Abstract
Human exposures to environmental toxicants have been associated with development of a number of diseases. Animal experiments have identified a number of cytoprotective enzymes under the transcriptional control of NF-E2-related factor 2 (Nrf2) including electrophile conjugation and antioxidative enzymes and enzymes responsible for the production of antioxidants, reducing equivalents and cofactors. The up-regulation of these enzymes represents an adaptive response which occurs in the face of exposure to electrophilic or oxidative compounds thereby leading to enhanced metabolism of these molecules or their reactive metabolites. This adaptive response is regulated by an interaction between Keap1 and Nrf2 in which the exposure to reactive molecules is sensed either directly by Keap1 or indirectly by cellular signaling cascades resulting in activation of Nrf2 transcriptional regulation. The Nrf2-mediated adaptive response has been shown to attenuate toxicity and carcinogenesis during electrophile or oxidative stress as well as inflammation in rodent models. The cytoprotective attributes of the Nrf2 signaling pathway have been targeted for chemoprevention as administration of Nrf2-inducing agents has been shown to result in decreased carcinogenesis in animal models and altered carcinogen metabolism in humans. On the other hand, polymorphisms in the Nrf2 signaling pathway can lead to differential susceptibility to disease while mutations in the Nrf2 signaling pathway have been shown to an effective mechanism for cancer cells to evade chemotherapy. Overall, the Nrf2 cytoprotective adaptive response has evolved to be a powerful protective strategy for organisms against exposure to environmental toxicants and may provide insight into differential disease susceptibilities across populations and responses to therapies designed to alleviate these conditions.
Humans are exposed to a diverse array of environmental toxicants associated with the development of a number of diseases including cancer, acute organ toxicity, and chronic inflammatory conditions. Many of these exposures are inadvertent while others are due to certain lifestyle factors. Examples include carcinogenic and pro-inflammatory chemicals in tobacco smoke and air pollution, pharmaceuticals, dietary carcinogens, and occupational hazards. The transcription factor, Nrf2, has been shown to regulate the expression of a network of cytoprotective enzymes resulting in protection against toxicity following exposure to electrophilic and oxidative chemicals. This pathway is highly conserved across vertebrates[1] and provides an excellent example of the evolution of a cytoprotective network of enzymes under the control of a sensory switch.
The present review will focus on the role of Nrf2 in protection against toxicity following exposure to environmental toxins and toxicants. The importance of the Nrf2-regulated cytoprotective adaptive response has been demonstrated in animal models of electrophile and oxidative toxicity and carcinogenesis, as well as acute inflammation and inflammation-associated carcinogenesis (Table 1). The role of Nrf2 induction of cytoprotective enzymes in modulation of human disease is becoming clearer. Targeting Nrf2 for chemoprevention of human environmental carcinogenesis has been shown to effectively modulate carcinogen metabolism by favoring elimination of reactive carcinogen metabolites. On the other hand, variant sequences of genes in the Nrf2 signaling pathway have been shown to adversely affect susceptibility of human disease progression as well as favor the selection of chemoresistant tumor cells.
Table 1.
Exposures in which increased susceptibility of Nrf2-deficient mice to toxicity has been demonstrated.
| Condition | Agent | Findings | Reference |
|---|---|---|---|
| Hepatotoxicity | acetaminophen | increased serum ALT values and centrilobular necrosis | [17,18] |
| pentachlorophenol | increased centrilobular necrosis, and serum ALT, liver TBARS and 8-oxo-dG levels | [58] | |
| Pneumotoxicity | BHT | increased lethality and acute respiratory distress syndrome | [59] |
| bleomycin | increased epithelial cell death and fibrosis | [60] | |
| hyperoxia | enhanced epithelial injury and inflammation | [28] | |
| Neurotoxicity | malonate | increased neuronal cell death and motor deficits | [29] |
| 3-NP | enhanced motor deficits and striatal lesions | [30] | |
| MPTP | increased parkinsonian phenotype – greater loss of dopamine transporter activity | [31] | |
| kainic acid | greater seizure severity and duration, hippocampal neuron death and mortality | [61] | |
| Carcinogenicity | diesel exhaust particles | enhanced oxidative DNA adduct formation and epithelial hyperplasia | [34] |
| B[a]P | increased multiplicity of gastric neoplasias | [23] | |
| DMBA/TPA | increased multiplicity of skin papillomas | [35] | |
| BBN | increased incidence and invasiveness of bladder carcinoma | [25] | |
| 2-amino-3-methylimidazo[4,5-f]-quinolone | greater incidence and multiplicity of liver tumors | [62] | |
| AOM/DSS | increased multiplicity of aberrant crypt foci due to more severe colitis | [43] | |
| Inflammation | tobacco smoke | increased alveolar inflammation and emphysema | [36] |
| elastase | enhanced severity of pulmonary inflammation and emphysema | [37] | |
| ovalbumin | greater airway hyperresponsiveness due to increased TH2 response | [38] | |
| LPS | increased lung oxidative stress, NF-κB activation and inflammation | [41] | |
| carageenan | increased and prolonged pleural cavity inflammation | [39] | |
| DSS | greater colonic inflammation and pro-inflammatory cytokine signaling | [40] |
The Nrf2 signaling pathway
Nrf2 is a member of the Cap-N-Collar transcription factor family and recognizes the antioxidant response element (ARE) in the promoter of target genes[2]. Normally, under basal conditions Nrf2 is bound to Keap1 in the cytoplasm due to an interaction between a single Nrf2 protein and a Keap1 dimer[3]. Keap1 serves as a substrate linker protein for interaction of the Cul3-based E3-ubiquitin ligase complex with Nrf2 leading to ubiquitination of Nrf2 and proteosomal degradation[4].
