Abstract
Interleukin-6 (IL-6) is a multifunctional cytokine that has been shown to play a pivotal role in centrally-mediated physiological responses including activation of the hypothalamic-pituitary-adrenal axis. Cerebral spinal fluid (CSF) concentrations of IL-6 are elevated in multiple pathophysiological conditions including Alzheimer’s disease, autoimmune disease, and meningitis. Despite this, the effect of IL-6 on central regulation of sympathetic nerve discharge (SND) remains unknown which limits understanding of sympathetic-immune interactions in health and disease. In the present study we determined the effect of intracerebroventricular (icv, lateral ventricle) administration of IL-6 on splenic SND in urethane-chloralose-anesthetized rats. A second goal was to determine if icv injected IL-6 enters the brain parenchyma and acts as a volume transmission signal to access areas of the brain involved in regulation of sympathetic nerve outflow. Icv administration of IL-6 (10 ng, 100 ng, and 400 ng) significantly and progressively increased splenic SND from control levels in baroreceptor denervated Sprague-Dawley rats. Administration of 100 ng and 400 ng IL-6 resulted in significantly higher SND responses when compared to those elicited with a 10 ng dose. Sixty minutes following icv administration, fluorescently labeled IL-6 was not distributed throughout the parenchyma of the brain but was localized to the periventricular areas of the ventricular system. Brain sections counter-stained for the IL-6 receptor (IL-6R) revealed that IL-6 and the IL-6R were co-localized in periventricular areas adjoining the third ventricle. These results demonstrate that icv IL-6 administration increases splenic SND, an effect likely achieved via signaling mechanisms originating in the periventricular cells.
INTRODUCTION
The existence of interactions between the immune system and the nervous system is now well established (Downing and Miyan, 2000; Kohm and Sanders, 2001; Licinio and Wong, 1997; Madden and Felten, 1995; Steinman, 2004). Although the sympathetic nervous system is thought to play a role in mediating bidirectional neuroimmune interactions (Elenkov et al., 2000; Kohm and Sanders, 2001; Sanders and Kohm, 2002), most information has been derived from studies focused on one arm of this interaction: sympathetic innervation of immune cells in various lymphoid organs. The release of immune cell products (i.e. cytokines) is a common physiological response to stress and disease, however, little is known regarding the influence of immunological factors on central neural circuits responsible for regulating sympathetic nerve discharge (SND).
Interleukin-6 (IL-6) is a multifunctional cytokine that belongs to a group of structurally related IL-6-type cytokines that includes IL-11, leukemia inhibitory factor, oncostatin M, ciliary neurotrophic factor, cardiotrophin-1, and cardiotrophin-like cytokine (Heinrich et al., 2003). Cerebral spinal fluid (CSF) concentrations of IL-6 are elevated in various diseases (e.g., Alzheimer’s disease, autoimmune disease, meningitis) (Blum-Degen et al., 1995; Frei et al., 1989; Hirohata and Miyamoto, 1990; Houssiau et al., 1988; Waage et al., 1989) and IL-6 plays a role in numerous centrally mediated physiological responses, including; hypothalamic expression of corticotrophin releasing factor, activation of the hypothalamic-pituitary-adrenal axis, fever, and memory formation (Balschun et al., 2004; LeMay et al., 1990; Lenczowski et al., 1999; Rothwell et al., 1991; Vallieres et al., 1997; Vallieres and Rivest, 1999; Zhou et al., 1996). However, the effect of CSF IL-6 administration on central regulation of SND remains unknown. This is a significant omission because elaboration of mechanisms by which the immune system alters SND is critical for understanding the role of sympathetic-immune interactions in physiological regulation and disease processes.
In the present study we determined the effect of intracerebroventricular (icv, lateral ventricle) administration of IL-6 on splenic SND in urethane-chloralose-anesthetized rats. Because the sympathetic innervation of the spleen provides a functional link between central sympathetic neural circuits and splenic immune cells (Nance and Burns, 1989; Wan et al., 1993), we hypothesized that central IL-6 administration would activate splenic SND. As the results reveal, icv administration of IL-6 dose-dependently increased splenic SND. Based on this finding, a second goal of this study was to determine if icv injected IL-6 enters the brain parenchyma and acts as a volume transmission signal to access areas of the brain involved in regulation of sympathetic nerve outflow.
METHODS
General Procedures
The Institutional Animal Care and Use Committee approved the experimental procedures and protocols used in the present study and all procedures were performed in accordance with the American Physiological Society’s guiding principles for research involving animals (APS, 2002). Male Sprague-Dawley rats (300–350 grams) were obtained from Harlan Sprague Dawley Inc (Indianapolis, IN). All rats were housed in 6 × 9 inch cages, received rat chow and water ad libitum, and were maintained in a 24°C room on a 12:12-h light-dark cycle. Anesthesia was induced by isoflurane (3%) and maintained during surgical procedures using isoflurane (1.5%–2.5%), α-chloralose (80 mg/kg, ip), and urethane (800 mg/kg, ip). A catheter was placed in the femoral vein for the intravenous administration of maintenance doses of α-chloralose (35–45 mg/kg/hr). Maintenance doses of urethane (200 mg/kg every 4 hours) were administered intraperitoneally. The trachea was cannulated with a polyethylene-240 catheter. Sinoaortic denervation was completed using previously published procedures (Ganta et al., 2005). Briefly, bilateral denervation of the aortic arch was completed by cutting the superior laryngeal nerve near its junction with the vagus nerve and removing the superior cervical ganglion. Bilateral carotid sinus denervation was completed by removing the adventitia from the area of the carotid sinus bifurcation. Baroreceptor denervation was confirmed by examining the coherence relationship between SND and arterial pressure. Femoral arterial pressure was recorded and heart rate (HR) was derived from the pulsatile arterial pressure output of the blood pressure analyzer. Colonic temperature was maintained between 37.8°C and 38.0°C during surgical procedures.
