Abstract
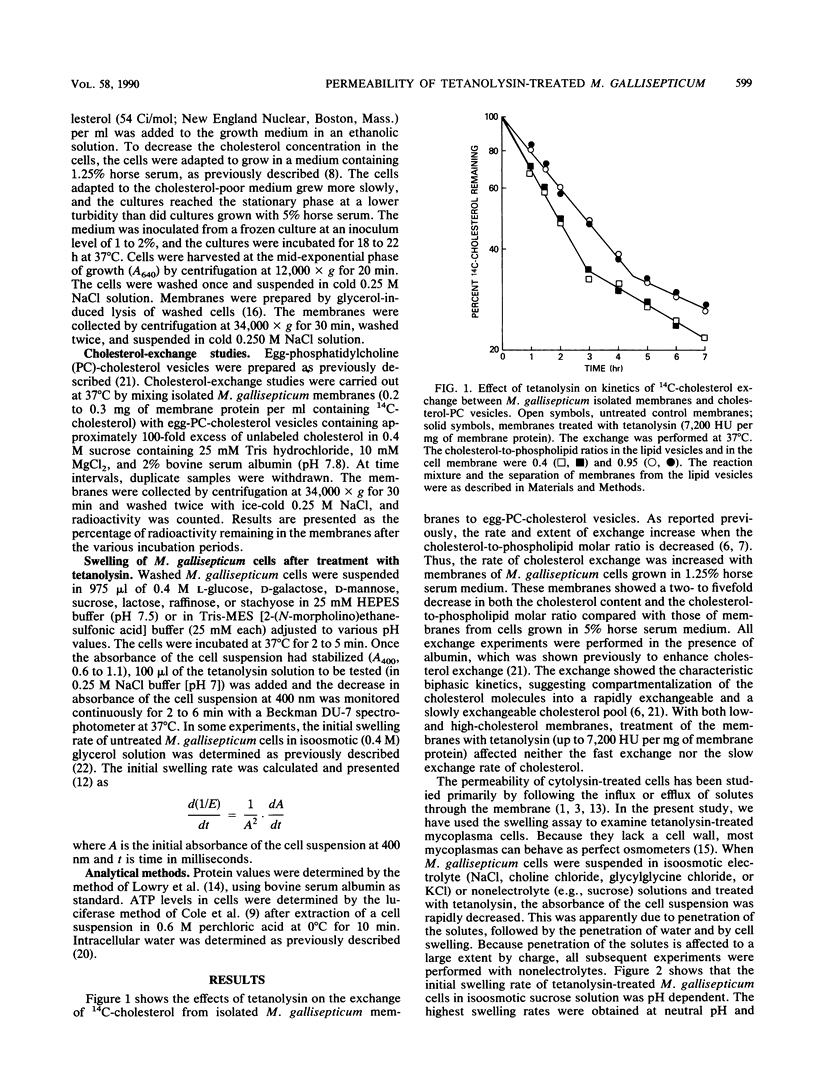
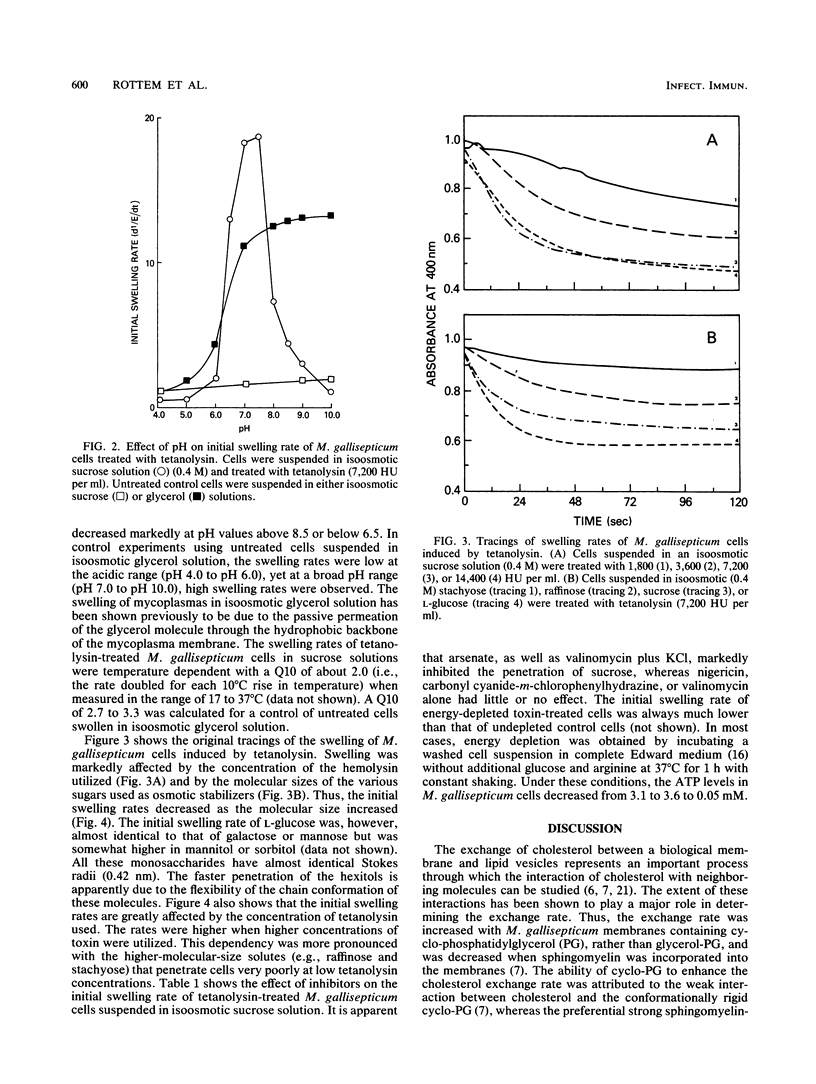
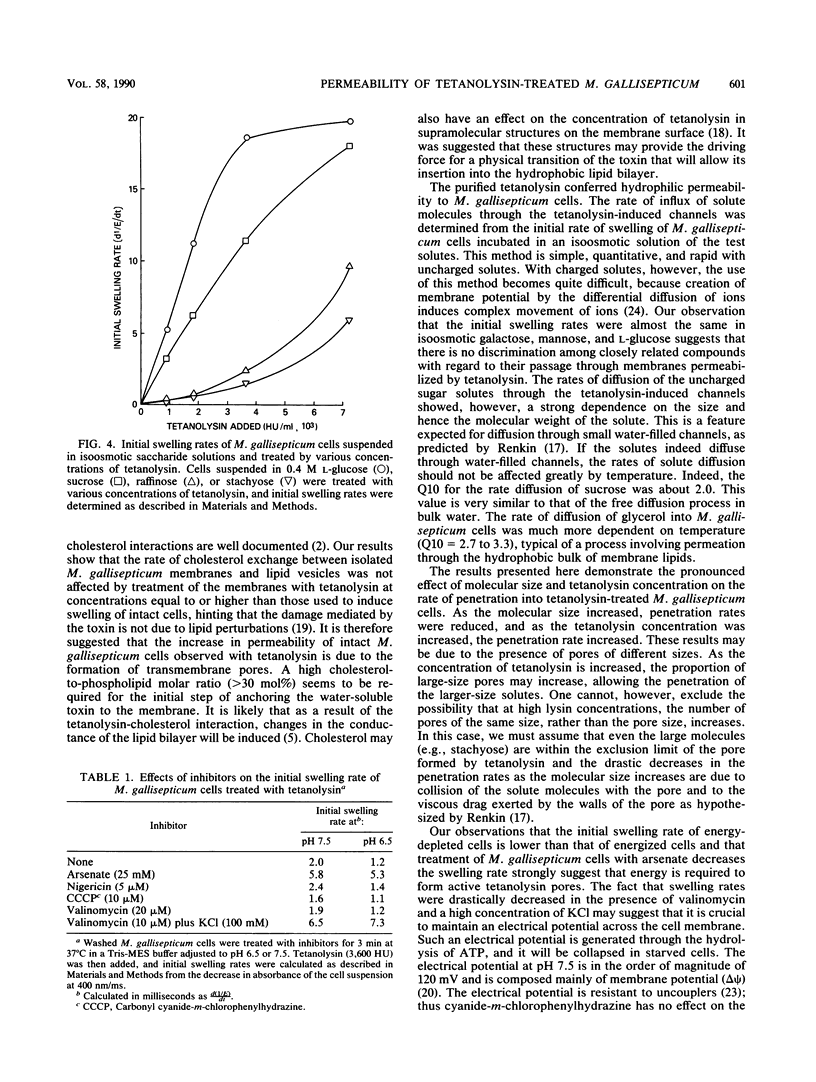
The permeability properties of Mycoplasma gallisepticum cells treated with a purified preparation of tetanolysin were investigated by determining the initial swelling rates of cells suspended in an isoosmotic solution of electrolytes or nonelectrolytes. The swelling, initiated by the tetanolysin, depended on the tetanolysin concentration and was markedly affected by the molecular size of the various osmotic stabilizers utilized. Thus, the initial swelling rates in an isoosmotic solution of monosaccharides were much higher than those in isoosmotic solutions of di-, tri-, or tetrasaccharides. Cell swelling induced by tetanolysin was much lower with energy-depleted M. gallisepticum cells, with arsenate-treated cells, or when the membrane potential (delta psi) was collapsed by valinomycin (10 microM) plus KCl (100 mM). Swelling was not affected by the proton-conducting ionophore carbonyl cyanide-m-chlorophenylhydrazone (1 to 10 microM) or by nigericin (5 microM). These results support the concept that the damage induced by tetanolysin is due to the formation of water-filled pores within the membranes of energized M. gallisepticum cells. Such pores allow the diffusion of hydrophilic molecules into the cells and may vary in size, depending on the tetanolysin concentration utilized.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Habig W. H., Urban K. A., Hardegree M. C. Cholesterol-dependent tetanolysin damage to liposomes. Biochim Biophys Acta. 1979 Feb 20;551(1):224–228. doi: 10.1016/0005-2736(79)90368-7. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R., Habig W. H. Mechanism of tetanolysin-induced membrane damage: studies with black lipid membranes. J Bacteriol. 