Abstract
The survival of naïve T cells is compromised in the absence of molecules encoded by the major histocompatibility complex (MHC) while antigen-experienced T cells survive. We hypothesized that survival pressures in an in vivo, MHC-deficient environment would permit enrichment of less frequent antigen-experienced autoreactive cells at the expense of the majority of antigen naïve T cells. To test this hypothesis, we generated MHC class I- and class II-deficient mice in NOD and C57Bl/6 (B6) backgrounds, and examined the capacity of adoptively transferred autoimmune-prone NOD T cells, or non-autoimmune prone naïve B6 T cells, respectively, to reject transplanted wild-type pancreatic islets or transplantable tumors in the MHC-deficient mice. In the MHC-deficient environment, CD4 T cells acquired self-hostile properties (islet rejection and tumor invasion) that were independent from their genetic propensity for autoreactivity, while CD8 T cells required appropriate prior exposure to antigen in order to survive and function (reject tumor) in this environment; however, disengagement of Tob1, a negative regulator of proliferation, led to a reverse phenotype with regard to persistence of CD4 and CD8 T cells in the MHC-deficient environment. Our data suggest that self-peptide/MHC interactions have dual roles to facilitate survival and restrain autoreactivity, thus acting as integral components of an intrinsic network of negative regulation that maintains tolerance.
Keywords: T cells, MHC, Sensitization, Desensitization, Negative regulation, Tolerance
Introduction
Peripheral T cells can be induced into what has been termed ‘lymphopenia-induced proliferation’ or ‘homeostatic proliferation’ (HP) by a T lymphopenic environment. Lessened competition for the cytokine interleukin-7 (IL-7) seems to be a major driving force for HP, although recent data suggest that HP may occur under more specialized conditions associated with increased concentrations of IL-2 and IL-15 [1–4]. The control of HP is multifactorial and often favors memory T cells over naïve T cells; for example, clonal competition promotes expansion of memory cells at the expense of naïve cells [5, 6], cytokine responsiveness similarly tilts the composition of the reconstituted population towards cells with memory phenotypes (MP) [7], and regulatory (CD4/CD25/FoxP3+) T cells (Treg) also can contribute to the balance of naïve and memory cells that repopulate a lymphopenic environment [8].
Specifically, the tempo of IL-7-mediated HP is slow [9], HP driven by constitutive levels of IL-15 is more rapid, generating memory phenotype cells [4], and HP driven by elevated levels of IL-2/IL-15 is rapid with differentiation into both effector and memory phenotype cells [2]. While the slow paced (IL-7-dependent) HP seems to be restrained by signals delivered through CD24 expressed in bone marrow-derived dendritic cells (DC) [10], differentiation from naïve T cells to MP T cells following rapid IL-2 and IL-15-dependent HP is comparable to events driven by encounter with foreign antigen. Presumably, HP leads to attenuation of intrinsic pathways that maintain T cells in a non-proliferative state, such as those controlled by Cbl-b [11], Krupple-like factor-2 (KLF-2) [12, 13], Tob1 [14], Fox-O [15], and nuclear factor of activated T cells-c2 (NFATc2) [16]. In particular, NFATc2 and Tob1 may have non-redundant functions to maintain naïve T cell quiescence, respectively, by preventing activation of CDK4 and by supporting p27 expression [14, 16].
T cell receptor (TCR)/major histocompatibility complex (MHC) interactions also are important determinants of HP. A majority of naïve T cells die or make at most a few divisions if the lymphopenic periphery is devoid of MHC [17–21], and HP may itself be driven by T cell recognition of self-peptide/MHC complexes. It is, therefore, not surprising that these self-reactive, HP-driven T cells have been associated with both autoimmunity and anti-tumor immunity [22, 23]. CD4 and CD8 T cells largely share these characteristics of HP, although CD4 T cells expand less in response to MHC and CD8 T cells are more sensitive to its absence. The different rates of erosion for CD4 and CD8 T cells in MHC-deficient environments can be explained at least partly by differential survival properties between these subsets, as the absence of MHC class I leads to death of naïve CD8 T cells by Fas-dependent and mitochondrial-independent, Bcl-xL-resistant mechanisms [24], but it also is likely that MP CD8 cells fail to proliferate efficiently in the absence of MHC [7, 25]. Nevertheless, once naïve T cells transit into MP, their dependence on MHC for persistence in the periphery is reduced or no longer apparent [17, 26–28].
Experiments using transgenic T cells show that HP applies to most TCR transgenic animals, although the intensity of proliferation varies from one TCR transgenic line to another [2]. The especially weak proliferation of HY transgenic T cells may reflect the observation that they have below average affinity for self-peptide/MHC and/or low TCR promiscuity [29, 30]. The capacity of TCR transgenic T cells to undergo HP once in the MP state is dissociated from the avidity of TCR to MHC-self complexes [28], which fits the properties of the MP in general.
At least two models have been proposed to explain the predilection for self-peptide/MHC reactivity to sustain HP. One model proposed by Grossman and Paul posits that even though thymic selection deletes T cells bearing receptors with high affinity for self-antigens, it ultimately selects for T cells with measurable reactivity for self-antigens. Grossman and Paul surmised that these T cells are ‘tuned’ or desensitized by continued interactions in the periphery with MHC and self-peptides against which they were selected in the thymus. Recent data from Singer’s lab suggests that this “tuning,” at least in CD8 cells, may be mediated at least partly by complementary signals from self-peptide MHC (delivered through the TCR), and from IL-7 to regulate the levels of co-receptor expression [31]. Conversely, Stefanova et al. [32] hypothesized that the self-peptide/MHC interactions in the periphery keep T cells in a state of partial activation or ‘sensitization’ in which survival is the outcome, and that allows T cells to respond to foreign antigen. These models are not necessarily contradictory when MHC is present, as the MHC-dependent desensitization or ‘tuning’ of naïve T cells proposed by Grossman and Paul could operate to control incipient self-reactive T cells (i.e., T cells with intrinsic potential for autoreactivity), while the MHC-dependent ‘sensitization’ proposed by Stefanova and Germain could enhance high-affinity T cell receptor interactions to non-self antigens [33].
However, the models appear to make different predictions in the absence of MHC: for desensitization as described by Grossman and Paul, self reactivity might be predicted to result in the absence of tuning when interactions with MHC are removed, while in the sensitization model of Stefanova and Germain, the absence of MHC-dependent sensitization should lead to anergy or death rather than the unleashing of autoimmunity.
The original rationale behind the experiments presented here was that the predilection for survival of memory, but not naïve T cells in the absence of MHC might provide an opportunity to enrich antigen experienced memory T cells in vivo [17, 34]—in our case, offering an opportunity to study the intrinsic properties of disease causing T cells and the role of intrinsic negative regulatory molecules in this process. Specifically, we reasoned that ‘parking’ T cells from diabetic NOD mice in an MHC-less environment would promote in vivo selection where the bulk of naïve (and presumably non-autoreactive) T cells would be unable to survive, leaving only the less frequent, antigen-experienced (including autoreactive) T cells. While the experiments in diabetes-prone NOD mice bore out this prediction, we unexpectedly encountered similar results when the experiments were performed in diabetes-resistant B6 mice. Therefore, it appeared that parking naïve T cells in an MHC-less environment led to the generation and/or survival of autoreactive T cells independent from the genetic background or the donor’s propensity for autoimmunity.
To further verify this, we developed a tumor rejection model to test the properties of naïve or memory T cells undergoing HP in an MHC-positive environment, or preferential survival without HP in an MHC-deficient environment. The results showed that naïve T cells that are allowed to undergo HP in an MHC positive, lymphopenic environment do not destroy tumor targets unless they are effectively primed, while the absence of MHC allows latently reactive cells to persist, leading to tumor destruction without priming. Finally, our data suggest that, as is true in the case of stimulation by CD24-deficient DC, CD4 T cells with impairments of intrinsic factors that negatively regulate cell cycle progression fail to survive and expand in MHC-deficient environments, yet this largely eliminates the MHC requirement for survival and expansion of CD8 T cells. We interpret the sum of our data as supporting the prediction from the ‘tuning’ model that MHC deficiency unleashes autoreactive cells (i.e., they are no longer desensitized in the absence of MHC).