A lateral ventricular cannula was surgically implanted after the rat was placed in a stereotaxic frame, the head leveled between lambda and bregma, and a small hole made in the skull (1.2–1.4 mm lateral to the midline and 0.8–1.0 mm posterior to bregma). A stainless steel guide cannula was lowered 4 mm below the surface of the skull, and an injector was introduced through the guide cannula to protrude 0.5 mm beyond the tip of the guide cannula (Ganta et al., 2005; Kenney and Bealer, 1993).
Sympathetic Nerve Recordings
Splenic SND were recorded as previously described (Ganta et al., 2005; Saindon et al., 2001). Briefly, nerve activity was recorded biphasically (bandpass 30–3000 Hz) with a platinum bipolar electrode. The splenic sympathetic nerve was isolated using a lateral approach and SND was full wave rectified and integrated (time constant 10 ms). For monitoring during the experiment and for subsequent data analysis, the filtered neurogram was routed to an oscilloscope and a nerve traffic analyzer. Total power in splenic SND was quantified as volts × seconds (V•s) and SND recordings were corrected for background noise after administration of the ganglionic blocker, chlorisondamine (5 mg/kg, iv).
Experimental Protocols
Experiments were completed in baroreceptor-denervated rats to eliminate the influence of baroreceptor afferent feedback mechanisms which can alter SND responses of central origin. Following completion of surgical procedures rats were allowed to stabilize for 60 min before initiation of the experimental protocols. Lateral ventricle infusions were completed using an injector that was connected via polyethylene tubing to a 100 µl microsyringe driven by a micropump (1 µl/min, 10 min). In the first experimental series, rats were treated with 10 min infusions of IL-6 dissolved in artificial CSF (aCSF) (10, 100, or 400 ng in 10 µl, each rat received a single dose) or aCSF alone (10 µl). Splenic SND, mean arterial pressure (MAP) and HR were recorded continuously before, during, and for 60 min after cessation of icv infusions.
In a second experimental series, similar icv infusion techniques were used with the exception that IL-6 (100 ng) was tagged with a fluorescent tracer molecule (Alexa-Fluor 594, Molecular Probes). Icv administration of artificial aCSF and unlabeled IL-6 served as controls. Experiments were terminated at 30, 60, or 150 min after icv infusions of IL-6 or aCSF to determine a timeline of IL-6 migration patterns. Brain tissue was imaged for localization of IL-6 in specific brain regions (forebrain and brainstem) using confocal microscopy. Using immunohistochemistry, the co-localization of labeled IL-6 and IL-6 receptor (IL-6R) in the brain was also determined.
Labeling of IL-6 and Imaging of Brain Slices
Prior to administration, carrier-free IL-6 (recombinant rat IL-6, Invitrogen, endotoxin <0.1 ng/µg) was labeled on primary amines with Alexa Fluor 594 dye using the Alexa Fluor Microscale Protein Labeling Kit (Molecular Probes) and excess dye removed by following the instructions supplied with the kit. Following the completion of experimental protocols, rats were transcardially perfused, brains were removed, fixed overnight, and cryoprotected in sucrose. Tissue was cut into coronal sections (40 µm), mounted on glass slides, cover-slipped, and imaged using a Zeiss 510 Meta Confocal Microscope with the appropriate filter and mirror setup.
IL-6 Receptor Antibody Immunohistochemistry
Following detection of IL-6, brain slices were quenched with glycine, permeabilized with 0.2% Triton X, and blocked for one hour in blocking buffer (0.2% Triton X, 10% goat serum in phosphate buffered saline, PBS). Slides were then incubated with primary antibody against IL-6R (1:50 diluted in blocking buffer, Santa Cruz Biotechnology) overnight at 4°C with agitation. Slides were washed with PBS, and agitated in the dark with Alexa Fluor 488 tagged secondary (1:250 in blocking buffer, Molecular Probes-Invitrogen) antibody for one hour at room temperature followed by washing in PBS. Slides were then cover-slipped and imaged to determine localization of the IL-6R as well as co-localization of IL-6 and IL-6R. Slides incubated without IL-6R but in the presence of Alexa Fluor 488 secondary served as immunohistochemistry controls.
Brain Histology
Fluorescent latex microspheres (50 nm diameter) were injected (with the exception of experiments involving the infusion of tagged IL-6) into the lateral ventricle, rats received an overdose of methohexital sodium (150 mg/kg, iv), and were transcardially perfused with 0.15 M NaCl (containing 3 IU/ml heparin) followed by a fixative solution consisting of 10% buffered neutral formalin. Brains were removed, blocked, post-fixed in buffered neutral formalin, and placed in 20% sucrose for cryoprotection. Brains were frozen sectioned at 40 µm in the coronal plane, collected into PBS, and mounted on slides in serial sequence. The sections were rinsed in distilled water, air dried, and cleared in xylenes. Lateral ventricular injection sites were confirmed by observing fluorescent microspheres in the ventricular system using brightfield or epifluoresence microscopy.
Data and Statistical Analysis
Values are means ± SE. Splenic SND data are expressed as percentage change from baseline. SND, MAP and HR responses were analyzed using analysis of variance techniques with a repeated-measures design followed by Bonferroni post hoc tests. The overall level of statistical significance was p<0.05.
