Abstract
Background
Several treatment strategies are available for children with severe immune thrombocytopenic purpura (ITP) and other immune cytopenias refractory to initial therapies. 6-Mercaptopurine (6MP) is one option, however it has not been well studied in children, especially as a single agent, and no pediatric case series have been reported since 1970.
Patients and Methods
We reviewed the experience at our institution over eight years, using 6MP as a steroid sparing treatment for children with ITP, auto-immune hemolytic anemia (AIHA) or Evans syndrome. A total of 29 pediatric patients were treated with 6MP from 2000-2007.
Results
Response was defined as a rise in hemoglobin by at least 1.5g/dL and to a level of 10 g/dL or greater in patients treated for anemia, or a platelet count ≥ 50 × 109/L in patients treated for thrombocytopenia. We found an overall response rate of 83% among all patients. Fourteen percent of patients stopped drug because of side effects.
Conclusions
These results suggest that 6MP can be an effective single-agent treatment for refractory immune cytopenias in children. Prospective studies are warranted to determine long term efficacy and toxicity and to more clearly define patient populations most likely to respond.
Keywords: 6MP, mercaptopurine, ITP, AIHA, Evans, pediatric
INTRODUCTION
Autoimmune cytopenias in children include idiopathic thrombocytopenic purpura (ITP), auto-immune hemolytic anemia (AIHA) and Evans syndrome, which result in immune mediated destruction of platelets, red cells or both. Traditional therapies include immune-modulators such as steroids or intravenous immune globulin (IVIG) for AIHA or ITP, anti-D immunoglobulin for ITP, and splenectomy. For a fraction of children these first line agents are ineffective, or clinically stable status can be maintained only by continuing steroids at the risk of long-term side effects.
Up to 20% of children with ITP will develop chronic disease and a percentage of those will have severe thrombocytopenia requiring treatment [1]. The fraction with chronic disease is substantially higher in adolescents [1]. Many approaches exist, and because all have some efficacy, treatments tend to be physician- or center specific. Choices for severe and refractory disease include combination strategies [2], rituximab [3,4], splenectomy [5,6], vincristine [2,7], azathioprine/6MP [8-13], mycophenylate mofetil [14,15], danazol [16,17], and in severe cases, cyclophosphamide [18]. Few comparative studies exist to determine a “best next-line” therapy for these patients [19].
6-Mercaptopurine, a purine antimetabolite, was developed in the 1950s as a chemotherapy agent along with thioguanine and the antifolate metabolites. Because of its immunosuppressant effects, as a suppressor of both B and T lymphocytes, it was tried in the 1960s as a treatment for auto-immune hemolytic anemia and ITP, and found to have activity [12]. Very little has been published about its effect in autoimmune cytopenias in children, especially as a single agent. Azathioprine, which is slowly but completely metabolized to 6MP, was felt to have fewer side effects then 6MP. Several small series reported response rates of 57-87% to azathioprine for patients with refractory ITP [8,9,13]. Subsequently, two larger series showed favorable response rates of 51-64% in adult patients with refractory ITP [10,11]. None of these series reported any serious toxicity, although they did find transient leukopenia or moderately elevated transaminases (3x normal) in several patients. There were no reported cases of serious infection or malignancy. Key results from prior series of 6MP/Azathioprine are summarized in Table I.
TABLE I.