Exposure to a number of stressors and inducing agents leads to dissociation of Nrf2 from Keap1 thereby rescuing Nrf2 from proteasomal degradation and allowing for entry into the nucleus (Figure 1). These include both endogenous activators such as reactive oxygen species (ROS), reactive nitrogen species, lipid aldehydes and 15-deoxy-D12,14-prostaglandin J2; and exogenous agents such as heavy metals and electrophilic xenobiotics and their metabolites[5]. Experiments have demonstrated two separate mechanisms responsible for dissociation of Nrf2 from Keap1. First, Keap1 contains reactive cysteines that form protein-protein crosslinks following reaction with electrophiles leading to disruption of the Keap1-Nrf2 interaction and release of Nrf2[6]. The second mechanism involves secondary sensor proteins and the activation of protein kinase signaling pathways resulting in phosphorylation of Nrf2, enhanced stability and/or release of Nrf2 from Keap1[7].
Figure 1.
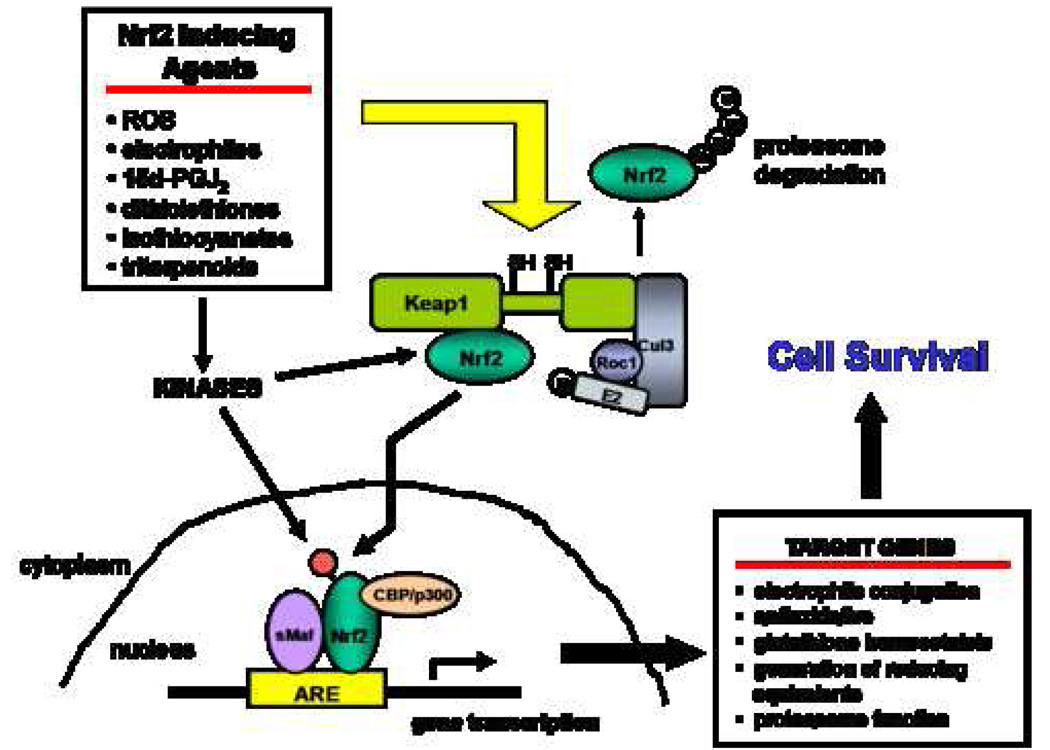
The Nrf2 signaling pathway.
Once inside the nucleus, Nrf2 dimerizes with small Maf proteins leading to binding of Nrf2 to AREs present in the promoter of Nrf2-target genes and transcriptional activation of these genes[8]. Additionally, other proteins have been identified that modulate Nrf2 transcription activation illustrating the influence of multiple signaling pathways on Nrf2 responses. Enhancement of Nrf2-mediated transcription has been demonstrated by CRE binding protein following the activation of upstream mitogen activated protein kinase signaling pathways[9]. On the other hand, both CCAAT enhancer-binding protein alpha[10] and p53[11] have been shown to negatively regulate the transcriptional activation of Nrf2 target genes.
Multiple microarray analyses of Nrf2 wild-type (WT) and Nrf2-deficient (N0) mice have identified a battery of Nrf2-regulated cytoprotective enzymes. The classes of these Nrf2-regulated genes include electrophile conjugating enzymes, antioxidative enzymes, glutathione (GSH) homeostasis, production of reducing equivalents and components of the proteasome. The protein products of these genes provide multiple layers of protection during cellular insults, collectively favoring cell survival.
Exposure of WT and N0 mice to Nrf2 inducing agents revealed that Nrf2 up-regulates the expression of a number of electrophile conjugation enzymes. These include glutathione-S-transferases (μ1, 2, and 3; α2 and 4; π1; and τ2), epoxide hydrolase, NADPH-quinone oxidoreductase, UDP-glucuronosyltransferase (1A1 and 2B5), aldehyde dehydrogenase 1A1 and aldoketo reductase (1B7 and 1B8)[12–15]. Additionally, a number of Nrf2-regulated antioxidative enzymes have been identified including glutathione reductase, peroxiredoxin, thioredoxin and thioredoxin reductase, catalase, copper/zinc superoxide dismutase and glutathione peroxidase[12–15]. Interestingly, Nrf2 also has been shown to play a role in up-regulating multiple components of the 20S proteasome catalytic core, responsible for ubiquitin-independent degradation of oxidized proteins implicated in a number of chronic diseases[14].