Methods
Animals and adoptive transfers
NOD/bdc (NOD) mice and C57BL/6-C2D mice (B6 C2D, deficient in MHC class II expression) were kindly provided by Dr. Ron Gill (Barbara Davis Center for Childhood Diabetes, Denver, CO); C57BL/6 (B6) mice, C57BL/6-β2m mice (B6 β2m, deficient in MHC class I expression), and B6 SCID mice were purchased from Jackson Laboratories (Bar Harbor, ME). MHC-deficient NOD mice (henceforth referred to as NOD DKO) and MHC-deficient B6 mice (B6 DKO) were bred in our facility according to the following scheme. First, we generated MHC class II-deficient NOD mice by backcrossing B6 C2D females with NOD males. Offspring from F1 × NOD breedings were screened using 22 microsatellite marker pairs associated with 15 defined Type 1 diabetes (iddm) loci as described in reports of NOD speed congenics [35]. Offspring were tested for inheritance of the disrupted I-A (C2D) allele, and DNA from C2D-positive female mice was further subjected to PCR analysis of the 15 iddm-associated loci. At each locus, mice were assigned as NOD or F1 to indicate inheritance pattern from the mixed strain parent. Females possessing the most NOD loci were backcrossed to NOD males. Subsequent generations were similarly analyzed and bred back to NOD. Following six backcross generations, 18 of the 22 loci were positive for NOD, three of the primer sets were indistinguishable for the NOD and F1 PCR products, and the C2D allele for primers associated with the MHC locus on chromosome 17 was present in the heterozygous state. These offspring were bred with NOD β2m KO mice (provided by D. Serreze, Jackson Laboratories, Bar Harbor, ME) and inheritance of disrupted MHC alleles was confirmed by PCR analysis using microsatellite markers used in congenic typing to verify disruption of the I-A region. B6 DKO mice were generated by breeding B6 C2D and B6 β2m mice, with inheritance of disrupted MHC alleles confirmed by PCR analysis. B6 DKO mice (Abb/B2m [36]) were also purchased from Taconic Farms (Germantown, NY); B6 DKO mice bred in our facility and those purchased from Taconic had comparable numbers of detectable T cells and B cells in peripheral blood and in the lymphoid compartments as determined by flow cytometry and immunohistology. Tob1 knockout mice (bred on a B6 background) were kindly provided by Dr. Tadashi Yamamoto (The Institute of Medical Science, The University of Tokyo, Tokyo, Japan).
For adoptive transfers, T cells were enriched from spleen and draining lymph nodes using a negative selection T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada). After selection, the populations routinely consisted of >96% CD3+ cells. Twenty million (2 × 107) CD3 cells were transferred to each mouse using a single donor to reconstitute each recipient [37]. For experiments evaluating islet autoreactivity, cells were allowed to reach equilibrium in the recipient hosts for 4 weeks prior to chemical ablation of the endogenous pancreatic islets by streptozotocin and implantation of wild-type pancreas under the kidney capsule [38].
For experiments evaluating tumor killing, cells were allowed to reach equilibrium in the recipient hosts for 2 weeks prior to subcutaneous injection of syngeneic Lewis lung (LL) carcinoma cells. Recovery of splenic and lymph node T cells from recipients was predictably higher in hosts after 2 weeks than after 4 weeks, although spleen sizes at the time mice were sacrificed, were not significantly different among the experimental groups whether mice did or did not receive T cells. The histomorphology of the spleens, however, was different among mice that had normal T cell development (B6), mice that did not have T cells (SCID, DKO), and mice that were reconstituted by adoptive T cell transfers. Spleens in SCID and DKO mice consisted mostly of red pulp surrounded by extramedullary hematopoiesis and leukocytes (neutrophils and histiocytes) without discrete white pulp or follicular structures. Adoptive T cell transfers into SCID or DKO mice did not restore the splenic architecture. Although lymphocyte repopulation of the spleen was readily evident, these cells only formed small lymphoid aggregates that did not organize into follicular patterns.
All mice were maintained in a specific pathogen-free animal satellite facility of the University of Colorado Health Sciences Center under normal housing conditions (B6, NOD, Tob1-k/o) or under barrier conditions (SCID, DKO). All protocols and procedures involving live animals were reviewed and approved by the UCDHSC IACUC.
Cells and cell lines
Lewis lung (LL) is a pulmonary carcinoma cell line from B6 mice that expresses Fas but is resistant to FasL-mediated apoptosis and that can be transplanted to form tumors in vivo [39]. Primary mouse spleen cells and lymph node cells were prepared as described [40]. Cells were grown in RPMI 1640 media (Gibco Life Technologies, Rockville, MD) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 10 µM HEPES, 50 µM 2-ME and 10 µg gentamycin in an atmosphere of 5% CO2 at 37°C.
Generation of recombinant adenoviruses
Viruses were prepared from mammalian expression plasmids encoding mutant forms of human FasL. Briefly, FasL and enhanced green fluorescent protein (EGFP) were inserted into the left end of the Ad5 replacing the E1 region and driven by the human ubiquitin promoter in all constructs. Recombinant viruses were generated by using the AdEasy System, confirmed by PCR and amplified in FasL resistant 293-crmA cells as described [41, 42].
Flow cytometry
Antibodies were purchased from BD Bioscience (San Diego, CA) and included anti-CD3 (2C11) conjugated to fluorescein isothiocyanate (FITC), anti-CD19 (6D5) conjugated to tricolor fluorochrome, anti-CD14 (rmC5-3) conjugated to phycoerythrin (PE), anti-CD4 (Gk1.5) conjugated to PE or FITC, anti-CD8 (53–6.7) conjugated to PE or FITC, anti-CD44 (IM7) conjugated to allophycocyanin (APC), anti-CD62L (MEL-14) conjugated to FITC, and anti-CD25 (7D4) conjugated to FITC. Anti-CD20 antibodies were kindly provided by Dr. Tom Tedder (Duke University) [43]. Staining was done using routine protocols with goat gamma globulin (Jackson ImmunoResearch Labs, West Grove, PA) as a blocking reagent [40]. As needed, gates were set to include lymphocytes (CD3+ and CD19+ cells) to determine the percentage of T cells in single cell suspensions from tumors. At least 10,000 cells per sample were collected for analysis of spleen and lymph node preparations, and >100,000 cells were collected for the analysis of tumor samples.
Tumor induction
For heterotopic tumor growth, 5 × 105 unmodified LL cells or 5 × 105 LL cells infected with Ad-FasL constructs were injected subcutaneously in the flank of B6 mice [37]. Tumor volume was estimated from two-dimensional measurements using the formula L × W 2 × 0.52. Mice used as donors for adoptive transfer were humanely sacrificed after 16 days; mice that were used to determine tumor burden were sacrificed between 15 and 22 days when tumors reached 15 mm in a single diameter. Tumor burden for all mice was determined at necropsy; the mean (±SD) three-dimensional tumor burdens in mm3 for each group was 622 (179) in B6 mice sacrificed at day 16 (T cell donors), 950 (440) in SCID mice without adoptive T cell transfers, 2,012 (903) in SCID mice that received naïve T cells, 1,030 (706) in SCID mice that received antigen-experienced T cells that did not reject tumors in the donor mice (AE-NR T cells), 1,189 (640) in DKO mice without adoptive T cell transfers, 907 (1,144) in DKO mice that received naïve T cells, and 570 (631) in DKO mice that received AE-NR T cells. Six to twelve mice per group were used for the experiments.
Histopathologic examination
Skin biopsy samples, spleens, lymph nodes, and tumors were collected and equally divided for staining, fixation in O.C.T. Tissue-TEK medium (stored at −80°C) or fixation in 10% neutral-buffered formalin. Tissues were sectioned in 5-μm slices and stained with hematoxylin and eosin for microscopic examination. Immunohistology test development and/or staining was contracted to IHC Services (Smithville, TX) and performed by Dr. J. Wojcieszyn. Antibodies used included anti-CD3 (Sigma), anti-CD4, anti-CD8, and anti-CD18 (Santa Cruz Biotechnology), anti-FoxP3 (eBioscience), and secondary reagents included rabbit anti-goat IgG (Bethyl Labs, Montgomery, TX) and goat anti-rat IgG (Serotec, Raleigh, NC). Semi-quantitative estimates of FoxP3 cells in tissues and tumors were done in consecutive images analyzed using ImageJ software (NIH). Briefly, digital images were captured at 400× magnification using an Olympus BH60 microscope with a cooled digital Penguin camera. Fields were deconvoluted into digital slices and a color replacement algorithm was applied to convert red and blue stains into distinct shades of gray in a linear 256 bit gray scale. Quantification was done over three slices encompassing the “top”, “middle”, and “bottom” of the image by pixel density over ten overlapping fields, each ~50 μm in diameter where the area was normalized to account for cellularity using “object counts” with each “object” identified by a nucleus and delineated by a cell membrane assigned a number or “event” equivalent to a cell. Red staining for FoxP3 and CD3 was quantified from Stack-2. Color images were converted into binary images, appropriate scaling was set for the magnification, a threshold was applied to recognize discrete round cells in the image, and a total count was obtained. Next, a threshold was applied to define positively stained cells in the native 8-bit image using the Threshold Colours Plugin for ImageJ and the count was repeated. The number of FoxP3 cells was normalized to the number of CD3 cells in each tissue analyzed.
