatodendritic region (9, 10). Therefore, a first aim of this article is to discover whether the inhibitory input is also involved in the activity-dependent competition for the PC innervation. In addition, following the prolonged administration of TTX, a synapse made by GABAergic terminals onto the recently formed PC spines appear in the proximal dendrites, spines that normally receive an excitatory input (6). This surprising observation has prompted us to study the glutamate and GABA receptor distribution in the GABAergic terminal-PC synapses, both on the shaft and on the spines.
Here we show that the density of GABAergic terminals is increased after the block of electrical activity, and that such an expansion takes place only in the PC proximal domain. Thus, the competition for the innervation of the PC is not limited to the two excitatory inputs and activity of the CF has a fundamental role in the maintenance of the proper synaptic excitatory and inhibitory architectural wiring. In the asymmetric spine synapses, in addition to the glutamate receptors and the GluRδ2 subunits, there is a surprising expression of GABAA receptor subunits. Finally, we also observed unexpected double symmetric/asymmetric postsynaptic densities replacing the typical symmetric phenotype of the GABAergic shaft synapses. Thus, in the adult cerebellum, electrical activity has a key role in shaping the correct synaptic transmitter match.
Results
Analysis of GABAergic Terminals After TTX Infusion.
First, we evaluated the density of the GABAergic terminals to verify a possible expansion of their territory of innervation. In vehicle- and TTX-treated cerebella, we calculated the number of the GABAergic terminals taking contact with the PC proximal and distal dendritic domains. The mean density values were expressed as the number of terminals per 100 μm of dendrite length [supporting information (SI) Table S1]. After TTX there is a significant increase of the number of GABAergic terminals in the proximal domain from a value of 6.8 ± 0.4 SE to a value of 15.6 ± 1.5 SE. Strikingly, nearly half of these ectopic contacts (41.6%) presented an additional synapse, also with the spines emerging from the proximal dendrite. The analysis of the distal dendritic compartment didn't show any significant difference in the GABAergic terminal density and all of the synapses were located exclusively on the dendritic shaft (see Table S1).
To confirm the expansion of the GABAergic input, we counted the number of GABAergic terminals per unit area of cerebellar molecular layer (Fig. 1A). We found that following a block of electrical activity, there is a significant increase from a mean value of 14.37 ± 5.69 SE to a value of 25.84 ± 5.11 SE (see Table S1).
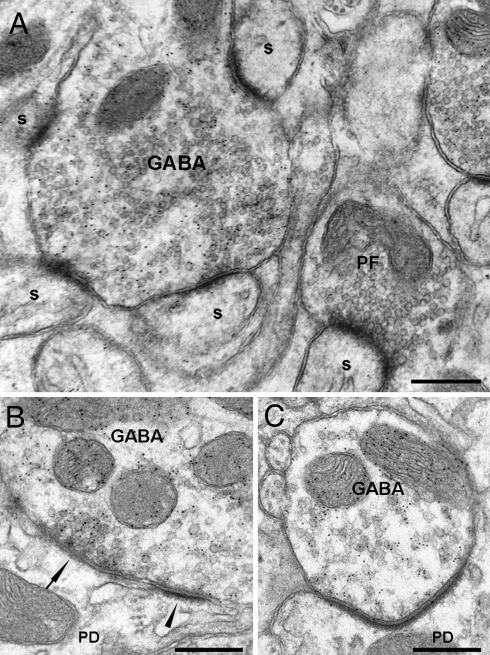
Fig. 1.
Ultrastructural features of GABAergic terminals labeled with the anti-GABA antibody in TTX-treated cerebella. (A) A GABAergic terminal contacts several spines with an asymmetrical phenotype; note the large size of such aberrant terminal. In the vicinity, an unlabeled PF terminal makes atypical asymmetric synapses with a spines. (Scale bar, 0.3 μm.) (B) Synapse between a GABAergic terminal and the dendritic shaft; note the presence of a double synapse with asymmetrical postsynaptic densities (PSDs) (arrowhead) close to a symmetrical region (arrow). The synaptic vesicles form a prominent cluster facing the symmetrical contact. (Scale bar, 0.15 μm.) (C) Asymmetrical GABAergic synapse characterized by a low vesicle density in the presynaptic region juxtaposed to the PSD. (Scale bar, 0.5 μm.) s, spine; PD, proximal dendrite; PF, parallel fiber.
