Abstract
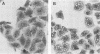
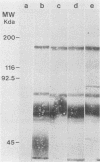
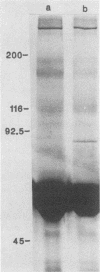
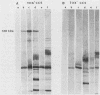
Helicobacter pylori infection is strongly associated with histologic gastritis and peptic ulcer disease. Broth culture supernatants from a subset of H. pylori strains induce vacuolization in cultured cells, a phenomenon that has been attributed to cytotoxin activity. Concentrated culture supernatants from 15 of 28 (53.6%) H. pylori strains tested induced vacuolization in HeLa cells in titers ranging from 1:10 to 1:180. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining of supernatants from these 28 strains and 2 control strains demonstrated an 82-kilodalton (kDa) protein band in 3 of 16 supernatants with vacuolizing activity, but in none of 14 supernatants without vacuolizing activity. By immunoblotting with human sera, a 128-kDa band was recognized in all 16 supernatants with vacuolizing activity, compared with 9 of 14 (64%) supernatants without vacuolizing activity (P = 0.014). Serologic recognition of the 128-kDa band in H. pylori culture supernatants was more prevalent among persons infected with vacuolizing H. pylori strains than among persons infected with nonvacuolizing strains, but the difference was not statistically significant (80 versus 45%; P = 0.079); human serologic recognition of the 82-kDa band was less common. The 128-kDa band was recognized by 100% of 31 serum samples from H. pylori-infected patients with duodenal ulcer disease, compared with 60.8% of 74 serum samples from H. pylori-infected persons without peptic ulcer disease (P = 0.0001). These data indicate that antigenic 128- and 82-kDa proteins are present in H. pylori broth culture supernatants with vacuolizing activity and that serologic responses to the 128-kDa protein are more prevalent among H. pylori-infected persons with duodenal ulceration than among infected persons without peptic ulceration.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthel J. S., Westblom T. U., Havey A. D., Gonzalez F., Everett E. D. Gastritis and Campylobacter pylori in healthy, asymptomatic volunteers. Arch Intern Med. 1988 May;148(5):1149–1151. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 1987 Aug;93(2):371–383. doi: 10.1016/0016-5085(87)91028-6. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. E., Gourley W. K., Lee W. K., Subramanyam K., Latimer J. M., DiNuzzo A. R. Relation of Campylobacter pyloridis to gastritis and peptic ulcer. J Infect Dis. 1986 Apr;153(4):664–669. doi: 10.1093/infdis/153.4.664. [DOI] [PubMed] [Google Scholar]
- Chen X. G., Correa P., Offerhaus J., Rodriguez E., Janney F., Hoffmann E., Fox J., Hunter F., Diavolitsis S. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am J Clin Pathol. 1986 Nov;86(5):575–582. doi: 10.1093/ajcp/86.5.575. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. The pathobiology of Campylobacter infections in humans. Annu Rev Med. 1989;40:269–285. doi: 10.1146/annurev.me.40.020189.001413. [DOI] [PubMed] [Google Scholar]
- Eaton K. A., Morgan D. R., Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989 Apr;57(4):1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons P. L., Dooley C. P., Cohen H., Appleman M. D. Prevalence of gastric metaplasia, inflammation, and Campylobacter pylori in the duodenum of members of a normal population. Am J Clin Pathol. 1988 Dec;90(6):711–714. doi: 10.1093/ajcp/90.6.711. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Armstrong J. A., Marshall B. J. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986 Apr;39(4):353–365. doi: 10.1136/jcp.39.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell S. L., Hennessy W. B., Borody T. J., Carrick J., Ralston M., Brady L., Lee A. Campylobacter pyloridis gastritis II: Distribution of bacteria and associated inflammation in the gastroduodenal environment. Am J Gastroenterol. 1987 Apr;82(4):297–301. [PubMed] [Google Scholar]
- Hupertz V., Czinn S. Demonstration of a cytotoxin from Campylobacter pylori. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):576–578. doi: 10.1007/BF01962619. [DOI] [PubMed] [Google Scholar]
- Johnston B. J., Reed P. I., Ali M. H. Campylobacter like organisms in duodenal and antral endoscopic biopsies: relationship to inflammation. Gut. 1986 Oct;27(10):1132–1137. doi: 10.1136/gut.27.10.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., McGechie D. B., Rogers P. A., Glancy R. J. Pyloric Campylobacter infection and gastroduodenal disease. Med J Aust. 1985 Apr 15;142(8):439–444. doi: 10.5694/j.1326-5377.1985.tb113444.x. [DOI] [PubMed] [Google Scholar]
- Megraud F., Bonnet F., Garnier M., Lamouliatte H. Characterization of "Campylobacter pyloridis" by culture, enzymatic profile, and protein content. J Clin Microbiol. 1985 Dec;22(6):1007–1010. doi: 10.1128/jcm.22.6.1007-1010.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Cortesia M. J., Rosenthal L. E., Jones B. D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988 May;26(5):831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Blaser M. J. Conservation and diversity of Campylobacter pyloridis major antigens. Infect Immun. 1987 May;55(5):1256–1263. doi: 10.1128/iai.55.5.1256-1263.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Dworkin B. M., Chodos J. E., Blaser M. J. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988 Jul 1;109(1):11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- Perez G. I., Hopkins J. A., Blaser M. J. Antigenic heterogeneity of lipopolysaccharides from Campylobacter jejuni and Campylobacter fetus. Infect Immun. 1985 May;48(2):528–533. doi: 10.1128/iai.48.2.528-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Sarosiek J., Slomiany A., Slomiany B. L. Evidence for weakening of gastric mucus integrity by Campylobacter pylori. Scand J Gastroenterol. 1988 Jun;23(5):585–590. doi: 10.3109/00365528809093916. [DOI] [PubMed] [Google Scholar]
- Tricottet V., Bruneval P., Vire O., Camilleri J. P., Bloch F., Bonte N., Roge J. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10(2):113–122. doi: 10.3109/01913128609014587. [DOI] [PubMed] [Google Scholar]