Nrf2 also plays a role in controlling the inducible expression of a number of enzymes responsible for the synthesis of GSH, direct acting antioxidants and reducing equivalents. Treatment with Nrf2-inducing agents resulted in increased Nrf2-dependent transcription of genes encoding glutamate cysteine ligase regulatory and catalytic subunits, heme oxygenase-1, UDP-glucose dehydrogenase, malic enzyme, and glucose-6-phosphate dehydrogenase [14,15]. In addition to the fact that some of the products of these enzymes have inherent antioxidative or electrophile conjugating activity, a number of these products are necessary cofactors for electrophile conjugating and antioxidative enzymes. Similarly, some of the products of these Nrf2-regulated enzymes can activate Nrf2 signaling, thereby potentiating the Nrf2 adaptive response. For example, Li, et al[16] demonstrated that carbon monoxide, produced by heme oxygenase-1 during nitrosative stress due to peroxynitrite exposure, activates Nrf2 resulting in increased expression of glutamate cysteine ligase catalytic subunit and replenishment of cellular GSH. Therefore, up-regulation of these genes represents a broad-based mechanism that ensures sustained activity of Nrf2-regulated cytoprotective enzymes in the face of toxic insults.
In addition to controlling inducible expression of cytoprotective enzymes, Nrf2 has been shown to regulate the basal expression of some electrophile conjugation and antioxidative enzymes. Higher transcript levels of UDP gluronosyltransferase (2B5 and 1A6), epoxide hydrolase, NADPH-quinone oxidoreductase, glutathione-S-transferase (μ1 and μ5), aldehyde dehydrogenase 1A1 and carbonyl reductase have been demonstrated in WT compared to N0 mice. Also, the basal expression of glutathione peroxidase, glutathione-S-transferase α4 and ferritin light chain subunit 1 has been shown to be higher in WT mice, compared to N0 mice[14,15]. Although not as extensive as the network of genes under inducible control by Nrf2, the differential basal expression of some Nrf2-regulated genes may partially explain underlying susceptibilities to toxicities from environmental insults due to an impaired initial capacity to metabolize reactive molecules.
Nrf2-dependent attenuation of electrophile toxicity
The importance of Nrf2 in protection against reactive electrophiles was first demonstrated using acetaminophen. N0 mice displayed greater hepatotoxicity, manifested in increased serum ALT values and altered hepatic histology, following acetaminophen exposure, relative to WT mice[17,18]. The hepatoprotective effect of Nrf2 was shown to be due to both increased expression of UDP-glucuronosyltransferase 1A6 presumably leading to increased glucuronidation and excretion of acetaminophen, and the activation of an Nrf2-dependent adaptive response[17].
Acetaminophen was also shown to activate the nuclear translocation of Nrf2 at non-toxic doses thereby illustrating the role of Nrf2 in coordinating an adaptive response leading to attenuated acetaminophen toxicity[19]. This adaptive response resulted in increased de novo synthesis of GSH and conjugation and excretion of reactive acetaminophen metabolites[17,19]. This observation was further confirmed by the use of hepatocyte-specific conditional Keap1 knockout mice, a model in which the inhibitory component of the Nrf2 signaling pathway is absent resulting in elevated and sustained nuclear accumulation of Nrf2. These conditional knockout mice were considerably more resistant to acetaminophen toxicity than WT mice due to higher levels of Nrf2-regulated cytoprotective enzymes[20].
The role of Nrf2 in protection against electrophilic stress has also been demonstrated using in vitro models of exposure to electrophiles. Zhu, et al demonstrated that bone marrow stromal cells from N0 mice displayed increased cytotoxicity, relative to cells from WT mice, following exposure to the electrophiles 4-hydroxynonenal, a reactive aldehyde formed during lipid peroxidation, and 1,4-benzoquinone, a reactive molecule formed during the metabolism of benzene[21]. Additionally, mouse embryonic fibroblast (MEF) cells from N0 mice exhibited increased cell death, compared to WT MEF cells, following exposure to menadione, an electrophilic substrate for NQO1[22]. Therefore, the Nrf2-mediated cytoprotective adaptive response represents a powerful mechanism for organisms and cells to cope with electrophile stress.
Typically, genotoxic carcinogens are either inherently DNA reactive or bioactivated to electrophilic, DNA-reactive species that can initiate the carcinogenic process by reacting with DNA to form adducts, possibly leading to DNA mutations. Conjugating and electrophile reduction enzymes regulated by Nrf2 can effectively metabolize reactive electrophiles into less reactive or more readily excreted species. Animal studies have demonstrated the impact of Nrf2-regulated genes in protecting against chemical carcinogenesis. Ramos-Gomez, et al[23] demonstrated that loss of Nrf2 signaling in N0 mice resulted in increased sensitivity to benzo[a]pyrene (BaP)-induced forestomach tumorigenesis. This protective effect of Nrf2 appeared to be due to up-regulation of cytoprotective enzymes as increased enzyme activity of glutathione-S-transferases and NQO1 were detected in the forestomachs of WT mice relative to N0 mice. A follow-up study revealed that N0 mice exposed to BaP displayed increased levels of BaP-DNA adducts in the forestomach mucosa, relative to WT mice, and that the levels of BaP adducts were positively correlated with tumor burden[24].