A further issue is to see whether the expansion of the GABAergic input is accompanied by a change in size of the axonal terminals. We measured area, perimeter, and major and minor axis length of the terminals, and we found a significant increase in all these parameters for the synapses occurring in the proximal dendritic compartment, but not in the distal one (Table S2). In conclusion, this first set of experiments demonstrates that the active CF is able to repress not only the PF synapses, but also the inhibitory wiring, because in the absence of activity, a high number of GABAergic terminals form ectopic contacts with newly formed spines in the proximal dendrites.
Finally, we analyzed the morphology of the inhibitory synapses to see whether they have symmetrical or asymmetrical profiles. In the synapses made by GABAergic terminals onto the spines we found that all of them were typically asymmetrical (n = 64). Concerning the GABAergic terminals ending on the dendritic shaft, we found that out of 54 synapses, only 25 (46%) were typically symmetrical. Out of the remaining 29 (54%), 14 were double synapses with asymmetrical postsynaptic densities (PSDs) close to symmetrical regions (Fig. 1B). Surprisingly, in all these atypical synapses, the synaptic vesicles are clearly oriented toward the symmetrical contact. The profile of the remaining 15 synapses (28%) appeared asymmetrical along the entire PSD length (Fig. 1C). All these synapses had a low vesicle density in the presynaptic region juxtaposed to the PSD.
Characterization of the Glutamate and GABAA Receptor Subunits in the GABAergic Synapses onto the PC Dendritic Spines and Shaft.
Spine synapses.
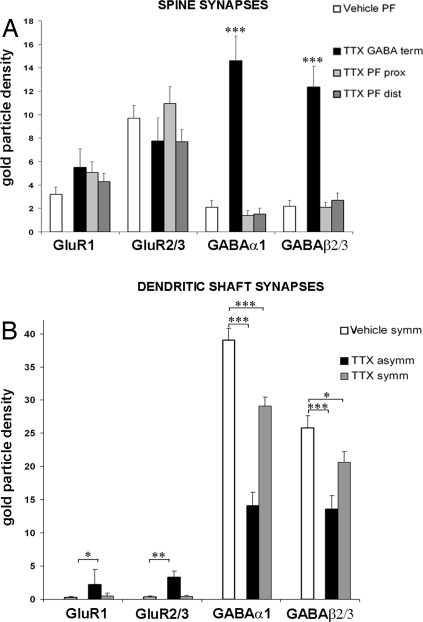
In a previous article, we showed that following TTX administration, the ectopic GABAergic terminals synapse onto the spines expressing the GluRδ2 subunit in the postsynaptic membrane (6). These synapses are to be considered atypical because they are formed by GABAergic neurons contacting spines that express at least one of the receptors that characterize the excitatory synaptic transmission. Here we investigate the possible presence in these synapses of other glutamate, but also GABA receptors. We used the freeze substitution and postembedding immunogold technique to evaluate the expression and the density of the glutamate receptor GluR1 and GluR2/3 and of the GABAA α1 and β2/3 subunits. Before approaching this issue, we confirmed the presence of the GluRδ2 subunit in all observed synapses (n = 15) (6). A total of 115 spines innervated by GABAergic terminals have been analyzed. We found a consistent presence of GluR2/3 (density per μm postsynaptic membrane 7.7 ± 1.9SE, n = 21; Fig. S1A) (Fig. 2A) and GluR1 receptors (5.5 ± 1.6 SE, n = 27) (Fig. 2A and 3A). We then determined the density of these receptors in spines innervated by the PFs in the proximal and distal dendrites of TTX-treated cerebella. For the GluR1, the values (respectively 5.06 ± 0.9 SE, n = 66 and 4.3 ± 0.7 SE, n = 76) are similar to those obtained in the vehicle-treated cerebella (3.2 ± 0.6 SE, n = 70). Similarly, for the GluR2/3 receptors, the mean density values in the proximal (10.93 ± 1.45 SE, n = 36) and in the distal dendrites (7.69 ± 1.06 SE, n = 47) are not significantly different from those found in the vehicle-treated animals (9.7 ± 1.1 SE, n = 48, P > 0.05). These density values in the PF-innervated spines are not significantly different from those of the GABAergic-innervated spines (P > 0.05). Thus, in the absence of activity the glutamate-receptor expression in the PC spines is not prevented by an ectopic GABAergic innervation.
