Abstract
OBJECTIVES:
To examine the effect of graded doses of testosterone on physical function (PF) and muscle performance in healthy, older men.
DESIGN:
Randomized, double-blind, placebo controlled clinical trial.
SETTING:
General Clinical Research Center
PARTICIPANTS:
Community-dwelling healthy older men aged 60-75 yr, N=44.
INTERVENTION:
Monthly treatment with a gonadotropin releasing hormone agonist plus 25, 50, 125, or 300 mg/wk testosterone enanthate IM for 20 weeks.
MEASUREMENTS:
Skeletal muscle mass (SMM) was estimated by DEXA. Leg press strength was measured by 1-RM, leg power by Nottingham Leg Rig, and muscle fatigability by repetitions to failure in the leg press exercise. Stair climbing, 6-m and 400-m walking speed, and a timed-up-and-go (TUG) were used to assess PF.
RESULTS:
Significant T dose- and concentration-dependent increases were observed in SMM (P<0.001) and maximal strength (P=0.001), but not muscle fatigability. Leg power also increased dose-dependently (P=0.048). In contrast, changes in self-selected normal and fast walking speed over 6-m or 400-m, stair climbing power, and time for the TUG were not significantly related to T-dose, T-concentrations, or changes in muscle strength or power, or SMM.
CONCLUSION:
Testosterone administration was associated with dose-dependent increases in SMM, leg strength and power, but did not improve muscle fatigability or physical function. The observation that physical function scores did not improve linearly with strength suggests that our high functioning older men were already in the asymptotic region of the curve describing the physical function – strength relationship.
Keywords: stair climb, timed up-and-go, timed walk test
INTRODUCTION
Testosterone therapy has been shown to consistently increase lean body mass, but the effects of testosterone administration on muscle performance and physical function have been inconsistent across trials. Muscle strength and skeletal muscle mass, important determinants of physical function, decrease with aging and are weakly associated with circulating testosterone concentrations.1-3 Walking speed, the ability to rise from a chair and to climb stairs have all been shown to be significantly but moderately correlated to muscle strength,4 although these correlations are complex and nonlinear.5 The strength of major muscle groups of the lower extremity and ankle is important for maintenance of normal gait.6 Not surprisingly, subjects in the lowest quartile of knee extensor strength have nearly twice the risk for developing mobility limitations7 than those in the upper quartile. Therefore, anabolic interventions that increase muscle mass and strength would be expected to improve physical function.
Resistance exercise training improves muscle mass, strength, and some measures of physical function in older individuals. In contrast, testosterone therapy in older men with low or low normal testosterone levels has been consistently shown only to increase lean body mass.8 In both young 9,10 and older healthy men,11 changes in lean body mass and muscle size during testosterone therapy are dependent on androgen dose and the circulating testosterone concentrations. However, while some androgen trials in older men have reported improvements in muscle strength10-15 and performance-based measures of physical function,16,17 other trials18,19 have failed to confirm these improvements with androgen administration.
The reasons for the failure to demonstrate consistent improvements in muscle strength and physical function measures in older men with testosterone supplementation are not clear. Of the multiple hypotheses that have been proposed, we considered the possibility that testosterone doses used in previous trials were relatively small and had resulted in only modest increments in serum testosterone levels in the participants whose average baseline testosterone levels were in the low normal range. We hypothesized that higher doses of testosterone when administered to healthy older men would be associated with greater increments in serum testosterone levels, skeletal muscle mass, and maximal voluntary strength, and consequently with greater improvements in measures of physical function. Accordingly, we determined the effects of graded testosterone doses on several measures of physical function in healthy, older men whose baseline testosterone production had been suppressed by administration of a long-acting gonadotropin releasing hormone (GnRH) agonist. We also evaluated the effects of testosterone administration in older men on maximal voluntary strength, leg power and muscle fatigability that are important proximal markers of muscle performance. A secondary objective was to determine whether changes in physical function are related to testosterone-induced changes in lower extremity voluntary muscle strength or muscle power. The study design and the main findings of this study have been published;11 this manuscript describes in detail the changes in measures of muscle performance and physical function.
METHODS
Study Design
This was a double-blind, randomized study, approved by the institutional review boards of Charles Drew University and Los Angeles Biomedical Research Institute. All subjects provided written, informed consent.
The details of the study design have been previously described 11. Briefly, the study consisted of a 4-week control period, 20 weeks of treatment, and a 16-week recovery phase. Treatment consisted of monthly injections of a long-acting GnRH agonist (Lupron® depot, 7.5 mg; TAP, North Chicago, IL) to suppress endogenous testosterone production, plus weekly injections following one of five testosterone dosing regimens.