Statistical analyses
Tumor-free survival and T cell reconstitutions were compared using 2 × 2 tables analyzed with no prior expectation or alternative for independence (i.e., 2-tails) with Fisher’s exact test. Tumor size, spleen size and rates of tumor growth between any two groups were compared using the Student’s t test. A value of P < 0.05 was considered statistically significant.
Results
An MHC-deficient environment leads to islet autoreactivity
The requirement for class I MHC in CD8 T cell survival was first inferred by the inability of CD8 thymic emigrants to seed MHC-deficient peripheral organs [44], with the differential capacity of naïve and memory CD8 T cells to survive in an environment devoid of class I MHC verified in a system using LCMV as a source of antigen to generate immunological memory [17]. Although largely similar, the survival of CD4 T cells in a class II-deficient environment showed some important differences [20, 26]. For example, unlike naïve CD8 cells that die rapidly in the absence of MHC class I, the half-life of naïve CD4 cells in the absence of MHC class II was estimated to be as long as 3–4 weeks.
We examined this paradigm with T cells from diabetes prone NOD mice. As predicted, NOD DKO mice had an approximately normal complement of B cells, but virtually no peripheral T cells. To validate the model, purified T cells from diabetic NOD mice were adoptively transferred to NOD DKO recipients. After allowing the cells to undergo selection in this environment, the animals’ pancreatic islets were ablated with streptozotocin, and the diabetic phenotype was reversed by transplantation of islets from MHC-positive NOD donors [38]. The function of transplanted islets was followed by monitoring blood sugar levels. Experimental controls included transfer of the same islet preparations into non-diabetic NOD and NOD SCID recipients that also had been adoptively transferred with T cells from diabetic NOD mice. The results of these experiments were clear. The transplanted pancreatic islets survived and functioned uneventfully for more than 60 days in normal NOD and NOD SCID recipients, but they were destroyed in 10–26 days (median 18 days) in the NOD DKO recipients (Table 1).
Table 1.
Time to islet rejectiona
| Recipient | Donor strain | |||
|---|---|---|---|---|
| NOD | C57Bl/6 | |||
| Median (N) | Mean (±SD) | Median | Mean (±SD) | |
| WT | >60 (7) | >60 | >60 (4) | >60 |
| SCID | 45 (4) | 45b (17) | >60 (4) | >60 |
| β2m (class I knockout) | >60 (5) | 54c (21.0) | >60 (2) | >60 |
| C2D (class II knockout) | >60 (2) | >60 | >60 (3) | >60 |
| DKO (class I + II knockout) | 18d (4) | 18.5d (9.0) | 9.5d (4) | 9.75d (3.3) |
aRecipient mice received 2 × 107 donor T cells by intravenous injection. After 4 weeks, endogenous pancreatic islets were ablated with streptozotocin, and diabetes was corrected by transplantation of wild-type islets under the kidney capsule. Blood glucose concentrations were used to monitor pancreatic islet function; values >6 mM were indicative of islet graft rejection. Experiments were terminated 60 days after islet transplantation, considered a hallmark of long-term graft survival
bPancreatic grafts in two of four mice functioned >60 days
cTwo animals rejected islet grafts in <60 days, one at 19 days and one at 52 days
dSignificantly different from control (P < 0.05)
We examined whether T cells from autoimmune-prone donors were a pre-requisite for islet destruction in the MHC-deficient environment by repeating the same experiments done in the NOD DKO animals, but instead using non-diabetic, wild-type B6 mice as donors and MHC-deficient B6 mice (B6 DKO) as recipients. The results of these experiments were remarkably similar to those seen in the NOD background. Adoptive transfer of naïve B6 T cells into B6 DKO animals resulted in rejection of pancreatic islets in 6–14 days (median 9.5 days, Table 1).
This raised two important possibilities. First, the DKO mice could be “leaky,” even though they lacked β2-microglobulin, which largely prevents development of CD8 T cells and most NK cells, while also harboring a targeted insertion that knocks out the I-Aβ locus that prevents development of CD4 cells. We did not detect archetypal class I (H-2Db and H-2Kb) and class II (I-A and I-E) molecules in spleen cells from these DKO mice by flow cytometry. Nevertheless, β2m-deficient mice have been reported to have a population of lymphocytes expressing CD8α homodimers (versus the more common CD8α/β heterodimers) with no or significantly reduced surface CD3 [45, 46]. We reasoned that, if these cells were responsible for the observed phenotype in the NOD DKO and in the DKO mice, we would observe similar results in β2m-single knockout (SKO) mice. Second, in the case of class II knockouts, deletion of the I-A β b locus can lead to assembly of atypical (Aα:Eβ) class II heterodimers [47], which may promote alloresponses in mice that are incompletely tolerized. Thus, transfer of CD4 T cells into the I-A bβ environment might generate systemic inflammation leading to islet rejection. If this were the reason for islet rejection in the B6 DKO, we anticipated we would observe similar results in B6 I-A bβ-SKO mice.
Contrary to these two predictions, the phenotype leading to rapid islet rejection was peculiar to the DKO environment. Only two of five MHC class I-deficient (MHC class II wild type) NOD mice rejected wild-type pancreatic islet transplants (one in 19 days, the other in 52 days). Islet rejection was not seen in either the MHC class II-deficient (MHC class I wild type) NOD mice, nor either SKO strain in the B6 background. Thus, we believe alloreactivity is an unlikely explanation for our findings, and rather, the MHC-deficient environment in the DKO mice may have unleashed histocompatible graft destruction that was independent of the donor’s genetic predisposition to autoimmunity.
The MHC-deficient environment enhances tumor reactivity
The apparent autoreactivity unleashed in the MHC-deficient environment was inherent in both autoimmune-prone and normal mouse strains. The islet graft reactivity was independent of lymphopenia per se, given the respective phenotypes in NOD DKO mice (rejection) and NOD SCID mice (no rejection).
If the pancreatic islet graft reactivity in the DKO MHC-deficient environment was a generalized occurrence associated with, for example, a fundamental break in tolerance [27] or simply due to reduced T cell numbers with exuberant MHC-independent expansion of MP cells, such as that reportedly driven by IL-21 that can select for autoreactive cells [48], it should occur with tissues other than islets. To address this, we developed a distinct and simpler (not requiring islet isolation) adoptive transfer model where we could also assess the requirements for antigenic priming and memory T cell generation.
We chose the transplantable, syngeneic Lewis lung (LL) carcinoma derived from B6 mice as a suitable ‘self’ target for analysis that was distinct from islets. LL tumors grow when implanted subcutaneously into normal B6 mice, B6 SCID, and B6 DKO recipients (Table 2) just as syngeneic islet grafts survived in these various B6 recipients. While LL cells do not express tumor antigens that elicit immune responses in normal mice (for example, T cells from tumor bearing mice are incapable of CTL responses in vitro), under appropriate conditions, a polyclonal population of tumor-specific T cells can be generated. Specifically, inoculation of tumor cells infected with an adenovirus containing the Fas ligand (FasL) gene leads to the destruction of the LL tumor in vivo [49]. The targets of FasL are Fas-sensitive host cells, not the LL tumor cells themselves (LL cells are resistant to Fas-dependent apoptosis [49, 50]). The destruction of Fas-positive host cells sets in motion an inflammatory response that destroys the Fas-resistant LL tumor cells, and up to this point, T cells are not required for this process to occur. Yet, the process primes an anti-tumor adaptive immune response. That is, wild-type B6 mice can reject a secondary LL tumor challenge, but B6 SCID mice cannot. Depletion of CD8 T cells or class I MHC deficiency prevents the development of such a response [49, 51]. Consistent with the dependence on CD8 T cells, this protective anti-tumor response generated by FasL gene transfer is specific, as it cannot protect mice from challenge with a tumor distinct from LL [51]. Together, these data indicate that destruction of the primary tumor results in a memory T cell response that is specific for the LL tumor, and which no longer requires FasL.
Table 2.
Growth of heterotopic LL tumors in C57Bl/6 mouse strainsa
| Strain | % Mice with tumors (N) | Median survivalb (days) |
|---|---|---|
| WT | 100 (70) | 17 |
| SCID | 100 (23) | 15 |
| DKO (class I + II knockout) | 100 (9) | 18 |
aMice were injected subcutaneously in the flank with 5 × 105 LL tumor cells. Tumor growth was monitored daily by palpation and verified microscopically at necropsy
bMice were sacrificed when a tumor diameter reached 15 mm
We took advantage of these observations to confirm that the anti-tumor memory response could be adoptively transferred into immunodeficient recipients. We used unchallenged (naïve) B6 mice, B6 mice challenged with unmodified (or mock adenovirus-infected) LL cells, or B6 mice challenged with LL-FasL as T cell donors for adoptive transfer experiments into B6 SCID mice. Each donor ostensibly had unique properties. The first contained T cells that had never seen the tumor, the second had T cells that failed to respond in a fashion leading to rejection (we will refer to these as “antigen-experienced–no rejection”, or AE-NR T cells), while the group treated with LL-FasL generated antigen-experienced T cells that productively responded to and destroyed the tumor. We will refer to these as “antigen-experienced–rejected”, or AE-R T cells. This latter group would be the only one with assured memory T cell responses to the tumor; upon transfer to lymphopenic SCID mice, the other two experimental groups would allow us to evaluate the ability of T cells undergoing HP to potentially overcome tolerance and mount anti-tumor responses.