Fig. 2.
Density distribution of receptors expressed as number of immunogold particles per micrometer of PSD. (A) Black columns show the glutamate and GABAA receptor density in spines innervated by GABAergic terminals under TTX (TTX GABA term). The GluR density values are similar to those obtained in vehicle PF-spine synapses (white column) and in TTX PF proximal (TTX PF prox; light gray columns) and distal spine synapses (TTX PF dist; gray columns). The GABAA receptors are highly expressed only in the spines contacted by GABAergic terminals in TTX-treated rats. (B) Distribution of glutamate and GABA receptors in the vehicle- and TTX-treated cerebella shaft synapses. GABAA receptor density decreases after the block of the electrical activity: for α1 the reduction is highly significant in both TTX symmetric (TTX symm; gray columns) and asymmetric (TTX asymm; black columns) synapses; for β2/3 the decrease is especially evident in the TTX asymmetric synapses. A significant increase of the GluR1 and 2/3 in the asymmetric dendritic shaft membrane occurs. Asterisks indicate the significant differences, ***, P < 0.001; **, P < 0.01; *, P < 0.05.
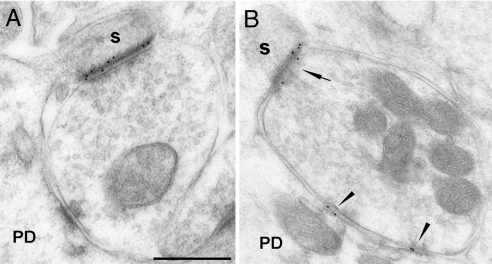
Fig. 3.
Immunogold labeling of GABA and glutamate receptors in spine and shaft synapses innervated by GABAergic terminals in TTX-treated cerebella. (A) GABAergic terminal showing two synapses: one with the shaft, devoid of labeling, and another one with a spine expressing GluR1. (B) GABAA α1 subunits are expressed in both shaft (arrowheads) and spine (arrow) synapses. s, spine; PD, proximal dendrite. (Scale bar, 0.5 μm.)
We also found a consistent presence of GABAA α1 (14.59 ± 2.1 SE, n = 54) (Fig. 3B) and β2/3 (12.3 ± 1.8 SE, n = 21) (Fig. S1 B and C) subunits in the spines (see Fig. 2A). The density of these receptors is 37.4% and 47.8%, respectively, relative to the density of the GABAergic shaft synapses in vehicle-treated cerebella. Double immunogold labeling for the the GABAA β2/3 subunits (20-nm gold particles) and for GluRδ2 subunit (10-nm gold particles) showed the coexistence of both these receptor subtypes in the same GABAergic-PC ectopic spine synapses (see Fig. S1C). Thus, in the absence of activity, the postsynaptic densities of the spines innervated by the GABAergic input acquire both an excitatory and an inhibitory phenotype.
PF-innervated spines are confined to the distal domain. Normally, the presence of GABA receptors is virtually absent. A weak immunolabeling for GABAA α1 and β2/3 subunits has been shown in 2.8% of the PC spines (11). Similarly, in vehicle-treated rats, sparse gold particles are observed in some of them and the mean density values for the α1 and β2/3 subunits are respectively 2.1 ± 0.6 SE, n = 60 and 2.2 ± 0.5 SE, n = 50. As shown in Fig. 2A, these values are not significantly different for those found in TTX-treated cerebella (1.5 ± 0.5 SE, n = 28 and 2.7 ± 0.6 SE, n = 27). We wondered whether the situation is different in the newly formed spines innervated by the PFs in the proximal dendritic domain. We found that the density of these subunits (1.4 ± 0.4 SE, n = 23 and 2.1 ± 0.4 SE, n = 25) is not significantly different from that reported above for the distal dendritic spines. Therefore, because the ectopic expression of GABAergic receptors in the spines is confined to those innervated by the GABAergic terminal and not by the PFs, it is reasonable to assume that the expression of the appropriate (GABA) receptors depends on the nature of the presynaptic compartment, even in the absence of activity.
