Abstract
We have restored the CoQ oxidative capacity of mouse mtDNA-less cells (ρ° cells) by transforming them with the alternative oxidase Aox of Emericella nidulans. Cotransforming ρ° cells with the NADH dehydrogenase of Saccharomyces cerevisiae, Ndi1 and Aox recovered the NADH DH/CoQ reductase and the CoQ oxidase activities. CoQ oxidation by AOX reduces the dependence of ρ° cells on pyruvate and uridine. Coexpression of AOX and NDI1 further improves the recycling of NAD+. Therefore, 2 single-protein enzymes restore the electron transport in mammalian mitochondria substituting >80 nuclear DNA-encoded and 11 mtDNA-encoded proteins. Because those enzymes do not pump protons, we were able to split electron transport and proton pumping (ATP synthesis) and inquire which of the metabolic deficiencies associated with the loss of oxidative phosphorylation should be attributed to each of the 2 processes.
Keywords: AOX, mouse, oxidative phosphorylation, NDI1, CoQ
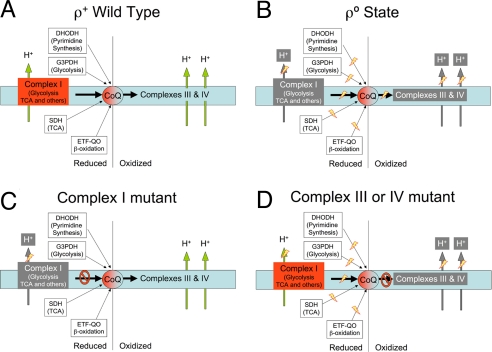
The mammalian mitochondrial electron transport chain (mtETC) couples NADH and FADH2 oxidation to proton pumping across the inner mitochondrial membrane. The resultant electrochemical gradient is used for ATP synthesis through the H+-ATP synthase (1). Coenzyme Q (CoQ) plays a central role in the electron flow through the mtETC. Mitochondrial CoQ reduction/oxidization is required in mammals for several pathways, including the synthesis of pyrimidines, the tricarboxylic acid cycle and β- oxidation, as well as aerobic ATP production (Fig. 1). Lower animals, plants and fungi can use alternative ways to reduce and oxidize CoQ, such as NADH-DH/CoQ reductase activity or CoQ oxidase activity, albeit without proton translocation (2, 3). Vertebrate cells lacking a functional mtETC owing to the absence of mtDNA (ρ° cells), can be maintained in culture if supplemented with uridine and pyruvate (4, 5). NDI1 and AOX are monopeptidic enzymes with NADH DH/CoQ reductase and CoQ/O2 oxidase activities, respectively, that do not translocate protons. NDI1 substitutes in yeast mitochondria the role of complex I, and AOX is an alternative electron transport system present in lower eukaryotes, plants and lower animals that can perform the overall oxidation of CoQH2 instead of complex III and complex IV. NDI1 protein was recently expressed in human cultured cells lacking complex I where it can restore NADH dependent respiration as well as the growth of the cells in galactose (6, 7). AOX expression is well tolerated in wild-type cultured human cells, where it confers resistance to cyanide, an inhibitor of complex IV (2). These studies highlight the potential use of NDI1 and AOX for gene therapy of respiratory chain deficiencies (2, 7).
Fig. 1.
CoQ role in the mitochondrial electron transport chain (mtETC). Schematic representation of the respiratory chain status (electron flow and proton pumping activities) in wild-type (ρ+) cells (A), mtDNA-less (ρ°) cells (B), and in cells with knockout mutations in either complex I (C) or in complexes III or IV (D). The pivotal role of CoQ as electron acceptor from different routes and as electron donor to complexes III and IV is highlighted. DHODH, dihydroorotate dehydrogenase; G3PDH, glycerol-3-phosphate dehydrogenase (glycerol-phosphate shuttle); SDH, succinate dehydrogenase; ETF-QO, electron-transfer flavoprotein-ubiquinone oxidoreductase; TCA, tricarboxylic acid cycle.