Also, a study by Iida, et al[25] demonstrated that Nrf2-deficiency resulted in increased tumor incidence in a mouse model of N-nitrosobutyl(4-hydroxybutyl)amine (BBN)-induced bladder carcinogenesis by modulating the levels of carcinogenic metabolites. In this experiment, the incidence of bladder tumors was greater in N0 mice, relative to WT mice. BBN detoxication is primarily accomplished by O-glucuronidation by UDP-glucuronosyltransferase (UGT) enzymes. However, if BBN is not conjugated, the compound can undergo oxidation catalyzed by aldehyde/alcohol dehydrogenase to form the proximate carcinogen, N-nitrosobutyl(3-carboxypropyl)amine (BCPN). Interestingly, the researchers demonstrated that N0 mice exhibited decreased expression of UGTs and a decreased potential for O-glucuronidation of BBN, which was manifested in increased urinary concentrations of BCPN. Therefore, it is apparent that the Nrf2-mediated cytoprotective adaptive response is effective in inhibiting chemical carcinogenesis through up-regulation of the transcription of cytoprotective genes and represents a potential molecular target for chemoprevention of chemical carcinogenesis.
Importance of Nrf2 signaling in protection against oxidative toxicity
Increased oxidative stress has been implicated in the etiology of a number of acute and chronic diseases linked to exposures to environmental toxicants. Reactive species can react with lipids, protein and DNA. In vitro studies have illustrated the importance of the Nrf2-regulated signaling pathway in protection against oxidative stress-mediated cytotoxicity following exposure to oxidants. For example, mouse embryonic fibroblasts (MEF) from N0 mice had increased sensitivity to superoxide anion generated from diquat, relative to MEF cells from WT mice[26]. Interestingly, MEF cells from WT and N0 mice had similar basal expression of antioxidative enzymes as well as similar levels of oxidative damage biomarkers. However, increased levels of ROS and oxidative damage biomarkers were detected over time in MEF cells from N0 but not WT cells following exposure to diquat. The resistance of MEF cells from WT mice to diquat-induced oxidative damage was due to a functional adaptive response resulting in increased expression of Nrf2-regulated antioxidative enzymes following exposure to diquat.
Gong and Cederbaum[27] demonstrated that knockdown of Nrf2 in a variant line of HepG2 cells, a human liver cell line, exposed to increased ROS due to overexpression of CYP2E1, resulted in decreased cell viability. The researchers compared the effects of siRNA directed against Nrf2 in a normal HepG2 cell line not overexpressing CYP2E1 to the CYP2E1 overexpressing cell line. They found that knockdown of Nrf2 in the normal HEPG2 cells resulted in minimally decreased expression of antioxidative enzymes and no changes in biomarkers of oxidative damage. Meanwhile, knockdown of Nrf2 in the CYP2E1-overexpressing HepG2 cells resulted in markedly decreased expression of antioxidative enzymes resulting in increased levels of oxidative damage biomarkers. Therefore, under non-stress situations the role of Nrf2 in combating basal levels of ROS is minimal. However, during oxidative stress an effective Nrf2 cytoprotective adaptive response is vital.
Cho, et al[28]demonstrated the in vivo importance of Nrf2 in protection against oxidative stress by demonstrating that N0 mice displayed markedly increased pneumotoxicity following exposure to hyperoxic concentrations of oxygen. While the expression of several antioxidative enzymes, such as glutathione peroxidase 2 and heme oxygenase-1, were similar in normal air-exposed WT and N0 mice, exposure to hyperoxic oxygen concentrations resulted in increased expression of these enzymes in the lungs of WT but not N0 mice leading to protection against hyperoxia-induced lung oxidative stress.
A number of researchers have demonstrated the ability of Nrf2 to control cytoprotective adaptive responses in the brain using models of oxidative neurodegeneration. Intrastriatal stereo-taxic injection of malonate or administration of 3-nitroproprionic acid (3-NP), both mitochondrial complex II inhibitors, resulted in increased neurotoxicity and lower motor function performance in N0 compared to WT mice[29,30]. Additionally, the neuroprotective function of Nrf2 in protection against oxidative stress-mediated neurodegeneration was demonstrated using the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. N0 mice displayed an increased Parkinsonian phenotype represented by increased tyrosine hydroxylase-positive cell death in the substantia nigra pars compacta relative to WT mice[31]. Taken together, these studies demonstrated the in vitro and in vivo importance of the Nrf2 signaling pathway in protection against oxidative stress.
Increased oxidative stress has been implicated in the carcinogenic process following exposure to a number of carcinogens, such as metals, asbestos, benzo[a]pyrene, ionizing radiation, and tobacco smoke[32,33]. Therefore, Nrf2-regulated antioxidative enzymes would be expected to contribute to the anti-carcinogenic properties of the Nrf2 signaling pathway by reducing levels of ROS-derived DNA adducts and redox sensitive signaling of tumor promotion.
Exposure to diesel exhaust particles has been postulated to be a probable risk factor for the development of lung cancer partially attributed to an increase in oxidative DNA damage and adduct formation[34]. Aoki, et al[34] sub-chronically exposed heterozygous WT and N0 mice to diesel exhaust particles and demonstrated an increase in the levels of 8-oxo-dG in bronchial epithelial cells from N0 mice only. This DNA damage was accompanied by an increase in the thickness of the bronchial epithelium in N0 mice representing greater epithelial hyperplasia in the lungs of these mice.