Symmetrical shaft synapses.
As described above, after TTX the GABAergic shaft synapses appear both with a typical symmetric and an atypical asymmetric profile. Therefore, we asked how glutamate and GABA receptors are involved in these synapses. As far as the symmetrical synapses are concerned, in TTX-treated cerebella the density of α1 (29.1 ± 1.3 SE, n = 106) (see Fig. 3B) and of β2/3 (20.6 ± 1.6 SE, n = 35) (see Fig. S1B) receptor subunits (see Fig. 2B) is 74.6% and 79.8% relative to the symmetrical shaft synapses of the vehicle-treated ones, both reductions being significant (P < 0.01). Concerning the glutamate receptors, both GluR1 and GluR2/3 subunits are expressed at a very low level in the vehicle- and TTX-treated cerebella, with no significant difference (P > 0.05) (see Fig. 2B).
Asymmetrical shaft synapses.
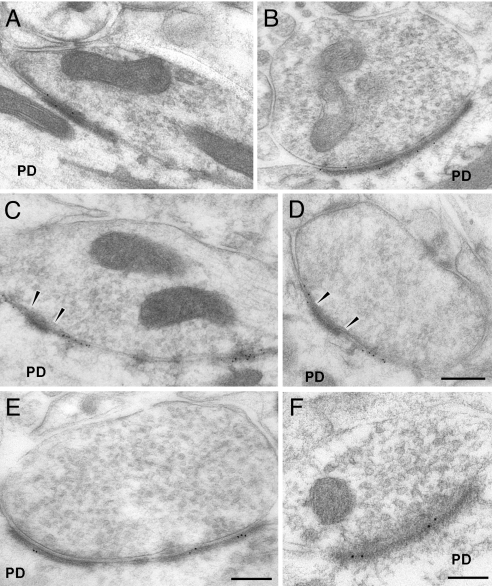
Inhibitory shaft synapses with an asymmetric profile are never observed in the normal cerebellar cortex and are very numerous following a prolonged block of electrical activity. In these synapses, the density of the GABAA α1 receptors (14.1 ± 2.0 SE, n = 45) (Fig. 4A) is very similar to that we found in the synapses made by the GABAergic terminals onto the spines. This density is 36.2% relative to the symmetrical shaft synapses of untreated cerebella and 48.4% relative to the symmetric synapses of the TTX-treated animals, both reductions being significant (see Fig. 2B). Similar significant differences have been found for the density of the β2/3 receptors (13.6 ± 2.0 SE, n = 16) (Fig. 4B), where the respective percentages were 52.7% and 66.0% (see Fig. 2B). Surprisingly, when the synapses are composed by both symmetrical and asymmetrical PSDs, it should be noted that the labeling for the GABAA subunits is restricted to the symmetric component of the PSD (Fig. 4 C and D). Concerning the glutamate receptors, we found that the density of the GluR1 (2.2 ± 2.3 SE, n = 33) and GluR2/3 (3.3 ± 0.9 SE, n = 21) receptors (Fig. 4 E and F) is significantly higher than that found in symmetrical shaft synapses both in vehicle and under TTX (see Fig. 2B).
Fig. 4.
Immunogold labeling of GABA and glutamate receptors in shaft synapses innervated by GABAergic terminals in TTX-treated cerebella. (A) GABAA α1 in asymmetrical PSD. (B) Same labeling for GABAA β2/3 subunits. (C) Synapses composed by both symmetrical and asymmetrical PSD; the labeling for the GABAA α1 subunits is restricted to the symmetric component of the PSD. (D) The same labeling for GABAA β2/3 subunits; arrowheads indicate the asymmetrical part of the PSD devoid of labeling. (Scale bars, A–D: 0.5 μm) (E) Immunolabeling for GluR1 subunit in asymmetrical shaft synapses. (Scale bar, 0.3 μm.) (F) The same labeling for GluR2/3 subunits. (Scale bar, 0.1 μm.)