1984 Jan;157(1):321–323. doi: 10.1128/jb.157.1.321-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clejan S., Bittman R. Kinetics of cholesterol and phospholipid exchange between Mycoplasma gallisepticum cells and lipid vesicles. Alterations in membrane cholesterol and protein content. J Biol Chem. 1984 Jan 10;259(1):441–448. [PubMed] [Google Scholar]
- Clejan S., Bittman R., Rottem S. Uptake, transbilayer distribution, and movement of cholesterol in growing Mycoplasma capricolum cells. Biochemistry. 1978 Oct 31;17(22):4579–4583. doi: 10.1021/bi00615a001. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Bernheimer A. W. Role of cholesterol in the action of cereolysin on membranes. Arch Biochem Biophys. 1978 Oct;190(2):603–610. doi: 10.1016/0003-9861(78)90316-8. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Kim K. S., Bernheimer A. W. Alteration by cereolysin of the structure of cholesterol-containing membranes. Biochim Biophys Acta. 1978 Feb 21;507(2):230–241. doi: 10.1016/0005-2736(78)90419-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linder R., Bernheimer A. W. Action of bacterial cytotoxins on normal mammalian cells and cells with altered membrane lipid composition. Toxicon. 1984;22(4):641–651. doi: 10.1016/0041-0101(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkin E. M. Some consequences of capillary permeability to macromolecules: Starling's hypothesis reconsidered. Am J Physiol. 1986 May;250(5 Pt 2):H706–H710. doi: 10.1152/ajpheart.1986.250.5.H706. [DOI] [PubMed] [Google Scholar]