Additionally, increased oxidative stress has been shown to have a non-genotoxic role in tumor promotion by activating or inhibiting redox-sensitive signaling pathways resulting in inappropriate cell proliferation and survival[32,33]. A study by auf dem Keller, et al[35] demonstrated the role of the Nrf2 signaling pathway in protection against 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-promoted skin tumorigenesis The researchers showed that mice expressing biallelic dominant-negative Nrf2 (Nrf2-silenced mice) experienced increased skin oxidative damage, relative to WT mice, during TPA promotion due to decreased expression of Nrf2-regulated antioxidative enzymes. This resulted in a more powerful promoting stimulus in Nrf2-silenced mice reflected by increased multiplicity of skin tumors in these mice. The results of these studies illustrate the potential of an Nrf2-regulated antioxidative adaptive response in protecting against oxidative stress-associated carcinogenesis by targeting both initiating and promoting events in the carcinogenic process.
Anti-inflammatory role of Nrf2 signaling
Numerous in vivo studies have illustrated that Nrf2 plays an important role in modulating inflammation in a variety of experimental models. N0 mice have been shown to exhibit increased susceptibility to tobacco smoke-[36] and elastase-[37] mediated emphysema, allergen-driven airway inflammation[38], carrageenan-induced pleurisy[39] and dextran sulfate sodium (DSS)-mediated colitis[40], compared to WT mice. For example, enlarged alveoli and increased lung compliance was detected in the lungs of N0 mice, indicative of the development of emphysema, following exposure to tobacco smoke while the lungs of WT mice were indistinguishable from normal air controls[36]. These effects were associated with a prolonged infiltration of neutrophils in the lungs of N0 mice compared to WT mice.
Increased expression of antioxidative enzymes and modulation of pro-inflammatory cytokine signaling, in an Nrf2-dependent manner, has been demonstrated in these acute inflammation animal models possibly explaining the decreased sensitivity to inflammatory oxidative damage seen in WT animals. Inflammation-mediated oxidative stress represents a potential stimulus for activation of the Nrf2-regulated cytoprotective response. For example, increased levels of oxidized GSH were detected in the lungs of N0 mice, relative to WT mice, following administration of lipopolysaccharide (LPS), a model of pulmonary sepsis, illustrating the effect of Nrf2-regulated antioxidative enzymes on inflammation-mediated oxidative stress[41]. Additionally, enhanced expression of Nrf2-regulated antioxidative enzymes and decreased expression of pro-inflammatory mediators, such as Cox-2, interleukin-1β, interleukin-6 and TNFα, was detected in colons from WT but not N0 mice exposed to DSS, a model of colitis[40]. Also, the Nrf2 signaling pathway has been shown to modulate NF-κB activation. Increased activation of NF-κB has been demonstrated in N0 mice, relative to WT mice, following LPS administration[41]. Taken together, these studies show that the Nrf2 signaling pathway can effectively attenuate pro-inflammatory stimuli leading to decreased inflammation and inflammatory damage.
It has been estimated that approximately 20% of all human cancers are due to chronic inflammation[42]. The Nrf2 adaptive response has already been shown to be protective against acute inflammation. Therefore this adaptive response would be expected to provide protection against cancers associated with chronic inflammation due to up-regulation of antioxidative enzymes leading to decreased inflammatory signaling and oxidative damage, all important in the progression of inflammation-associated carcinogenesis. It was recently demonstrated that an active Nrf2 signaling pathway inhibited the development of aberrant crypt foci in a model of colitis-associated colorectal cancer. Increased levels of aberrant crypt foci were detected in N0 mice following exposure to both azoxymethane (AOM) and dextran sulfate sodium (DSS) compared to N0 mice exposed to AOM only while no effect of DSS was demonstrated in WT mice[43]. Severe mucosal damage, increased infiltration of inflammatory cells, up-regulated pro-inflammatory cytokine signaling as well as increased oxidative damage was detected in colons from N0 but not WT mice during the DSS-mediated inflammatory component of the model. These results demonstrated that the Nrf2 signaling pathway is a protective factor against inflammation-associated tumorigenesis and illustrates a potential strategy for chemoprevention of inflammation-associated carcinogenesis.
Targeting the Nrf2 signaling pathway for chemoprevention
The activation of the Nrf2 signaling by administration of Nrf2-inducing small molecules has been shown to be chemoprotective in a number of animal models of carcinogenesis and the potential for this strategy in humans is beginning to be demonstrated. The cancer chemopreventive activity of oltipraz, a drug originally developed for the treatment of schistosomiasis, was first demonstrated by Wattenburg and Bueding. Oltipraz administration resulted in decreased tumor formation in a mouse model of B[a]P-induced forestomach and pulmonary carcinogenesis[44]. This was followed by demonstration of the chemopreventive activity of oltipraz and other dithiolethiones against aflatoxin-mediated hepatocarcinogenesis in rats[45]. These results led to human trials examining the effect of oltipraz on aflatoxin metabolism. A study conducted in Qidong, People’s Republic of China, an area of endemic high aflatoxin exposure, demonstrated that low-dose administration of oltipraz effectively modulated the metabolism of aflatoxin by increasing the rate of excretion of aflatoxin-GSH conjugation products[46]. Thus, the chemopreventive potential of oltipraz appears to be related to the effect of up-regulation of cytoprotective enzymes on carcinogen metabolism in humans.