Subjects
Sixty healthy, eugonadal men, aged 60-75 years, participated in this study. Men were excluded if they had an American Urological Association symptom score >7, a prostate-specific antigen (PSA) level >4 ng/ml, a history of prostate cancer, hematocrit >48%, diabetes mellitus, congestive heart failure, severe sleep apnea, or myocardial infarction in the preceding 6 months. All participants performed a maximal cardiopulmonary cycle ergometer test with electrocardiogram monitoring to exclude those with cardiovascular symptoms during exercise. We excluded volunteers who had taken androgenic steroids, growth hormone, or other anabolic agents within the previous year. Men who were participating in resistance exercise training or moderate to heavy endurance exercise training were also excluded.
Subjects meeting eligibility criteria were randomly assigned using a block size of four to receive testosterone enanthate intramuscularly weekly in one of five dose regimens, 25, 50, 125, 300, or 600 mg/wk. All men received monthly injections of long-acting GnRH agonist during the treatment phase, starting on day 1. The five testosterone doses were chosen such that, when administered weekly in combination with GnRH agonist, they would produce nadir testosterone concentrations below, within, and above the physiological range. Subsequent to the Data Safety Monitoring Board discontinuing the 600 mg/wk dose group, new volunteers were randomized into the remaining four groups. As only six men had been randomized to 600 mg/wk dose at the time of discontinuation and because of high frequency of adverse events in those included in this group, data from the 600 mg/wk dose group are not included in this report.
Controlling Exercise and Nutritional Intake
Subjects were advised to refrain from all resistance exercise training and intense endurance exercise, but were allowed to continue other habitual activities throughout the study. Two weeks prior to randomization, subjects were prescribed a diet standardized for energy intake at 150 kJ/kg/d and protein intake at 1.3 g/kg body weight per day. These instructions were reinforced every 4-weeks by a dietitian and subject's nutrient intake was verified by analysis of 3-day food logs.
Performance-Based Measures of Physical Function
We selected tests of physical function that reflect common, everyday activities including stair climbing and walking. However, we recognized the possibility that the baseline performance of these healthy men in commonly used measures of physical function might already be in the asymptotic region of the curve describing the relationship between physical ability and physical performance. Consequently, we attempted to choose or modify physical function tests that had somewhat higher effort requirements.
Stair Climb
Two stair climb tests were administered; a four-step stair climb that has been shown to be responsive to resistance training in frail elderly20 and a more rigorous version that was a modification of the Margaria stair climb.21 In the former, subjects were instructed to climb four steps (total rise 0.66 meters) to a platform as quickly as possible. Handrail support was not permitted except as needed for balance. Subjects started six feet from a switch mat located at the base of the first step; a second switch mat was positioned on the fourth step and both mats were interfaced with an electronic digital timer (Lafayette Instruments, Lafayette, IN). After demonstration and practice, subjects ascended the four steps as rapidly as possible. Three trials were performed with one minute rest periods between trials. The fastest time achieved in the climb, subjects' body weight, total rise height, and the acceleration of gravity were used to calculate stair climbing power (watts). We chose this approach to normalize for the likely changes in body mass during testosterone administration. The modified Margaria stair climb consisted of a 12-step ascent up a 14 step staircase that ended on an 8 foot platform; the time was recorded over the middle four steps (4-8) of the climb (rise for middle four steps 0.69 meters) and power was calculated as above.
Timed up-and-go (TUG)
This test was used as an assessment of functional mobility 22. Subjects rose from a standard, armless, chair, walked 10 meters, turned around a marker, and returned to the seated position, performing these maneuvers as fast as possible. A pressure switch placed on the seat started a timer when subject rose from the chair and stopped the clock when the subject returned. Five trials were given with one minute rest between trials; the fastest time was accepted for the TUG score.
Walking speed
(m/s) was determined to the nearest 0.01 second using photoelectric cells and timers over the middle 6-m of a 10-m flat course.23 Subjects were instructed to walk the measured course under two conditions: at their usual walking speed or as fast as possible. The fastest of three trials was used to calculate walking speed under each of the two conditions. Subjects also completed a 400-meter course around a measured track with instructions to complete the course as fast as possible. No further instructions or encouragement were provided. A single trial was given with time to complete the course measured with a handheld stopwatch.
Assessment of Skeletal Muscle Mass
Dual energy x-ray absorptiometry (DEXA, Hologic QDR 4500A, Waltham, MA) was used to determine appendicular lean soft tissue (ALST) calculated from the sum of bilateral arm and leg fat-free masses. The DEXA scanner was calibrated before each measurement by using a soft tissue phantom. We used prediction models linking DEXA derived ALST to whole body skeletal muscle mass (SMM) as quantified by multi-slice magnetic resonance imaging (MRI). 24 Values for SMM were reported in kg and as a skeletal muscle mass index (SMMI, SMM/Ht2) to account for possible differences in size.