RESULTS
Splenic SND responses to icv IL-6 (unlabeled) and aCSF infusions
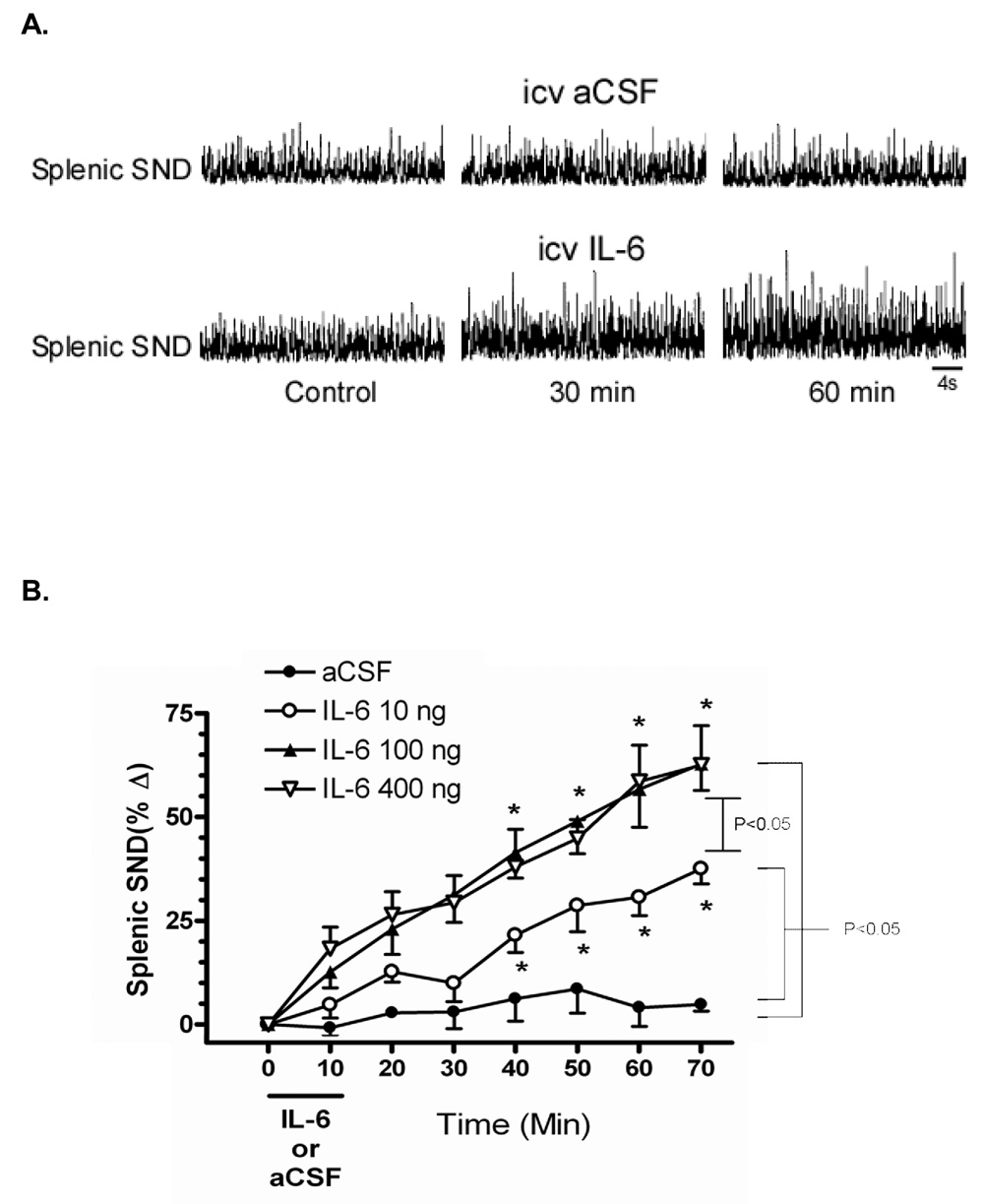
Figure 1A shows splenic SND traces recorded before (Control), and 30 and 60 min after icv infusions of either aCSF (top) or 100 ng IL-6 (bottom) in sinoaortic-denervated rats. Splenic SND remained unchanged from pretreatment levels after aCSF infusion but was increased from pretreatment levels at 30 min and 60 min after IL-6 infusion. Figure 1B summarizes changes from control (time 0) for splenic SND in response to 10-min icv infusions of IL-6 and aCSF. Splenic SND was progressively and significantly increased from control after icv infusions of IL-6 at doses of 10 ng, 100 ng, and 400 ng. In contrast, splenic SND remained unchanged from control after icv aCSF administration. Splenic SND responses were significantly higher in IL-6-treated (10, 100, and 400 ng) compared to aCSF-treated rats. Intracerebroventricular doses of 100 ng and 400 ng IL-6 resulted in significantly higher SND main effect responses when compared to the 10 ng dose of IL-6. Splenic SND main effect responses to icv infusion of 100 ng and 400 ng IL-6 did not differ. Icv administration of labeled (+62%, 60 min after IL-6 infusion) and unlabeled (+68%, 60 min after IL-6 infusion) IL-6 produced similar splenic sympathoexcitatory responses (data not shown).
Figure 1.
Panel A - Representative splenic sympathetic nerve discharge (SND) responses at control, 30, and 60 minutes after intracerebroventricular (icv) administration of artificial cerebrospinal fluid (aCSF) (top) and IL-6 (bottom). Panel B - Percent change in splenic sympathetic nerve discharge (SND) following icv injection of IL-6 and aCSF. Brackets indicate significant ANOVA main effects. The same bracket designates 100 ng and 400 ng doses of IL-6 versus aCSF or versus 10 ng of IL-6. *Change in splenic SND significantly different from time 0 at P < 0.05, the same asterisks represent both 100 ng and 400 ng doses.