The consumption of cruciferous vegetables has consistently been associated with a decreased risk of cancer development[47]. Sulforaphane, an isothiocyanate compound formed following myrosinase-catalyzed metabolism of glucosinolates present in high concentrations in broccoli sprouts and other crucifers, was found to be a potent inducer of the Nrf2-regulated cytoprotective adaptive response[48]. Studies have demonstrated the chemopreventive activity of sulforaphane in a number of rodent cancer models including 7,12-dimethylbenzanthracene (DMBA) -mediated mammary tumorigenesis[49], B[a]P-induced forestomach carcinogenesis[50] and DMBA/TPA-induced skin carcinogenesis[51]. These findings prompted the initiation of human clinical trials investigating the effect of a glucosinolate-rich broccoli sprout hot water extract on the genotoxicity of aflatoxin. Administration of this extract to study participants in Qidong, People’s Republic of China was not shown, as a whole, to modulate urinary excretion of aflatoxin-DNA adducts[52]. However, large inter-individual differences in the metabolism of glucosinolates to sulforaphane were demonstrated across the study population. Therefore, some individuals were being exposed to lower levels of sulforaphane due to decreased metabolism of glucosinolates to sulforaphane or other factors affecting bioavailability or disposition. When these differences were taken into account, a significant inverse relationship between sulforaphane elimination and urinary aflatoxin-DNA adduct excretion was seen in individual participants, thereby illustrating the capacity of sulforaphane to modulate aflatoxin metabolism, possibly affecting the carcinogenic potential of aflatoxin exposure.
Nrf2 signaling pathway polymorphisms
Recently, Marzec, et al[53] identified a number of single nucleotide polymorphisms (SNP) in the promoter region of Nrf2 present in human subjects across multiple ethnic groups. Functional analysis of these polymorphisms showed that one of the SNPs resulted in decreased in vitro binding of Nrf2 to an ARE promoter following exposure to Nrf2-inducing stresses[53]. Importantly, individuals with this SNP were found to be more likely to develop acute lung injury, relative to individuals with a normal Nrf2 sequence, following major trauma[53]. This was the first study to demonstrate the effect of abnormal Nrf2 activity on the risk of developing acute disease in humans and additional experiments have documented SNPs in AREs present in the promoter of a number of Nrf2-regulated cytoprotective genes[54], so Nrf2 polymorphisms may potentially adversely affect the progression of a host of other diseases.
Conversely, it is becoming apparent that constitutive activation of Nrf2 may also have negative effects as it may provide a protective mechanism that can be highjacked by cancer cells to evade chemotherapy. Variant Keap1 gene sequences have been found in human lung tumor tissue that results in loss of Keap1 repressor activity and constitutive Nrf2 transactivation. Mutations in the Nrf2 binding region of Keap1 were identified in lung tumor tissue removed from lung cancer patients[3,55]. These mutations did not alter the protein levels of Keap1, but instead resulted in loss of the ability of Keap1 to repress Nrf2 activity leading to an accumulation of Nrf2 in the nucleus and constitutive up-regulation of Nrf2-regulated cytoprotective genes[3,55]. Singh, et al also identified lung tumor cell lines containing similar mutations and they demonstrated that these cell lines had increased resistance to chemotherapeutic drugs than normal lung cells[55]. Therefore, it appears that the protection afforded by Nrf2-Keap1 system is a double-edged sword[56]. On one hand, the Nrf2-mediated cytoprotective effect protects against the progression of acute and chronic diseases, but on the other hand, constitutive Nrf2 activation may provide a growth advantage to cancer cells in that it can lead to chemoresistance. This necessitates careful consideration as to the context in which strategies capitalizing on activation of the Nrf2 signaling pathway are to be utilized. It is worth pointing out however that the inducers of clinical utility in chemoprevention do not induce expression of Nrf2-regulated genes to the levels achieved by knocking out Keap1 in cells or mice, and exert no additional inductive response above the genotype-induced response when administered to Keap1 knockout mice. Thus, the cytoprotective actions of inducers may be lost in cancer cells harboring Keap1 mutations, but appears unlikely to provoke additional harm.
Conclusions
Up-regulation of Nrf2 cytoprotective enzymes possesses great potential to effectively attenuate toxicity following exposure to environmental toxicants. The protein products of Nrf2-regulated cytoprotective genes can target multiple steps along the pathway of toxicity following exposure to electrophilic and oxidative xenobiotics, as illustrated in Figure 2. This adaptive response is energetically favorable in that enhanced transcription of cytoprotective genes only occurs through the cellular sensing ability of the Keap1-Nrf2 complex when the organism needs a greater capacity for electrophile or ROS metabolism. However, issues remain in regards to translating these targets to improved public health. The main issue revolves around the ability to deliver effective, non-toxic doses of Nrf2 inducing agents to the general population. Part of this challenge was illustrated in the clinical trial in Qidong, People’s Republic of China with broccoli sprouts. While no concerns with safety arose, large inter-individual differences in the ability of study participants to convert the glucosinolates to suforaphane were found to exist. Perhaps the development of more potent Nrf2-inducing agents will result in more effective chemoprevention strategies. Indeed, an Nrf2-inducing synthetic triterpenoid, CDDO-Im, has recently been identified with approximately 100-fold greater potency than dithiolethiones in chemoprevention of aflatoxin-mediated hepatocarcinogenesis in rats[57]. Clinical development of triterpenoids for disease prevention and treatment is just beginning. However, due to the strong dependency of Nrf2 in protection against both electrophilic and oxidative toxicities following environmental exposures, the development of strategies targeting the Nrf2 signaling pathway for attenuating environmental disease, in addition to cancer, is highly warranted.
Figure 2.