In conclusion, the GABAergic asymmetrical shaft synapses on the PCs, which appear following a block of electrical activity, show some basic excitatory profiles in their postsynaptic domain, with the presence of the glutamate receptors but also an inhibitory profile with GABA receptors. Because all changes are reversible after the TTX removal (12), we suggest that electrical activity regulates the right matching between presynaptically released transmitter and both postsynaptically expressed receptors and typical symmetric postsynaptic specialization.
Analysis of Glutamate Expression in the GABAergic Terminals.
The appearance of asymmetrical synapses made by GABAergic terminals on the spines and on the shaft of the PCs under TTX requires checking whether glutamate transmitter is present in these GABAergic terminals to match the presence of glutamate receptors in the postsynaptic membrane. Immunolabeling for glutamate (Fig. S2) was performed to determine whether profiles forming hybrid synapses express a dual GABAergic/glutamatergic phenotype. We compared the number of gold particles per area of presynaptic terminals in control excitatory synapses (PF boutons) (see Fig. S2A) with the density in GABAergic terminals in control and after TTX treatment (see Fig. S2 C and D). The density of labeling in the presynaptic GABAergic terminals forming symmetric and asymmetric shaft synapses (34.8 ± 5.6 SE, n = 10 and 47.7 ± 10 SE, n = 11) was similar to that of the GABAergic synapses in control conditions (39.2 ± 4.1 SE, n = 10) and nearly half that of the PF terminals in vehicle- and TTX-treated cerebella (71 ± 9 SE, n = 10 and 91 ± 11 SE, n = 10, respectively, P < 0.05) (see Fig. S2B). This result shows that ectopic synapses do not over-express glutamate for neurotransmission and that in these terminals glutamate only serves metabolic or precursor roles. Therefore, the presence of glutamate receptors in spines and shaft asymmetric postsynaptic specializations in the presence of GABAergic innervation suggests that this expression does not depend on the nature of the presynaptic terminal.
Discussion
We had previously shown that the two excitatory inputs to the PC are competitors in terms of occupied territory and in adulthood. However, in addition to excitatory inputs, the PC also receives inhibitory inputs from basket and stellate cells and from their recurrent collaterals (9, 10, 13).
We asked whether following a block of electrical activity the remarkable ectopic extension of the PF induces regressive phenomena toward the GABAergic input. Surprisingly, we found that both the number and the size of the GABAergic terminals are increased. This remodeling is confined in the proximal dendritic domain, the territory where the CF activity exerts an inhibition on the synaptogenesis in the regions surrounding its synaptic contacts. When this repressive action is removed in the mature cerebellum, the proximal domain becomes a territory with an intense synaptogenesis. This model allows the description of the entire process of new synapse formation. Therefore, although cellular and subcellular selectivity of GABAergic axons seem to be genetically determined (14), our results support the view that the density of GABAergic innervation is regulated by neuronal activity and by experience, both necessary to prevent the reconfiguration of circuits (15–18).
A second issue raised in this article stems from the serendipitous observation made in previous experiments under the activity block. We observed that in the proximal dendritic domain, some spines were innervated by GABAergic terminals (6). Here, we found that nearly half of the ectopic GABAergic contacts occurred on dendritic spines. Innervation of spines by GABAergic terminals have also been found in the barrel cortex of adult rats. Knott et al. (16) observed an increase of 36% of the total density of synapses in the corresponding barrel cortex after single-whisker stimulation. The increase affects excitatory and inhibitory synapses and both of them occur on spines and on the dendritic shaft. PC spines innervated by GABAergic terminals have been already described in GABAA receptor α1 subunit deficient mice (19). In these mice, the GABAA receptor mediated neurotransmission from PC is abolished. The vast majority of GABAergic terminals forms mismatched synapses with PC spines characterized by a typical asymmetric PSD, whereas the number of symmetric synapses is reduced by about 75%. These spines bear the GluRδ2 but no AMPA receptors. Because GABAergic synapses normally form during development, synapse formation is independent from neurotransmitters, but the maintenance requires the functional synaptic machinery. Ectopic synapses have also been previously reported in the cerebellar cortex of reeler mice, characterized by a mutation that causes a malposition of different neuronal classes. In these mice, type I synapses between Golgi axons and PC spines were observed (20, 21). It has been suggested that lack of competition between afferent inputs and the attraction of noninnervated postsynaptic sites could be responsible for the appearance of these unconventional synaptic connections. In our model, it is possible that the high number of newly formed spines exert a synaptogenic action also on GABAergic terminals that are able to form ectopic inactive contacts.