Prior Studies of Anti-Metabolite Therapy for Auto-Immune Cytopenias
| Authors | Patients (children) Diagnosis Treatment |
Response | Comments |
|---|---|---|---|
| Schwartz and Damesheck [12] |
14 patients (1 child) AIHA 6MP (3), 6TG (11) |
9 “responded” 5 failed to respond |
3 stopped due to side effects |
| Bouroncle and Doan [8] |
7 patients (1 child) refractory ITP azathioprine |
4 “excellent” (complete remission off therapy) 2 “fair” (some improvement) 1 failed to respond |
|
| Sussman [13] | 8 adults refractory ITP azathioprine |
7 “satisfactory” | |
| Hilgartner, Lanzkowsky and Smith [9] |
5 children refractory ITP azathioprine |
3 “excellent” (complete remission off therapy) 1 “good” (complete remission, ongoing therapy) 1 failure |
Treated for 8 months before declaring failure |
| Pizzuto and Ambriz [10] |
41 adults refractory ITP azathioprine |
8 “prolonged complete remission” (remission 6 months off therapy) 4 “complete remission with relapse” 9 “partial remission“ (platelets > 50 × 109/L for at least 3 months) 20 failures |
|
| Quiquandon, Fenaux, Caulier et al [11] |
53 adults refractory ITP azathioprine |
24 “complete response” (platelets > 150 × 109/L for 3 months) 3 “partial response” (platelets 100-150 × 109/L for 3 months) 7 “modest response” (platelets > 50 × 109/L for 3 months) |
Median time to response: 4 months No predictors of response |
| Schiavotto, Castaman, Rodeghiero [17] |
11 adults refractory ITP azathioprine, danazol, vinca alkaloids |
5 responded |
In this study, we reviewed the records of all 29 children with severe, refractory ITP, AIHA or Evans syndrome treated with 6MP at our institution in the past 8 years to determine the overall response rate, complication rate and to examine possible predictors of response.
PATIENTS AND METHODS
The retrospective review was approved by the Committee on Clinical Investigation at Children's Hospital, Boston. We identified all children treated with 6MP for auto-immune cytopenias at our institution through a comprehensive search of the hospital data warehouse cross-referenced with our hematology clinical and research databases to ensure we captured all patients. The hospital database was searched from 1/1/2000 to 10/1/2007 for patients who 1) were seen in any of our hematology outpatient locations, 2) had a diagnosis code corresponding to ITP, Evans syndrome, AIHA, thrombocytopenia or hemolytic anemia and 3) had the terms mercaptopurine, 6MP, Imuran or azothioprine mentioned in free text in their clinic note. After eliminating duplicates, 170 patients were identified. Their electronic charts were reviewed to define their hematologic diagnosis, whether they received 6MP and whether they were treated at our institution. Patients were included if they had a diagnosis of ITP, AIHA or Evans syndrome, and if they received 6MP under the direction of our hematology department. As a tertiary care center, a number of patients were seen on a second opinion basis and these patients were excluded, because detailed clinical records were not available.
Twenty-nine patients who received 6MP under these conditions were identified and their electronic medical records were reviewed. The database was closed as of 12/1/07. Subsequent patients started on 6MP or changes in outcome status after that date are not included. Data were collected on standardized case report forms drawn from an ongoing longitudinal study of ITP/Evans syndrome. All data were either collected or reviewed by a single author (AS) to ensure consistency. SPSS software was used for data collection and analysis.
Patients were treated either because of a low platelet count or low hemoglobin and responses were categorized to reflect this difference. Platelet response was defined as a platelet count of 50 × 109/L or greater. Hemoglobin response was defined as a rise in hemoglobin by at least 1.5 g/dL and to a level of 10 g/dL or greater.
Hemoglobin or platelet nadir was defined as the lowest measurement just prior to starting 6MP. Because the response to 6MP is not immediate, the best response was defined as the best hemoglobin or platelet count 1 - 4 months after starting 6MP that was not clearly attributable to another therapy. However follow-up counts were not obtained at standard intervals and for some older charts, not every outside CBC was available for review.
Relapse was defined as recrudescence of disease; a fall in platelet count to under 50 × 109/L for patients treated for thrombocytopenia, or a drop in hemoglobin to under 10 g/dL for patients treated for anemia. Steroid sparing was defined as the ability to discontinue or reduce steroid dose without relapse.
Treatment regimen: the typical dosing regimen at our center was 50-75 mg/m2 body surface area (BSA) once daily, with mercaptopurine/metabolite levels after 5-7 days of therapy to assess for slow metabolizers. Thiopurine methyltransferase gene status was not routinely tested. PCP prophylaxis was at the discretion of the treating physician, and generally limited to those on multiple immunosuppresants or with underlying immunodeficiency. Children under 1 m2 BSA were treated with 2.5 mg/kg.