- Rottem S., Cole R. M., Habig W. H., Barile M. F., Hardegree M. C. Structural characteristics of tetanolysin and its binding to lipid vesicles. J Bacteriol. 1982 Nov;152(2):888–892. doi: 10.1128/jb.152.2.888-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hardegree M. C., Grabowski M. W., Fornwald R., Barile M. F. Interaction between tetanolysin and Mycoplasma cell membrane. Biochim Biophys Acta. 1976 Dec 14;455(3):876–888. doi: 10.1016/0005-2736(76)90057-2. [DOI] [PubMed] [Google Scholar]
- Rottem S., Linker C., Wilson T. H. Proton motive force across the membrane of Mycoplasma gallisepticum and its possible role in cell volume regulation. J Bacteriol. 1981 Mar;145(3):1299–1304. doi: 10.1128/jb.145.3.1299-1304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Shinar D., Bittman R. Symmetrical distribution and rapid transbilayer movement of cholesterol in Mycoplasma gallisepticum membranes. Biochim Biophys Acta. 1981 Dec 21;649(3):572–580. doi: 10.1016/0005-2736(81)90161-9. [DOI] [PubMed] [Google Scholar]
- Shirvan M. H., Rottem S., Ne'eman Z., Bittman R. Isolation of mycoplasma membranes by dicyclohexylcarbodiimide-induced lysis. J Bacteriol. 1982 Mar;149(3):1124–1128. doi: 10.1128/jb.149.3.1124-1128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvan M. H., Schuldiner S., Rottem S. Volume regulation in Mycoplasma gallisepticum: evidence that Na+ is extruded via a primary Na+ pump. J Bacteriol. 1989 Aug;171(8):4417–4424. doi: 10.1128/jb.171.8.4417-4424.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H. Ionic permeability and osmotic swelling of cells. Science. 1954 Jul 16;120(3107):104–105. doi: 10.1126/science.120.3107.104. [DOI] [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. Lipid composition and permeability of liposomes. Biochim Biophys Acta. 1968 Jun 11;150(4):666–675. doi: 10.1016/0005-2736(68)90056-4. [DOI] [PubMed] [Google Scholar]