Twenty million purified T cells (>98% CD3+) derived from spleens and draining lymph nodes were adoptively transferred from these donors (unchallenged, AE-NR, AE-R) into B6 SCID recipients, and the T cells were allowed to undergo HP followed by challenge with wild-type LL tumor cells. We reasoned that, as shown for other tumors, the SCID recipients transferred with T cells from donors challenged with LL-FasL (AE-R) would reject the unmodified LL cells because they had been primed by rejecting the LL-FasL tumor. Whether the T cells from the other donors (unchallenged or AE-NR) would be able to do so with the added impetus of the SCID lymphopenic environment was the experiment under test.
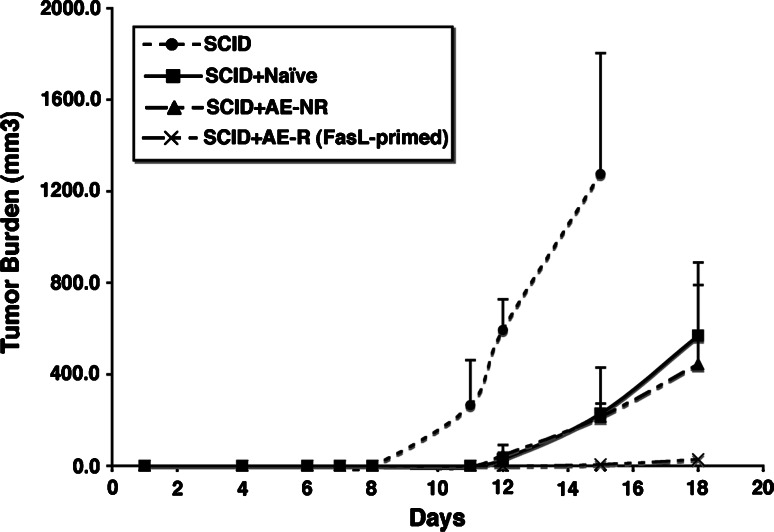
Figure 1 shows that the kinetics of tumor growth in SCID mice that received T cell adoptive transfers were delayed; i.e., the tumor was under immune attack as compared to control, T cell-deficient mice SCID mice. However, there was no difference in the response to the tumor between mice that received unchallenged T cells and the mice that received antigen-experienced T cells that did not reject tumor in the donor mice (AE-NR group). In other words, prior antigen exposure provided no additional advantage for tumor rejection compared to simply having a population of unchallenged (naïve) T cells that underwent HP. On the other hand, SCID mice adoptively transferred with antigen-experienced T cells that rejected FasL-bearing tumors in the donors (AE-R group) were themselves able to reject (or in one case, significantly delay) challenge of wild-type LL cells.
Fig. 1.
Deliberate priming induces anti-tumor immunity that can be adoptively transferred by T cells. B6 mice (five per group) were challenged with saline (naïve), with 5 × 105 unmodified LL cells (“antigen-experienced, no rejection” or AE-NR) or with 5 × 105 LL cells infected with adenovirus-FasL (500 pfu/cell, “antigen-experienced, rejected” or AE-R). After 16 days, mice were sacrificed and T cells were purified from the spleen and draining lymph node. These T cells were then transferred into B6 SCID recipients. A control group (N = 9) received saline injections in lieu of T cells (SCID). The experimental groups were adoptively transferred with pooled T cells from each group of B6 mice above (Naïve, AE-NR, and AE-R). Two weeks later, mice were injected with 5 × 105 LL cells subcutaneously in the flank. Tumor growth was monitored by palpation, and palpable tumors were measured across two diameters. At the end of 2 weeks, mice were sacrificed to evaluate T cell reconstitution and lymphoid infiltrates into the tumors. Data show the mean (±SD) tumor burden as measured by volume (mm3). One mouse from the AE-R group that had a palpable tumor (25 mm3) at day 15 was maintained for an additional 4 days before it was sacrificed to assess T cell reconstitution and lymphoid infiltrates into the tumor
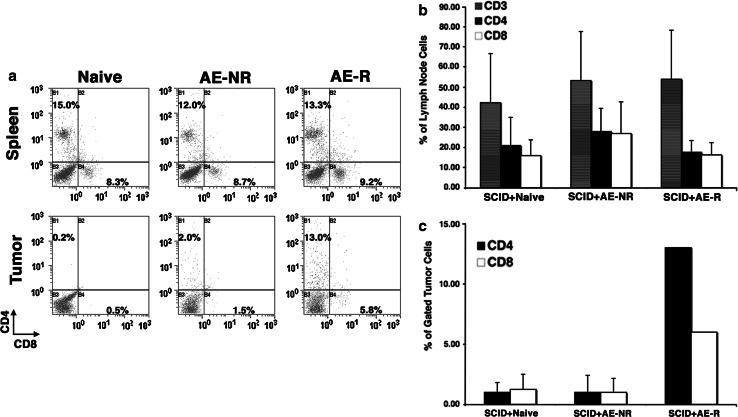
The differences in tumor rejection among these groups were not related to levels of engraftment or T cell survival. CD4 and CD8 T cell populations in spleens and lymph nodes from the recipients were comparable (Fig. 2). However, in the mice that were unable to reject the tumors, “AE-NR” T cells (that were exposed to tumor in the donor but were unable to reject it) appeared to largely ignore these tumors, as evidenced by the paucity of CD4 and CD8 cells found infiltrating the tumor mass. In contrast, “AE-R” T cells obtained from mice primed with FasL-bearing tumors were readily detectable infiltrating the tumor in the SCID recipient that developed a small mass (Fig. 2). Unlike previous reports showing infiltration of tumors by CD4/CD8 double negative T cells with suppressor function (DN TIL) [52], the cumulative number of CD3-positive T cells infiltrating tumors in each group of mice in these experiments was not measurably greater than the additive number of CD4- and CD8-positive T cells.
Fig. 2.
Reconstitution of SCID mice by adoptive T cell transfer and homing to tumors. a Spleens, lymph nodes, and tumors from mice of each group that was adoptively transferred (see Fig. 1) were evaluated for the presence of CD4 and CD8 T cells as a proportion of all viable cells. The top panels show staining on representative spleen samples. The bottom panels show staining for T cells in tumors from the same mice in each group. Tumors were harvested at day 15 for the mice reconstituted with naïve T cells and with antigen-experienced T cells that did not lead to rejection in the donors (AE-NR), and at day 18 from the single mouse that developed a tumor from the group reconstituted with antigen-experienced T cells from donors that rejected their tumors (AE-R, FasL-primed). A region was created around the area where lymphocytes scatter light. CD3+, CD4+, and CD8+ cells were analyzed within that population. Light scatter signatures of CD3+ cells in tumors were comparable to those seen in spleens and lymph nodes. CD4 T cells are shown on the Y-axis and CD8 cells in the X-axis. b Graphical representation of means ± SD (measured in three mice from each group) for the percentage of CD3, CD4, and CD8 T cells as a function of all viable cells in reconstituted lymph nodes. These cell frequencies were equivalent to those found in wild-type mice. Similar data were obtained from spleens from four additional mice examined (not shown). c Graphical representation of means ± SD (measured at day 15 in four mice from each control group, and at day 19 in the single mouse from the FasL-T group that developed a tumor) for CD4, and CD8 T cells in tumors
These results suggested that by itself, HP in the lymphopenic SCID environment was unable to promote rejection of the syngeneic LL tumor, even when T cells were obtained from donors that had been previously exposed to the tumor (but failed to reject it). Yet, it provided an appropriate series of controls to examine how the absence of MHC might influence self-reactivity against syngeneic tumors. If the results observed in the islet transplantation experiments were due to loss of tuning and thus could be generalized to other self-peptide/MHC interactions, the prediction would be that adoptively transferred T cells from donors that had not previously been exposed to tumor would be able to delay or reject growth of LL cells in DKO recipients.
