Abstract
Background
Doxorubicin is considered one of the most potent established chemotherapeutics in the treatment of liposarcoma; however, the response rates usually below 30%, are still disappointing. This study was performed to identify gene expression changes in liposarcoma after doxorubicin treatment.
Methods
Cells of 19 primary human liposarcoma were harvested intraoperatively and brought into cell culture. Cells were incubated with doxorubicin for 24 h, RNA was isolated and differential gene expression was analysed by the microarray technique.
Results
A variety of genes involved in apoptosis were up and down regulated in different samples revealing a heterogeneous expression pattern of the 19 primary tumor cell cultures in response to doxorubicin treatment. However, more than 50% of the samples showed up-regulation of pro-apoptotic genes such as TRAIL Receptor2, CDKN1A, GADD45A, FAS, CD40, PAWR, NFKBIA, IER3, PSEN1, RIPK2, and CD44. The anti-apoptotic genes TNFAIP3, PEA15, Bcl2A1, NGFB, and BIRC3 were also up-regulated. The pro-apoptotic CD14, TIA1, and ITGB2 were down-regulated in more than 50% of the tumor cultures after treatment with doxorubicin, as was the antiapoptotic YWHAH.
Conclusion
Despite a correlation of the number of differentially regulated genes to the tumor grading and to a lesser extent histological subtype, the expression patterns varied strongly; however, especially among high grade tumors the responses of selected apoptosis genes were similar. The predescribed low clinical response rates of low grade liposarcoma to doxorubicin correspond to our results with only little changes on gene expression level and also divergent findings concerning the up- and down-regulation of single genes in the different sarcoma samples.
Background
Together with malignant fibrous histiocytoma (not otherwise specified sarcoma, NOS), liposarcoma represents the most common entity of soft tissue sarcomas and accounts for approximately 20% of sarcomas in adults [1-3]. Although surgery and radiation therapy could achieve good results concerning local control, distant metastatic disease remains a therapeutic dilemma limiting survival [4,5]. With a maximum response rate of approximately 20% the effects of cytostatics on liposarcoma are still disappointing [6-8]. The most favoured chemotherapeutics for treatment of advanced soft tissue sarcoma, including liposarcoma, are ifosfamide and doxorubicin, but the data for ifosfamide differ with respect to improvement of local control and survival [9-11]. Although meta-analysis of 14 randomised trials found that doxorubicin treatment was associated with a 10% improvement of recurrence free survival, the overall survival could not be improved [12-14]. In carcinomas, multiple mechanisms of drug resistance on the molecular level have been characterized [15,16] including over-expression of p53 [17-20], MDR1 (multidrug resistance gene 1) [20-22], MRP1 (multidrug resistance-associated protein), the induction of DNA repair [20] and many others involving tumor suppressor genes, oncogenes, cell cycle regulators, transcription factors, growth factor receptors, and cell death regulators. Only little is known about the molecular basis of drug resistance in soft tissue sarcomas and studies on the effect of cytostatics on gene expression, especially in liposarcomas [23-27], are rare. Comprehensive knowledge of the differential expression patterns induced by cytotoxic drugs may be useful for examining the molecular basis of drug effects and also drug resistance. Because of the limited comparability of established purchasable sarcoma cell lines to in vivo tumors, we primarily harvested liposarcoma cells from resection specimens, incubated the cultured cells with doxorubicin and evaluated the changes in gene expression with a focus on genes related to apoptotic pathways. To the authors' knowledge, to date there are no studies that examined the effects of doxorubicin on primary human liposarcoma on a molecular basis.
Methods
Primary human liposarcoma tumor samples of at least 1 cm3 were harvested intraoperatively from patients undergoing resection of an already diagnosed liposarcoma and immediately processed under sterile conditions. Seven atypical lipomas (low grade sarcomas), four dedifferentiated, four pleomorphic, three myxoid/round cell, and one myxoid liposarcoma were included. The grading of the tumors ranged from GI to GIII (GI: 4, GII: 8, GIII: 7). The probes were derived from primary tumors in 12, from recurrent tumors in six, and from metastasis in one case. Nineteen primary human liposarcoma cultures were isolated by dissecting the tumor and digesting the minced samples enzymatically with 10 ml each of collagenase and dispase (10 mg/ml). The single cell suspension was depleted of red blood cells and cellular debris by centrifugation through a Ficoll-Hypaque density gradient. Liposarcoma cells were diluted and cultured during the whole experiment with Leibovitz's L-15 medium, supplemented with 2.0 mM glutamine and 10% fetal bovine serum in a humidified atmosphere in free air exchange with atmospheric air. Cells were seeded at a density of 2 × 106 in 25 cm2flasks; 24 h later, after having grown to a subconfluent layer, cell cultures were incubated with doxorubicin (0.5 μg/ml) for 24 h and equal volume of PBS as control [28-30].
Oligonucleotide microarray analysis
For microarray analyses we used the Affymetrix Gene Chip platform employing a standard protocol for sample preparation and microarray hybridization that has been described in detail previously [26,31]. Briefly, total RNA was converted into double-stranded cDNA using an oligo-deoxythymidine primer containing the T7 RNA polymerase binding site (5'-GCATTAGCGGCCGCGAAATTAATACGACTCACTATAGGGAGA – (dT)21V-3') (MWG Biotech, Ebersberg, Germany) for first strand synthesis. After generation of double-stranded cDNA from the first-strand cDNA, biotinylated cRNA was synthesized by in vitro transcription using the BioArray High Yield RNA Transcript Labeling Kit (Enzo Diagnostics, New York, USA). Labeled cRNA was purified on RNeasy columns (Qiagen, Hilden, Germany) and fragmented and hybridized to HG-U133A microarrays (Affymetrix, Santa Clara, USA). The arrays were washed and stained according to the manufacturer's recommendation and finally scanned in a GeneArray scanner 2500 (Agilent, Santa Clara, USA).
Array images were processed to determine signals and detection calls (Present, Absent, Marginal) for each probeset using the Affymetrix Microarray Suite 5.0 software (MAS 5.0; statistical algorithm). The clustering was performed unsupervised. Pairwise comparisons of treated versus control samples were carried out with MAS 5.0, which calculates the significance (change p-value) of each change in gene expression based on a Wilcoxon ranking test. To limit the number of false positives, we restricted further target identification to those probesets, which received at least one present detection call in the treated/control pair. Each single microarray analysis was derived from one cell culture. The treated cells were compared to the control. Probesets exhibiting a signal log2 ratio > 1.0 and a change p-value < 0.004 or a signal log2 ratio < -1.0 and a change p-value > 0.996 (corresponding to 2-fold up- or down-regulation) were identified by filtering using the Affymetrix Data Mining Tool 3.0 (Table 1). Additionally unsupervised clustering was performed between the 19 control tumor samples.
Table 1.
Summarized patients' data
| Patient | Gender | Age at operation | Site | Size in cm | Histological subtype | Responder type | Grading | Specimen character | Previous radiation | Previous chemotherapy |
| 1 | female | 69 years | lower arm | 4.5 × 3.5 × 2.2 | atypical lipoma with partly dedifferentiated areas | low | G2 | local recurrence | no | no |
| 2 | female | 74 years | Thigh | 14.5 × 7.5 × 9 | myxoid/roundcell liposarcoma | high | G3 | primary tumor | no | no |
| 3 | female | 70 years | upper arm | 3 × 5 × 6 | atypical lipoma | low | G1 | primary tumor | no | no |
| 4 | male | 74 years | Thigh | 16.5 × 9 × 7 | dedifferentiated liposarcoma | medium | G2 | primary tumor | no | no |
| 5 | male | 38 years | Knee | 8.3 × 4 × 7 | myxoid liposarcoma | high | G3 | primary tumor | yes | no |
| 6 | female | 58 years | pelvis retro-peritoneal | 4 × 7 × 9 | myxoid/roundcell liposarcoma | high | G3 | metastasis | no | no |
| 7 | male | 37 years | Thigh | 7 × 14 × 9 | myxoid/roundcell liposarcoma | medium | G2 | primary tumor | no | yes |
| 8 | female | 85 years | lower arm | 11 × 8 × 4 | pleomorphic liposarcoma | high | G3 | primary tumor | no | no |
| 9 | male | 53 years | Thigh | 10 × 3 × 5 | atypical liposarcoma | low | G2 | local recurrence | yes | no |
| 10 | male | 76 years | Thigh | 3.5 × 3 × 3 | dedifferentiated liposarcoma | high | G3 | local recurrence | yes | no |
| 11 | female | 57 years | Thorax | 4.9 × 4 × 3 | pleomorphic liposarcoma | high | G3 | local recurrence | no | no |
| 12 | female | 76 years | Thigh | 38.5 × 17.5 × 6 | atypical lipoma | low | G1 | primary tumor | no | no |
| 13 | female | 74 years | Thigh | 7 × 6 × 4 | dedifferentiated liposarcoma | medium | G2 | primary tumor | no | no |
| 14 | female | 70 years | Thorax | 1.9 × 1.3 × 1 | pleomorphic liposarcoma | medium | G2 | residual tumor | no | no |
| 15 | male | 70 years | Thigh | 9 × 3 × 6 | atypical lipoma | medium | G1 | primary tumor | no | no |
| 16 | male | 60 years | Thigh | 7.5 × 6 × 5.5 | pleomorphic liposarcoma | high | G3 | primary tumor | no | no |
| 17 | female | 78 years | Thigh | 13 × 10 × 6 | atypical lipoma with partly dedifferentiated areas | high | G2 | local recurrence | yes | no |
| 18 | female | 67 years | Thigh | 35 × 15 × 12 | atypical lipoma | low | G1 | primary tumor | no | no |
| 19 | male | 60 years | upper arm | 9,9 × 7 × 7 | dedifferentiated liposarcoma | medium | G2 | local recurrence | yes | no |
Genes associated with apoptotic pathways were selected based on Gene Ontology (GO)-analysis [32].