We have then analyzed in detail the morphological and the neurotransmitter/receptor profile of the GABAergic synapses that are present both on spines and shaft following a block of activity. At the morphological level, we found that these synapses may be symmetrical, like those in control conditions, but also surprisingly asymmetrical. The latter type is consistently present when the GABA terminals are located on spines. In contrast, in the PC dendritic shaft both the normal symmetrical and the abnormal asymmetrical profiles are present. The two profiles are sometimes present in the same synapse and in this case the vesicle density prevails near the symmetric component of the postsynaptic specialization (see Fig. 1B and Fig. 5).
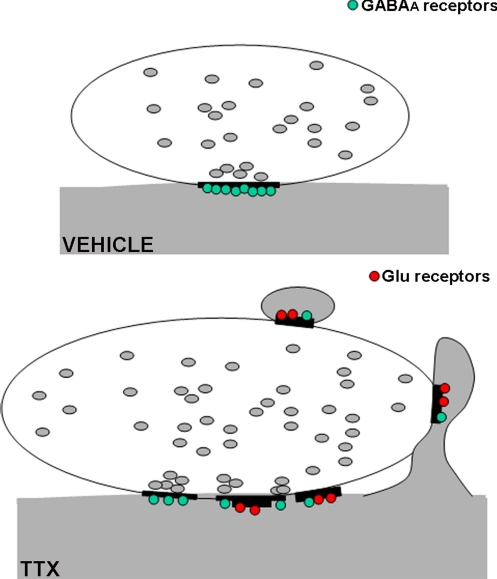
Fig. 5.
Schematic representation of the GABAergic terminal-PC synaptic remodeling after a block of the electrical activity. (Upper) In the vehicle-treated cerebella, GABAergic terminals contact the dendritic shaft forming symmetric synapses expressing the GABA receptors (green). (Lower) After TTX infusion, the terminals increase in size and present ectopic contacts on spines. In addition, the synapses on the dendritic shaft may be symmetrical, but also totally or in part asymmetrical. Glutamate receptors (red) are expressed by both spines and asymmetrical shaft synapses; GABAA receptors are downregulated in the shaft synapses and appear in the spines.
We then evaluated in these two types of synapses the distribution of the glutamate and GABA receptors at postsynaptic sites and verified the possible presence of glutamate transmitter in the GABAergic terminals. In all asymmetrical synapses, both in the spines and in the dendritic shaft, we found a consistent presence of glutamate receptors with a density that is similar to that of the PF-innervated spines (see Figs. 2 and 5). However, this expression is not correlated with an up-regulation of glutamate neurotransmitter inside the presynaptic terminals. These results suggest that in the absence of activity the PC dendrites are prone to develop a postsynaptic assembly of excitatory synapses, independently of the nature of the presynaptic partner, leading to a transmitter/receptor mismatch.
Concerning the GABA receptors, they are present, although with a density that is lower than that of the active inhibitory synapses in the symmetric and in the asymmetric synapses, both in the spine and in the shaft membrane (see Figs. 2 and 5). The possible influence of the GABAergic presynaptic terminals in inducing the expression of their proper receptors, even in the absence of activity, is supported by the observation that under TTX, the PF synapses are devoid of such an expression. On the other hand, normal levels of GABA receptor expression require the activity of presynaptic terminals.