Assessment of Muscle Performance
Muscle Strength
Maximal voluntary strength in the leg press exercise was measured as the one repetition maximum, 1-RM,25 using a Keiser Seated Leg Press machine (Keiser Sport, Fresno, CA) with pneumatic resistance. Since maximal voluntary strength measurements are highly effort dependent, several strategies were used to assure reliability and to minimize the confounding influence of the learning effect. Tests were performed in duplicate or triplicate, with careful attention to standardization of starting knee flexion (approximately 90° by goniometry), foot placement, and completion of full range of motion. The familiarization effect was avoided by instructing subjects in the proper execution of the exercise and allowing practice trials. Following familiarization, subjects completed a warm up including 5 minutes of cycle ergometer or treadmill exercise. Details of the 1-RM test procedure have been described 10. Briefly, subjects performed progressively fewer lifts at heavier loads prior to attempts at 1-RM. Initial loads were set at 50% of the subject's estimated 1-RM using reference values established for this equipment. Attempts at 1-RM were interspersed with two-minute rest intervals and continued until 1-RM was identified as the greatest amount of weight lifted through the complete range of motion. Strength tests were conducted in duplicate within one week on non-consecutive days with scores required to be within 5%. Failure to meet this criterion required a third test. Only 10% of our subjects required a third test and none required a fourth. The highest value in the trials was taken as the 1-RM.
Power
Lower extremity knee and hip extensor muscle power was determined using a validated 26 leg power instrument (University of Nottingham Medical College, Nottingham, UK). The movement pattern for this exercise is similar to the leg press exercise except that subjects push as hard and as fast as possible using only the right leg the with the foot precisely positioned on a foot pedal. Peak power (watts and watts per kg body weight) was calculated using the mass of the flywheel, and its revolution frequency. The subjects were familiarized with the procedures, asked to complete a warm-up, and positioned so that the knee was flexed to 90°. Trials were continued until a plateau was reached. Peak power was typically identified after five to 15 trials. Thirty seconds of rest was provided between trials.
Fatigability
Local muscle endurance of the leg and hip extensors was determined with the leg press exercise. Subjects performed as many full range of motion leg press repetitions as possible using loads of 80% of their individual baseline 1-RM. The same familiarization and warm up employed in the 1-RM tests were used.
Pattern of Testing
All muscle function and physical performance testing was carried out over three days within a seven-day period. The first day of testing was used to identify initial values for leg press strength. On the second day of testing, subjects first performed the four-step stair climb, the two walking tests, and the timed-up-and-go each interspersed with 5 minutes rest. After 10 minutes rest, initial 1-RM values for the leg press were confirmed. Following additional rest, the subjects completed the 12-step stair climb and the fast faced 400-meter walk. Leg power and fatigability were assessed on the third day of testing. This schedule was well tolerated by all the men with no complaints or outward signs of fatigue.
Hormone Assays
Serum testosterone (immunoassay) and free testosterone (equilibrium dialysis) were measured as previously reported.9,11 The sensitivities, and intra- and inter-assay coefficients of variation, respectively, of these assays were 0.6 ng/dL, 8.2% and 13.2% for total testosterone and 0.22 pg/ml, 4.2% and 12.3% for free testosterone.
Statistical Analysis
Subject characteristics were analyzed with descriptive statistics and displayed as mean values ± standard deviation (sd). Differences among the groups were evaluated with analysis of variance (ANOVA). If the ANOVA was significant, the source of the difference was isolated using a Newman-Keuls post-hoc analysis. Changes from baseline in the measures of muscle performance and physical function due to testosterone dose were analyzed with dependent t-tests. Relationships between serum testosterone level and measures of muscle function and physical performance are described with Pearson Product-Moment correlations. A probability level of P≤0.05 was required for statistical significance.
RESULTS
Flow of subjects through the study
As described previously11, of the 60 men who were randomized, 52 completed all phases of the study; 13 in the 25 mg/wk group, 12 in the 50 mg/wk group, 11 in the 125 mg/wk group, 10 in the 300 mg/wk group and 6 in the 600 mg/wk group. The 600 mg/wk dose was discontinued by the Data Safety Monitoring Board (DSMB) because of the high frequency of erythrocytosis and leg edema. Because of its small size and the high frequency of adverse events experienced by subjects in this group, we excluded the 600 mg/wk group from our analyses. Of the 46 men who completed the study in the other four groups, 44 men who underwent both baseline and end-of-treatment evaluations of muscle performance and physical function are the subject of this analysis.
Baseline Characteristics of the Subjects
The baseline characteristics of the entire sample of 60 men have been reported previously; the 44 men included in this analysis did not differ significantly from the total sample in any of the baseline characteristics. Additionally, there were no significant differences among the four treatment groups at baseline for age, height, weight, serum total or free testosterone, or for any muscle performance or physical function variables. Baseline characteristics of the 44 men included in this report are presented in Table 1.
Table 1.