RESULTS
Patient Characteristics
A total of 29 patients met inclusion criteria and were analyzed. Thirteen patients had ITP (10 primary, 3 secondary), 7 had AIHA (5 primary, 2 secondary) and 9 had Evans syndrome (7 primary, 2 secondary). Non-hematologic primary diagnoses included two patients with inflammatory bowel disease (IBD), two patients with systemic lupus erythematosus (SLE), and one patient each with polyserositis, autoimmune lymphoproliferative syndrome and Kabuki syndrome. Of the patients with Evans syndrome, 6 were treated mainly for thrombocytopenia and 3 were treated mainly for anemia. The majority of children were female (59%) and the mean age at diagnosis was 9.9 years. Most of the patients had been heavily pre-treated; in addition to 6MP, 25 patients received 2 or more different therapies, 12 received 3 or more and 6 received 4 or more. The mean duration of disease from the time of diagnosis to the time of starting 6MP was 18.7 months. A brief clinical summary of each patient is available in the Supplementary Table.
Overall Response
Twenty-four patients had a platelet or hemoglobin response to 6MP for an overall response rate of 83% (95%CI 65%-92%). Patient response to 6MP by hematologic diagnosis and overall platelet and hemoglobin responses are shown in Figure 1. When assessed by hematologic diagnosis, the response rate was 11/13 for patients with ITP (85%, 95% CI 58%-96%), 7/7 for patients with AIHA (100%; 95% CI 65%-100%), and 6/9 for patients with Evans syndrome (67%; 95% CI 35%-88%). For patients with Evans syndrome there was a response rate of 4/6 for platelets alone and 2/3 for hemoglobin alone.
Figure 1.
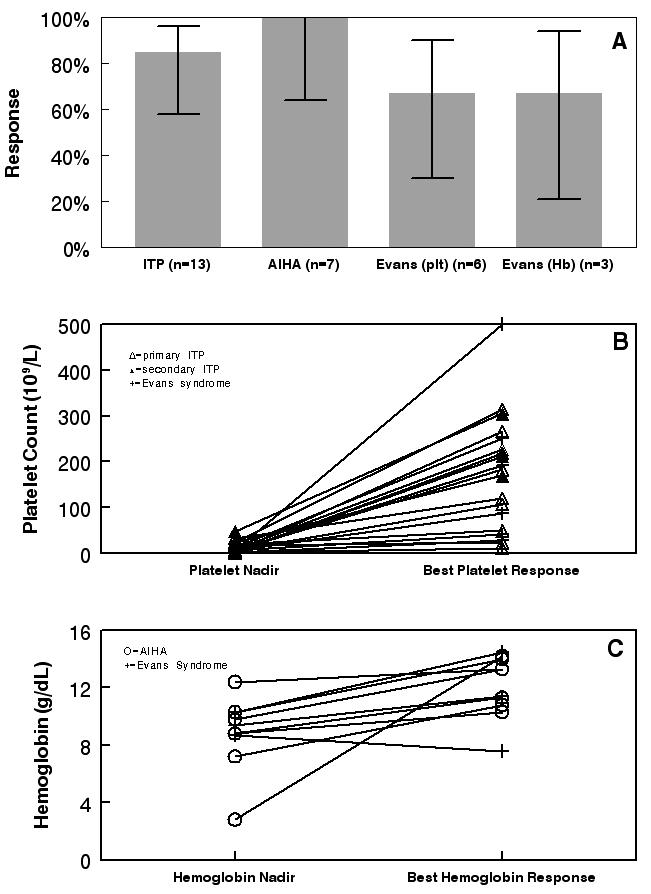
Among patients treated for thrombocytopenia the mean platelet nadir prior to starting 6MP was 12 × 109/L (range 2 – 34 × 109/L); for responders the mean platelet nadir was 13 × 109/L. The mean increase in platelet count from nadir to response was 162 × 109/L (range 6 - 493 × 109/L) overall and 200 × 109/L among responders. All 15 responders treated for thrombocytopenia at least doubled their platelet count on 6MP. Among patients treated for anemia, the mean hemoglobin nadir was 8.8 g/dL (range 2.8-12.4) with a mean rise in hemoglobin of 3.7g/dL (range 0.9-11.4).