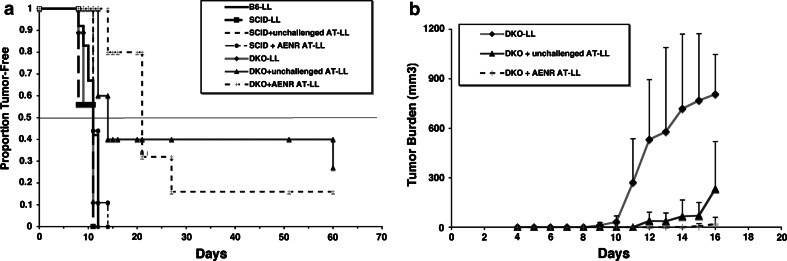
Hence, we repeated the experiment using B6 mice as donors, in this case, unchallenged (naïve) or exposed to unmodified LL cells (the AE-NR condition), and B6 DKO mice as recipients. As was true before, tumor rejection was not observed in the challenged donor group (antigen-experienced, no rejection). After 16 days, naïve and tumor-bearing donors were humanely sacrificed and 20 million purified T cells obtained from spleens and draining lymph nodes were adoptively transferred into the B6 DKO recipients. The T cell grafts were allowed to undergo selection for persistence of MHC-independent subsets, after which the mice were challenged with wild type, unmodified LL cells. The results showed convincing differences vis-à-vis the presence of MHC in the recipient. The median time to tumor formation in both groups of adoptively transferred B6 DKO mice was delayed as compared to the B6 donors or the comparable B6 SCID recipient groups (Fig. 3). In fact, 2/5 mice that received unchallenged T cells and 8/10 mice that received AE-NR T cells survived >21 days without tumor (Fig. 3a). The rate of progression as measured by average of measurable tumor size was slower in the “AE-NR” B6 DKO recipients than in the unchallenged T cell B6 DKO recipients. In addition, while survival between these two groups was not significantly different, both survival and tumor growth rate were slower in both as compared to all other groups (P < 0.05, Fig. 3b).
Fig. 3.
Rejection or delayed tumor growth by adoptive transfer of T cells into an MHC-deficient environment. a Groups of B6 mice were treated with saline (unchallenged) or with 5 × 105 unmodified LL cells (AE-NR) as in Fig. 1. After 16 days, mice were sacrificed and T cells were purified from the spleen and draining lymph nodes. SCID mice or DKO mice then received saline injections or were adoptively transferred (AT) with pooled unchallenged or antigen-experienced T cells as indicated in the legends. Two weeks later, mice were injected with 5 × 105 LL cells subcutaneously in the flank. Tumor growth was monitored by palpation. Data show the proportion of tumor-free mice followed for 60 days. b Mice (groups of 8–12 for WT and groups of 5–11 for DKO) were followed for the first 18 days as in Fig. 2. Tumor burden (in mm3) was determined by measuring two diameters
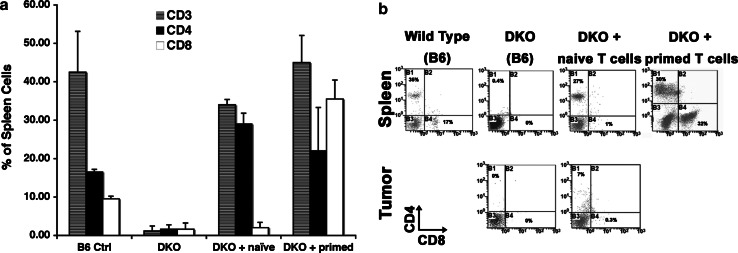
The phenotype of surviving cells in these mice was also different from what we observed in SCID recipients. As predicted from results reported by Mathis’s group [20], a proportion of CD4 cells that had not been exposed to antigen survived in the MHC-deficient environment (i.e., >3 weeks after tumor challenge, compare “DKO” and “DKO+ naïve” panels in Fig. 4), and the cells had a surface phenotype that was consistent with a ‘memory’ population, i.e., they expressed high levels of CD44 (not shown). In addition, these surviving CD4 cells also appeared to infiltrate transplanted tumors (Fig. 4).
Fig. 4.
Reconstitution of DKO mice by adoptive T cell transfer and homing to tumors. a T cell reconstitution in DKO mice receiving no cells, naïve (unchallenged) T cells, or primed T cells (from WT animals that were initially challenged with tumor) was determined as a proportion of all viable cells using spleen samples as in Fig. 2. Data show the means ± SD (measured in four WT mice and three mice from each DKO group) for CD3 (gray), CD4 (black), and CD8 (white) T cells in reconstituted spleens. Similar results were obtained when the analysis was performed in lymph nodes. b Representative dot plots for CD4 and CD8 staining in spleens of control B6, B6 DKO, or B6 DKO mice transferred with naïve T cells, and B6 DKO mice transferred with T cells primed by exposure to tumor in the absence of FasL (AE-NR). Tumors were available for staining from the same DKO and DKO+ naïve mice, and are shown in the bottom middle and right panels. Percentages for CD4 and CD8 cells, respectively, in each panel were: 35/17 (WT B6 spleen), 0.4/0 (DKO spleen + no adoptive transfer), 27/1 (DKO spleen + naïve T cells), 30/21 (DKO spleen + primed AE-NR T cells), 0/0 (DKO tumor + no adoptive transfer), 7/0.3 (DKO tumor + naïve T cells)
In contrast, CD8 cells that had not been exposed to antigen were unable to survive in this environment, but as predicted by the results of Ahmed and colleagues [17], a subpopulation of antigen-experienced CD8 cells survived in the MHC-deficient environment (Fig. 4), despite their inability to reject the primary tumor challenge in the donors. In the animals from this group that developed tumors, the size of the tumors at the time the mice were sacrificed precluded analysis by flow cytometry (cell yields were insufficient), so we can only presume that infiltration of T cells accounted for delayed tumor growth or tumor rejection in B6 DKO mice that received T cells that were primed in the donor by exposure to tumor in the absence of FasL (AE-NR).
In addition to the possibility that MHC “tunes” the CD4 population and contributes to survival of naïve cells, we considered several alternative explanations for our data. First, it has been reported that residual NK cells in DKO strains are highly active and may reject adoptively transferred T cells [26, 53–55]. It is unlikely that this accounts for the differences we observed, unless rejection is highly selective, or peculiar subsets of cells (for e.g., unchallenged wild-type CD4 cells and antigen-experienced CD8 cells) are highly resistant. Also, Treg cells may have led to the altered tumor rejection phenotype in the DKO recipients. When we consider rejection in the SCID mice that received “AE-R” T cells, it is possible that FasL might have depleted Treg cells in the donor prior to transfer [56, 57], but DKO mice received T cells that were not primed by exposure to FasL in the donor. Survival of Treg cells also seems to be dependent on self-peptide/MHC [27, 58], so they may have undergone preferential depletion from the adoptively transferred CD4 subset in the DKO recipients. To address this, we examined the persistence of Treg cells in tissues and tumors from adoptive recipients using immunohistochemistry. The presence of FoxP3-positive cells was examined in samples from three mice for each of the adoptive SCID and DKO recipients as described in “Methods”. The number of FoxP3-positive cells in the spleen and the skin was not significantly different among the recipient groups (0–2 cells/1,000 CD3 cells), and no FoxP3-positive T cells were seen within or along the periphery of any tumor. Intriguingly, rare FoxP3-positive cells (at a similar frequency of 1–3/1,000 nucleated cells) present in normal skin adjacent to the tumors were identified as mast cells by the presence of toluidine blue-positive granules.
Tob1 has differential effects to restrain activation and promote survival of CD4 and CD8 T cells
The erosion of adoptively transferred T cells reflects a balance between HP and cell death. Recent data suggest that HP is restrained by negative regulatory factors such as CD24 in host DCs [10], which has different effects on proliferation and survival of CD4 and CD8 subsets. We thus examined whether modulation of Tob1, an intrinsic negative regulatory molecule that controls transition from G0 to G1 in T cells, had similar effects on survival and autoreactivity in the DKO environment. Both Tob1- and NFATc2-deficient cells show a reduced threshold for activation similar to that reported in IAN5 (lyp)-deficient T cells [59, 60]. At least part of this phenotype was due to constitutively elevated levels of CDK4 expression, an indicator of transition through the G0/G1 boundary [16, 60, 61].
We next examined if there was an in vivo corollary to the reduced threshold of Tob1-k/o cell activation by testing whether Tob1-deficient mice would reject or delay transplantable tumors. In previous experiments, we had noticed that growth of B16 melanoma was delayed in Tob1-k/o mice, which was associated with increased inflammatory infiltrates, reduced capillary density, and consequent necrosis [60]. We thus surmised these cells might generate a more dramatic tumor rejection phenotype when adoptively transferred to MHC-deficient mice. We adoptively transferred unchallenged, Tob1-k/o cells to B6 DKO (N = 9) mice as described above, and 2 weeks later, we injected the adoptive recipients with LL tumor cells. Intriguingly, all the mice that were transferred with Tob1-k/o cells developed tumors by 11 days (which is the same as control mice that receive no T cells, see Fig. 3). In sharp contrast to what we observed after adoptive transfer of unchallenged wild-type T cells into the MHC-deficient environment where there was rapid erosion of CD8 cells and protracted survival of CD4 cells, adoptive transfer of unchallenged Tob1-k/o cells resulted in a reverse phenotype with rapid erosion of CD4 cells and protracted survival of CD8 cells (Fig. 5). Results from MHC class I SKO recipients (N = 2) suggest these animals may readily reject tumors (both survived >40 days). As illustrated in Fig. 5, both CD4 and CD8 T cells survived in peripheral lymphoid organs from these mice. These results suggest that, much like absence of CD24 in DCs, the loss of intrinsic negative regulation created by knocking out the Tob1 gene shifts the balance of proliferation and death to allow for persistence of CD8 cells while leading to rapid erosion of CD4 cells.