Baseline Subject Characteristics for the Four Testosterone Dose Groups.
| GnRH Agonist plus: | 25 mg/wk | 50 mg/wk | 125 mg/wk | 300 mg/wk | ||||
|---|---|---|---|---|---|---|---|---|
| mean | sd | mean | sd | mean | sd | mean | sd | |
| Age, yr | 65 | 4 | 66 | 4 | 66 | 5 | 67 | 4 |
| Height, cm | 177 | 6 | 177 | 5 | 176 | 8 | 178 | 5 |
| Weight, kg | 85 | 11 | 80 | 6 | 81 | 11 | 86 | 15 |
| SMM, kg | 29.5 | 3.7 | 28.5 | 3.7 | 28.4 | 3.7 | 28.8 | 3.1 |
| Total Testosterone, ng/dL | 372 | 128 | 328 | 77 | 388 | 129 | 320 | 110 |
| Free Testosterone, pg/dL | 37 | 14 | 36 | 13 | 45 | 12 | 33 | 9 |
| Leg press force, N | 2932 | 657 | 2717 | 422 | 2718 | 786 | 2947 | 448 |
| Power, watts | 187 | 30 | 171 | 30 | 162 | 28 | 175 | 29 |
| Fatigue, repetitions | 20 | 6 | 19 | 8 | 17 | 8 | 17 | 6 |
| 4-Step stair climb power, watts | 421 | 93 | 343 | 89 | 447 | 107 | 426 | 100 |
| Modified Margaria stair climb power, watts |
385 | 95 | 362 | 44 | 371 | 85 | 453 | 145 |
| TUG, s | 6.26 | 1.52 | 5.88 | 1.33 | 7.14 | 1.60 | 5.78 | 1.52 |
| 400-m, m/s | 2.43 | 0.60 | 2.64 | 0.67 | 2.75 | 1.09 | 2.20 | 0.75 |
| 6-m usual pace walk, m/s | 1.54 | 0.12 | 1.59 | 0.25 | 1.48 | 0.19 | 1.49 | 0.35 |
| 6-m fast pace walk, m/s | 2.30 | 0.24 | 2.42 | 0.36 | 2.44 | 0.45 | 2.70 | 0.62 |
There were no significant differences among the testosterone dose groups for any of the variables, P > 0.05.
GnRH is gonadotropin releasing hormone
SMM is skeletal muscle mass; TUG is timed up-and-go
Changes in Serum Total and Free Testosterone
Baseline values for serum total and free testosterone are shown in Table 1. Changes in nadir levels for serum total and free testosterone increased dose dependently (r =0.66, P<0.001 and r=0.57, P<0.001, respectively). After treatment, nadir levels for serum total testosterone were 176±34, 274±18, 852±111, and 1784±173 ng/dL for the 25, 50, 125, and 300 mg/wk dose groups, respectively, mean ± SD. After treatment, serum free testosterone levels for the same four dose groups were 19±4, 63±33, 80±11, and 215±25 pg/dL.
Skeletal Muscle Mass
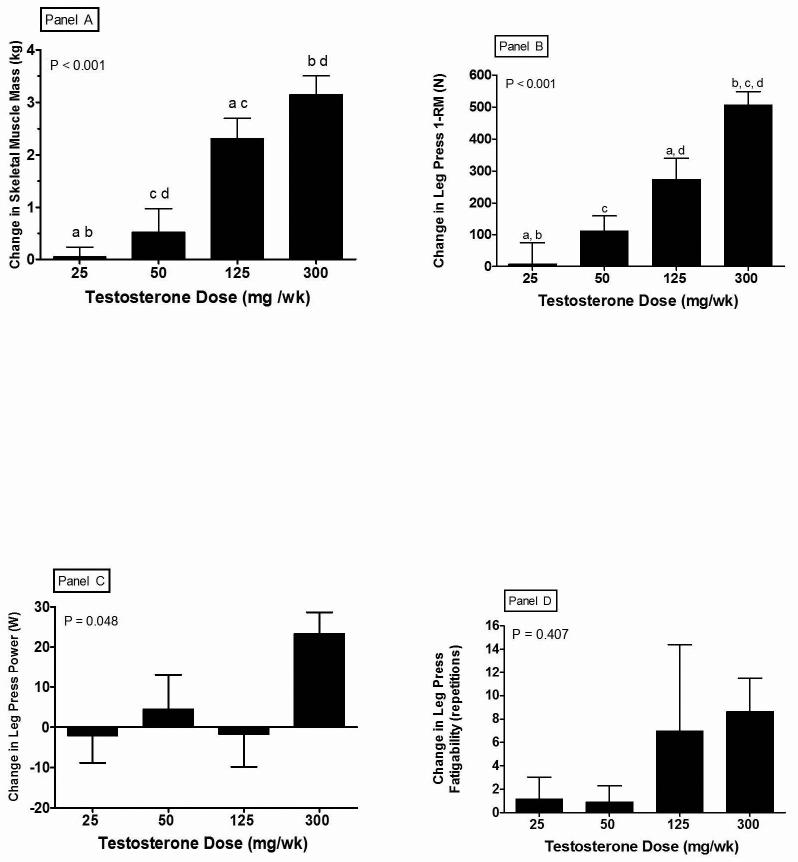
Increases in skeletal muscle mass were dose-dependent (Figure 1, Panel A). Skeletal muscle mass increased significantly from baseline in both the 125 (8.5 ± 4.0%) and 300 (11.1 ± 4.3%) mg/wk dose groups. Skeletal muscle mass index (skeletal muscle mass divided by height squared) also increased dose-dependently with testosterone administration (ANOVA P<0.0001). The changes in skeletal muscle mass and skeletal muscle mass index were significantly related to changes in leg press 1-RM strength, r = 0.64, P = 0.0001 and r = 0.52, P = 0.0006, respectively. However, neither SMM nor SMMI were significantly related to leg power or any of the physical function measures.