A total of 11 responders (46%) were treated with 6MP as monotherapy. Twenty-one of the 24 responders were on steroids at the time that 6MP was started. From a hematologic perspective all of those patients were able to either wean off steroids entirely (9 patients, 43%) or taper their dose (12 patients, 57%). Two of the patients who were able to wean steroids for their cytopenia had to increase them again because of their underlying disease (IBD, BOOP). A total of 5 patients received additional immunosuppressive therapy for co-morbidities (2 patients with IBD, one patient each with solid organ transplant, SLE and ALPS).
Of the 24 responders, 18 (75%) had an ongoing response at the time of last contact with a mean duration of response of 16 months (range 1-60). The mean length of treatment with 6MP was 10 months (range 2-60) for all 29 patients. Responders were treated for a mean of 10.4 months and non-responders for a mean of 8.4 months.
Seven patients were still on 6MP at the time of last contact, 8 were taken off 6MP when they demonstrated a sufficient response (of those patients 3 remained in remission off-therapy, and 5 relapsed but responded again when 6MP was restarted), 4 had to stop because of side effects, 1 patient had an initial response but then relapsed while on treatment, 5 stopped when it was determined that the drug was not having the desired effect and 4 patients who had responded stopped 6MP for other reasons (one was put back on high-dose steroids for auto-immune lung disease, one required a change in immunosuppression for his heart transplant, one patient underwent splenectomy after being unable to sufficiently wean steroids, and one patient was stopped due to concern for drug interaction).
Response to Other Therapies
Table II displays the patient responses to other therapies. All 29 patients had previously been treated with steroids. In addition, at various times in their courses, 20 received IVIG, 8 received anti-D immunoglobulin and 9 received rituximab. Using the same criteria to define response, among the 24 responders to 6MP, 3/24 (12.5%) had previously failed to respond to steroids, 6/15 (40%) failed to respond to IVIG, 5/7 (71.4%) failed to respond to anti-D immunoglobulin and there were no treatment failures among the 5 patients who received rituximab. The majority of patients (19, 65%) were started on 6MP to spare steroid toxicity, 8 patients (28%) received 6MP because they were refractory to other treatments, and 2 patients (7%) were not able to continue steroids because of contraindications (cataracts, severe mood disturbance).
TABLE II.
Other Therapies
| ITP (n=13) | AIHA (n=7) | Evans (n=9) | Total Patients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Therapy | Received | Response | Received | Response | Received | Response | Received | Response | |
| Steroids | 13 (100%) | 9/13 (69%) | 7 (100%) | 7/7 (100%) | 9 (100%) | 8/9 (89%) | 29 (100%) | 24/29 (83%) | |
| IVIG | 9 (69%) | 4/9 (44%) | 3 (43 %) | 2/3 (67%) | 8 (89%) | 6/8 (75%) | 20 (69%) | 12/20 (60%) | |
| Anti-D | 8 (62%) | 3/8 (38%) | 0 | n/a | 0 | n/a | 8 (28%) | 3/8 (38%) | |
| Rituximab | 3 (23%) | 2/3 (67%) | 2 (29%) | 2/2 (100%) | 4 (44%) | 3/4 (75%) | 9 (31%) | 7/9 (78%) | |
| Splenectomy | 1 | 1 | 1 | ||||||
| Mycophenolate | 2 | 2 | |||||||
Side Effects
Of the 29 patients treated with 6MP, 8/29 (27.6%) had a documented increase in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) while on the medication. Only 1 patient had to stop 6MP for this reason, while the remaining 7 patients were able to remain on the drug at a reduced dose. One patient was taken off of 6MP for presumed drug-related neutropenia. Two additional patients had 6MP stopped for other side effects (one for pancreatitis and one who developed bronchiolitis obliterans with organizing pneumonia (BOOP)), although the causal relationship to 6MP was not ascertained. Ultimately, 4/29 (14%) of patients stopped 6MP because of side effects.