Fig. 5.
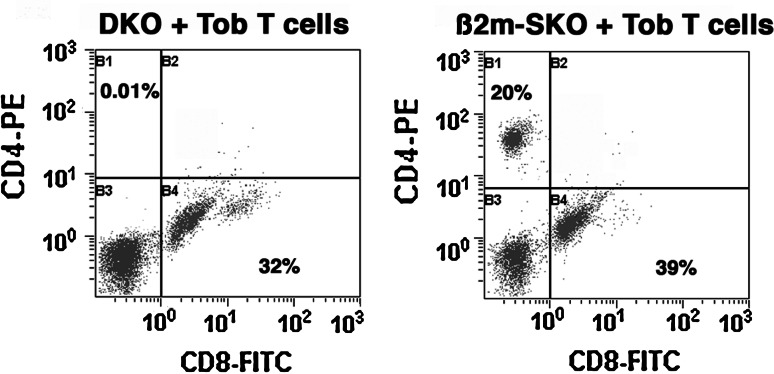
Reconstitution of DKO mice by adoptive T cell transfer from Tob1-deficient donors. T cell reconstitution in B6 DKO mice (left) or from B6 SKO (β2m class I-negative) mice (right) receiving naïve (unchallenged) Tob1-deficient T cells was determined as a proportion of all viable cells using spleen samples as in Fig. 2. Data show representative dot plots for CD4 and CD8 staining
Discussion
This study was prompted by an expanding literature concluding that memory T cells, but not naïve T cells, preferentially survived in an MHC-deficient environment [2, 17–20, 26, 27, 34, 62–64]. We initially predicted that such an environment would provide a means to enrich autoreactive cells and study their intrinsic properties, for example, by ‘parking’ diabetogenic NOD T cells in an MHC-less NOD environment (the NOD DKO model). We also sought to use this model to explore potential differences between CD4 and CD8 T cell survival under these conditions, to address the role of MHC in desensitizing (‘tuning’) or in sensitizing T cell responses against self-antigens, and to begin to address how intrinsic negative regulatory factors influence this process.
The results of adoptive transfer of NOD T cells into the MHC-deficient NOD environment supported our expectations, as the majority of donor T cells died within 4 weeks of transfer into NOD DKO animals, but a few T cells that remained were associated with fulminant destruction of syngeneic, MHC-positive NOD islet grafts. Islet destruction was not simply due to T cells undergoing HP in the lymphopenic recipient, since it did not occur in the 60-day window when we adoptively transferred T cells into lymphopenic SCID mice. Nor could this be explained simply by use of T cells from an autoimmune-prone donor, as the results of experiments in the non-autoimmune-prone B6 background were essentially indistinguishable from the results in the NOD background. Finally, graft rejection was probably not due to alloreactivity, as it was not apparent in single MHC knockout recipients. While we cannot completely rule out the possibility that this is because of a requirement for cooperative interactions between CD4 and CD8 T cells subsets, the response appeared to hinge on the concurrent absence of class I and class II MHC.
We used a syngeneic tumor model [49] as a complementary model to examine how loss of self-influenced T cell survival and activation. The B6 DKO model recapitulated results from single class I and single class II knockout mice with respect to T cell survival [17, 20, 27]. In addition, naïve T cells delayed LL tumor growth in the MHC-deficient environment, and this effect was enhanced when we used antigen-experienced cells from tumor-bearing donors (i.e., they were somehow ‘primed’), even though the donors themselves were unable to reject the tumors. The observation that tumor rejection did not occur in MHC-positive, lymphopenic SCID animals that were adoptively transferred with naïve T cells, or with antigen-experienced T cells that did not reject tumor in the donor (AE-NR), indicates that the absence of MHC was essential to uncover the tumor reactive population, i.e., HP in the MHC-replete environment alone could not provide sufficient impetus for activation of quiescent self-(tumor) reactive T cells. Furthermore, failure to reject tumors was not due to infiltration by suppressive double negative (CD4−/CD8−) tumor infiltrating lymphocytes as has been reported for other syngeneic murine tumors [52].
On the other hand, T cells that were deliberately primed under conditions of productive inflammation (by ectopic expression of FasL in tumors), were able to destroy secondary tumors even when MHC was present, indicating that the functional phenotype of memory T cells generated by canonical innate immune responses or by exposure to exosomes harboring apoptotic bodies (such as the seen with AE-R T cells) behaves differently than that generated by HP (i.e., by transfer of naïve T cells or AE-NR T cells to SCID mice).
It is worth noting that there were three distinct points of event-free survival for the groups tested. First, it took 10–11 days for 50% of wild-type B6 mice, B6 DKO mice (without T cells) or any of the SCID groups (with or without T cells) to develop tumors, and 100% of mice had tumors 14 days after challenge, although the rate of tumor growth in SCID mice that received unchallenged T cells or AE-NR T cells was slower than it was in mice with no T cells. In contrast, it took 14 and 21 days, respectively, for 50% of B6 DKO mice that received unchallenged T cells and B6 DKO mice that received “AE-NR” T cells to develop palpable tumors (significantly different from controls to P < 0.04 and P < 0.01). Moreover, while tumor growth was significantly slower in DKO mice that received unchallenged T cells than in the control or SCID groups, the slowest tumor growth rate was recurrently observed in the “AE-NR” B6 DKO recipients.
The sum of our results suggests that complete absence of MHC (class I and class II) incites T cell autoreactivity, or rather, removes a component of intrinsic negative regulation that maintains tolerance to self. There are conflicting data regarding loss of MHC and T cell reactivity (reviewed in [33]). Several reports indicate that self-interactions (TCR-MHC) maintain a high threshold for self-antigens [27, 28, 30, 33], whereas others show that these interactions promote modest receptor clustering and CD3ζ chain phosphorylation [20, 32] and in the case of CD4 cells, MHC class II availability promotes activation of Rap1 and Rac1, enhancing motility [65], suggesting self-interactions are responsible for partial activation that sensitizes the TCR as well as for increasing the potential for these cells to encounter and respond to antigen. One possibility that remains to be explored is that quantum signaling through the TCR might be responsible for sensitization and for tuning in T cells with varying avidity for self-peptides. Quantum signaling refers to the difference in signal magnitude that can be achieved by the number of receptors that are bound by ligand (determined by receptor density and ligand concentration) and the temporal occupancy of each receptor (determined by affinity).
The phenotype was dependent on loss of both MHC class I and MHC class II, as it was not evident in the single knockout mice. Unlike our results in the single class II knockout mice that did not reject pancreatic islets after adoptive transfer with naïve T cells, Bhandoola et al. reported rejection of MHC-positive skin grafts in approximately one third of MHC Class II-deficient animals that received naïve CD4 T cells [27]. Given that skin is particularly sensitive to T cell-mediated attack, rejection of only a fraction of the grafts suggests that the response unleashed by the absence of MHC class II alone was not very robust (as one might expect if the response is derived from T cells bearing low affinity receptors for self), even though lymphocytic infiltration into the skin was observed in all the animals.
Our results showing survival and autoreactivity of adoptively transferred, naïve CD4 T cells resembled those reported for the skin graft experiments into MHC class II SKO mice [27], with two of five B6 DKO mice that were adoptively transferred with naïve T cells showing delayed tumor growth for >50 days, and all of the mice examined containing CD4 T cell infiltrates in the tumors. In contrast, both CD4 and CD8 T cells survived in the MHC-deficient environment after they were exposed to tumor in the donor (antigen-experienced), and both the CD4 and the CD8 populations infiltrated LL tumors in the recipients. Hence, the results support current dogma that CD4 T cells persist longer than CD8 T cells in an MHC-deficient environment [20, 26], and the improved outcomes seen in mice that received antigen-experienced T cells were expected because these would contain MP CD8 T cells that are less dependent on MHC for survival.