Our results support the hypothesis that the GABA-mediated activity has a fundamental role in the maintenance of properly matched symmetric synapses, but it is dispensable for the initial synapse formation. Basic machinery for synapse formation is shared by all neuronal cells, and a general signal attracts postsynaptic protein to nascent PSD independent of the nature of the presynaptic terminal. Mismatched synapses with GABAA receptor clusters facing glutamatergic afferents are formed in autapses of hippocampal pyramidal cells (22). In addition, GABAergic neurones cultured in the absence of glutamatergic innervation express clusters of NMDA receptor and PSD95 scaffolding protein, suggesting that glutamate is not necessary to induce aggregates of these postsynaptic components. Moreover, cerebellar granule cell cultures express GABAergic receptors and the scaffolding protein gephyrin, irrespective of the presence of GABAergic axons and in mismatched synapses formed by glutamatergic afferents (23, 24). Furthermore, synapse assembly occurs and morphological normal synapses form in mutant mice that are deficient in synaptic vesicle fusion (25–27), suggesting that other factors provide cues for proper sorting and targeting of synaptic molecules. Several cell adhesion molecules, such as SynCAM and neurexin, can initiate synapse formation, even in non-neuronal cells (28–30). However, an activity-dependent change in the neurotransmitter/receptor profile has been recently demonstrated in the neuromuscular junction and hippocampal granule cells. Embryonic striated muscle cells initially express several classes of transmitter receptors, but during normal muscle innervation acetylcholine-receptor expression prevails. Altering calcium-spike activity leads to retention of noncholinergic receptors (3). Adult hippocampal granule cells can re-express their latent GABA phenotype in response to sustained or repeated depolarization (31). Therefore, neuronal activity ensures matching of transmitters and receptors in the assembly of functional synapses. Correct sorting of neurotransmitter and postsynaptic receptors is not required initially, but nonfunctional synapses are replaced during neuronal maturation (2, 32).
We report an abnormal neurotransmitter/receptor matching because of an altered synaptic transmission in the adult cerebellum. On the whole, our results support the view that the CFs are the main determinant of the correct basic architectural wiring of the cerebellar cortex. The mechanism might involve peptides, such as corticotropin releasing factor or urocortin released from CF. It has been recently shown how corticotropin releasing factor modulates the physiological properties of basket and stellate cells (33). In the absence of activity, the proximal dendritic region acquires a high degree of plasticity and forms a high number of spines innervated by both a glutamatergic and a GABAergic input. In addition, a thick postsynaptic specialization, that in the PCs is present only in the spines, is also expressed in the dendritic shaft and, in this case, the postsynaptic region is endowed with AMPA and GluRδ2 subunits. These abnormalities disappear when the electrical activity is restored and the pruning effect on the spines by CF activity is mediated through the AMPA receptors (8). Therefore, electrical activity plays important roles for the maintenance of both a properly matched pre- and postsynaptic structure and a correct balance between excitation and inhibition.
Methods
Toxin Delivery.
Adult male Wistar albino rats were infused for 7 days with TTX (80 μM). Control animals were infused only with physiological solution. See SI Methods for further details. The experiments were designed according to the European Community Council Directive 86/609/EEC for care and use of experimental animals and approved by the Bioethical Committee of Turin University.
Electron Microscopy.
Proximal and distal dendrites were identified according to described morphological features (12). See SI Methods for further details.
Postembedding for GABA and Glutamate Labeling.
Sections were labeled with the antibodies against GABA and glutamate. GABAergic terminal density was expressed as number per μm of dendrite membrane length and per area. Glutamate labeling intensity was assessed by determining the number of gold particles per area. Student's t test was used for statistical evaluation. See SI Methods for further details.
Freeze Substitution and Lowicryl Embedding.
Lowicryl-embedded sections were labeled for AMPA receptor GluR1 or GluR2/3, GluRδ2, GABAA receptors α1 and β2/3. Receptor densities were quantified as number of immunogold particles per μm of PSD length. See SI Methods for further details.
Supplementary Material
Acknowledgments.
We thank Mrs. A. Renna for excellent technical assistance. This work was supported by grants from the Italian Space Agency, the Italian Ministry of University and Research, the Ministry of Health, the European Community (contract number 512039), Regione Piemonte, and the Compagnia San Paolo Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806979105/DCSupplemental.