Expression changes were correlated to the grading and the histological sub-entity of the tumors. Only tumor samples were included in the final analysis whose gross sections were diagnosed as liposarcomas by an experienced soft tissue pathologist.
According to the number of differentially expressed genes after incubation with doxorubicin, liposarcomas were categorized into high (n > 2000), intermediate (100 < n < 1000) and low responders (n < 100). The results were uploaded to NCBI GEO, number GSE12972 http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE12972.
Real-time PCR for microarray data validation
Microarray data validation was performed for selected gene products with relevant up-regulation in more than ten out of the 19 liposarcoma probes (CD40, CDKN1A, FAS, GADD45A, NFKBIA, PAWR, TNFAIP3, and TNFRSF10B) or relevant down-regulation in at least ten probes (YWHaH, PPP3CA, and ITGB2). CD14 and TIA were not tested because no high quality PCR assays were purchasable.
Total RNA (2 μg) was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems). Realtime PCR was done with a 7900HT SDS system (Applied Biosystems) in 20 μl reaction volume containing 1× Master Mix, 1 μl assay and cDNA equivalent to 2 ng total RNA. All reagents and realtime PCR assays (m1 type only; CD40_Hs00386848_1, CDKN1A_Hs00355782_m1, FAS_Hs00236330_m1, GADD45A_Hs00169255_m1, ITGB2_Hs00164957_m1, NFKBIA_Hs00153283_m1, PAWR_Hs00169332_m1, PPP3CA_Hs00174223_m1, TNFAIP3_Hs00234713_m1, TNFRSF10B_Hs00366272_m1, YWHaH_Hs00607046_m1) used were purchased from Applied Biosystems. Reactions were performed in duplicates and analysed by the deltadeltaCT method. Human GAPD was used for normalization. Pearson correlation of the microarray and the rtPCR data was calculated using SPSS Version 15.0 for Windows (SPSS Inc., Chicago, USA).
Results
Sixteen tumors were located at the extremities, three at the trunk. The tumors diameters ranged from 1 cm to 38,5 cm. Twelve primary cultures were harvested from primary tumors, 6 from local recurrences, and 1 from a metastatic tumor. The majority of tumors were high grade (G2-3); only 4 were diagnosed low grade (G1). Atypical lipoma or highly differentiated liposarcoma was the most common histological subentity (n = 7) followed by dedifferentiated (n = 4) and pleomorphic (n = 4), myxoid/rundcell (n = 3) and myxoid (n = 1) liposarcoma. All low grade tumors were diagnosed as atypical lipoma, whereas 3 atypical lipomas were categorized as G2 tumors because of aggressive growth or localized areas of dedifferentiation. The other subentities were all categorized as high grade tumors (G2-3). A summary of the patients' data is given in table 1.
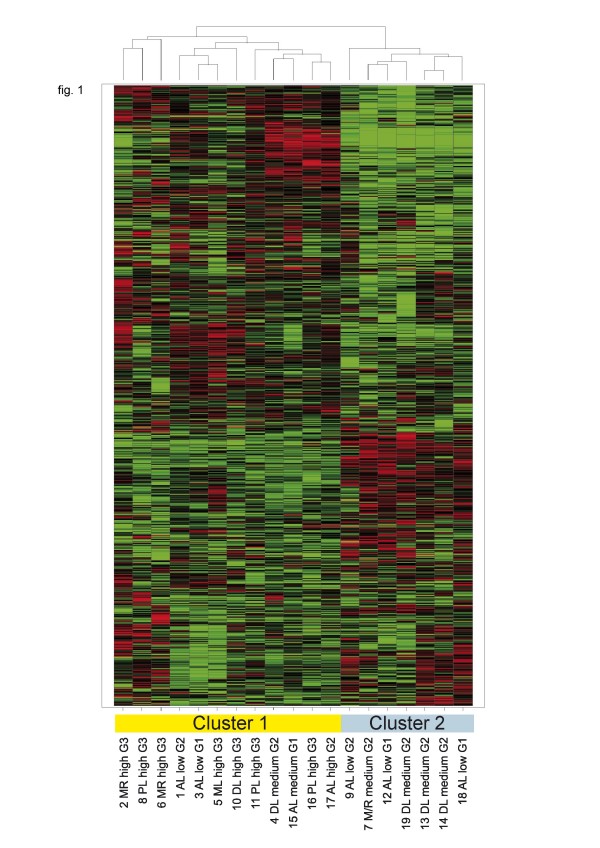
Hierarchical clustering of expression profiles from untreated samples did not clearly separate according to histological subtype or grading, but revealed two major branches, which showed some enrichment for G3 (left) and G2 tumors (right) (fig. 1).
Figure 1.
Unsupervised hierarchical cluster analysis of the 19 primary tumor cell cultures without doxorubin treatment. Gene signal intensities were normalized to the mean signal of all samples, log2 transformed and subjected to hierarchical clustering (UPGMA, Spotfire) and correlation as a similarity measure. Horizontal rows represent individual genes; vertical columns represent individual samples. Black indicates average signal intensity, brightest red ≥ 4-fold up-regualtion, brightest green ≥ 4-fold down-regulated gene expression relative to the mean. Only probesets receiving P detection calls in 6 or more samples and a stander deviation of normalized signals > 0.2 were considered. (7239 retained). The dendogram at the top of the matrix indicates the degree of similarity between tumor samples (the higher the dendogramm, the lower the similarity). Two major clusters were identified as indicated. MR: Myxoid/Roundcell Liposarcoma, PL: Pleomorphic Liposarcoma, AL: Atypical Lipoma, ML: Myxoid Liposarcoma, DL: Dedifferentiated Liposarcoma, low: low responder group, medium: medium responder group, high: high responder group.
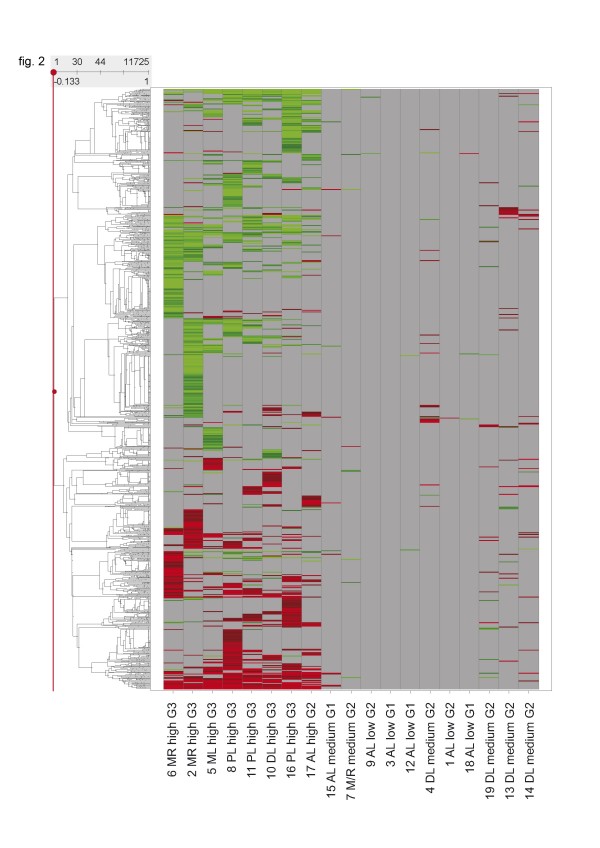
According to the number of differentially expressed genes n after incubation with doxorubicin, the 19 liposarcomas were categorized into high (n > 2000), intermediate (100 < n < 1000) and low responders (n < 100). All poorly differentiated (G3) tumors were high responders; G2 tumors were predominantly intermediate responders; and, most of the G1 tumors were low responders. A heatmap of all differentially expressed genes after 24 h of doxorubicin tratment shows heterogeneous response patterns (fig. 2).
Figure 2.
Heatmap of total gene expression changes and cluster analysis after incubation with doxorubicin for 24 h. Horizontal rows represent individual genes; vertical columns represent individual samples. Color range: Brightest red (Change call increased (change p-value < 0.002) and Signal Log Ratio > 1): SLR = >2 (4× or higher), Black: SLR = 0 (no change); not visible as a consequence of the filtering process, Brightest green (Change call decreased (change p-value > 0.998) and Signal Log Ratio < -1): SLR < -2 (0.25× or smaller). Grey: no value (requirements for a reliably measured target not met). Calculated in Affymetrix comparison analysis (MAS5.0 algorithm) and at least one present call in the two síngle array analyses compared in the comparison analysis. The dendogram at the left side indicates the degree of similarity among the selected genes according to their expression patterns (the higher the dendogramm, the lower the similarity). MR: Myxoid/Roundcell Liposarcoma, PL: Pleomorphic Liposarcoma, AL: Atypical Lipoma, ML: Myxoid Liposarcoma, DL: Dedifferentiated Liposarcoma, low: low responder group, medium: medium responder group, high: high responder group.
Correlating the overall expression changes with the histological subtype showed that most of the atypical lipomas were low responders (5 low, 1 medium, 1 high), dedifferentiated were predominantly medium responders (3 medium, 1 high), most myxoid/roundcell and myxoid liposarcomas as well as the pleomorphic liposarcomas were high responders (3 high, 1 medium each). The high grade sarcomas (G3) clustered closely together.