MAP and HR responses to icv IL-6 (unlabeled) and aCSF infusions
MAP remained unchanged from control values for 60 min after icv infusions of aCSF, 10 ng IL-6, and 100 ng IL-6; however, it was significantly but modestly increased 60 min after icv infusion of 400 ng IL-6 (Control, 98±3 mmHg; 60 min after 400 ng IL-6 infusion, 112±5 mmHg). HR remained unchanged from control values for 60 min after icv infusions of aCSF, 10 ng IL-6, and 100 ng IL-6, but was significantly increased 60 min after icv infusion of 400 ng IL-6 (Control, 337±21 bpm; 60 min after 400 ng IL-6 infusion, 374±16 bpm).
Brain distribution of labeled IL-6 following icv administration
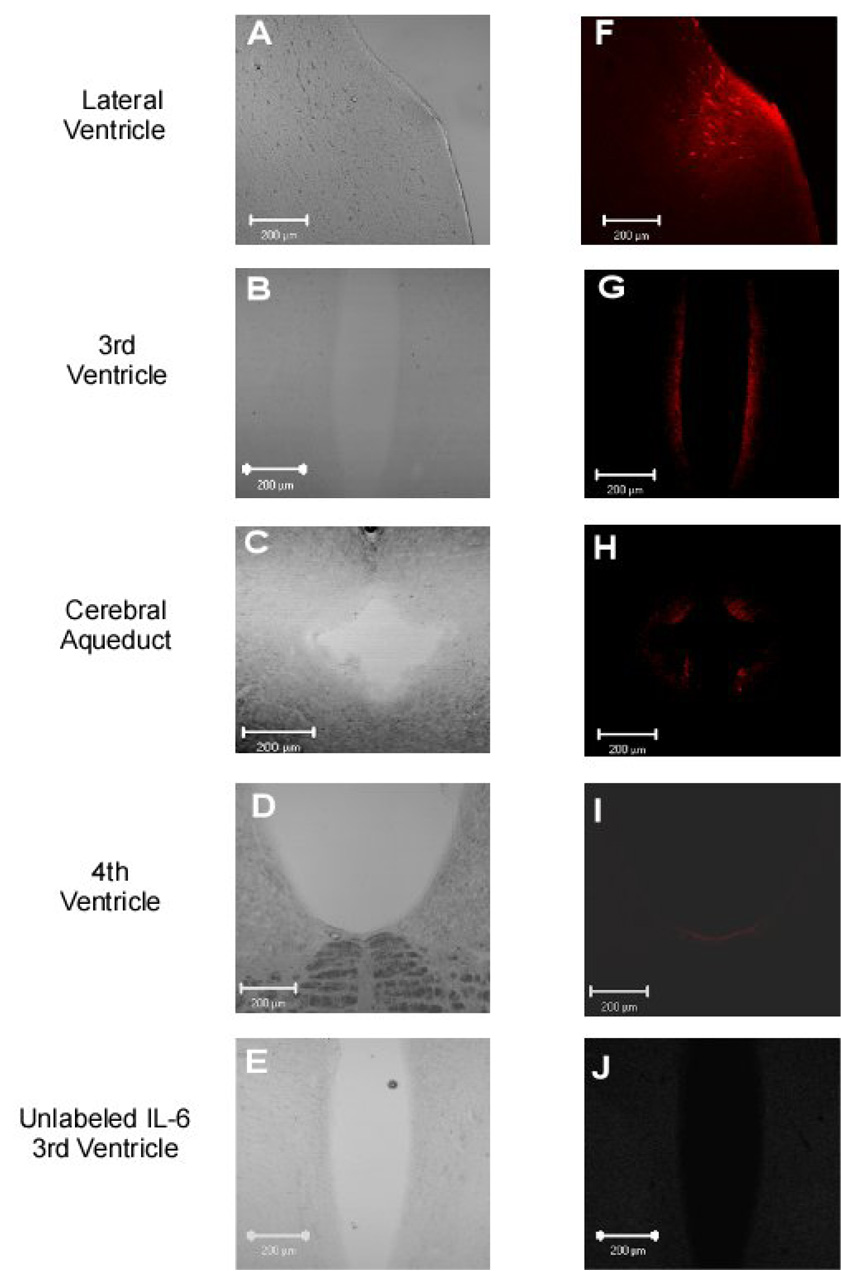
The left side of Figure 2 shows brightfield images of the lateral ventricle (A), third ventricle (B), cerebral aqueduct (C), and the fourth ventricle (D) and adjoining periventricular areas, whereas the right side shows the distribution of labeled IL-6 (100 ng) at the same sites 60 minutes after infusion of labeled IL-6 into the lateral ventricle. Although parenchyma and ventricle regions of brain slices from the lateral ventricle to the 4th ventricle were analyzed for IL-6, the most intense signal was located in the periventricular areas of the lateral (Figure 2F) and third ventricles (Figure 2G). Sixty minutes after icv infusion, labeled IL-6 demonstrated little or no migration beyond the periventricular areas into the parenchyma of the brain (Figure 2F–I). Unlabeled IL-6, administered into the lateral ventricle, was utilized as a negative control for localization studies. Confocal analysis revealed the absence of auto-fluorescence in parenchyma and ventricle regions of brain slices following icv administration of unlabeled IL-6 (Figures 2E and 2J show the 3rd ventricle area). In addition, there was no auto-fluorescence in parenchyma and ventricle regions of brain slices following icv administration of aCSF.
Figure 2.
IL-6 migration patterns through the ventricular system and associated periventricular tissue 60 minutes following icv injection of 100 ng of labeled IL-6. Images A-D are brightfield images at the level of the lateral ventricle, 3rd ventricle, cerebral aqueduct, and 4th ventricle (10x zoom), respectively. Images F-I show labeled IL-6 at the level of the lateral ventricle, 3rd ventricle, cerebral aqueduct, and 4th ventricle regions, respectively. Negative control images of unlabeled IL-6 are shown in Panels E (brightfield) and J (fluorescence) at the level of the 3rd ventricle. Scale bar is 200 µm.