However, these proteins have an unexplored highly significant interest because they may be able to restore electron transfer in cells completely lacking the mt-ETC. By doing that, they have the potential to decipher the physiological role of mt-ETC several activities, including electron flux, redox balance and generation of the electrochemical gradient. Understanding the contribution of these activities to cell metabolism is essential to gauge their role in oxidative phosphorylation (OXPHOS) system defects in humans.
Results and Discussion
ρ°AOX Cells Recover the Capacity to Oxidize CoQH2.
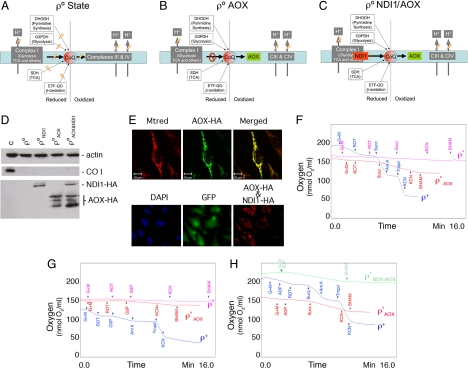
We engineered mouse ρ° cells to recover the capacity to oxidize CoQH2 by transforming them with AOX. Fig. 2 A and B illustrates the changes in the mitochondrial metabolic properties that are predicted by the expression of AOX in ρ° cells. AOX was efficiently expressed in transformed ρ° cells (ρ°AOX) and targeted to mitochondria (Fig. 2 D and E Upper). Polarographic measurements showed that both succinate (Fig. 2F) and glycerol-3-phosphate (Fig. 2G) are able to stimulate respiration in ρ°AOX that is insensitive to antimycin A (data not shown) and cyanide (complex III and IV inhibitors, respectively). However, this respiration is sensitive to salicylhydroxamic acid (SHAM), an inhibitor of AOX (Fig. 2 F and G) demonstrating that CoQH2 oxidization by AOX is sufficient to recover succinate dehydrogenase activity (SDH) and hence the tricarboxylic acid cycle (TCA) as well as the proper work of the glycerol shuttle (G3PDH activity). We inferred that also the electron-transfer flavoprotein-ubiquinone oxidoreductase (ETF-QO) and dihydroorotate dehydrogenase (DHODH) activities are recovered (see below).
Fig. 2.
Partial or total reconstruction of the mitochondrial respiratory chain in ρ° cells with exogenous enzymes. (A–E) MtETC status in ρ° cells (A) and in the same cells expressing Emericella nidulans alternative oxidase AOX (B) or AOX plus Saccharomyces cerevisiae NADH dehydrogenase NDI1 (C). Western blots showing the expression of actin, of mtDNA encoded COX subunit I or of the exogenous enzymes NDI1 and/or AOX in the indicated cell line, where C represented ρ+ cells (D). (E) (Upper) Subcellular localization of AOX in ρ°AOX cells immunostained for the HA epitope (green) and costained for mitochondria with Mito Tracker red. (Lower) Subcellular localization of NDI1 and AOX in ρ°NDI1/AOX cells immunostained for the HA epitope (red). Green color shows the expression of GFP as a marker for cell transfection with NDI1 expressing vector and blue color shows the staining of cell nucleus with DAPI. (F–H) Polarographic traces showing the respiratory activity of the indicated cell lines (F and G) ρ+: 5 × 106 cells, ρ° and ρ°AOX: 107 cells or isolated mitochondria (H) (0.5–1.0 mg of total protein) in the presence of different substrates and inhibitors. DHODH, dihydroorotate dehydrogenase; G3PDH, glycerol-3-phosphate dehydrogenase (glycerol-phosphate shuttle); SDH, succinate dehydrogenase; ETF-QO, electron-transfer flavoprotein-ubiquinone oxidoreductase; TCA, tricarboxylic acid cycle; Mtrred, Mito Tracker red, HA, hemagglutinin epitope; G+M, glutamate plus malate; ROT, rotenone; Succ, succinate; Ant A, antimycin A; Tmpd, N,N,N′, N′-tetramethyl-p-phenylenediamine; KCN, potassium cyanide; SHAM, salicylhydroxamic acid.