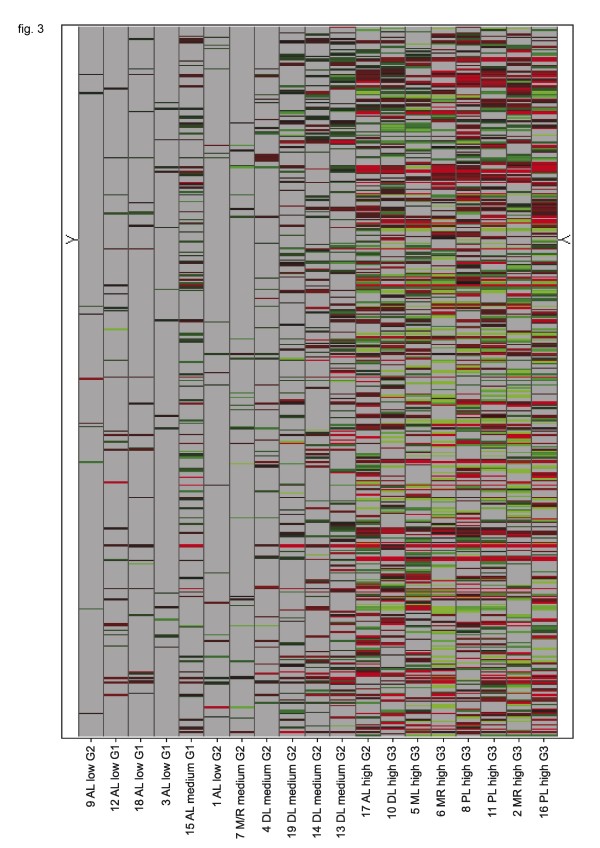
The alteration of gene expression related to apoptotic pathways correlated to the categorisation given above. Low responders also did also not respond with relevant gene expression changes of "apoptosis genes" whereas the high responders showed a significantly different gene expression profile concerning apoptosis related genes compared to the untreated control. In all, we found 464 genes with expression changes that are related to apoptotic pathways. The single genes that were differentially expressed in the medium and high responder group only partly overlapped with the low responder group. The heterogeneity of the response patterns of apoptotis related genes is illustrated in figure 3.
Figure 3.
Heatmap of expression changes of genes related to apoptotic pathways after incubation with doxorubicin for 24 h. Horizontal rows represent individual genes; vertical columns represent individual samples (left to right: low responders to high responders). Color range: Brightest red (Change call increased (change p-value < 0.002) and Signal Log Ratio > 1): SLR = >2 (4× or higher), Black: SLR = 0 (no change); not visible as a consequence of the filtering process, Brightest green (Change call decreased (change p-value > 0.998) and Signal Log Ratio < -1): SLR < -2 (0.25× or smaller). Grey: no value (requirements for a reliably measured target not met). Calculated in Affymetrix comparison analysis (MAS5.0 algorithm) and at least one present call in the two síngle array analyses compared in the comparison analysis.
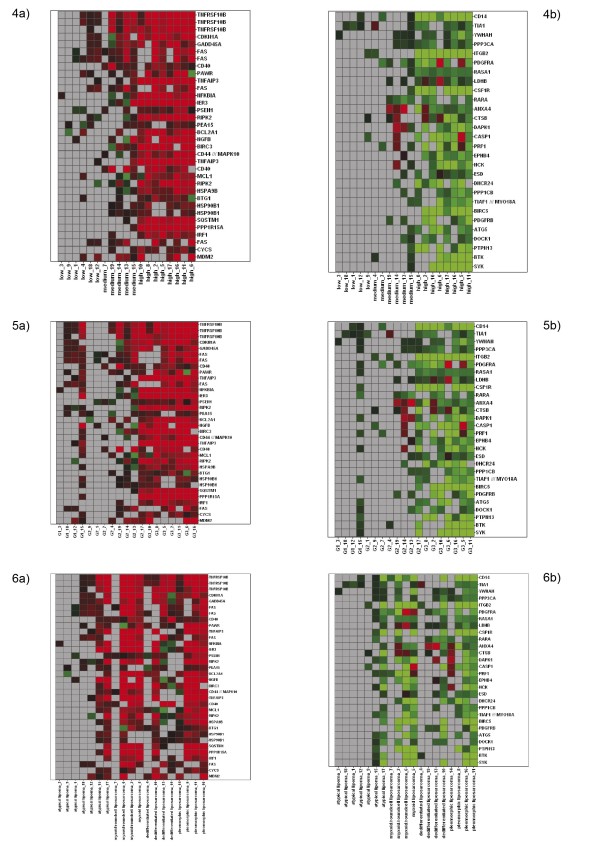
Although the diversity of changes in gene expression was large, some apoptosis related genes showed similar expression changes in the tumor samples, especially the high grade tumors (G2 and G3) or high responders. Figure 4 focus on expression changes in these genes. The apoptosis related genes most often affected by doxorubicin treatment are mentioned below. Due to their large number, we only refer to the genes that were differentially (up- or down-) regulated in more than 50% of the probes (samples). Some of the genes that were found up-regulated in the majority of the probes (tumors) could be found down-regulated in some other samples and vice versa. The heatmaps provided illustrate the similarity of the expression of these selected genes in correlation to responder group, grading, and histological subtype (fig. 4. The intrinsic and the extrinsic apoptotic pathway, as well as transcription factors and genes, so far only marginally associated with apoptotic pathways. A summary of the genes and their expression changes is given in table 2 and 3.
Figure 4.
Heatmaps of expression changes of selected genes associated with apoptotic pathways after incubation with doxorubicin for 24 h ordered by responder group (5 a,b), grading (6 a,b), and histologic subtype (7 a/b). Expression changes as determined by comparison analysis were considered only if the probeset showed at least one P detection in untreated/treated sample pairs. Excluded expression changes are shown in grey. Only probesets with expression changes in 7 or more samples are given. Horizontal rows represent individual probesets. Vertical columns represent individual samples (left to right: low responders to high responders); gene symbols are indicated to the right. Gene expression changes are indicated by a continuous scale; the brightest red indicating ≥ 4-fold up-regulation; and, brightest green ≥ 4-fold down-regulated gene expression relative to the untreated control sample.
Table 2.
Summary of the genes that were up-regulated by doxorubicin treatment, including the log ratios
| Samples with increased expression (n) | Gene symbol | Mean log ratio | Range of log ratio | Samples with decreased expression (n) | Mean log ratio | (Range of) log ratio |
| 15 | TNFRSF10B | 1.96 | 0.41/4.49 | 0 | - | - |
| 12 | CDKN1A | 1.85 | 0.59/4.10 | 1 | - | -0.99 |
| 12 | GADD45A | 1.44 | 0.58/2.88 | 1 | - | -0.59 |
| 12 | FAS | 1.36 | 0.27/3.87 | 1 | - | -0.28 |
| 12 | CD40 | 0.94 | 0.39/2.11 | 0 | - | - |
| 11 | PAWR | 1.16 | 0.33/2.51 | 1 | - | -1.25 |
| 11 | TNFAIP3 | 2.16 | 0.23/3.67 | 0 | - | - |
| 10 | NFKBIA | 2.04 | 0.43/3.34 | 2 | -0.57 | -0.45/-0.69 |
| 10 | IER3 | 3.81 | 0.71/6.08 | 2 | -0.60 | -0.36/-0.84 |
| 10 | PSEN1 | 0.84 | 0.23/1.61 | 2 | -0.46 | -0.46 |
| 10 | RIPK2 | 2.02 | 0.42/3.65 | 2 | -0.60 | -0.46/-0.74 |
| 10 | PEA15 | 0.61 | 0.23/0.96 | 1 | - | -0.68 |
| 10 | BCL2A1 | 2.18 | 1.03/5.08 | 1 | - | -0.98 |
| 10 | NGFB | 3.06 | 0.40/5.80 | 1 | - | -1.12 |
| 10 | BIRC3 | 2.06 | 0.63/3.23 | 1 | - | -0.93 |
| 10 | CD44 | 1.57 | 0.26/3.09 | 1 | - | -0.56 |
| 9 | MCL1 | 1.46 | 0.67/2.08 | 3 | -0.65 | -0.26/-1.21 |
| 9 | HSPA9 | 1.25 | 0.46/1.95 | 2 | -0.50 | -0.21/-0.79 |
| 9 | BTG1 | 0.77 | 0.31/1.50 | 1 | - | -1.06 |
| 9 | HSP90B1 | 0.80 | 0.21/1.10 | 0 | - | - |
| 9 | SQSTM1 | 2.03 | 0.27/3.23 | 0 | - | - |
| 9 | PPP1R15A | 3.16 | 0.65/4.40 | 0 | - | - |
| 9 | IRF1 | 1.38 | 0.60/2.94 | 0 | - | - |
| 9 | CYCS | 0.82 | 0.34/1.37 | 0 | - | - |
| 9 | MDM2 | 1.76 | 0.33/3.58 | 0 | - | - |
Negative log ratios stand for down-regulated genes.
Table 3.