Figure 1.
Changes from baseline after 20 weeks of treatment with a GnRH agonist plus one of five weekly doses of testosterone enanthate. Error bars are SE. Means with the same letter are significantly different. Panel A: Skeletal muscle mass: Overall ANOVA, P < 0.001; a = P < 0.001; b = P < 0.001; c = P < 0.01; d = P < 0.001. (Panel B), 1-RM leg press strength: Overall ANOVA, P < 0.001; a = P < 0.01; b = P < 0.001; c = P < 0.001; d = P < 0.05. Panel C: Leg press power: Overall ANOVA, P = 0.048; there were no pairs of mean changes that were significantly different, P > 0.05. Panel D Leg press fatigability: Overall ANOVA, P = 0.407.
Muscle Performance Measures
There was no relationship between baseline total (r=0.06, P=0.68) or free (r=0.21, P=0.17) testosterone level and baseline leg press strength. Significant testosterone dose- and concentration-dependent increases were observed for changes in maximal voluntary muscle strength (Panel B, Figure 1). Subjects receiving 300 mg T (nadir serum T=1784 ng/dL) demonstrated a significant increase in leg press strength (+19%) while no change was observed in subjects receiving 25 mg T (nadir serum T=176 ng/dL).
Figure 1, Panel C illustrates significant differences for change in leg power (watts) among the four testosterone dose groups. These changes were positively correlated with testosterone dose, r =0.36, P=0.027. Changes from baseline in leg power for the four treatment groups were −2±24%, 5±29%, −2±27%, and 24±18%, respectively. The increase from baseline seen in the 300 mg/wk dose group was statistically significant. No significant relationship was observed between changes in leg power and changes in serum total or free testosterone.
Muscle fatigability determined by leg press repetitions to failure at 80% of baseline leg press 1-RM did not change significantly in any group (Figure 1, Panel D). Changes in fatigability were not significantly correlated to testosterone dose, changes in total or free testosterone (Table 2).
Table 2.
Relationships Between Changes in Measures of Muscle Function, Physical Performance, and Serum Testosterone Levels Resulting from Different Testosterone Dose Regimens.
| Change in Measures of Muscle Performance | r | P | |
|---|---|---|---|
| 1-RM Leg press (N) | Dose | <0.001 | |
| Change in TT | 0.40 | 0.006 | |
| Change in FT | 0.40 | 0.007 | |
| Leg press Power (watts) | Dose | 0.048 | |
| Change in TT | 0.10 | 0.478 | |
| Change in FT | 0.05 | 0.706 | |
| Fatigability | Dose | 0.407 | |
| Change in TT | 0.13 | 0.402 | |
| Change in FT | 0.11 | 0.472 | |
| Change in Measures of Physical Function | r | P | |
| Modified Margaria Stair Climb Power (watts) | Dose | 0.61 | |
| Change in TT | 0.03 | 0.85 | |
| Change in FT | 0.04 | 0.79 | |
| Standard 4-Step Star Climb Power (watts) | Dose | 0.25 | |
| Change in TT | 0.27 | 0.08 | |
| Change in FT | 0.29 | 0.06 | |
| Timed Up-and-Go | Dose | 0.26 | |
| Change in TT | 0.06 | 0.67 | |
| Change in FT | 0.03 | 0.84 | |
| 400-m walk (m/s) | Dose | 0.58 | |
| Change in TT | 0.16 | 0.33 | |
| Change in FT | 0.09 | 0.71 | |
| 6-m walk, Usual Pace (m/s) | Dose | 0.75 | |
| Change in TT | 0.09 | 0.56 | |
| Change in FT | 0.03 | 0.85 | |
| 6-m walk, Fast Pace (m/s) | Dose | 0.33 | |
| Change in TT | 0.07 | 0.65 | |
| Change in FT | 0.02 | 0.91 | |
Changes in Performance Based Measures of Physical Function
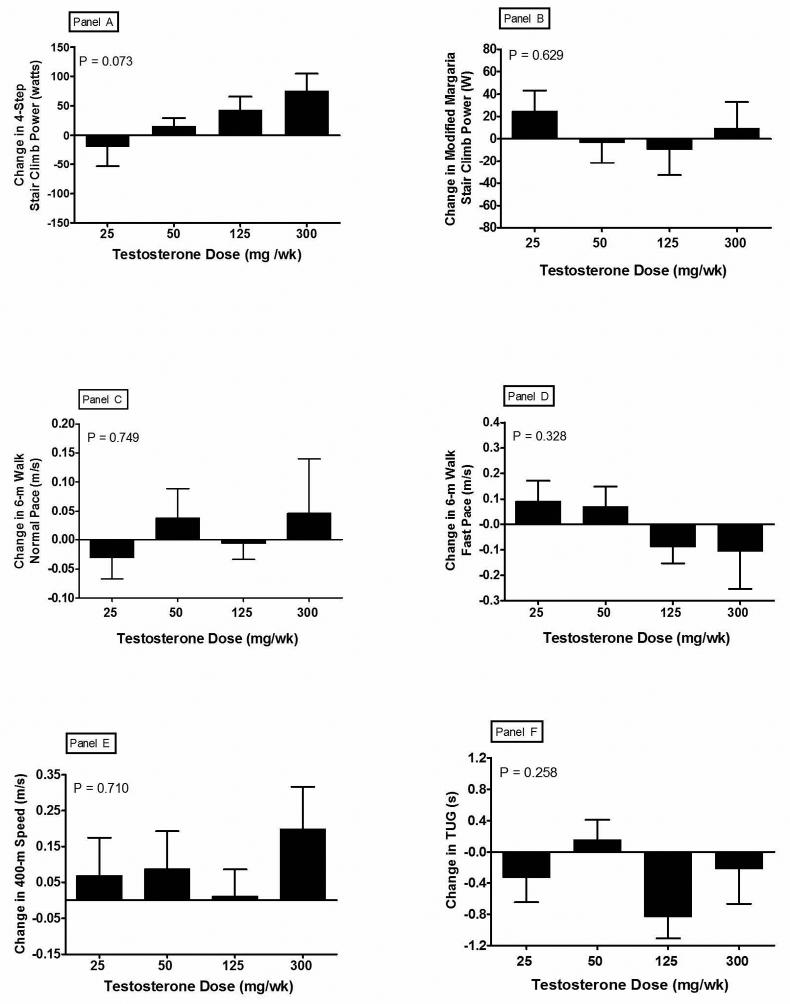