Unlike what we observed in MHC-positive SCID mice, thus, unchallenged T cells and antigen-experienced T cells from donors that did not reject the tumor challenge could be induced to destroy LL cells following adoptive transfer into the MHC-deficient environment. This indicates that absence of MHC released a previously quiescent population with effector potential that was controlled by the presence of MHC in the unchallenged donors, and it also released a tumor sensitized (antigen-experienced) population that had been controlled by the presence of MHC in donors that had been challenged with, but failed to reject tumors. This latter conclusion is possible both because of the delay in the median survival and the rate of tumor growth.
These observations could be explained by release of T cells from intrinsic negative regulation in the absence of MHC. In other words, the same mechanisms that restrain T cell proliferation might control “tuning”. Among various candidates, we elected to study the effects of Tob1, which acts to silence the IL-2 promoter [66], and also modulates the activity of SMAD transcription factors, which are responsible for most of the anti-proliferative effects of transforming growth factor-β (TGF-β) [66]. CDK inhibitors including Ink4 proteins and p27 are upregulated by TGF-β [67–69], and at least p27 also is strongly induced by Tob1 [14]. The data from adoptive transfers using Tob1-deficient cells were initially counterintuitive, as the recipients showed no propensity to reject syngeneic tumors and the resulting peripheral T cell phenotypes were reversed from mice that received wild-type T cells. Tob1-k/o CD8 cells were able to persist in spleen and draining lymph nodes in MHC-deficient recipients, suggesting that Tob1 deletion was sufficient to overcome the requirement for MHC of naïve CD8 T cells (and thus these cells survived in both DKO and class I SKO mice), whereas CD4 cells did not tolerate the simultaneous loss of MHC and Tob1.
The response of naïve and memory cells to a lymphopenic environment has been the subject of several recent studies. Under normal conditions of HP, memory cells have an advantage due to their lower threshold of response for IL-7, clonal competition, and reduced sensitivity to the effects of Treg cells. It is reasonable to assume this balance would be even more noticeable in the MHC-deficient environment where there are additional obstacles that impair survival of naïve T cells. However, the ability of naïve T cells to undergo HP is different when there are abundant levels IL-2 and IL-15. Not only do these cytokines allow for expansion of naïve cells, but they also generate functionally competent cells [2]. The peripheral polyclonal T cell populations from donor mice in our experiments did not contain appreciably changed frequencies of CD44hi cells that might reflect an alteration in phenotype dictated by the immune status of the donor. Nor were the ratios of CD4/CD8 subsets altered, an expected result given that the T cell enrichment approach was a negative rather than positive selection approach, but the origin of the expanded population cannot be ascertained from this fact as the cells acquired a memory-like phenotype in the recipient mice.
Finally, we cannot completely exclude a role for CD4/CD25/FoxP3+ Treg cells. Independent studies indicate Treg cells are deficient in MHC-less recipients [27, 58], and our preliminary data suggest that the MHC-deficient environment does not strongly support survival of Treg cells. We quantified FoxP3 cells in samples from the mice a posteriori, and thus relied on immunohistochemistry of formalin-fixed and paraffin-embedded tissues. No difference was apparent in SCID mice that received naïve, AE-NR, or AE-R T cells or in DKO mice that received naïve or AE-NR T cells. The absence (or loss) of Treg cells may reflect their formation in the thymus at the boundary of TCR affinities for self, separating cells that are high affinity and subject to negative selection from those that are low affinity and subject to positive selection. The proclivity of Treg cells for self probably makes them particularly dependent on MHC, both for survival and for tuning; thus, they might die readily in a peripheral environment that is devoid of MHC.
Nevertheless, if regulatory T cells were the main reason why tolerance was maintained in adoptively transferred SCID mice, as compared to DKO mice, we might expect them to similarly dampen anti-self (tumor) responses in animals that received antigen-experienced T cells primed in the presence of FasL (AE-R), since survival of Treg cells should be assured in the MHC-positive SCID environment. Similarly, while the observation that class I SKO did not universally reject pancreatic islet grafts could be consistent with survival of Treg cells in the MHC class II-positive environment, the prediction would then be that Treg cells should die in class II SKO mice and that these animals would reject islet grafts, and this was not the case. Hence, the sum of our data implicates intrinsic mechanisms that are at least partially independent of Treg cells for the generation of islet and tumor rejection phenotypes in DKO mice.
In summary, our results are consistent with the interpretations that MHC class I is required for survival of naïve CD8 T cells, but not memory CD8 T cells, and that MHC class II provides ‘tuning’ signals that restrain autoreactivity by CD4 T cells. It is widely assumed that most tumors express only self-antigens and are therefore immunologically silent. Hence, unresponsiveness to tumors, or to pancreatic islets in non-autoimmune-prone animals, is reversed by the absence of ligands that limits self-reactivity, i.e., self-MHC.
Acknowledgments
The authors wish to thank Susan Fosmire, Dr. Angela Pierce, Dr. Ashley Frazer-Abel, and Dr. John Wojcieszyn for assistance with experiments, Dr. Tadashi Yamamoto for the Tob1 knockout mice, and Drs. Anne Avery, Kristin Hogquist, and Stephen Jameson for review of the manuscript and helpful discussions. This work was supported by a grant from The Cancer League of Colorado, Inc., by grants R21DK63410, P30CA46934, R01DK58722, and PO1HD38129 from the National Institutes of Health, by a grant from the Monfort Family Foundation, and by discretionary funds from the Integrated Department of Immunology and the University of Colorado Cancer Center. ARL was the recipient of a post-doctoral fellowship from the University of Colorado Cancer Center.
Footnotes
Donald Bellgrau and Jaime F. Modiano contributed equally to this work.
Contributor Information
Donald Bellgrau, Phone: +1-303-3981955, Email: Don.Bellgrau@UCHSC.edu.
Jaime F. Modiano, Phone: +1-612-6257436, Email: modiano@umn.edu
References
- 1.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med. 2007;204:1787–1801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti-IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandau MM, Winstead CJ, Jameson SC. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J Immunol. 2007;179:120–125. doi: 10.4049/jimmunol.179.1.120. [DOI] [PubMed] [Google Scholar]
- 5.Troy AE, Shen H. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. J Immunol. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- 6.Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci USA. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen S, Ding Y, Tadokoro CE, Olivares-Villagomez D, Camps-Ramirez M, Curotto de Lafaille MA, Lafaille JJAG. Control of homeostatic proliferation by regulatory T cells. J Clin Invest. 2005;115:3517–3526. doi: 10.1172/JCI25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 10.Li O, Chang X, Zhang H, Kocak E, Ding C, Zheng P, Liu Y. Massive and destructive T cell response to homeostatic cue in CD24-deficient lymphopenic hosts. J Exp Med. 2006;203:1713–1720. doi: 10.1084/jem.20052293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangachari M, Penninger JM. Negative regulation of T cell receptor signals. Curr Opin Pharmacol. 2004;4:415–422. doi: 10.1016/j.coph.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Buckley AF, Kuo CT, Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nat Immunol. 2001;2:698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CT, Veselits ML, Leiden JM. LKLF: a transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 14.Tzachanis D, Freeman GJ, Hirano N, van Puijenbroek AA, Delfs MW, Berezovskaya A, Nadler LM, Boussiotis VA. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat Immunol. 2001;2:1174–1182. doi: 10.1038/ni730. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. doi: 10.1016/S1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 16.Baksh S, Widlund HR, Frazer-Abel AA, Du J, Fosmire S, Fisher DE, DeCaprio JA, Modiano JF, Burakoff SJ. NFATc2-mediated repression of cyclin-dependent kinase 4 expression. Mol Cell. 2002;10:1071–1081. doi: 10.1016/S1097-2765(02)00701-3. [DOI] [PubMed] [Google Scholar]
- 17.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 18.Boursalian TE, Bottomly K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J Immunol. 1999;162:3795–3801. [PubMed] [Google Scholar]