Summary of the genes that were down-regulated by doxorubicin treatment, including the log ratios
| Samples with decreased expression (n) | Gene symbol | Mean log ratio | Range of log ratio | Samples with increased expression (n) | Mean log ratio | Range of log ratio |
| 11 | CD14 | -2.99 | -0.27/-5.38 | 0 | - | - |
| 10 | TIA1 | -0.68 | -0.24/-1.05 | 1 | - | 0.77 |
| 10 | YWHAH | -0.72 | -0.27/-1.80 | 0 | - | - |
| 10 | PPP3CA | -0.69 | -0.34/-1.14 | 0 | - | - |
| 10 | ITGB2 | -2.87 | -0.58/-5.11 | 0 | - | - |
| 9 | PDGFRA | -1.32 | -0.42/-3.30 | 2 | 2.62 | 1.21/4.03 |
| 9 | RASA1 | -0.93 | -0.42/-1.17 | 0 | - | - |
| 8 | LDHB | -0.60 | -0.32/-1.25 | 2 | 1.11 | 0.64/1,58 |
| 8 | CSF1R | -3.05 | -0.63/-5.08 | 0 | - | - |
| 8 | RARA | -0.95 | -0.51/-1.23 | 0 | - | - |
| 7 | ANXA4 | -1.10 | -0.64/-1.16 | 4 | 1.37 | 0.84/2.33 |
| 7 | CTSB | -0.93 | -0.23/-1.97 | 3 | 0.94 | 0.82/1.13 |
| 7 | DAPK1 | -1.35 | -0.59/-2.07 | 2 | 1,40 | 1.19/1.60 |
| 7 | CASP1 | -1.69 | -0.71/-2.90 | 2 | 1.44 | 0.97/1.90 |
| 7 | PRF1 | -1.68 | -0.54/-3.68 | 2 | 0.79 | 0.67/0.91 |
| 7 | EPHB4 | -1.30 | -0.68/-2.34 | 1 | - | 0.30 |
| 7 | HCK | -1.79 | -0.39/-3.65 | 1 | - | 1.08 |
| 7 | ESD | -0.76 | -0.44/-0.95 | 1 | - | 0.36 |
| 7 | DHCR24 | -1.97 | -0.75/-4.34 | 0 | - | - |
| 7 | PPP1CB | -0.99 | -0.62/-1.56 | 0 | - | - |
| 7 | MYO18A/TIAF1 | -1.23 | -0.67/-2.02 | 0 | - | - |
| 7 | BIRC5 | -2.95 | -1.17/-4.91 | 0 | - | - |
| 7 | PDGFRB | -1.28 | -0.61/-2.26 | 0 | - | - |
| 7 | ATG5 | -1.39 | -0.61/-3.19 | 0 | - | - |
| 7 | DOCK1 | -0.86 | -0.40/-1.23 | 0 | - | - |
| 7 | PTPN13 | -1.97 | -0.69/-3.17 | 0 | - | - |
| 7 | BTK | -1.78 | -0.39/-3.28 | 0 | - | - |
| 7 | SYK | -4.26 | -0.34/-8.06 | 0 | - | - |
Positive log ratios stand for up-regulated genes.
The results of the microarray analysis were validated using rtPCR. The general Pearson correlation of the expression changes measured in all selected genes in the tumor probes was high (0.913). The correlation coefficients for the single candidate genes are given in table 4.
Table 4.
Pearson coefficient calculated for the candidate genes describing the correlation of the gene expression changes measured microarray and rtPCR.
| Gene symbol | Pearson coefficient |
| CD40 | 0.945 |
| CDKN1A | 1.000 |
| FAS | 0.813 |
| GADD45A | 0.848 |
| ITGB2 | 0.999 |
| NFKBIA | 0.882 |
| PAWR | 0.913 |
| PPP3CA | 0.638 |
| TNFAIP3 | 0.997 |
| TNFRSF10B | 0.998 |
| YWHAH | 0.370 |
Discussion
Gene expression profiling has already been helpful in categorizing distinct subtypes of sarcomas by profile clustering [33-35] and identifying subtype specific changes in gene expression in liposarcoma, e.g. abnormal expression of cell cycle regulators in FUS-DDIT3 carrying liposarcomas [34,36-43] and even provided potential targets for new therapeutic agents like important mediators in cell cycle regulation, e.g. MDM2 [44-48].
Gene expression profiling studies on liposarcomas have already shown that this entity presents a somewhat similar expression pattern with malignant fibrous histiocytoma and leimyosarcoma [31,49] and that highly differentiated lesions cluster with lipoma whereas the dedifferentiated tumors cluster with myxoid/round cell liposarcomas [50]; however no clear correlation between expression patterns and histological subtype could be detected [37]. Another difficulty in the assessment of gene expression profiles is the inter- and intra-tumoral heterogeneity. Several subtypes with different expression patterns and histologic features can often be found within one same tumor [51,52].
Liposarcomas are classified into several types based on histological findings and cytological aberrations – well differentiated (atypical lipoma), dedifferentiated, myxoid, round cell tumors and pleomorphic. The risk of distant metastasis grows with the grading of the lesion to up to 75% in pleomorphic sarcoma. Myxoid tumors with a greater than 5% round cell component, most dedifferentiated, and pleomorphic liposarcomas are considered high grade lesions [4,53-55].
There are no markers to clearly identify liposarcoma cells. S100, CD34, and others may be helpful (as well as cytogenetic techniques) as they can identify aberrations indicating myxoid/round cell sarcoma; however, they cannot identify liposarcoma cells with absolute certainty [56]. In our series, we relied on a proper tumor dissection and preparation of the specimens to ensure, the isolated tissue mainly consisted of liposarcoma cells as previously described by Sreekantaiah et al. [57] and Lehnhardt et al [58], but have to admit that results may be partly falsified by residual tumor stroma cells accidentally co-cultivated within the liposarcoma samples.
Liposarcoma cells showed diverse gene expression patterns before and after incubation with doxorubicin. Tumors of the same histologic subgroup did not cluster together concerning their overall gene expression. The correlation of the number of differentially regulated genes to the tumor grading, and, to a lesser extent, to the histological subgroup after doxorubicin incubation, may be caused by the tumor associated up-regulation of cell metabolism and the therefore greater effect of any interference. The overall expression patterns and the ones of the apoptosis related genes were also very heterogenous (fig. 2, 3). This finding is concordant to the results of other studies with soft tissue sarcoma cells [59] and may partly be explained by the known inter- and intratumoral heterogeneity in soft tissue sarcomas [51,60].
Interestingly the myxoid and the myxoid/round cell liposarcomas clustered together in figure 2 and 3 except from the tumor 7 that was pre-treated with chemotherapy. If that is a coincidence or may be interpreted as a kind of selectional process that could have eliminated the high grade parts of the tumor leaving the residual to cluster closer to the low grade sarcomas can not be determined.
The predescribed low clinical response rates of low grade sarcoma correlate to our findings that low grade liposarcoma, especially atypical lipoma, showed almost no response to doxorubicin on gene expression level [61]. However, some expression changes in response to doxorubicin treatment, observed especially in the high responder and in the high grade group were similar and are focussed on the figure 4. According to the large number of apoptosis related genes, we identified the first five ones that were differentially expressed in more than 50% of the samples and limit further explanation to these with special reference to apoptotic function in sarcoma or previous reports concerning doxorubicin treatment, although the understanding of their exact functions is limited. The up-regulated genes beyond the mentioned five are summarized in table 5.
Table 5.
Summary of genes beyond the ones already mentioned in the text that were found to be up-regulated by doxorubicin treatment in more than 50% of the probes.
| Gene symbol | Gene name | Probes upregulated | Additional information | Apoptotic function |
| PAWR | PRKC (protein kinase C) apoptosis WT1 (Wilms tumor gene) regulator protein |
11 | STS with high WT1 mRNA expression levels have poorer outcome than those with low levels [89,90]. Ecteinascidin that has been shown to be effective against STS also increases expression of PAWR [92]. PAWR inhibits the PKC (atypical protein kinase)-NF-(kappa)B (nuclear factor-(kappa)B)-XIAP pathway [91]. |
proapoptotic |
| TNFAIP3 | tumor necrosis factor, alpha-induced protein 3 | 11 | TNFAIP3 down-regulates the TNF-α-induced NFκB signalling pathway [93,94]. | antiapoptotic |
| NFKBIA | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha | 10 | The doxorubicin analogon DA-125 reduces proliferation in HT1080 fibrosarcoma cells through a NFKB dependent pathway [96,97]. MDM2 (upregulated in 9 samples in our series) is overexpressed in several liposarcoma subtypes [58] and increases NFKB activity in a p53 dependent manner and thereby leads to doxorubicin resistance [98]. |
proapoptotic/antiapoptotic |
| IER3 | immediate early response 3 | 10 | IER3 function is increased by p53, that is induced by doxorubicin. IER3 is involved in cell cycle arrest and programmed cell death [99,100]. | proapoptotic |
| PSEN1 | presenilin 1 | 10 | Effects are mediated via Bcl-2 interaction [101]. | proapoptotic |
| RIPK2 | receptor-interacting serine-threonine kinase 2 | 10 | RIPK2 is cell death inducing and NFKB activating, via caspase 1 activation [102,103]. | proapoptotic |
| PEA15 | phosphoprotein enriched in astrocytes 15 | 10 | RPEA15 is regulating caspase-3 function in epidermal cells [104], but has not yet been associated with doxorubicin treatment or apoptosis in sarcoma cells. | antiapoptotic |
| BCL2A1 | BCL2 (B-cell CLL/lymphoma 2)-related protein A1 | 10 | BCL2A1 stabilizes the mitochondrial membrane [105,106]. | antiapoptotic |
| NGFB | nerve growth factor, beta polypeptide | 10 | NGF reduces apoptosis induced by chemotherapeutics in sarcoma cells [109]. | antiapoptotic |
| BIRC3 | baculoviral IAP repeat-containing 3 | 10 | BIRC3 is associated with chemotherapy resistance in Ewing sarcoma, rhabdomyosarcoma [110] and prostatic cancer [111]. | antiapoptotic |
| CD44 | cell surface glycoprotein CD44 | 10 | CD44 is a proapoptotic factor in FAS mediated apoptosis in sarcoma cells [112], but is also connected to cancer drug resistance [113]. CD44 has successfully been used as a target for liposomal encapsuled doxorubicin [114]. |
proapoptotic/antiapoptotic |
Up-regulated genes
As previously shown, doxorubicin could overcome TRAIL resistance in a variety of sarcoma cell lines. An up-regulation of the TRAIL-R2, increasing the susceptibility to apoptosis inducing agents such as TRAIL, may be a possible explanation [62-65], as we found the Trail receptor 2 gene expression increased in 15 probes.