The stair climb time and power for the 4-step stair climb and the modified Margaria stair climb did not change significantly at any dose (Figure 2; Panels A and B). Stair climb power was not related to testosterone dose or concentrations.
Figure 2.
Changes from baseline in six measures of physical performance after 20 weeks of treatment with a GnRH agonist plus one of five weekly doses of testosterone enanthate. Error bars are SE. Panel A: 4-step stair climb; Panel B modified Margaria stair climb; Panel C: 6-m walk, usual pace; Panel D: 6-m walk, fast pace; Panel E: 400-m fast pace; Panel F: timed up-and-go. There were no significant differences between any of the mean changes for any of the six measures of physical performance, P > 0.05.
Changes in the three walk tests (usual and fast pace over 6-m and fast pace over 400 m) for the four testosterone dose groups are exhibited in Panels C-E of Figure 2. There was no significant change at any dose, nor was there a significant correlation between testosterone dose or concentrations and walking speed.
Time to complete the TUG test did not differ among groups. There was a significant change from baseline in the 125 mg/wk (12% faster, P=0.01 Figure 2 Panel F). However, this change was not statistically different from the other groups.
There was no significant relationship between changes in any of the six measures of physical function (4-step stair climb power, Modified Margaria stair climb power, TUG time, 6-m walk (usual pace) , 6-m walk (fast pace), 400-m walk, with testosterone dose, change in serum total or free testosterone concentration (Table 2). Similarly, changes in skeletal muscle mass, maximal voluntary strength, leg power, or fatigability were not correlated with changes in physical function measures (Table 3).
Table 3.
Relationships Between Changes in Leg Press Strength, Power, and Fatigability with Changes in Selected Measures of Physical Performance.
|
Change in Measures of Muscle Performance |
r | P | |
|---|---|---|---|
| 1-RM Leg press (N) | 4-Step stair climb | 0.20 | 0.78 |
| Modified Margaria stair climb | 0.48 | 0.33 | |
| 6-m walk, usual pace | 0.32 | 0.53 | |
| 6-m walk, fast Pace | 0.50 | 0.10 | |
| 400-m speed | 0.42 | 0.25 | |
| Timed Up-and-Go | 0.26 | 0.62 | |
| Leg Power (watts) | 4-Step stair climb | 0.07 | 0.66 |
| Modified Margaria stair climb | 0.07 | 0.67 | |
| 6-m walk, usual pace | 0.14 | 0.36 | |
| 6-m walk, fast Pace | 0.02 | 0.88 | |
| 400-m speed | 0.19 | 0.26 | |
| Timed Up-and-Go | 0.17 | 0.24 | |
| Fatigability (repetitions to failure) | 4-Step stair climb | 0.09 | 0.54 |
| Modified Margaria stair climb | 0.02 | 0.91 | |
| 6-m walk, usual pace | 0.06 | 0.71 | |
| 6-m walk, fast Pace | 0.06 | 0.71 | |
| 400-m speed | 0.10 | 0.54 | |
| Timed Up-and-Go | 0.04 | 0.81 |
DISCUSSION
Testosterone administration to healthy older men without mobility limitations was associated with dose-dependent changes in skeletal muscle mass, maximal voluntary leg press strength, and leg power. Testosterone administration did not improve leg muscle fatigability. Healthy older men experienced no detectable improvements in performance-based measures of physical function such as walking, climbing stairs, or rising from a chair and walking a distance even after receiving supraphysiologic doses of testosterone that induced substantial gains in skeletal muscle mass and maximal voluntary strength. Changes in testosterone concentrations and muscle strength induced by testosterone administration were not correlated with changes in physical function measures in healthy older men.