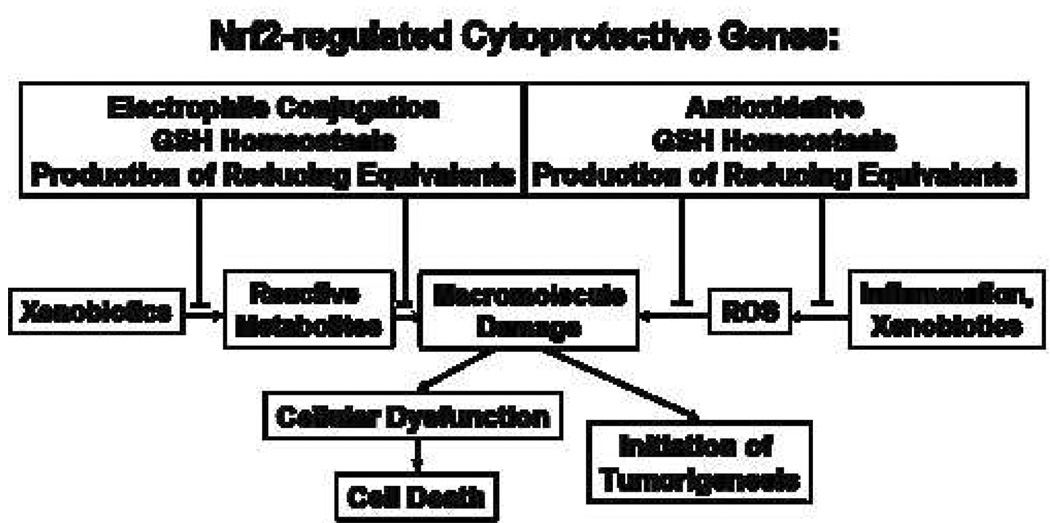
Effect of Nrf2-regulated cytoprotective enzymes on the progression of cellular injury.
Acknowledgements
Our work on cancer chemoprevention is supported by NIH grants RO1CA39416, RO1CA94076, PO1ES06052 and P50CA88843. William O. Osburn is supported by NIEHS training grant ES07141.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 2.Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Tong K, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs:characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2890. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullinan S, Gordan J, Jin J, Harper J, Diehl J. The keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of keap1 in cellular protective responses. Chem. Res. Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 6.Wakabayashi N, Dinkova-Kostova A, Holtzclaw W, Kang M, Kobayashi A, Yamamoto M, Kensler T, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Nguyen T, Pickett C. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 8.Motohashi H, Katsuoka F, Engel JD, Yamamoto M. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci U S A. 2004;101:6379–6384. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, Keum Y, Han J, Gallo M, Kong A. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004;279:23052–23060. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda H, Omoteyama K, Yoshida K, Nishi S, Sakai M. CCAAT enhancer-binding protein alpha suppresses the rat placental glutathione S-transferase gene in normal liver. J Biol Chem. 2006;281:6734–6741. doi: 10.1074/jbc.M513014200. [DOI] [PubMed] [Google Scholar]
- 11.Faraonio R, Vergara P, Marzo DD, Pierantoni M, Napolitano M, Russo T, Cimino F. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem. 2006;281:39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- 12.Leonard M, Kieran N, Howell K, Burne M, Varadarajan R, Dhakshinamoorthy S, Porter A, O'Farrelly C, Rabb H, Taylor C. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- 13.Hu R, Xu C, Shen G, Jain M, Khor T, Gopalkrishnan A, Lin W, Reddy B, Chan J, Kong A. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 14.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 15.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 16.Li M, Jang J, Na H, Cha Y, Surh Y. Carbon monoxide produced by heme oxygenase-1 in response to nitrosative stress induces expression of glutamate-cysteine ligase in PC12 cells via activation of phosphatidylinositol 3-kinase and Nrf2 signaling. J Biol Chem. 2007;282:28577–28586. doi: 10.1074/jbc.M701916200. [DOI] [PubMed] [Google Scholar]
- 17.Chan K, Han X, Kan Y. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 19.Goldring C, Kitteringham N, Elsby R, Randle L, Clement Y, Williams D, McMahon M, Hayes J, Itoh K, Yamamoto M, Park B. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 2004;39:1267–1276. doi: 10.1002/hep.20183. [DOI] [PubMed] [Google Scholar]
- 20.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler T, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Zhang L, Itoh K, Yamamoto M, Ross D, Trush M, Zweier J, Li Y. Nrf2 controls bone marrow stromal cell susceptibility to oxidative and electrophilic stress. Free Radic Biol Med. 2006;41:132–143. doi: 10.1016/j.freeradbiomed.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Kwak M, Ramos-Gomez M, Wakabayashi N, Kensler T. Chemoprevention by 1,2-dithiole-3-thiones through induction of NQO1 and other phase 2 enzymes. Methods Enzymol. 2004;382:414–423. doi: 10.1016/S0076-6879(04)82022-6. [DOI] [PubMed] [Google Scholar]
- 23.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos-Gomez M, Dolan P, Itoh K, Yamamoto M, Kensler T. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24:461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 25.Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 26.Osburn W, Wakabayashi N, Misra V, Nilles T, Biswal S, Trush M, Kensler T. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454:7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong P, Cederbaum A. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43:144–153. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- 28.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 29.Calkins M, Jakel R, Johnson D, Chan K, Kan Y, Johnson J. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih A, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy T. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22935–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- 31.Burton N, Kensler T, Guilarte T. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Kensler T, Egner P, Taffe B, Trush M. Role of free radicals in tumor promotion and progression. Prog Clin Biol Res. 1989;298:233–248. [PubMed] [Google Scholar]