The second most frequently up-regulated gene was cyclin-dependent kinase inhibiton 1A (CDKN1A), a downstream target of p53, has has already been shown to be involved in cell cycle arrest and apoptosis induction by doxorubicin in sarcoma cells. CDKN1A can act as apositive regulator of senescence-like terminal proliferation arrest, but its function seems neither sufficient nor absolutely required for a treatment response to doxorubicin in tumor cells, especially soft tissue sarcoma [66-70].
GADD45A, a potent inhibitor of the c-Jun N-terminal kinase (JNK) cascade and NFKBIA, inhibits transcription factors associated with tumor growth [71-74] and was up-regulated by doxorubicin in 12 probes. In a variety of soft tissue sarcoma cell lines GADD45A was found to increase cell cycle arrest and apoptosis {Zhu, 2008 #165}. For rhabdomyosarcoma, increased GADD45A has previously been associated with less aggressive tumor behaviour [75].
FAS, a member of the tumor necrosis factor receptor family, seems to be an important mediator in doxorubicin induced apoptosis. Its effects were shown to be dependant on metalloproteinases in soft tissue sarcoma and Ewing sarcoma. These metalloproteinases have been associated with aggressive tumor behaviour and may promote invasiveness and the occurrence metastasis of malignant cells [76-78].
Another member of the TNF receptor superfamily, CD40, was also up-regulated by doxubicin in our experiments. To the authors' knowledge, CD40 has not yet been associated with doxorubicin treatment in liposarcoma, but its expression in soft tissue sarcoma was associated with an unfavourable outcome [79], whereas Lodge et al. could show a beneficial effect of antibody mediated CD40 activation in elimination of fibrosarcoma in nude mice [80].
Down-regulated genes
Among the down-regulated genes related to apoptotic pathways CD14, a receptor marking apoptotic cells was found in 8 probes. Increased CD14 has been associated with apoptosis induction and cell clearance, especially mediated by macrophages, but is expressed by a variety of other cells too [81-83]. Its role in terms of cell death mediation in sarcoma cells has not yet been examined, therefore interpretation of this finding remains difficult.
TIA-1, which encodes an RNA-binding protein with translation-regulatory functions has already been reported to be up-regulated in tumor specimens post-treatment with TNF alpha in soft tissue sarcomas. It was further hypothesized that TIA-1 could mediate death receptor mediated apoptosis in soft tissue sarcoma and that its overexpression might sensitize endothelial cells to proapoptotic stimuli present in the tumor microenvironment and enhance NK cell cytotoxic activity against cancer cells [84].
YWHAH, or 14-3-3 eta, is a member of the dimeric 14-3-3 family of signal transduction proteins that specifically binds to phosphorylated serine on a variety of signalling molecules, such as Bcl-2, MDMX, and Bax, thereby promoting cell survival and acting antiapoptotic in several tumor cells [85-87]. On the other hand, it is supposed to be associated with tumorigenesis through its binding interaction with gremlin1 [88]. Therefore the issue of further studies should be awaited before interpreting this finding.
PPP3CA (CCN1/Cyr61) is susceptible to various growth factors and promotes cell proliferation, adhesion, and differentiation and plays important roles in angiogenesis. Additionally, PPP3CA has been associated with tumorigenesis. It was reported that PPP3CA exerts its functions via interacting with integrins as well as heparan sulfate proteoglycan. By activating NF-kappaB and tyrosine kinase signalling pathways, PPP3CA is not only able to control cell growth, but also induce or suppress apoptosis in a cell type-specific manner [89,90]. To the authors' knowledge, it has so far not been reported in context with liposarcoma or doxorubicin treatment.
Integrin B2 (ITGB2) is known to play a role in mediating apoptosis [91] and chemotherapy resistance. Although it is widely attributed to white blood cells, it is also expressed in a variety of other benign and malignant cells and seems to play a major role in cell invasion and migration [92].
Conclusion
In summary, pro- and antiapoptotic genes were found up- as well as down-regulated with a dominance of up-regulation of proapoptotic genes. The heterogeneous expression profiles reflect the heterogeneous reaction of liposarcomas to doxorubicin therapy. A lot of genes we found differentially expressed have not yet been associated with apoptosis in liposarcoma or doxorubicin treatment. Therefore it is not possible to relate our findings to other studies. Our study shows that the low clinical response rates of highly differentiated liposarcoma correlate to minimal changes in the expression patterns and that only high grade tumors, especially myxoid/roundcell and pleomorphic tumors, respond to doxorubicin on gene expression level. In most cases, this response seems to be based on an increase of the extrinsic pathway such as TRAIL Receptor 2 and FAS but also members of the intrinsic pathway such as BCL2A1 were found to be differentially expressed. Interestingly several factors (NFKBIA, GADD45A, RIPK2, and PAWR) point to the NFKB transcriptional factor as possible mediator of doxorubicin effects.
Our results indicate that gene expression profiling may be a promising approach to improve the understanding of the diverse modes of programmed cell death in liposarcoma following doxorubicin treatment and can provide a molecular basis for new chemotherapeutic strategies.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AD developed the study design, coordinated the work, interpreted the data and prepared the manuscript. LKH carried out and interpreted the microarrays. AMC carried out statistical analyses, have given substantial contribution to conception and design as well manuscript preparation. OM improved the study design and corrected the manuscript. HJ prepared the figures and gathered patients data. HHH was helpful in preparing the manuscript and conceived the work. HUS was helpful in preparing the manuscript and conceived the work. ML carried out cell culture and developed the idea, study design and conceived the work
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
We thank Amanda Daigeler for her formal English revision of the manuscript.
Contributor Information
Daigeler Adrien, Email: adrien.daigeler@rub.de.
Klein-Hitpass Ludger, Email: ludger.klein-hitpass@uk-essen.de.
Chromik Ansgar Michael, Email: ansgar_chromik@gmx.de.
Müller Oliver, Email: oliver.mueller@mpi-dortmund.mpg.de.
Hauser Jörg, Email: joerg.hauser@rub.de.
Homann Heinz-Herbert, Email: heinz.homann@rub.de.
Steinau Hans-Ulrich, Email: hans-ulrich.steinau@bergmannsheil.de.
Lehnhardt Marcus, Email: marcus.lehnhardt@rub.de.
References
- Lehnhardt M, Kuhnen C, Drucke D, Homann HH, Joneidi Jafari H, Steinau HU. [Liposarcoma of the extremities: recent developments in surgical therapy – analysis of 167 patients] Chirurg. 2004;75:1182–1190. doi: 10.1007/s00104-004-0900-2. [DOI] [PubMed] [Google Scholar]
- Kuhnen C, Lehnhardt M, Steinau HU, Muller KM. [liposarcoma. Aspects of pathomorphology – an analysis of 209 tumos] Chirurg. 2004;75:1151–1158. doi: 10.1007/s00104-004-0901-1. [DOI] [PubMed] [Google Scholar]
- Mack TM. Sarcomas and other malignancies of soft tissue, retroperitoneum, peritoneum, pleura, heart, mediastinum, and spleen. Cancer. 1995;75:211–244. doi: 10.1002/1097-0142(19950101)75:1+<211::AID-CNCR2820751309>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Dei Tos AP. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol. 2000;4:252–266. doi: 10.1053/adpa.2000.8133. [DOI] [PubMed] [Google Scholar]
- Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244:381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel T, Fletcher CD. Lipomatous tumours of soft tissues: an update. Virchows Arch. 1995;427:353–363. doi: 10.1007/BF00199383. [DOI] [PubMed] [Google Scholar]
- Hartmann JT, Patel S. New drug developments for patients with metastatic soft tissue sarcoma. Curr Oncol Rep. 2005;7:300–306. doi: 10.1007/s11912-005-0054-5. [DOI] [PubMed] [Google Scholar]