Colocalization of IL-6R
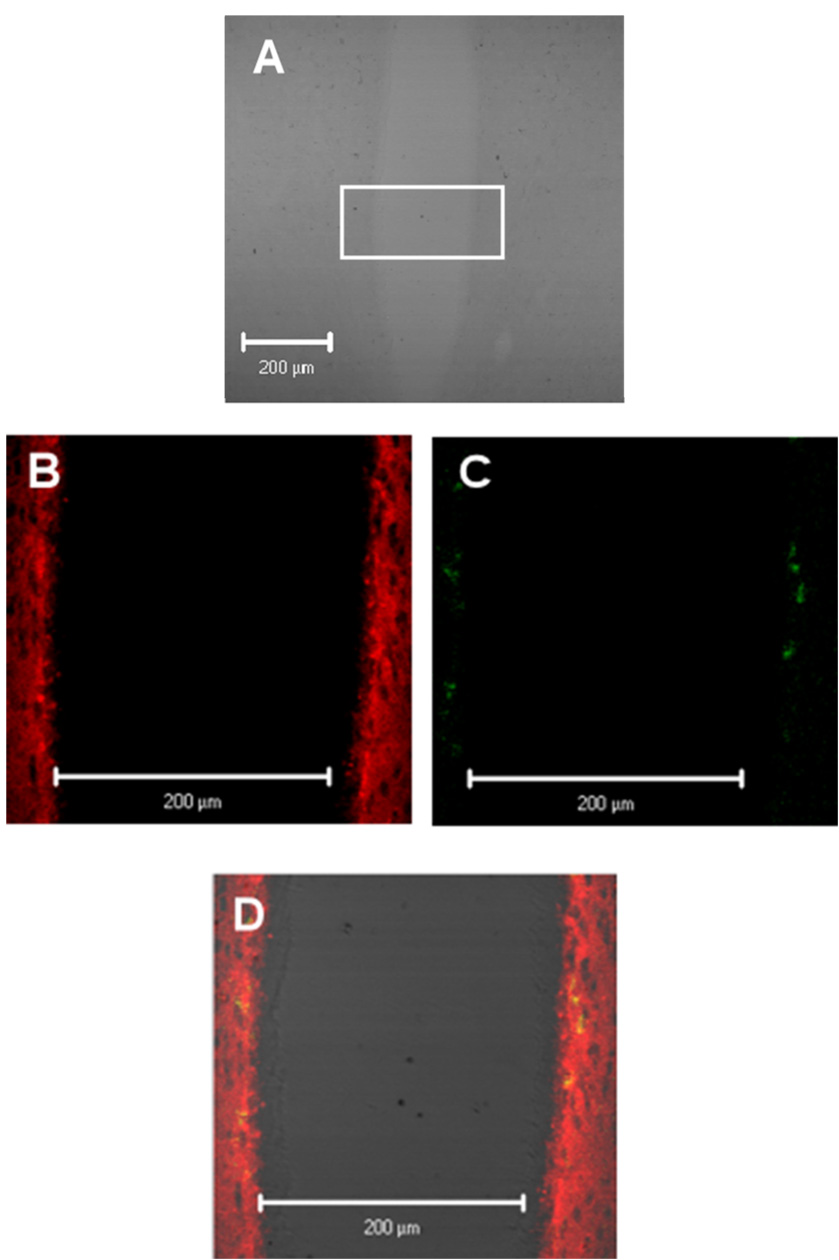
Based on the periventricular distribution of labeled IL-6, ventricular regions were analyzed for co-localization of the IL-6R. Figure 3A shows a brightfield image of the third ventricle and adjoining periventricular region. A selected section of the third ventricle, as indicated by the rectangle, is enlarged in Figure 3B–3D. Localization of labeled IL-6 is shown in Figure 3B (red) and expression of the IL-6R is shown in Figure 3C (green). Co-localization of receptor and ligand are shown by the yellow areas in Figure 3D. Areas of the third ventricle where labeled IL-6 and IL-6R are not co-localized are shown in red and green respectively. IL-6R and IL-6 were determined to be co-localized in the periventricular areas adjoining the third ventricle but not at other regions of the ventricular system.
Figure 3.
IL-6 and IL-6R co-localization patterns in the 3rd ventricle region. Panel A is a 20x zoom of image 2B. Localization of IL-6 (red) and IL-6 receptor (IL-6R; green) are shown in Figure 3B and Figures 3B and 3C respectively. Overlay of figure 3B and figures 3C and 3C are shown in figure 3D with yellow representing co-localization of IL-6 and the IL-6R.
DISCUSSION
The current study revealed three new findings. First, icv injections of IL-6 produced significant dose-dependent increases in splenic SND. Second, fluorescently labeled IL-6 was not distributed throughout the brain parenchyma following icv administration, suggesting that IL-6 likely does not act as a volume transmission signal to access brain nuclei involved in sympathetic nerve regulation. Rather, the current results indicated that following icv administration, labeled IL-6 was localized to periventricular tissue at all levels of the ventricular system, with the strongest signal evident at the level of the lateral and third ventricles. Third, immunohistochemistry revealed co-localization of IL-6 with the IL-6R in periventricular tissue at the level of the third ventricle.