DISCUSSION
Our data suggests that 6MP is a reasonable single-agent treatment for children with refractory ITP, AIHA or Evans syndrome. Response rates in Evans syndrome tended to be lower, as expected for this highly refractory disease. Furthermore, this therapy is effective in reducing steroid exposure for patients with Evans syndrome and AIHA. While 14% of patients stopped the drug due to side effects, all side effects clearly related to 6MP (i.e., elevated liver enzymes, neutropenia) were reversible with dose reduction or cessation of drug.
A potential challenge to our interpretation of the data is that in disorders such as these, which wax and wane spontaneously, it is difficult to confirm that an improvement in hematologic parameters is due to any specific treatment and is not a coincidental spontaneous remission. However, there are several observations which support our conclusion that 6MP was responsible for disease response. First, the patients included in this study had received multiple prior therapies without effect, making it less likely that the observed response after 6MP therapy was merely coincidence. Second, 21 of 24 responders were steroid dependant when 6MP was started, and all showed reduced steroid requirements after 6MP. This finding strongly suggests 6MP effect and in a pediatric population where chronic steroid exposure is particularly problematic, this effect alone is highly beneficial. Third, the protracted duration of symptomatic disease in these patients (mean of 18 months and up to many years) makes it unlikely that the observed responses were unrelated to 6MP therapy. Lastly, 5 patients demonstrated multiple responses to 6MP when it was given for more than one course, which is further evidence of true response.
This study has some potential limitations. It was a small series, so results are somewhat limited by sample size, however our robust response rate is very suggestive of 6MP benefit. We were not able to identify specific predictors of response to 6MP, mainly due to high response rates in all groups and small sample size. In addition, this was a retrospective study where patients were started on 6MP at the recommendation of their hematologist without prospective criteria, and therefore selection bias may be present. However, the ITP patients in this study are representative of typical chronic ITP patients in our center overall, as reported in a large retrospective cohort, so significant selection bias is unlikely [20].
These results strongly suggest that 6MP is active in pediatric patients with auto-immune cytopenias without significant toxicity. As a non-surgical, reversible treatment option 6MP has some distinct advantages as second-line therapy; it is relatively inexpensive, oral, and does not require a hospital or clinic visit to administer. For these reasons we recommend a prospective, multi-center trial to more clearly define the response rate to 6MP along with subsets of patients who would most benefit from this treatment.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grant K24HL04184 to EJN, by a grant to CMB from the St. Giles Foundation, and by generous support of the Windsor Family philanthropic fund. We thank Dr. Joseph McNamara for the suggested treatment regimen of 6MP monotherapy.
REFERENCES
- 1.Kuhne T, Buchanan GR, Zimmerman S, et al. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143(5):605–608. doi: 10.1067/s0022-3476(03)00535-3. [DOI] [PubMed] [Google Scholar]