- Jones RL, Fisher C, Al-Muderis O, Judson IR. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer. 2005;41:2853–2860. doi: 10.1016/j.ejca.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Gortzak E, Azzarelli A, Buesa J, Bramwell VH, van Coevorden F, van Geel AN, Ezzat A, Santoro A, Oosterhuis JW, van Glabbeke M, et al. A randomised phase II study on neo-adjuvant chemotherapy for 'high-risk' adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. doi: 10.1016/S0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- Frustaci S, Gherlinzoni F, De Paoli A, Bonetti M, Azzarelli A, Comandone A, Olmi P, Buonadonna A, Pignatti G, Barbieri E, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19:1238–1247. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- Eilber FC, Eilber FR, Eckardt J, Rosen G, Riedel E, Maki RG, Brennan MF, Singer S. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann Surg. 2004;240:686–695. doi: 10.1097/01.sla.0000141710.74073.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meta-analysis-Group Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350:1647–1654. doi: 10.1016/S0140-6736(97)08165-8. [DOI] [PubMed] [Google Scholar]
- Mack LA, Crowe PJ, Yang JL, Schachar NS, Morris DG, Kurien EC, Temple CL, Lindsay RL, Magi E, DeHaas WG, et al. Preoperative chemoradiotherapy (modified Eilber protocol) provides maximum local control and minimal morbidity in patients with soft tissue sarcoma. Ann Surg Oncol. 2005;12:646–653. doi: 10.1245/ASO.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, Verweij J, Santoro A, Buesa J, Tursz T. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens – a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53:153–159. doi: 10.1007/s00262-003-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- Hostanska K, Vuong V, Rocha S, Soengas MS, Glanzmann C, Saller R, Bodis S, Pruschy M. Recombinant mistletoe lectin induces p53-independent apoptosis in tumour cells and cooperates with ionising radiation. Br J Cancer. 2003;88:1785–1792. doi: 10.1038/sj.bjc.6600982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan M, Yu D, Lang A, Li L, Pollock RE. Wild type p53 sensitizes soft tissue sarcoma cells to doxorubicin by down-regulating multidrug resistance-1 expression. Cancer. 2001;92:1556–1566. doi: 10.1002/1097-0142(20010915)92:6<1556::AID-CNCR1482>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Coley HM. Drug resistance studies using fresh human ovarian carcinoma and soft tissue sarcoma samples. Keio J Med. 1997;46:142–147. doi: 10.2302/kjm.46.142. [DOI] [PubMed] [Google Scholar]
- Toffoli G, Frustaci S, Tumiotto L, Talamini R, Gherlinzoni F, Picci P, Boiocchi M. Expression of MDR1 and GST-pi in human soft tissue sarcomas: relation to drug resistance and biological aggressiveness. Ann Oncol. 1992;3:63–69. doi: 10.1093/oxfordjournals.annonc.a058073. [DOI] [PubMed] [Google Scholar]
- Komdeur R, Molenaar WM, Zwart N, Hoekstra HJ, Berg E van den, Graaf WT van der. Multidrug resistance proteins in primary and metastatic soft-tissue sarcomas: down-regulation of P-glycoprotein during metastatic progression. Anticancer Res. 2004;24:291–295. [PubMed] [Google Scholar]
- Kuhnen C, Muller KM, Steinau HU, Lehnhardt M. [Therapy-induced tumor regression in adult soft tissue sarcomas-morphological findings] Pathologe. 2004 doi: 10.1007/s00292-004-0720-7. [DOI] [PubMed] [Google Scholar]
- Skubitz KM, Skubitz AP. Differential gene expression in leiomyosarcoma. Cancer. 2003;98:1029–1038. doi: 10.1002/cncr.11586. [DOI] [PubMed] [Google Scholar]
- Hwang RF, Hunt KK. Experimental approaches to treatment of soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12:499–521. doi: 10.1016/S1055-3207(03)00006-1. [DOI] [PubMed] [Google Scholar]
- Schofield D, Triche TJ. cDNA microarray analysis of global gene expression in sarcomas. Curr Opin Oncol. 2002;14:406–411. doi: 10.1097/00001622-200207000-00007. [DOI] [PubMed] [Google Scholar]
- Lehnhardt M, Klein-Hitpass L, Kuhnen C, Homann HH, Daigeler A, Steinau HU, Roehrs S, Schnoor L, Steinstraesser L, Mueller O. Response rate of fibrosarcoma cells to cytotoxic drugs on the expression level correlates to the therapeutic response rate of fibrosarcomas and is mediated by regulation of apoptotic pathways. BMC Cancer. 2005;5:74. doi: 10.1186/1471-2407-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22:2805–2811. doi: 10.1038/sj.onc.1206366. [DOI] [PubMed] [Google Scholar]
- Rebbaa A, Chou PM, Emran M, Mirkin BL. Doxorubicin-induced apoptosis in caspase-8-deficient neuroblastoma cells is mediated through direct action on mitochondria. Cancer Chemother Pharmacol. 2001;48:423–428. doi: 10.1007/s002800100375. [DOI] [PubMed] [Google Scholar]
- Rebbaa A, Chou PM, Mirkin BL. Factors secreted by human neuroblastoma mediated doxorubicin resistance by activating STAT3 and inhibiting apoptosis. Mol Med. 2001;7:393–400. [PMC free article] [PubMed] [Google Scholar]
- Nielsen TO, West RB, Linn SC, Alter O, Knowling MA, O'Connell JX, Zhu S, Fero M, Sherlock G, Pollack JR, et al. Molecular characterisation of soft tissue tumours: a gene expression study. Lancet. 2002;359:1301–1307. doi: 10.1016/S0140-6736(02)08270-3. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RB, Harvell J, Linn SC, Liu CL, Prapong W, Hernandez-Boussard T, Montgomery K, Nielsen TO, Rubin BP, Patel R, et al. Apo D in soft tissue tumors: a novel marker for dermatofibrosarcoma protuberans. Am J Surg Pathol. 2004;28:1063–1069. doi: 10.1097/01.pas.0000126857.86186.4c. [DOI] [PubMed] [Google Scholar]
- Skubitz KM, Cheng EY, Clohisy DR, Thompson RC, Skubitz AP. Differential gene expression in liposarcoma, lipoma, and adipose tissue. Cancer Invest. 2005;23:105–118. doi: 10.1081/CNV-50432. [DOI] [PubMed] [Google Scholar]
- Baird K, Davis S, Antonescu CR, Harper UL, Walker RL, Chen Y, Glatfelter AA, Duray PH, Meltzer PS. Gene expression profiling of human sarcomas: insights into sarcoma biology. Cancer Res. 2005;65:9226–9235. doi: 10.1158/0008-5472.CAN-05-1699. [DOI] [PubMed] [Google Scholar]
- Goransson M, Elias E, Stahlberg A, Olofsson A, Andersson C, Aman P. Myxoid liposarcoma FUS-DDIT3 fusion oncogene induces C/EBP beta-mediated interleukin 6 expression. Int J Cancer. 2005;115:556–560. doi: 10.1002/ijc.20893. [DOI] [PubMed] [Google Scholar]
- Fritz B, Schubert F, Wrobel G, Schwaenen C, Wessendorf S, Nessling M, Korz C, Rieker RJ, Montgomery K, Kucherlapati R, et al. Microarray-based copy number and expression profiling in dedifferentiated and pleomorphic liposarcoma. Cancer Res. 2002;62:2993–2998. [PubMed] [Google Scholar]
- Italiano A, Cardot N, Dupre F, Monticelli I, Keslair F, Piche M, Mainguene C, Coindre JM, Pedeutour F. Gains and complex rearrangements of the 12q13-15 chromosomal region in ordinary lipomas: the "missing link" between lipomas and liposarcomas? Int J Cancer. 2007;121:308–315. doi: 10.1002/ijc.22685. [DOI] [PubMed] [Google Scholar]
- Bassett MD, Schuetze SM, Disteche C, Norwood TH, Swisshelm K, Chen X, Bruckner J, Conrad EU, 3rd, Rubin BP. Deep-seated, well differentiated lipomatous tumors of the chest wall and extremities: the role of cytogenetics in classification and prognostication. Cancer. 2005;103:409–416. doi: 10.1002/cncr.20779. [DOI] [PubMed] [Google Scholar]
- Meis-Kindblom JM, Sjogren H, Kindblom LG, Peydro-Mellquist A, Roijer E, Aman P, Stenman G. Cytogenetic and molecular genetic analyses of liposarcoma and its soft tissue simulators: recognition of new variants and differential diagnosis. Virchows Arch. 2001;439:141–151. doi: 10.1007/s004280100423. [DOI] [PubMed] [Google Scholar]
- Sandberg AA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: liposarcoma. Cancer Genet Cytogenet. 2004;155:1–24. doi: 10.1016/j.cancergencyto.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Engstrom K, Willen H, Kabjorn-Gustafsson C, Andersson C, Olsson M, Goransson M, Jarnum S, Olofsson A, Warnhammar E, Aman P. The myxoid/round cell liposarcoma fusion oncogene FUS-DDIT3 and the normal DDIT3 induce a liposarcoma phenotype in transfected human fibrosarcoma cells. Am J Pathol. 2006;168:1642–1653. doi: 10.2353/ajpath.2006.050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanska J, Virolainen M, Tarkkanen M, Wiklund T, Asko-Seljavaara S, Tukiainen E, Elomaa I, Blomqvist C, Knuutila S. Overrepresentation of 1q21-23 and 12q13-21 in lipoma-like liposarcomas but not in benign lipomas: a comparative genomic hybridization study. Cancer Genet Cytogenet. 1997;99:14–18. doi: 10.1016/S0165-4608(96)00436-0. [DOI] [PubMed] [Google Scholar]
- Fukukawa C, Nakamura Y, Katagiri T. Molecular target therapy for synovial sarcoma. Future Oncol. 2005;1:805–812. doi: 10.2217/14796694.1.6.805. [DOI] [PubMed] [Google Scholar]
- Ishibe T, Nakayama T, Okamoto T, Aoyama T, Nishijo K, Shibata KR, Shima Y, Nagayama S, Katagiri T, Nakamura Y, et al. Disruption of fibroblast growth factor signal pathway inhibits the growth of synovial sarcomas: potential application of signal inhibitors to molecular target therapy. Clin Cancer Res. 2005;11:2702–2712. doi: 10.1158/1078-0432.CCR-04-2057. [DOI] [PubMed] [Google Scholar]