References
- 1.Lardi-Studler B, Fritschy JM. Matching of pre- and postsynaptic specializations during synaptogenesis. Neuroscientist. 2007;13:115–126. doi: 10.1177/1073858406296803. [DOI] [PubMed] [Google Scholar]
- 2.Spitzer NC, Borodinsky LN. Implications of activity-dependent neurotransmitter-receptor matching. Philos Trans R Soc Lond B Biol Sci. 2008;363:1393–1399. doi: 10.1098/rstb.2007.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc Natl Acad Sci USA. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunelli G, et al. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc Natl Acad Sci USA. 2005;102:8752–8757. doi: 10.1073/pnas.0500530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gómez-Lira G, Lamas M, Romo-Parra H, Gutiérrez RJ. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morando L, Cesa R, Rasetti R, Harvey R, Strata P. Role of glutamate δ2 receptors in activity-dependent competition between heterologous afferent fibers. Proc Natl Acad Sci USA. 2001;98:9954–9959. doi: 10.1073/pnas.171098398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesa R, Morando L, Strata P. Glutamate receptor δ2 subunit in activity-dependent heterologous synaptic competition. J Neurosci. 2003;23:2363–2370. doi: 10.1523/JNEUROSCI.23-06-02363.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesa R, Scelfo B, Strata P. Activity-dependent presynaptic and postsynaptic structural plasticity in the mature cerebellum. J Neurosci. 2007;27:4603–4611. doi: 10.1523/JNEUROSCI.5617-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eccles JC, Sasaki K, Strata P. A comparison of the inhibitory actions of Golgi cells and of basket cells. Exp Brain Res. 1967;3:81–94. doi: 10.1007/BF00234471. [DOI] [PubMed] [Google Scholar]
- 10.Ito M. The Cerebellum and Neural Control. New York: Raven; 1984. [Google Scholar]
- 11.Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABA(A) receptors concentrate on the glial processes that wrap inhibitory synapses. J Neurosci. 2002;22:10720–10730. doi: 10.1523/JNEUROSCI.22-24-10720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bravin M, Morando L, Vercelli A, Rossi F, Strata P. Control of spine formation by electrical activity in the adult rat cerebellum. Proc Natl Acad Sci USA. 1999;96:1704–1709. doi: 10.1073/pnas.96.4.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayama C. Formation of GABAergic synapses in the cerebellum. Cerebellum. 2005;4:171–177. doi: 10.1080/14734220510008012. [DOI] [PubMed] [Google Scholar]
- 14.Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- 15.Micheva KD, Beaulieu C. An anatomical substrate for experience-dependent plasticity of the rat barrel field cortex. Proc Natl Acad Sci USA. 1995;92:11834–11838. doi: 10.1073/pnas.92.25.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 17.Chattopadhyaya B, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoè-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the alpha1 subunit in Purkinje cells. J Neurosci. 2006;26:3245–3255. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson L, Sotelo C, Caviness VS., Jr Heterologous synapses upon Purkinje cells in the cerebellum of the Reeler mutant mouse: an experimental light and electron microscopic study. Brain Res. 1981;213:63–82. doi: 10.1016/0006-8993(81)91248-8. [DOI] [PubMed] [Google Scholar]
- 21.Sotelo C. Cerebellar synaptogenesis: what we can learn from mutant mice. J Exp Biol. 1990;153:225–249. doi: 10.1242/jeb.153.1.225. [DOI] [PubMed] [Google Scholar]
- 22.Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Studler B, Fritschy JM, Brünig I. GABAergic and glutamatergic terminals differentially influence the organization of GABAergic synapses in rat cerebellar granule cells in vitro. Neuroscience. 2002;114:123–133. doi: 10.1016/s0306-4522(02)00206-3. [DOI] [PubMed] [Google Scholar]
- 24.Anderson TR, Shah PA, Benson DL. Maturation of glutamatergic and GABAergic synapse composition in hippocampal neurons. Neuropharmacology. 2004;47:694–705. doi: 10.1016/j.neuropharm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 26.Varoqueaux F, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci USA. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- 28.Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 29.Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 30.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutiérrez R. Activity-dependent expression of simultaneous glutamatergic and GABAergic neurotransmission from the mossy fibers in vitro. J Neurophysiol. 2002;87:2562–2570. doi: 10.1152/jn.2002.87.5.2562. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Tian JB, King JS, Bishop GA. Stimulation of the inferior olivary complex alters the distribution of the type 1 corticotropin releasing factor receptor in the adult rat cerebellar cortex. Neuroscience. 2008;153:308–317. doi: 10.1016/j.neuroscience.2008.01.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.