Electron Transfer from NADH to Oxygen is Restored in ρ° NDI1/AOX Cells.
Both ρ° and ρ°AOX cells lack the ability of recycling NADH by CoQ reduction in mitochondria. To test the relevance of this limitation, we engineered again mouse ρ° cells to recover the CoQH2 oxidative capacity by transforming them with AOX but also to recover the CoQ reducing capacity from NADH by additionally transforming them with NDI1. Both enzymes were synthesized and targeted to mitochondria (Fig. 2 D and E). Together with succinate and glycerol-3-phospate dependent respiration, ρ°NDI1/AOX cells also recovered NADH-dependent respiration that is sensitive to the AOX inhibitor SHAM (Fig. 2H), indicating that the transfer of electrons from NADH to CoQ and from this to oxygen was effectively restored via NDI1/AOX. Flavone is an inhibitor of NDI1 activity but we could not use it for our polarographic assays because it also inhibits AOX activity.
Metabolic Capabilities of ρ°AOX and ρ° NDI1/AOX Cell Lines.
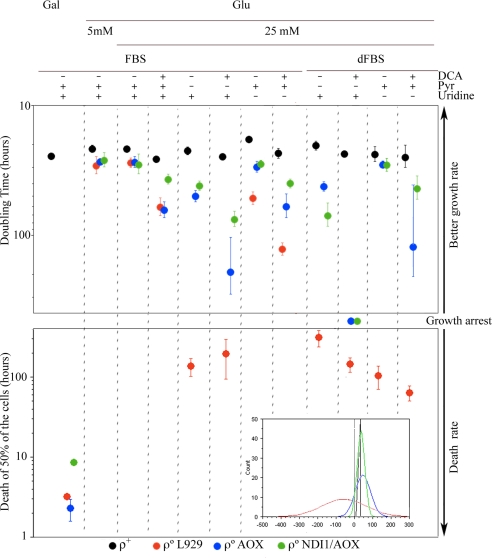
To determine the extent of the improvements in their metabolism, ρ°AOX and ρ°NDI1/AOX cells were cultivated under a variety of conditions. The growth of ρ° cells depends on glucose used through lactic fermentation, therefore growth (i) in the presence of high (25 mM) glucose, the most commonly used in cell culture, or 5 mM glucose (more physiological) was assayed; (ii) in the same vein glucose was replaced by galactose (5 mM) as the carbon source; (iii) the effect of limiting the cells' capacity to perform lactic fermentation was assessed by supplementing the medium with sodium dichloro acetate (DCA), an inhibitor of the pyruvate dehydrogenase kinase (PDHK); and cells were cultured without pyruvate (iv); or uridine (v) supplementation. For that last assay (v), dialyzed fetal bovine serum (dFBS) has to be used, and therefore the effect of dialyzed vs. nondialyzed fetal bovine serum was also analyzed. As an overall picture of the growth behavior of the different cell types we pooled all of the growth and/or death rates (as negative values) obtained per each cell type in the different conditions and we adjusted the obtained data to a normal distribution (Fig. 3 Inset). In this way we could see how respiratory competent cells containing mtDNA (ρ+) were able to grow in all cases with a doubling time (DT) that does not vary very much from the mean (black profile in Fig. 3 Inset). On the contrary, ρ° cells showed a very broad variability (red profile in Fig. 3 Inset). Interestingly, the expression of AOX in ρ°AOX cells largely decreased this variability approaching it to that of the control (blue profile in Fig. 3 Inset). Moreover ρ°NDI1/AOX showed a profile clearly distinct from ρ°AOX and closer to control (green profile in Fig. 3 Inset). Therefore, the restoration of the CoQH2 oxidation capability by AOX exerts the greatest effect on the performance of ρ° cells but the recovery of the mitochondrial NADH oxidation further improves it.
Fig. 3.