It is surprising that in spite of substantial gains in skeletal muscle mass and maximal voluntary strength, testosterone administration did not improve physical function. The performance in physical tasks, such as walking, is related to the strength and power of the muscle groups that are required to execute that physical task. At very low levels of physical function, this relationship is more or less linear: as strength increases, the performance in that physical function improves.27 At a certain level of muscle strength, performance in the physical task reaches an asymptote; further gains in strength beyond this asymptote would not be expected to improve performance in that physical task.27-29 As an example, in healthy young and older men, rising from a chair requires only a small fraction of the maximal voluntary strength. In these individuals, the physical performance is already in the asymptotic region of the strength - function relationship curve. Therefore, we posit that further gains in strength by testosterone therapy in healthy older men would not be expected to improve performance in this measure of physical function.
In addition to muscle strength and power, there are other determinants of performance in physical tasks. Neuromuscular adaptations play an important role in improvements in function during resistance exercise training. We do not know whether testosterone administration alone is sufficient to induce neurovascular or neuromuscular, i.e., motor control, adaptations that transform newly accrued muscle into a functional muscular unit. It is possible that the neuromuscular adaptations required to translate muscle mass gain into functional improvements may take longer than 20 weeks. Indeed, Page and colleagues noted improvements in a composite measure of physical function after one year of treatment with testosterone or a combined regimen of testosterone and finasteride.17
Others have suggested that the translation of strength gains into improvements in physical function may require cognitive, behavioral, or functional training. A systematic review of the effectiveness of progressive resistance strength training in older adults 30 revealed that resistance exercise training improves muscle strength, a proximal measure of muscle performance, but it does not reduce physical disability. The authors suggested that resistance exercise training may need to be combined with functional/behavioral training to translate strength gains into functional improvements. Similar caveats may apply to other anabolic therapies such as testosterone whose primary effect is to increase skeletal muscle mass. Additional vascular and/or biochemical mechanisms may be required for achieving functional improvements, and it is possible that testosterone therapy failed to induce these vascular and biochemical adaptations.
The study was powered primarily to determine the relationship between testosterone dose and changes in skeletal muscle mass and muscle strength, and it may not have had sufficient power to detect small but significant differences in the changes in physical function measures among groups. However, we observed neither treatment-related differences in physical function measures, nor any significant correlation between changes in testosterone concentrations and any physical function measure.
The potential applications of testosterone as a function promoting therapy should be tempered by recognition of its potential adverse effects, especially in older men.31, 32 Erythrocytosis is the most frequent adverse effect of testosterone therapy in randomized clinical trials of middle-aged and older men. Additional adverse effects include acne, oiliness of skin, breast tenderness and enlargement, leg edema, and exacerbation of sleep apnea. Testosterone therapy may promote the growth of metastatic prostate cancer. The long term adverse effects of testosterone on the prostate and cardiovascular events are unknown. Supraphysiologic doses of testosterone might be associated with even a higher frequency of adverse events than replacement doses.
These data have important implications for the design of future trials of anabolic therapies in older men and women. First, these trials should be conducted in older individuals who have functional limitations and whose baseline strength and physical performance is in steep ascending portion of the strength - function curve so that there is room for demonstrable improvement in function with increased muscle strength induced by anabolic therapies. Our subjects were healthy, eugonadal men who were unusually fit for their age (65 (±4) years) and had no functional limitations or symptomatic disease. This is clearly evidenced in the robust scores for the TUG and the walk tests, especially over the 400-m course. We acknowledge, however, that our instructions to subjects for the two 6-meter walks tests and for the 400-meter walk test did not specify heel-toe patterns, nor that one foot must always be in contact with the ground. While we are quite confident that this pattern was exhibited in the usual and fast paced 6-meter walks, our failure to specify heel-toe patterns and require one foot to be always in contact with the ground may have resulted in 400-meter speeds that were faster than the 6-meter walking speed.