- Lubieniecka JM, Nielsen TO. cDNA microarray-based translational research in soft tissue sarcoma. J Surg Oncol. 2005;92:267–271. doi: 10.1002/jso.20409. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Fukukawa C, Katagiri T, Okamoto T, Aoyama T, Oyaizu N, Imamura M, Toguchida J, Nakamura Y. Therapeutic potential of antibodies against FZD 10, a cell-surface protein, for synovial sarcomas. Oncogene. 2005;24:6201–6212. doi: 10.1038/sj.onc.1208780. [DOI] [PubMed] [Google Scholar]
- Singer S, Socci ND, Ambrosini G, Sambol E, Decarolis P, Wu Y, O'Connor R, Maki R, Viale A, Sander C, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res. 2007;67:6626–6636. doi: 10.1158/0008-5472.CAN-07-0584. [DOI] [PubMed] [Google Scholar]
- Segal NH, Pavlidis P, Antonescu CR, Maki RG, Noble WS, DeSantis D, Woodruff JM, Lewis JJ, Brennan MF, Houghton AN, et al. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am J Pathol. 2003;163:691–700. doi: 10.1016/S0002-9440(10)63696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji T, Kanda H, Kitagawa T, Kadota K, Asai R, Takahashi K, Kawaguchi N, Matsumoto S, Hayashizaki Y, Okazaki Y, et al. Clinico-molecular study of dedifferentiation in well-differentiated liposarcoma. Biochem Biophys Res Commun. 2004;314:1133–1140. doi: 10.1016/j.bbrc.2003.12.203. [DOI] [PubMed] [Google Scholar]
- Kim JI, Choi KU, Lee IS, Moon TY, Lee CH, Kim HW, Kim JY, Park DY, Sol MY. Gene expression in mixed type liposarcoma. Pathology. 2006;38:114–119. doi: 10.1080/00313020600557078. [DOI] [PubMed] [Google Scholar]
- Bui NB, Coindre JM, Maree D, Trojani M. Liposarcoma: patterns of tumor differentiation following induction chemotherapy. Oncology. 1984;41:170–173. doi: 10.1159/000225816. [DOI] [PubMed] [Google Scholar]
- Gebhard S, Coindre JM, Michels JJ, Terrier P, Bertrand G, Trassard M, Taylor S, Chateau MC, Marques B, Picot V, et al. Pleomorphic liposarcoma: clinicopathologic, immunohistochemical, and follow-up analysis of 63 cases: a study from the French Federation of Cancer Centers Sarcoma Group. Am J Surg Pathol. 2002;26:601–616. doi: 10.1097/00000478-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21:271–281. doi: 10.1097/00000478-199703000-00002. [DOI] [PubMed] [Google Scholar]
- McCormick D, Mentzel T, Beham A, Fletcher CD. Dedifferentiated liposarcoma. Clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994;18:1213–1223. doi: 10.1097/00000478-199412000-00004. [DOI] [PubMed] [Google Scholar]
- Kindblom LG. Lipomatous tumors-how we have reached our present views, what controversies remain and why we still face diagnostic problems: a tribute to Dr Franz Enzinger. Adv Anat Pathol. 2006;13:279–285. doi: 10.1097/01.pap.0000213053.00060.5a. [DOI] [PubMed] [Google Scholar]
- Sreekantaiah C, Karakousis CP, Leong SP, Sandberg AA. Cytogenetic findings in liposarcoma correlate with histopathologic subtypes. Cancer. 1992;69:2484–2495. doi: 10.1002/1097-0142(19920515)69:10<2484::AID-CNCR2820691017>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Lehnhardt M, Muehlberger T, Kuhnen C, Brett D, Steinau HU, Jafari HJ, Steinstraesser L, Muller O, Homann HH. Feasibility of chemosensitivity testing in soft tissue sarcomas. World J Surg Oncol. 2005;3:20. doi: 10.1186/1477-7819-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner DE, Bastar JD, Randall RL. Doxorubicin induces cell senescence preferentially over apoptosis in the FU-SY-1 synovial sarcoma cell line. J Orthop Res. 2006;24:1163–1169. doi: 10.1002/jor.20169. [DOI] [PubMed] [Google Scholar]
- Francis P, Fernebro J, Eden P, Laurell A, Rydholm A, Domanski HA, Breslin T, Hegardt C, Borg A, Nilbert M. Intratumor versus intertumor heterogeneity in gene expression profiles of soft-tissue sarcomas. Genes Chromosomes Cancer. 2005;43:302–308. doi: 10.1002/gcc.20191. [DOI] [PubMed] [Google Scholar]
- Goss G, Demetri G. Medical management of unresectable, recurrent low-grade retroperitoneal liposarcoma: integration of cytotoxic and non-cytotoxic therapies into multimodality care. Surg Oncol. 2000;9:53–59. doi: 10.1016/S0960-7404(00)00023-2. [DOI] [PubMed] [Google Scholar]
- Evdokiou A, Bouralexis S, Atkins GJ, Chai F, Hay S, Clayer M, Findlay DM. Chemotherapeutic agents sensitize osteogenic sarcoma cells, but not normal human bone cells, to Apo2L/TRAIL-induced apoptosis. Int J Cancer. 2002;99:491–504. doi: 10.1002/ijc.10376. [DOI] [PubMed] [Google Scholar]
- Komdeur R, Meijer C, Van Zweeden M, De Jong S, Wesseling J, Hoekstra HJ, Graaf WT van der. Doxorubicin potentiates TRAIL cytotoxicity and apoptosis and can overcome TRAIL-resistance in rhabdomyosarcoma cells. Int J Oncol. 2004;25:677–684. doi: 10.3892/ijo.25.3.677. [DOI] [PubMed] [Google Scholar]
- Bouralexis S, Findlay DM, Atkins GJ, Labrinidis A, Hay S, Evdokiou A. Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: resensitisation with chemotherapy. Br J Cancer. 2003;89:206–214. doi: 10.1038/sj.bjc.6601021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouralexis S, Clayer M, Atkins GJ, Labrinidis A, Hay S, Graves S, Findlay DM, Evdokiou A. Sensitivity of fresh isolates of soft tissue sarcoma, osteosarcoma and giant cell tumour cells to Apo2L/TRAIL and doxorubicin. Int J Oncol. 2004;24:1263–1270. doi: 10.3892/ijo.24.5.1263. [DOI] [PubMed] [Google Scholar]
- Ganjavi H, Gee M, Narendran A, Freedman MH, Malkin D. Adenovirus-mediated p53 gene therapy in pediatric soft-tissue sarcoma cell lines: sensitization to cisplatin and doxorubicin. Cancer Gene Ther. 2005;12:397–406. doi: 10.1038/sj.cgt.7700798. [DOI] [PubMed] [Google Scholar]
- Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, Roninson IB. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18:4808–4818. doi: 10.1038/sj.onc.1203078. [DOI] [PubMed] [Google Scholar]
- Van Valen F, Fulda S, Schafer KL, Truckenbrod B, Hotfilder M, Poremba C, Debatin KM, Winkelmann W. Selective and nonselective toxicity of TRAIL/Apo2L combined with chemotherapy in human bone tumour cells vs. normal human cells. Int J Cancer. 2003;107:929–940. doi: 10.1002/ijc.11503. [DOI] [PubMed] [Google Scholar]
- Li W, Fan J, Bertino JR. Selective sensitization of retinoblastoma protein-deficient sarcoma cells to doxorubicin by flavopiridol-mediated inhibition of cyclin-dependent kinase 2 kinase activity. Cancer Res. 2001;61:2579–2582. [PubMed] [Google Scholar]
- Vigneron A, Roninson IB, Gamelin E, Coqueret O. Src inhibits adriamycin-induced senescence and G2 checkpoint arrest by blocking the induction of p21waf1. Cancer Res. 2005;65:8927–8935. doi: 10.1158/0008-5472.CAN-05-0461. [DOI] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, et al. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- Lauricella M, Emanuele S, D'Anneo A, Calvaruso G, Vassallo B, Carlisi D, Portanova P, Vento R, Tesoriere G. JNK and AP-1 mediate apoptosis induced by bortezomib in HepG2 cells via FasL/caspase-8 and mitochondria-dependent pathways. Apoptosis. 2006;11:607–625. doi: 10.1007/s10495-006-4689-y. [DOI] [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. Distinct signaling pathways in TRAIL-versus tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2006;26:8136–8148. doi: 10.1128/MCB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T, Koyama T, Koyama T, Imakiire A, Yamamoto K, Furuhata M, Toyota H, Mizuguchi J. C-jun N-terminal kinase activation is required for apoptotic cell death induced by TNF-related apoptosis-inducing ligand plus DNA-damaging agents in sarcoma cell lines. Anticancer Res. 2006;26:1153–1160. [PubMed] [Google Scholar]
- Kappler R, Bauer R, Calzada-Wack J, Rosemann M, Hemmerlein B, Hahn H. Profiling the molecular difference between Patched- and p53-dependent rhabdomyosarcoma. Oncogene. 2004;23:8785–8795. doi: 10.1038/sj.onc.1208133. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001;61:577–581. [PubMed] [Google Scholar]
- Poulaki V, Mitsiades CS, Mitsiades N. The role of Fas and FasL as mediators of anticancer chemotherapy. Drug Resist Updat. 2001;4:233–242. doi: 10.1054/drup.2001.0210. [DOI] [PubMed] [Google Scholar]
- Li W, Bertino JR. Fas-mediated signaling enhances sensitivity of human soft tissue sarcoma cells to anticancer drugs by activation of p38 kinase. Mol Cancer Ther. 2002;1:1343–1348. [PubMed] [Google Scholar]
- Ottaiano A, De Chiara A, Perrone F, Botti G, Fazioli F, De Rosa V, Mozzillo N, Ravo V, Morrica B, Gallo C, et al. Prognostic value of CD40 in adult soft tissue sarcomas. Clin Cancer Res. 2004;10:2824–2831. doi: 10.1158/1078-0432.CCR-0139-03. [DOI] [PubMed] [Google Scholar]