Consequences on cell growth due to the mtETC recovery by AOX or NDI1 and AOX expression. (Inset) Frequency distribution of the growth and/or death rates (as negative values) obtained for each cell type and adjusted to a normal distribution. ρ+ cells, black profile; ρ° cells, red profile; ρ°AOX cells, blue profile; ρ°NDI1/AOX, green profile. Effect of AOX or NDI1+AOX expression on cell growth (DT) and survival under the indicated culture conditions. Data for different media are expressed as mean ± standard deviation being n (from left to right in the figure) = 3, 3, 4, 4, 5, 5, 3, 3, 3, 3, 4, 5 for ρ+; n = 3, 3, 4, 4, 4, 4, 3, 3,3, 3, 4, 5 for ρ°; n = 3, 3, 4, 3, 5, 3, 3, 3, 3, 3, 4, 4 for ρ°AOX; n = 3, 3, 4, 3, 4, 3, 3, 3, 3, 3,4, 4 for ρ°NDI1/AOX. The significance of the mean differences was determined by the ANOVA, Fisher's PLSD test. Gal, galactose; Glu, glucose; DCA, sodium dichloro acetate (15 mM); Uri, uridine (0.2 mM); Pyr, pyruvate (1 mM); CM, complete medium containing uridine and pyruvate; dFBS, dialyzed fetal bovine serum.
AOX Restores Uridine and Pyruvate Autotrophy in ρ° Cells.
When focusing in particular conditions, it is noticeable that ρ° cells maintained their growth rate in 5 mM glucose, indicating that this concentration of glucose is sufficient for the survival of the cells unable to generate ATP by mitochondria. However, DCA (15 mM) increased their DT 2-fold (Fig. 3). On the contrary, ρ° cells were unable to grow in galactose or in the absence of exogenous addition of pyruvate (Fig. 3) regardless of whether the serum was dialyzed or not. The growth of ρ° cells also depended on exogenous addition of uridine, being significantly decreased in nondialyzed serum [from 27.5 to 51.5 h, P < 0.0001, ANOVA, Fisher's probable least-squares difference (PLSD) test]. Absence of supplemented uridine causes the death of the cells in dialyzed serum, where its supply by the serum is prevented. AOX expression enabled ρ° cells to grow without uridine and pyruvate but their growth was still affected by DCA and they were unable to grow in galactose medium (Fig. 3). The recovery of the uridine autotrophy demonstrates our prediction that the dihydroorotate dehydrogenase activity should be restored when AOX is able to efficiently oxidize CoQH2. It is noticeable that the doubling time of ρ°AOX cells was similar in medium lacking uridine than in complete medium regardless of whether the serum was dialyzed or not. The expression of AOX also restored the pyruvate autotrophy. In this case, however, the doubling time of ρ°AOX cells was slower in medium lacking pyruvate than in complete medium. The observation that some respiratory-compromised cells require added pyruvate for growth was reported earlier (8) and it was later confirmed for human ρ° cells (4). Pyruvate would be necessary to maintain the adequate rate of lactic fermentation and may also be needed as a substrate for the synthesis of oxalacetate within mitochondria. ρ° cells cannot generate oxalacetate via TCA because SDH activity is blocked when CoQH2 cannot be oxidized. In the ρ°AOX cells SDH activity is resumed and cell growth becomes independent of pyruvate supporting the notion of its need for oxalacetate synthesis via pyruvate carboxylase in ρ° cells.
Therefore, AOX was capable, in respiratory compromised mammalian cells, of reaching mitochondria and being incorporated into the mitochondrial inner membrane maintaining its activity. The restored oxidation of CoQH2 in ρ° cells by AOX reverts the metabolic impairment in DHODH, SDH, G3PDH and presumably ETF-QO activities and hence pyrimidine synthesis, TCA activity, glycerol phosphate shuttle and β-oxidation. However, ρ°AOX cells are still incapable to recover from the growth impairment induced by DCA to ρ° cells. Moreover, the presence of DCA severely affects the growth of ρ°AOX cells if deprived of pyruvate or uridine (Fig. 3). We reasoned that DCA prevented the recycling of NAD+ by lactic fermentation and therefore reduced the glycolytic activity to a point that compromises ATP production and therefore cell growth. A similar, although more severe, process could be underlying the inability of ρ°AOX cells to grow in galactose. Because the conversion of galactose into glucose is a very slow process, glycolysis is not working at a rate fast enough to produce the minimum amount of ATP required for growth. Wild-type cells were capable to grow at normal rate in the presence of DCA and in galactose. It is assumed that they can do it because they are able to generate ATP from mitochondria when the glycolytic rate is reduced.