The primary goal of the current study was to determine the acute effect of icv IL-6 administration on the level of splenic SND. Splenic SND was recorded because the sympathetic innervation to the spleen is an important source of immune system modulation and represents a direct link between the central nervous system and splenic lymphocytes (Nance and Burns, 1989; Wan et al., 1993). In the rat, icv administration of IL-6 in doses from 1–100 ng alters physiological responses including oxygen consumption (Rothwell et al., 1991; Wallenius et al., 2002a; Wallenius et al., 2002b), core temperature (Lenczowski et al., 1999; Rothwell et al., 1991), and caloric intake (Plata-Salaman et al., 1996). Moreover, icv administration of 400 ng IL-6 in rats stimulates hypothalamic corticotrophin releasing factor expression and increases plasma ACTH and cortisol concentrations (Wallenius et al., 2002a), but does not affect social investigatory behavior or induce immobility (Lenczowski et al., 1999; Wallenius et al., 2002a). Based on these results, the effects of icv administration of 10 ng, 100 ng, and 400 ng doses of IL-6 on splenic SND were determined in the current study. While each of the three doses of IL-6 used produced splenic sympathoexcitation, the 100 ng and 400 ng doses of the cytokine resulted in significantly greater splenic sympathoexcitation than did the 10 ng dose of IL-6. Splenic SND responses to 100 ng and 400 ng doses of IL-6 did not significantly differ during the 60 min following icv administration. In contrast to the splenic sympathoexcitation produced by the icv administration of 10, 100, and 400 ng doses of IL-6, modest but significant increases in MAP and HR were observed only in rats receiving 400 ng of icv administered IL-6. Although SND responses can be affected by anesthesia, the present experiments were completed in chloralose-urethane anesthetized rats, an anesthetic regimen used widely in studies concerned with regulation of the sympathetic nervous and cardiovascular systems. Moreover, the use of an anesthetized model eliminates modulations in SND responses that can arise from behavioral modifications. Because splenic SND recordings were terminated 60 min after cessation of icv IL-6 infusions, the present results do not provide information regarding the magnitude or duration of the splenic sympathoexcitatory response to icv IL-6 administration.
In contrast to the IL-6-induced splenic sympathoexcitatory responses observed in the present study, Terao et al. (Terao et al., 1994) reported that the icv administration of IL-6 did not increase splenic norepinephrine turnover in rats, leading these investigators to speculate that central IL-6 may not activate splenic SND. One explanation for these conflicting results may be related to the timecourse of the sampling procedures used in the different studies. Specifically, direct recordings of splenic SND were completed for 60 min following icv IL-6 administration in the present study whereas splenic norepinephrine turnover was assessed 6 hours after icv IL-6 administration in the study by Terao et al. (Terao et al., 1994). Alternatively or in addition, it must be considered that the norepinephrine turnover method may be limited with regards to sensitivity. For example, Niijima et al. (Niijima et al., 1991) reported that the intravenous administration of interleukin-1β increased adrenal SND in anesthetized rats, however, Terao et al. (Terao et al., 1994) stated that they failed to observe changes in the adrenal gland turnover of norepinephrine and epinephrine in response to IL-1β.
A second goal of the current study was to determine if IL-6 injected into the ventricular space enters the brain parenchyma and acts as a volume transmission signal to access areas of the brain involved in regulation of sympathetic nerve outflow (e.g. paraventricular nucleus, rostral ventral lateral medulla, A5 region). Fluorescence microscopy revealed that icv administered IL-6 was present within the first 50 microns of periventricular tissue from the lateral ventricle, through the third ventricle, cerebral aqueduct, and fourth ventricle, however, fluorescently labeled IL-6 was not distributed throughout the brain parenchyma. Additional experiments were completed to determine IL-6 migration into brain parenchyma at 30 and 150 minutes after icv administration of the cytokine. The periventricular distribution pattern was similar in experiments terminated 30 min, 60 min, and 150 min after icv IL-6 infusion. IL-6 mediated physiological responses are initiated by binding of the cytokine to the soluble or tissue form of the receptor (Heinrich et al., 2003). IL-6R protein expression and co-localization with IL-6 was present in the ependymal cell region of the third ventricle but not at other levels of the ventricular system or within the brain parenchyma of any slice analyzed. The present results build on c-fos expression studies demonstrating that the ependymal cells lining the ventricular system are activated following icv administration of IL-6 (Vallieres et al., 1997).
Although the specific central nervous system pathways involved in mediating splenic SND responses to icv IL-6 remain to be determined, several potential sites should be considered. Schobitz et al. (Schobitz et al., 1993) reported the localization of IL-6 mRNA and IL-6 receptor mRNA under basal (non-inflammatory) conditions in the dorsomedial hypothalamus, ventromedial hypothalamus, medial preoptic nucleus, and the anterior tip of the lateral ventricle, whereas neither transcript was detected in the paraventricular nucleus. Vallieres and Rivest (Vallieres and Rivest, 1997) reported low levels of IL-6 receptor mRNA under basal conditions in the ependymal cells of the ventricles, bed nucleus of the stria terminalis, subfornical organ, and median eminence. Based on the marked distribution of labeled IL-6 along the periventricular tissue of the third ventricle following icv administration in the present study, and the presence of IL-6 receptor mRNA at similar locations, it is tempting to speculate that medial hypothalamic nuclei may play a key role in mediating SND responses to icv IL-6 infusion.