- 19.Viret C, Janeway CA., Jr MHC and T cell development. Rev Immunogenet. 1999;1:91–104. [PubMed] [Google Scholar]
- 20.Witherden D, van Oers N, Waltzinger C, Weiss A, Benoist C, Mathis D. Tetracycline-controllable selection of CD4(+) T cells: half-life and survival signals in the absence of major histocompatibility complex class II molecules. J Exp Med. 2000;191:355–364. doi: 10.1084/jem.191.2.355. [DOI] [PubMed] [Google Scholar]
- 21.Martin B, Bourgeois C, Dautigny N, Lucas B. On the role of MHC class II molecules in the survival and lymphopenia-induced proliferation of peripheral CD4+ T cells. Proc Natl Acad Sci USA. 2003;100:6021–6026. doi: 10.1073/pnas.1037754100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 24.Markiewicz MA, Brown I, Gajewski TF. Death of peripheral CD8+ T cells in the absence of MHC class I is Fas-dependent and not blocked by Bcl-xL. Eur J Immunol. 2003;33:2917–2926. doi: 10.1002/eji.200324273. [DOI] [PubMed] [Google Scholar]
- 25.Dorfman JR, Germain RN. MHC-dependent survival of naive T cells? a complicated answer to a simple question. Microbes Infect. 2002;4:547–554. doi: 10.1016/S1286-4579(02)01571-X. [DOI] [PubMed] [Google Scholar]
- 26.Grandjean I, Duban L, Bonney EA, Corcuff E, Di Santo JP, Matzinger P, Lantz O. Are major histocompatibility complex molecules involved in the survival of naive CD4+ T cells? J Exp Med. 2003;198:1089–1102. doi: 10.1084/jem.20030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhandoola A, Tai X, Eckhaus M, Auchincloss H, Mason K, Rubin SA, Carbone KM, Grossman Z, Rosenberg AS, Singer A. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17:425–436. doi: 10.1016/S1074-7613(02)00417-X. [DOI] [PubMed] [Google Scholar]
- 28.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–1016. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Y, Legrand N, Freitas AA. The clone size of peripheral CD8 T cells is regulated by TCR promiscuity. J Exp Med. 2006;203:1643–1649. doi: 10.1084/jem.20052174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 31.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 32.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 33.Hogquist KA, Starr TK, Jameson SC. Receptor sensitivity: when T cells lose their sense of self. Curr Biol. 2003;13:R239–241. doi: 10.1016/S0960-9822(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 34.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 35.Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grusby MJ, Auchincloss H, Jr, Lee R, Johnson RS, Spencer JP, Zijlstra M, Jaenisch R, Papaioannou VE, Glimcher LH. Mice lacking major histocompatibility complex class I and class II molecules. Proc Natl Acad Sci USA. 1993;90:3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modiano JF, Sun J, Lang J, Vacano G, Patterson D, Chan D, Franzusoff A, Gianani R, Meech SJ, Duke R, Bellgrau D. Fas ligand-dependent suppression of autoimmunity via recruitment and subsequent termination of activated T cells. Clin Immunol. 2004;112:54–65. doi: 10.1016/j.clim.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Kelemen K, Crawford ML, Gill RG, Hutton JC, Wegmann D. Cellular immune response to phogrin in the NOD mouse: cloned T cells cause destruction of islet transplants. Diabetes. 1999;48:1529–1534. doi: 10.2337/diabetes.48.8.1529. [DOI] [PubMed] [Google Scholar]
- 39.Bertram JS, Janik P. Establishment of a cloned line of Lewis lung carcinoma cells adapted to cell culture. Cancer Lett. 1980;11:63–73. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- 40.Lang J, Bellgrau D. A T cell functional phenotype common among autoimmune-prone rodent strains. Scand J Immunol. 2002;55:546–559. doi: 10.1046/j.1365-3083.2002.01086.x. [DOI] [PubMed] [Google Scholar]
- 41.Leon RP, Hedlund T, Meech SJ, Li S, Schaack J, Hunger SP, Duke RC, DeGregori J. Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Yang Y, Horton JL, Samoilova EB, Judge TA, Turka LA, Wilson JM, Chen Y. Amelioration of collagen-induced arthritis by CD95 (Apo-1/Fas)-ligand gene transfer. J Clin Invest. 1997;100:1951–1957. doi: 10.1172/JCI119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, Bowen K, Steeber DA, Haas KM, Poe JC, Tedder TF. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 44.Nesic D, Vukmanovic S. MHC class I is required for peripheral accumulation of CD8+ thymic emigrants. J Immunol. 1998;160:3705–3712. [PubMed] [Google Scholar]
- 45.Nesic D, Santori FR, Vukmanovic S. Alpha beta TCR+ cells are a minimal fraction of peripheral CD8+ pool in MHC class I-deficient mice. J Immunol. 2000;165:1896–1901. doi: 10.4049/jimmunol.165.4.1896. [DOI] [PubMed] [Google Scholar]
- 46.Raulet DH. MHC class I-deficient mice. Adv Immunol. 1994;55:381–421. doi: 10.1016/S0065-2776(08)60514-3. [DOI] [PubMed] [Google Scholar]
- 47.Ge Q, Bai A, Shen CH, Eisen HN, Chen J. CD4+ T-cell responses to self-peptide–MHC. Trends Immunol. 2003;24:186–189. doi: 10.1016/S1471-4906(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 48.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/S0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 49.Modiano JF, Lamerato-Kozicki AR, Jubala CM, Coffey D, Borakove M, Schaack J, Bellgrau D. Fas ligand gene transfer for cancer therapy. Cancer Ther. 2004;2:561–570. [Google Scholar]
- 50.Lee SH, Bar-Haim E, Goldberger O, Reich-Zeliger S, Vadai E, Tzehoval E, Eisenbach L. Expression of FasL by tumor cells does not abrogate anti-tumor CTL function. Immunol Lett. 2004;91:119–126. doi: 10.1016/j.imlet.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu M, Fontana A, Takeda Y, Yagita H, Yoshimoto T, Matsuzawa A. Induction of antitumor immunity with Fas/APO-1 ligand (CD95L)-transfected neuroblastoma neuro-2a cells. J Immunol. 1999;162:7350–7357. [PubMed] [Google Scholar]
- 52.Prins RM, Incardona F, Lau R, Lee P, Claus S, Zhang W, Black KL, Wheeler CJ. Characterization of defective CD4−CD8− T cells in murine tumors generated independent of antigen specificity. J Immunol. 2004;172:1602–1611. doi: 10.4049/jimmunol.172.3.1602. [DOI] [PubMed] [Google Scholar]
- 53.Denkers EY, Gazzinelli RT, Martin D, Sher A. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med. 1993;178:1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, Smyth MJ. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 55.Smyth MJ, Snook MB. Perforin-dependent cytolytic responses in beta2-microglobulin-deficient mice. Cell Immunol. 1999;196:51–59. doi: 10.1006/cimm.1999.1536. [DOI] [PubMed] [Google Scholar]
- 56.Chen A, Liu S, Park D, Kang Y, Zheng G. Depleting intratumoral CD4+CD25+ regulatory T cells via FasL protein transfer enhances the therapeutic efficacy of adoptive T cell transfer. Cancer Res. 2007;67:1291–1298. doi: 10.1158/0008-5472.CAN-06-2622. [DOI] [PubMed] [Google Scholar]
- 57.Fritzsching B, Oberle N, Eberhardt N, Quick S, Haas J, Wildemann B, Krammer PH, Suri-Payer E. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand- but not to TCR-mediated cell death. J Immunol. 2005;175:32–36. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 59.Lang JA, Kominski D, Bellgrau D, Scheinman RI. Partial activation precedes apoptotic death in T cells harboring an IAN gene mutation. Eur J Immunol. 2004;34:2396–2406. doi: 10.1002/eji.200324751. [DOI] [PubMed] [Google Scholar]
- 60.Modiano JF, Johnson LDS, Bellgrau D (2008) Negative regulators in homeostasis of naive peripheral T cells. Immunol Res. doi:10.1007/s12026-008-8017-1 [DOI] [PMC free article] [PubMed]
- 61.Modiano JF, Mayor J, Ball C, Fuentes MK, Linthicum DS. Cdk4 expression and activity are required for cytokine responsiveness in T cells. J Immunol. 2000;165:6693–6702. doi: 10.4049/jimmunol.165.12.6693. [DOI] [PubMed] [Google Scholar]
- 62.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self-peptide/MHC ligands. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medema JP, Borst J. T cell signaling: a decision of life and death. Hum Immunol. 1999;60:403–411. doi: 10.1016/S0198-8859(99)00008-7. [DOI] [PubMed] [Google Scholar]
- 64.Di Rosa F, Ramaswamy S, Ridge JP, Matzinger P. On the lifespan of virgin T lymphocytes. J Immunol. 1999;163:1253–1257. [PubMed] [Google Scholar]
- 65.Fischer UB, Jacovetty EL, Medeiros RB, Goudy BD, Zell T, Swanson JB, Lorenz E, Shimizu Y, Miller MJ, Khoruts A, Ingulli E. MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo. Proc Natl Acad Sci USA. 2007;104:7181–7186. doi: 10.1073/pnas.0608299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hua X, Thompson CB. Quiescent T cells: actively maintaining inactivity. Nat Immunol. 2001;2:1097–1098. doi: 10.1038/ni1201-1097. [DOI] [PubMed] [Google Scholar]
- 67.Massague J, Weinberg RA. Negative regulators of growth. Curr Opin Genet Dev. 1992;2:28–32. doi: 10.1016/S0959-437X(05)80317-X. [DOI] [PubMed] [Google Scholar]
- 68.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 69.Quelle DE, Ashmun RA, Hannon GJ, Rehberger PA, Trono D, Richter KH, Walker C, Beach D, Sherr CJ, Serrano M. Cloning and characterization of murine p16INK4a and p15INK4b genes. Oncogene. 1995;11:635–645. [PubMed] [Google Scholar]