- 2.Williams JA, Boxer LA. Combination therapy for refractory idiopathic thrombocytopenic purpura in adolescents. J Pediatr Hematol Oncol. 2003;25(3):232–235. doi: 10.1097/00043426-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CM, Rogers ZR, Kinnamon DD, et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107(7):2639–2642. doi: 10.1182/blood-2005-08-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Wiley JM, Luddy R, et al. Chronic immune thrombocytopenic purpura in children: assessment of rituximab treatment. J Pediatr. 2005;146(2):217–221. doi: 10.1016/j.jpeds.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Vesely SK, Perdue JJ, Rizvi MA, et al. Management of adult patients with persistent idiopathic thrombocytopenic purpura following splenectomy: a systematic review. Ann Intern Med. 2004;140(2):112–120. doi: 10.7326/0003-4819-140-3-200402030-00012. [DOI] [PubMed] [Google Scholar]
- 6.Vianelli N, Galli M, de Vivo A, et al. Efficacy and safety of splenectomy in immune thrombocytopenic purpura: long-term results of 402 cases. Haematologica. 2005;90(1):72–77. [PubMed] [Google Scholar]
- 7.Sikorska A, Slomkowski M, Marlanka K, et al. The use of vinca alkaloids in adult patients with refractory chronic idiopathic thrombocytopenia. Clin Lab Haematol. 2004;26(6):407–411. doi: 10.1111/j.1365-2257.2004.00643.x. [DOI] [PubMed] [Google Scholar]
- 8.Bouroncle BA, Doan CA. Refractory idiopathic thrombocytopenic purpura treated with azathioprine. N Engl J Med. 1966;275(12):630–635. doi: 10.1056/NEJM196609222751202. [DOI] [PubMed] [Google Scholar]
- 9.Hilgartner MW, Lanzkowsky P, Smith CH. The use of azathioprine in refractory idiopathic thrombocytopenic purpura in children. Acta paediatrica Scandinavica. 1970;59(4):409–415. doi: 10.1111/j.1651-2227.1970.tb15536.x. [DOI] [PubMed] [Google Scholar]
- 10.Pizzuto J, Ambriz R. Therapeutic experience on 934 adults with idiopathic thrombocytopenic purpura: Multicentric Trial of the Cooperative Latin American group on Hemostasis and Thrombosis. Blood. 1984;64(6):1179–1183. [PubMed] [Google Scholar]
- 11.Quiquandon I, Fenaux P, Caulier MT, et al. Re-evaluation of the role of azathioprine in the treatment of adult chronic idiopathic thrombocytopenic purpura: a report on 53 cases. Br J Haematol. 1990;74(2):223–228. doi: 10.1111/j.1365-2141.1990.tb02569.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz R, Dameshek W. The treatment of autoimmune hemolytic anemia with 6-mercaptopurine and thioguanine. Blood. 1962;19:483–500. [PubMed] [Google Scholar]
- 13.Sussman LN. Azathioprine in refractory idiopathic thrombocytopenic purpura. Jama. 1967;202(4):259–263. [PubMed] [Google Scholar]
- 14.Kotb R, Pinganaud C, Trichet C, et al. Efficacy of mycophenolate mofetil in adult refractory auto-immune cytopenias: a single center preliminary study. Eur J Haematol. 2005;75(1):60–64. doi: 10.1111/j.1600-0609.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- 15.Provan D, Moss AJ, Newland AC, et al. Efficacy of mycophenolate mofetil as single-agent therapy for refractory immune thrombocytopenic purpura. Am J Hematol. 2006;81(1):19–25. doi: 10.1002/ajh.20515. [DOI] [PubMed] [Google Scholar]
- 16.Maloisel F, Andres E, Zimmer J, et al. Danazol therapy in patients with chronic idiopathic thrombocytopenic purpura: long-term results. Am J Med. 2004;116(9):590–594. doi: 10.1016/j.amjmed.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Schiavotto C, Castaman G, Rodeghiero F. Treatment of idiopathic thrombocytopenic purpura (ITP) in patients with refractoriness to or with contraindication for corticosteroids and/or splenectomy with immunosuppressive therapy and danazol. Haematologica. 1993;78(6 Suppl 2):29–34. [PubMed] [Google Scholar]
- 18.Reiner A, Gernsheimer T, Slichter SJ. Pulse cyclophosphamide therapy for refractory autoimmune thrombocytopenic purpura. Blood. 1995;85(2):351–358. [PubMed] [Google Scholar]
- 19.George JN. Management of patients with refractory immune thrombocytopenic purpura. J Thromb Haemost. 2006;4(8):1664–1672. doi: 10.1111/j.1538-7836.2006.02013.x. [DOI] [PubMed] [Google Scholar]
- 20.Bennett CM, Aydinoz S, Boardman P, et al. Single center retrospective review of pediatric immune thrombocytopenic purpura (ITP): 522 subjects over 12 years at Children's Hospital, Boston (CHB) Pediatr Blood Cancer. 2006;46(6):693. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