The progressive but delayed increase in splenic SND following icv IL-6 administration suggests that binding of the cytokine to the soluble or tissue form of the receptor likely activates a cascade of responses that ultimately result in activation of splenic SND. The results of several studies suggest possible cellular mechanisms that may play a role in mediating central IL-6-induced increases in splenic SND. For example, icv administration of prostaglandin E2 (PGE2) results in an immediate increase in splenic SND (Ando et al., 1995; MacNeil et al., 2003). Intravenous LPS administration also increases splenic SND responses; the onset of which is slower (MacNeil et al., 1996) than that achieved following icv administration of either PGE2 or IL-6. Conversely splenic nerve responses to intravenous LPS administration can be blocked via icv administration of the nonsteroidal anti-inflammatory indomethacin, indicating a role for PGE2 in LPS-mediated sympathetic nerve responses (MacNeil et al., 1997). Additionally, c-fos staining has demonstrated that neuronal activation occurs in brain regions that regulate SND responses following icv administration of LPS (Wan et al., 1993). LPS increases the concentration of PGE2 in the preoptic area of the hypothalamus and plasma levels of PGE2 and IL-6 in the guinea pig, indicating a central role of PGE2 in LPS-induced febrile responses (Li et al., 2006; Sehic et al., 1996). IL-6 is known to be involved in communication between neurons, glia, and microglia (Gruol and Nelson, 1997), activation of the HPA axis, and release of paracrine factors from supporting glia; while astrocytes, microglia, neurons, and endothelial cells of the blood vessels in the brain have all been shown to produce IL-6 (Vallieres and Rivest, 1999).
Why study the effects of icv administration of IL-6 on splenic SND? Central IL-6 has been shown to induce fever (LeMay et al., 1990; Lenczowski et al., 1999), modulate immune responses (Schobitz et al., 1994), activate the hypothalamic-pituitary-adrenal axis (Vallieres and Rivest, 1999; Zhou et al., 1996), and has been hypothesized to compromise the blood brain barrier allowing for the infiltration of leukocytes (Schobitz et al., 1994). Furthermore Alzheimer’s disease (Blum-Degen et al., 1995), autoimmune disease (Hirohata and Miyamoto, 1990), CNS infection (Houssiau et al., 1988) and meningitis (Waage et al., 1989) are all characterized by increased levels of IL-6 in the CSF.
In summary, the current results demonstrate that elevations in CSF IL-6 levels can alter efferent sympathetic nerve outflow suggesting that, in pathophysiological conditions characterized by alterations in SND activity (Algotsson et al., 1995; Del Rey et al., 2003; Pascualy et al., 2000; Vayssettes-Courchay et al., 2005; Vitiello et al., 1993), increased levels of CSF IL-6 may contribute to the sympathetic dysregulation.
ACKNOWLEDGEMENTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-65346 and HL-69755. The authors acknowledge the Kansas State University COBRE Confocal Microscopy Facility supported by NIH grant P20-RR017686.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–R283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- Algotsson A, Viitanen M, Winblad B, Solders G. Autonomic dysfunction in Alzheimer's disease. Acta Neurol Scand. 1995;91:14–18. doi: 10.1111/j.1600-0404.1995.tb05836.x. [DOI] [PubMed] [Google Scholar]
- Ando T, Ichijo T, Katafuchi T, Hori T. Intracerebroventricular injection of prostaglandin E2 increases splenic sympathetic nerve activity in rats. Am J Physiol. 1995;269:R662–R668. doi: 10.1152/ajpregu.1995.269.3.R662. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. Faseb J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Kabiersch A, Petzoldt S, Besedovsky HO. Sympathetic abnormalities during autoimmune processes: potential relevance of noradrenalineinduced apoptosis. Ann N Y Acad Sci. 2003;992:158–167. doi: 10.1111/j.1749-6632.2003.tb03146.x. [DOI] [PubMed] [Google Scholar]
- Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–H1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Nelson TE. Physiological and pathological roles of interleukin- 6 in the central nervous system. Mol Neurobiol. 1997;15:307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohata S, Miyamoto T. Elevated levels of interleukin-6 in cerebrospinal fluid from patients with systemic lupus erythematosus and central nervous system involvement. Arthritis Rheum. 1990;33:644–649. doi: 10.1002/art.1780330506. [DOI] [PubMed] [Google Scholar]
- Houssiau FA, Bukasa K, Sindic CJ, Van Damme J, Van Snick J. Elevated levels of the 26K human hybridoma growth factor (interleukin 6) in cerebrospinal fluid of patients with acute infection of the central nervous system. Clin Exp Immunol. 1988;71:320–323. [PMC free article] [PubMed] [Google Scholar]
- Kenney MJ, Bealer SL. Arterial baroreceptors and brain histamine contribute to bradycardia to peripheral hyperosmolality. Am J Physiol. 1993;265:H1149–H1154. doi: 10.1152/ajpheart.1993.265.4.H1149. [DOI] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- LeMay LG, Vander AJ, Kluger MJ. Role of interleukin 6 in fever in rats. Am J Physiol. 1990;258:R798–R803. doi: 10.1152/ajpregu.1990.258.3.R798. [DOI] [PubMed] [Google Scholar]
- Lenczowski MJ, Bluthe RM, Roth J, Rees GS, Rushforth DA, van Dam AM, Tilders FJ, Dantzer R, Rothwell NJ, Luheshi GN. Central administration of rat IL-6 induces HPA activation and fever but not sickness behavior in rats. Am J Physiol. 1999;276:R652–R658. doi: 10.1152/ajpregu.1999.276.3.R652. [DOI] [PubMed] [Google Scholar]
- Li Z, Perlik V, Feleder C, Tang Y, Blatteis CM. Kupffer cell-generated PGE2 triggers the febrile response of guinea pigs to intravenously injected LPS. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1262–R1270. doi: 10.1152/ajpregu.00724.2005. [DOI] [PubMed] [Google Scholar]