ρ° NDI1/AOX Cells Survive in the Presence of Sodium Dichloro Acetate (DCA).
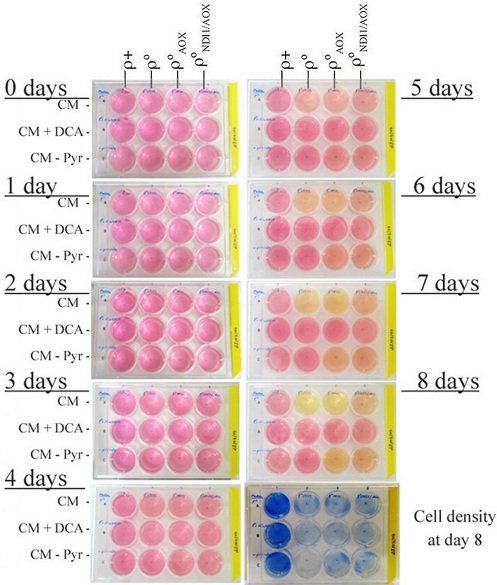
Interestingly the rate of growth of ρ°NDI1/AOX cells in the absence of pyruvate was improved from that of ρ°AOX cells (from 49.7 to 41.2 h, P = 0.0259, ANOVA, Fisher's PLSD test; Fig. 3). More important, ρ°NDI1/AOX cells also showed a significant improvement, compared with ρ°AOX cells, when grown in the presence of DCA (from 63.6 to 36.7 h, P < 0.001, ANOVA, Fisher's PLSD test; Fig. 3). DCA induced a higher reduction in the growth rate when pyruvate was not supplied both in ρ° and ρ°AOX cells, reduction that was partially compensated when NDI1 is coexpressed with AOX (Fig. 3). This was likely due to the restoration of NADH dependent respiration that allowed the recycling of NAD+ when lactic fermentation is compromised. In fact, the acidification in complete medium by ρ°NDI1/AOX cells was less pronounced than that of the ρ° or ρ°AOX cells (Fig. 4). In agreement with this interpretation, ρ°NDI1/AOX acidification rate in the absence of pyruvate was lower than that of ρ°AOX cells (Fig. 4). Therefore, ρ°NDI1/AOX cells recover the flux of electrons from NADH to oxygen, but are unable to generate a proton gradient for ATP synthesis regardless of the fact that they cannot assemble a wild-type ATPase because its 2 mtDNA-encoded subunits are absent. Despite of that, these cells recover pyrimidine synthesis (uridine autotrophy), TCA activity, and the glycerol phosphate shuttle. In addition, ρ°NDI1/AOX cells do not exclusively rely on lactic fermentation for the recycling of NAD+ for glycolysis maintenance because the full restoration of the mitochondrial electron transport chain is also contributing. Therefore, glycolysis seems to be sufficient to provide energy for the survival and growth of the cells. Interestingly, in galactose medium ρ°NDI1/AOX cells showed a substantial decrease in their death rate (Fig. 3), indicating that in the absence of mitochondrial ATP production, a threshold glycolytic rate is imperative for cell growth and that this cannot be achieved by metabolizing galactose.
Fig. 4.
Lactic fermentation of the different cell lines. The levels of medium acidification (yellow color) and cell density (methylene blue staining) achieved by the different cell lines in 3 culture conditions and after 8 days in culture is shown as a representative experiment. Gal, galactose; Glu, glucose; DCA, sodium dichloro acetate (15 mM); Uri, uridine (0.2 mM); Pyr, pyruvate (1 mM); CM, complete medium containing uridine and pyruvate.