- Lodge A, Yu P, Nicholl MB, Brown IE, Jackson CC, Schreiber K, Sugg SL, Schreiber H, Shilyansky J. CD40 ligation restores cytolytic T lymphocyte response and eliminates fibrosarcoma in the peritoneum of mice lacking CD4+ T cells. Cancer Immunol Immunother. 2006;55:1542–1552. doi: 10.1007/s00262-006-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power CP, Wang JH, Manning B, Kell MR, Aherne NF, Wu QD, Redmond HP. Bacterial lipoprotein delays apoptosis in human neutrophils through inhibition of caspase-3 activity: regulatory roles for CD14 and TLR-2. J Immunol. 2004;173:5229–5237. doi: 10.4049/jimmunol.173.8.5229. [DOI] [PubMed] [Google Scholar]
- Gregory CD, Devitt A. CD14 and apoptosis. Apoptosis. 1999;4:11–20. doi: 10.1023/A:1009673914340. [DOI] [PubMed] [Google Scholar]
- Mills KI, Woodgate LJ, Gilkes AF, Walsh V, Sweeney MC, Brown G, Burnett AK. Inhibition of mitochondrial function in HL60 cells is associated with an increased apoptosis and expression of CD14. Biochem Biophys Res Commun. 1999;263:294–300. doi: 10.1006/bbrc.1999.1356. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Provenzano M, Lise M, Nitti D, Rossi CR. Increased TIA-1 gene expression in the tumor microenvironment after locoregional administration of tumor necrosis factor-alpha to patients with soft tissue limb sarcoma. Int J Cancer. 2003;107:317–322. doi: 10.1002/ijc.11369. [DOI] [PubMed] [Google Scholar]
- Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nat Rev Neurosci. 2003;4:752–762. doi: 10.1038/nrn1197. [DOI] [PubMed] [Google Scholar]
- Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Dai MS, Lu SZ, Xu Y, Luo Z, Zhao Y, Lu H. 14-3-3gamma binds to MDMX that is phosphorylated by UV-activated Chk1, resulting in p53 activation. Embo J. 2006;25:1207–1218. doi: 10.1038/sj.emboj.7601010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkoong H, Shin SM, Kim HK, Ha SA, Cho GW, Hur SY, Kim TE, Kim JW. The bone morphogenetic protein antagonist gremlin 1 is overexpressed in human cancers and interacts with YWHAH protein. BMC Cancer. 2006;6:74. doi: 10.1186/1471-2407-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Du XY. Functional properties and intracellular signaling of CCN1/Cyr61. J Cell Biochem. 2007;100:1337–1345. doi: 10.1002/jcb.21194. [DOI] [PubMed] [Google Scholar]
- Rees H, Williamson D, Papanastasiou A, Jina N, Nabarro S, Shipley J, Anderson J. The MET receptor tyrosine kinase contributes to invasive tumour growth in rhabdomyosarcomas. Growth Factors. 2006;24:197–208. doi: 10.1080/08977190600759923. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, Cullere X. Neutrophil beta2 integrins: moderators of life or death decisions. Trends Immunol. 2005;26:388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Sotobori T, Ueda T, Oji Y, Naka N, Araki N, Myoui A, Sugiyama H, Yoshikawa H. Prognostic significance of Wilms tumor gene (WT1) mRNA expression in soft tissue sarcoma. Cancer. 2006;106:2233–2240. doi: 10.1002/cncr.21861. [DOI] [PubMed] [Google Scholar]
- Coosemans A, Nik SA, Caluwaerts S, Lambin S, Verbist G, Van Bree R, Schelfhout V, de Jonge E, Dalle I, Jacomen G, et al. Upregulation of Wilms' tumour gene 1 (WT1) in uterine sarcomas. Eur J Cancer. 2007;43:1630–1637. doi: 10.1016/j.ejca.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Martinez N, Sanchez-Beato M, Carnero A, Moneo V, Tercero JC, Fernandez I, Navarrete M, Jimeno J, Piris MA. Transcriptional signature of Ecteinascidin 743 (Yondelis, Trabectedin) in human sarcoma cells explanted from chemo-naive patients. Mol Cancer Ther. 2005;4:814–823. doi: 10.1158/1535-7163.MCT-04-0316. [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Duran A, Collado M, Carrascosa MJ, Martin-Caballero J, Flores JM, Diaz-Meco MT, Moscat J, Serrano M. Tumour-suppression activity of the proapoptotic regulator Par4. EMBO Rep. 2005;6:577–583. doi: 10.1038/sj.embor.7400421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissing D, Mouritzen H, Jaattela M. TNF-induced mitochondrial changes and activation of apoptotic proteases are inhibited by A20. Free Radic Biol Med. 1998;25:57–65. doi: 10.1016/S0891-5849(98)00043-4. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Yamamoto M, Miyagishi M, Taira K, Nakanishi M, Fujita T, Akira S, Yamamoto N, Yamaoka S. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol. 2005;174:1507–1512. doi: 10.4049/jimmunol.174.3.1507. [DOI] [PubMed] [Google Scholar]
- Campbell KJ, O'Shea JM, Perkins ND. Differential regulation of NF-kappaB activation and function by topoisomerase II inhibitors. BMC Cancer. 2006;6:101. doi: 10.1186/1471-2407-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Chung HJ, Min HY, Park EJ, Hong JY, Kim WB, Kim SH, Lee SK. Inhibitory effect of DA-125, a new anthracyclin analog antitumor agent, on the invasion of human fibrosarcoma cells by down-regulating the matrix metalloproteinases. Biochem Pharmacol. 2005;71:1–2. doi: 10.1016/j.bcp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Hu Q, Zhou MX, Liu SY, Zhang LQ, Liu AQ, Guo YJ, Song Y. [Regulation of NF-kappaB/P65 by MDM2 in acute lymphoblastic leukemia in childhood] Zhonghua Er Ke Za Zhi. 2003;41:921–924. [PubMed] [Google Scholar]
- Grobe O, Arlt A, Ungefroren H, Krupp G, Folsch UR, Schmidt WE, Schafer H. Functional disruption of IEX-1 expression by concatemeric hammerhead ribozymes alters growth properties of 293 cells. FEBS Lett. 2001;494:196–200. doi: 10.1016/S0014-5793(01)02344-4. [DOI] [PubMed] [Google Scholar]
- Schafer H, Trauzold A, Sebens T, Deppert W, Folsch UR, Schmidt WE. The proliferation-associated early response gene p22/PRG1 is a novel p53 target gene. Oncogene. 1998;16:2479–2487. doi: 10.1038/sj.onc.1201788. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Nakaya Y, Du Z, Yamane T, Shirane M, Kudo T, Takeda M, Takebayashi K, Noda Y, Nakayama KI, et al. Interaction of presenilins with FKBP38 promotes apoptosis by reducing mitochondrial Bcl-2. Hum Mol Genet. 2005;14:1889–1902. doi: 10.1093/hmg/ddi195. [DOI] [PubMed] [Google Scholar]
- McCarthy JV, Ni J, Dixit VM. RIP2 is a novel NF-kappaB-activating and cell death-inducing kinase. J Biol Chem. 1998;273:16968–16975. doi: 10.1074/jbc.273.27.16968. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Duncan M, Hart J, Hertlein E, Guttridge DC, Wewers MD. ASC directs NF-kappaB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J Immunol. 2006;176:4979–4986. doi: 10.4049/jimmunol.176.8.4979. [DOI] [PubMed] [Google Scholar]
- Formisano P, Perruolo G, Libertini S, Santopietro S, Troncone G, Raciti GA, Oriente F, Portella G, Miele C, Beguinot F. Raised expression of the antiapoptotic protein ped/pea-15 increases susceptibility to chemically induced skin tumor development. Oncogene. 2005;24:7012–7021. doi: 10.1038/sj.onc.1208871. [DOI] [PubMed] [Google Scholar]
- Nagy B, Lundan T, Larramendy ML, Aalto Y, Zhu Y, Niini T, Edgren H, Ferrer A, Vilpo J, Elonen E, et al. Abnormal expression of apoptosis-related genes in haematological malignancies: overexpression of MYC is poor prognostic sign in mantle cell lymphoma. Br J Haematol. 2003;120:434–441. doi: 10.1046/j.1365-2141.2003.04121.x. [DOI] [PubMed] [Google Scholar]
- Ushizawa K, Takahashi T, Kaneyama K, Hosoe M, Hashizume K. Cloning of the bovine antiapoptotic regulator, BCL2-related protein A1, and its expression in trophoblastic binucleate cells of bovine placenta. Biol Reprod. 2006;74:344–351. doi: 10.1095/biolreprod.105.042655. [DOI] [PubMed] [Google Scholar]
- Astolfi A, Nanni P, Landuzzi L, Ricci C, Nicoletti G, Rossi I, Lollini PL, De Giovanni C. An anti-apoptotic role for NGF receptors in human rhabdomyosarcoma. Eur J Cancer. 2001;37:1719–1725. doi: 10.1016/S0959-8049(01)00190-3. [DOI] [PubMed] [Google Scholar]
- Kilic M, Kasperczyk H, Fulda S, Debatin KM. Role of hypoxia inducible factor-1 alpha in modulation of apoptosis resistance. Oncogene. 2007;26:2027–2038. doi: 10.1038/sj.onc.1210008. [DOI] [PubMed] [Google Scholar]
- Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep. 2005;14:993–997. [PubMed] [Google Scholar]
- Hauptschein RS, Sloan KE, Torella C, Moezzifard R, Giel-Moloney M, Zehetmeier C, Unger C, Ilag LL, Jay DG. Functional proteomic screen identifies a modulating role for CD44 in death receptor-mediated apoptosis. Cancer Res. 2005;65:1887–1896. doi: 10.1158/0008-5472.CAN-04-3571. [DOI] [PubMed] [Google Scholar]
- Misra S, Ghatak S, Toole BP. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem. 2005;280:20310–20315. doi: 10.1074/jbc.M500737200. [DOI] [PubMed] [Google Scholar]
- Eliaz RE, Nir S, Marty C, Szoka FC., Jr Determination and modeling of kinetics of cancer cell killing by doxorubicin and doxorubicin encapsulated in targeted liposomes. Cancer Res. 2004;64:711–718. doi: 10.1158/0008-5472.CAN-03-0654. [DOI] [PubMed] [Google Scholar]