- 33.Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 35.Keller Uad, Huber M, Beyer T, Kumin A, Siemes C, Braun S, Bugnon P, V VM, Johnson D, Johnson J, Hohl D, Werner S. Nrf transcription factors in keratinocytes are essential for skin tumor prevention but not for wound healing. Mol Cell Biol. 2002;26:3773–3784. doi: 10.1128/MCB.26.10.3773-3784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii Y, Itoh K, Morishima Y, Kimura T, Kiwamoto T, Iizuka T, Hegab A, Hosoya T, Nomura A, Sakamoto T, Yamamoto M, Sekizawa K. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol. 2005;175:6968–6975. doi: 10.4049/jimmunol.175.10.6968. [DOI] [PubMed] [Google Scholar]
- 38.Rangasamy T, Guo J, Mitzner W, Roman J, Singh A, Fryer A, Yamamoto M, Kensler T, Tuder R, Georas S, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2) Mol Cell Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khor T, Huang M, Kwon K, Chan J, Reddy B, Kong A. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium–induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 41.Thimmulappa R, Lee H, Rangasamy T, Reddy S, Yamamoto M, Kensler T, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marzo AD, Platz E, Sutcliffe S, Xu J, Gronberg H, Drake C, Nakai Y, Isaacs W, Nelson W. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osburn W, Karim B, Dolan P, Li Q, Yamamoto M, Huso D, Kensler T. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer. 2007;121:1883–1891. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 44.Wattenberg L, Bueding E. Inhibitory effects of 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (Oltipraz) on carcinogenesis induced by benzo[a]pyrene, diethylnitrosamine and uracil mustard. Carcinogenesis. 1986;7:1379. doi: 10.1093/carcin/7.8.1379. [DOI] [PubMed] [Google Scholar]
- 45.Kensler T, Egner P, Dolan P, Groopman J, Roebuck B. Mechanism of protection against aflatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer Res. 1987;47:4271–4277. [PubMed] [Google Scholar]
- 46.Wang J, Shen X, He X, Zhu Y, Zhang B, Wang J, Qian G, Kuang S, Zarba A, Egner P, Jacobson L, Munoz A, Helzlsouer K, Groopman J, Kensler T. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 47.Brigelius-Flohe R, Banning A. Part of the series: from dietary antioxidants to regulators in cellular signaling and gene regulation. Sulforaphane and selenium, partners in adaptive response and prevention of cancer. Free Radic Res. 2006;40:775–787. doi: 10.1080/10715760600722643. [DOI] [PubMed] [Google Scholar]
- 48.Fahey J, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Kensler T, Cho C, Posner G, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fahey J, Haristoy X, Dolan P, Kensler T, Scholtus I, Stephenson K, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu C, Huang M, Shen G, Yuan X, Lin W, Khor T, Conney A, Kong AT. Inhibition of 7,12-Dimethylbenz(a)anthracene-Induced Skin Tumorigenesis in C57BL/6 Mice by Sulforaphane Is Mediated by Nuclear Factor E2-Related Factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 52.Kensler T, Chen J, Egner P, Fahey J, Jacobson L, Stephenson K, Ye L, Coady J, Wang J, Wu Y, Sun Y, Zhang Q, Zhang B, Zhu Y, Qian G, Carmella S, Hecht S, Benning L, Gange S, Groopman J, Talalay P. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 53.Marzec J, Christie J, Reddy S, Jedlicka A, Vuong H, Lanken P, Aplenc R, Yamamoto T, Yamamoto M, Cho H, Kleeberger S. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Tomso D, Chorley B, Cho H, Cheung V, Kleeberger S, Bell D. Identification of polymorphic antioxidant response elements (AREs) in the human genome. Hum Mol Genet. 2007 doi: 10.1093/hmg/ddm066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh A, Misra V, Thimmulappa R, Lee H, Ames S, Hoque M, Herman J, Baylin S, Sidransky D, Gabrielson E, Brock M, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayes J, McMahon M. The double-edged sword of Nrf2: subversion of redox homeostasis during the evolution of cancer. Mol Cell. 2006;21:732–734. doi: 10.1016/j.molcel.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Yates M, Kwak M, Egner P, Groopman J, Bodreddigari S, Sutter T, Baumgartner K, Roebuck B, Liby K, Yore M, Honda T, Gribble G, Sporn M, Kensler T. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 58.Umemura T, Kuroiwa Y, Kitamura Y, Ishii Y, Kanki K, Kodama Y, Itoh K, Yamamoto M, Nishikawa A, Hirose M. A crucial role of Nrf2 in in vivo defense against oxidative damage by an environmental pollutant, pentachlorophenol. Toxicol Sci. 2006;92:111–119. doi: 10.1093/toxsci/kfj076. [DOI] [PubMed] [Google Scholar]
- 59.Chan K, Kan Y. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho H, Reddy S, Yamamoto M, Kleeberger S. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 61.Kraft A, Lee J, Johnson D, Kan Y, Johnson J. Neuronal sensitivity to kainic acid is dependent on the Nrf2-mediated actions of the antioxidant response element. J Neurochem. 2006;98:1852–1865. doi: 10.1111/j.1471-4159.2006.04019.x. [DOI] [PubMed] [Google Scholar]
- 62.Kitamura Y, Umemura T, Kanki K, Kodama Y, Kitamoto S, Saito K, Itoh K, Yamamoto M, Masegi T, Nishikawa A, Hirose M. Increased susceptibility to hepatocarcinogenicity of Nrf2-deficient mice exposed to 2-amino-3-methylimidazo[4,5-f]quinoline. Cancer Sci. 2007;98:19–24. doi: 10.1111/j.1349-7006.2006.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