Our analyses have demonstrated that it is possible to reconstruct in vivo the flux of electrons within the inner membrane of mammalian mitochondria from ρ° cultured cells, independently of the proton pumping activity. This has allowed for splitting the roles of the mitochondrial electron transport chain in ATP synthesis and in CoQ oxidoreduction balance for the proper activity of critical cellular metabolic pathways. Thus, we could restore most of the metabolic stress induced to the cells by the impairment of the OXPHOS system, implying that those were due more to the alteration in the CoQ oxidoreduction flux, rather than to the loss of mitochondrial ATP synthesis capacity.
Materials and Methods
Cell Lines.
All cell lines were grown in DMEM (GIBCO–BRL) supplemented with 5% FBS (fetal bovine serum, GIBCO-BRL). mtDNA-less mouse cells (ρ° 929) were generated by long-term growth of L929 mouse cell line (CCL-1; ATCC) in the presence of high concentrations of ethidium bromide (EthBr) as previously described (9). Control ρ+ cells (TmC57BL/6J) were generated by transference of mitochondria from platelets to ρ° 929neo cells as described in refs. 10 and 11.
NDI1HA and AOXHA Constructs.
Mouse codon-usage optimized version of Ndi1 and Aox genes, were recodified by using the program Backtranslation-tool (Entelechon) and were ordered from GenScript. The hemagglutinin epitope (HA tag) (YPYDVPDYA) was added to the C-terminus. Aox gene was subcloned by using XbaI/MluI sites in the lentiviral vector p156RRLsinPPThCMVMCSpre, and Ndi1 gene into the PmeI site of pWPI lentiviral vector bicistronic with GFP (from Tronolab).
Lentiviral Vectors Production.
Human 293T cells (2.5 × 106) were plated 24 h before cotransfection with 10 μg of transfer vector (AOXHA- p156RRLsinPPThCMVMCSpre or NDI1HA-pWPI), 7.5 μg of second-generation packaging plasmid (pCMVdR8.74) and 3 μg of envelope plasmid (pMD2.VSVG). FuGENE 6 Transfection Reagent (Roche) was used as transfectant reagent. Infectious particles were collected 24 and 48 h after transfection (12).
Generation of ρ°AOX and ρ°NDI1/AOX Cell Lines.
ρ°929 80% confluent were transduced with lentiviral particles carrying the Aox gene. The pool of cells expressing AOX (ρ°AOX) was isolated by selection in DMEM with 5% dFBS (serum without uridine). ρ°AOX cell line was subsequently transduced with lentiviral particles carrying the Ndi1gene. AOX-NDI1 expressing cells (ρ°NDI1/AOX) were isolated by 1-week selection in DMEM supplemented with 5% dFBS, (without uridine), and 15 mM DCA (Sigma).
Immunological Techniques.
For immunofluorescence, cells were incubated with 200 nM mitochondrial dye Mito Tracker red (Invitrogen) for 30 min, primary antibody anti-HA (Roche) and secondary antibody Alexa Fluor 488 IgG anti-rat (Invitrogen) were used. For Western-blot, cell proteins were extracted in RIPA buffer (Pierce). Total protein (20 μg) was separated in 12.5% acrylamide/bisacrylamide SDS/PAGE, electroblotted onto PVDF filter, and sequentially probed with specific antibodies: anti-HA (Roche), anti-COI (Molecular Probes), and anti-actin (Sigma).
Growth Rates.
Growth capacity was determined in 12-well test plates in which 5 × 104 cells were plated per well, in 1 ml of the indicated medium and incubated at 37 °C for 5 days. Cells were daily counted by using a Neubauer chamber and Trypan blue exclusion. The culture media used were: DMEM with 25 mM or 5 mM of either glucose or galactose, supplemented with either 5% FBS or 5% dFBS as indicated. Sodium pyruvate (110 μg/ml), uridine (50 μg/ml) or DCA (sodium dichloro acetate) 15 mM, were included when indicated. Cell density was determined by staining with methylene blue solution (0.3% methylene blue in methanol) for 10 min.
Oxygen Consumption Measurements.
O2 consumption determinations in digitonin-permeabilized cells or in isolated mitochondria (13), were carried out in an oxytherm Clark-type electrode (Hansatech) as previously described (14) with small modifications (11).