- Licinio J, Wong ML. Pathways and mechanisms for cytokine signaling of the central nervous system. J Clin Invest. 1997;100:2941–2947. doi: 10.1172/JCI119846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil BJ, Jansen AH, Greenberg AH, Nance DM. Activation and selectivity of splenic sympathetic nerve electrical activity response to bacterial endotoxin. Am J Physiol. 1996;270:R264–R270. doi: 10.1152/ajpregu.1996.270.1.R264. [DOI] [PubMed] [Google Scholar]
- MacNeil BJ, Jansen AH, Greenberg AH, Nance DM. Neuropeptide specificity of prostaglandin E2-induced activation of splenic and renal sympathetic nerves in the rat. Brain Behav Immun. 2003;17:442–452. doi: 10.1016/s0889-1591(03)00050-3. [DOI] [PubMed] [Google Scholar]
- MacNeil BJ, Jansen AH, Janz LJ, Greenberg AH, Nance DM. Peripheral endotoxin increases splenic sympathetic nerve activity via central prostaglandin synthesis. Am J Physiol. 1997;273:R609–R614. doi: 10.1152/ajpregu.1997.273.2.R609. [DOI] [PubMed] [Google Scholar]
- Madden KS, Felten DL. Experimental basis for neural-immune interactions. Physiol Rev. 1995;75:77–106. doi: 10.1152/physrev.1995.75.1.77. [DOI] [PubMed] [Google Scholar]
- Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun. 1989;3:281–290. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Niijima A, Hori T, Aou S, Oomura Y. The effects of interleukin-1 beta on the activity of adrenal, splenic and renal sympathetic nerves in the rat. J Auton Nerv Syst. 1991;36:183–192. doi: 10.1016/0165-1838(91)90042-2. [DOI] [PubMed] [Google Scholar]
- Pascualy M, Petrie EC, Brodkin K, Peskind ER, Wilkinson CW, Raskind MA. Hypothalamic pituitary adrenocortical and sympathetic nervous system responses to the cold pressor test in Alzheimer's disease. Biol Psychiatry. 2000;48:247–254. doi: 10.1016/s0006-3223(00)00879-9. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Sonti G, Borkoski JP, Wilson CD, French-Mullen JMb. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- Rothwell NJ, Busbridge NJ, Lefeuvre RA, Hardwick AJ, Gauldie J, Hopkins SJ. Interleukin-6 is a centrally acting endogenous pyrogen in the rat. Can J Physiol Pharmacol. 1991;69:1465–1469. doi: 10.1139/y91-219. [DOI] [PubMed] [Google Scholar]
- Saindon CS, Blecha F, Musch TI, Morgan DA, Fels RJ, Kenney MJ. Effect of cervical vagotomy on sympathetic nerve responses to peripheral interleukin-1beta. Auton Neurosci. 2001;87:243–248. doi: 10.1016/s1566-0702(00)00280-0. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Kohm AP. Sympathetic nervous system interaction with the immune system. Int Rev Neurobiol. 2002;52:17–41. doi: 10.1016/s0074-7742(02)52004-3. [DOI] [PubMed] [Google Scholar]
- Schobitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Prog Neurobiol. 1994;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Schobitz B, de Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur J Neurosci. 1993;5:1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Sehic E, Szekely M, Ungar AL, Oladehin A, Blatteis CM. Hypothalamic prostaglandin E2 during lipopolysaccharide-induced fever in guinea pigs. Brain Res Bull. 1996;39:391–399. doi: 10.1016/0361-9230(96)00037-8. [DOI] [PubMed] [Google Scholar]
- Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- Terao A, Oikawa M, Saito M. Tissue-specific increase in norepinephrine turnover by central interleukin-1, but not by interleukin-6, in rats. Am J Physiol. 1994;266:R400–R404. doi: 10.1152/ajpregu.1994.266.2.R400. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Lacroix S, Rivest S. Influence of interleukin-6 on neural activity and transcription of the gene encoding corticotrophin-releasing factor in the rat brain: an effect depending upon the route of administration. Eur J Neurosci. 1997;9:1461–1472. doi: 10.1111/j.1460-9568.1997.tb01500.x. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. J Neurochem. 1997;69:1668–1683. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Rivest S. Interleukin-6 is a needed proinflammatory cytokine in the prolonged neural activity and transcriptional activation of corticotropin-releasing factor during endotoxemia. Endocrinology. 1999;140:3890–3903. doi: 10.1210/endo.140.9.6983. [DOI] [PubMed] [Google Scholar]
- Vayssettes-Courchay C, Bouysset F, Verbeuren TJ. Sympathetic activation and tachycardia in lipopolysaccharide treated rats are temporally correlated and unrelated to the baroreflex. Auton Neurosci. 2005;120:35–45. doi: 10.1016/j.autneu.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Veith RC, Molchan SE, Martinez RA, Lawlor BA, Radcliffe J, Hill JL, Sunderland T. Autonomic dysfunction in patients with dementia of the Alzheimer type. Biol Psychiatry. 1993;34:428–433. doi: 10.1016/0006-3223(93)90233-4. [DOI] [PubMed] [Google Scholar]
- Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenius K, Wallenius V, Sunter D, Dickson SL, Jansson JO. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002a;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002b;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wan W, Janz L, Vriend CY, Sorensen CM, Greenberg AH, Nance DM. Differential induction of c-Fos immunoreactivity in hypothalamus and brain stem nuclei following central and peripheral administration of endotoxin. Brain Res Bull. 1993;32:581–587. doi: 10.1016/0361-9230(93)90158-8. [DOI] [PubMed] [Google Scholar]
- Wan W, Vriend CY, Wetmore L, Gartner JG, Greenberg AH, Nance DM. The effects of stress on splenic immune function are mediated by the splenic nerve. Brain Res Bull. 1993;30:101–105. doi: 10.1016/0361-9230(93)90044-c. [DOI] [PubMed] [Google Scholar]
- Zhou D, Shanks N, Riechman SE, Liang R, Kusnecov AW, Rabin BS. Interleukin 6 modulates interleukin-1-and stress-induced activation of the hypothalamic-pituitary-adrenal axis in male rats. Neuroendocrinology. 1996;63:227–236. doi: 10.1159/000126962. [DOI] [PubMed] [Google Scholar]