Second, the physical function tests that require an effort that is close to the maximal voluntary strength for maximal performance in the physical task are more likely to demonstrate changes in physical function with strength improvements. Third, sample size estimates should take into account the considerable variability in measures of muscle strength, leg power, and even more so in the physical function measures. Consideration also should be given to incorporation of functional/behavioral training as an adjunct to anabolic therapy. Implementation of these strategies in the design of future trials would maximize the chances of demonstrating functional improvement with testosterone administration.
ACKNOWLEDGMENTS
The Corresponding author affirms that all individuals who contributed significantly to this study are listed on the title page as co-authors.
Financial Disclosure: This study was supported primarily by a grant from the National Institute of Aging (1RO1AG14369-01) awarded to Shalender Bhasin, MD. Additional support was provided by grants 1RO1DK59627-01, 2RO1DK49296-02A, 1RO1HD043348-01, U54HD041748-1, RCMI grants P20RR11145, G12RR03026, and UARP DrewCares HIV Center grant, GCRC grant MO-00425 also awarded to Shalender Bhasin, MD. TAP pharmaceuticals provided the GnRH agonist and BioTechnology General provided testosterone enanthate.
Sponsor's Role: The sponsors of this study played no role in the design, methods, subject recruitment, data collection, analysis, or preparation of this paper.
Footnotes
Conflict of Interest:
The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Shalender Bhasin, MD
1. Grants/Funds: Dr. Bhasin is the Principal Investigator of an investigator-initiated research project “Testosterone Effects on Atherosclerosis Progression in Older Men with Low Testosterone Levels” which is supported by a research grant from Solvay Pharmaceuticals to Boston University. However, Solvay provided no support for the research reported in this manuscript and did not participate in the design or conduct of this study or in the preparation of this manuscript.
2. Honoraria: None
3. Speaker Forum: None
REFERENCES
- 1.Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ, 3rd, Khosla S, Crowson CS, et al. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- 3.Roy TA, Blackman MR, Harman SM, et al. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283:E284–294. doi: 10.1152/ajpendo.00334.2001. [DOI] [PubMed] [Google Scholar]
- 4.Knutzen KM, Brilla L, Caine D, et al. Absolute vs. relative machine strength as predictors of function in older adults. J Strength Cond Res. 2002;16:628–640. [PubMed] [Google Scholar]
- 5.Ferrucci L, Guralnik JM, Buchner D, et al. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M275–285. doi: 10.1093/gerona/52a.5.m275. [DOI] [PubMed] [Google Scholar]
- 6.Perry J. Gait analysis: normal and pathological function. SLACK Inc.; New Jersey: 1992. [Google Scholar]
- 7.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S, Calof OM, Storer TW, et al. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2:146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 10.Storer TW, Magliano L, Woodhouse L, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 11.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 12.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 13.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder ET, Singh A, Bhasin S, et al. Effects of an oral androgen on muscle and metabolism in older, community-dwelling men. Am J Physiol Endocrinol Metab. 2003;284:E120–128. doi: 10.1152/ajpendo.00363.2002. [DOI] [PubMed] [Google Scholar]
- 15.Urban RJ, Bodenburg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 16.Brill KT, Weltman AL, Gentili A, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87:5649–5657. doi: 10.1210/jc.2002-020098. [DOI] [PubMed] [Google Scholar]
- 17.Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 18.Clague JE, Wu FC, Horan MA. Difficulties in measuring the effect of testosterone replacement therapy on muscle function in older men. Int J Androl. 1999;22:261–265. doi: 10.1046/j.1365-2605.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- 19.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 20.Bassey EJ, Fiatarone MA, O'Neill EF, et al. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 21.Margaria R, Aghemo P, Rovelli E. Measurement of muscular power (anaerobic) in man. J Appl Physiol. 1966:21. doi: 10.1152/jappl.1966.21.5.1662. [DOI] [PubMed] [Google Scholar]
- 22.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 23.Visser M, Pluijm SM, Stel VS, et al. Physical activity as a determinant of change in mobility performance: The Longitudinal Aging Study Amsterdam. J Am Geriatr Soc. 2002;50:1774–1781. doi: 10.1046/j.1532-5415.2002.50504.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Wang Z, Heymsfield SB, et al. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 25.Baechle T, Earle R, Wathen D. Resistance training. In: Baechle T, Earle R, editors. Essentials of strength training and conditioning. Human Kinetics; Champaign: 2000. pp. 407–409. [Google Scholar]
- 26.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 27.Buchner DM, Larson EB, Wagner EH, et al. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–391. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 28.Kwon IS, Oldaker S, Schrager M, et al. Relationship between muscle strength and the time taken to complete a standardized walk-turn-walk test. J Gerontol A Biol Sci Med Sci. 2001;56:B398–404. doi: 10.1093/gerona/56.9.b398. [DOI] [PubMed] [Google Scholar]
- 29.Rantanen T, Guralnik JM, Izmirlian G, et al. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998;77:299–305. doi: 10.1097/00002060-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Latham NK, Bennett DA, Stretton CM, et al. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 32.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: A meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]