Data Analysis.
The significance of the mean differences in growth rate in all cell lines was determined by the ANOVA and by the post hoc Fisher's PLSD test. All test and calculations were done with the statistical package StatView 5.0 for Macintosh (SAS Institute).
Acknowledgments.
We thank Dr. I. Holt for critical reading of the manuscript and Nieves Movilla and Santiago Morales for their technical assistance. Our work was supported by Spanish Ministry of Education Grants SAF2006-00428 and CSD2007-00020, Instituto de Salud Carlos III Grant PI 04/2647, European Union Grant EUMITOCOMBAT-LSHM-CT-2004-503116, Group of Excellence Grant DGA (B55). E.P.-C. was supported by a predoctoral fellowship from the Spanish Ministry of Education.
Footnotes
The authors declare no conflict of interest.
References
- 1.Lenaz G, Genova ML. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: Random collisions vs. solid state electron channeling. Am J Physiol. 2007;292:C1221–1239. doi: 10.1152/ajpcell.00263.2006. [DOI] [PubMed] [Google Scholar]
- 2.Hakkaart GA, Dassa EP, Jacobs HT, Rustin P. Allotopic expression of a mitochondrial alternative oxidase confers cyanide resistance to human cell respiration. EMBO Rep. 2006;7:341–345. doi: 10.1038/sj.embor.7400601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yagi T, et al. Can a single subunit yeast NADH dehydrogenase (Ndi1) remedy diseases caused by respiratory complex I defects? Rejuvenation Res. 2006;9:191–197. doi: 10.1089/rej.2006.9.191. [DOI] [PubMed] [Google Scholar]
- 4.King MP, Attardi G. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 5.Morais R, Desjardins P, Turmel C, Zinkewich-Peotti K. Development and characterization of continuous avian cell lines depleted of mitochondrial DNA in vitro. Cell Dev Biol. 1988;24:649–658. doi: 10.1007/BF02623602. [DOI] [PubMed] [Google Scholar]
- 6.Bai Y, et al. Lack of complex I activity in human cells carrying a mutation in MtDNA-encoded ND4 subunit is corrected by the Saccharomyces cerevisiae NADH-quinone oxidoreductase (NDI1) gene. J Biol Chem. 2001;276:38808–38813. doi: 10.1074/jbc.M106363200. [DOI] [PubMed] [Google Scholar]
- 7.Yagi T, et al. Possibility of transkingdom gene therapy for complex I diseases. Biochim Biophys Acta. 2006;1757:708–714. doi: 10.1016/j.bbabio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Howell N, Sager R. Cytoplasmic genetics of mammalian cells: Conditional sensitivity to mitochondrial inhibitors and isolation of new mutant phenotypes. Somatic Cell Genet. 1979;5:833–845. doi: 10.1007/BF01542645. [DOI] [PubMed] [Google Scholar]
- 9.Tiranti V, et al. Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am J Hum Genet. 1998;63:1609–1621. doi: 10.1086/302150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomyn A, et al. Platelet-mediated transformation of mtDNA-less human cells: analysis of phenotypic variability among clones from normal individuals—and complementation behavior of the tRNALys mutation causing myoclonic epilepsy and ragged red fibers. Am J Hum Genet. 1994;54:966–974. [PMC free article] [PubMed] [Google Scholar]
- 11.Bayona-Bafaluy MP, et al. Revisiting the mouse mitochondrial DNA sequence. Nucleic Acids Res. 2003;31:5349–5355. doi: 10.1093/nar/gkg739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Vizarra E, Lopez-Perez MJ, Enriquez JA. Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods. 2002;26:292–297. doi: 10.1016/S1046-2023(02)00034-8. [DOI] [PubMed] [Google Scholar]
- 14.Hofhaus G, Shakeley RM, Attardi G. Use of polarography to detect respiration defects in cell cultures. Methods Enzymol. 1996;264:476–483. doi: 10.1016/s0076-6879(96)64043-9. [DOI] [PubMed] [Google Scholar]