Abstract
Acid-sensing ion channels (ASICs) are cationic channels activated by extracellular acidosis that are expressed in both central and peripheral nervous systems. Although peripheral ASICs seem to be natural sensors of acidic pain (e.g., in inflammation, ischaemia, lesions or tumours), a direct demonstration is still lacking. We show that ∼60% of rat cutaneous sensory neurons express ASIC3-like currents. Native as well as recombinant ASIC3 respond synergistically to three different inflammatory signals that are slight acidifications (∼pH 7.0), hypertonicity and arachidonic acid (AA). Moderate pH, alone or in combination with hypertonicity and AA, increases nociceptors excitability and produces pain suppressed by the toxin APETx2, a specific blocker of ASIC3. Both APETx2 and the in vivo knockdown of ASIC3 with a specific siRNA also have potent analgesic effects against primary inflammation-induced hyperalgesia in rat. Peripheral ASIC3 channels are thus essential sensors of acidic pain and integrators of molecular signals produced during inflammation where they contribute to primary hyperalgesia.
Keywords: acid, ASIC3, DRG, inflammation, pain
Introduction
Acid-sensing ion channels (ASICs) are cationic channels activated by extracellular protons (Waldmann et al, 1997a, 1997b; Wemmie et al, 2006; Lingueglia, 2007). Four genes encoding seven subunits (ASIC1a, ASIC1b, ASIC1b2, ASIC2a, ASIC2b, ASIC3 and ASIC4) have been identified so far in mammals. Functional channels result from the association of the different ASIC subunits into trimers (Jasti et al, 2007) leading to homomeric or heteromeric channels (Lingueglia et al, 1997; Alvarez de la Rosa et al, 2002; Benson et al, 2002; Hesselager et al, 2004). ASICs are predominantly neuronal channels, expressed in central (CNS) and peripheral (PNS) nervous systems. Whereas ASIC1a and ASIC2 are widely present in both CNS and PNS, the expression of ASIC1b and ASIC3 is restricted to peripheral sensory neurons (Waldmann et al, 1997a; Chen et al, 1998; Bassler et al, 2001).
As extracellular acidosis correlates with pain sensations (Steen et al, 1995a; Issberner et al, 1996; Reeh and Steen, 1996), ASICs have been proposed to sense extracellular acidifications occurring in pathological conditions such as inflammation, ischaemia, haematomas, fractures and lesions as well as in postoperative states (Krishtal and Pidoplichko, 1981; Waldmann et al, 1997a, 1997b; Waldmann and Lazdunski, 1998; Woo et al, 2004). Indeed, experiments performed in healthy human volunteers (Ugawa et al, 2002; Jones et al, 2004) using non-specific blockers such as amiloride or non-steroidal anti-inflammatory drugs (NSAIDs) (Waldmann et al, 1997b; Voilley et al, 2001) and behavioural experiments in rats using a non-discriminative ASIC blocker (A-317567) (Dube et al, 2005) both support a function of ASICs in acid-induced cutaneous pain.
However, data obtained from ASIC knockout mice have failed to demonstrate a clear function of these channels in acidic or primary inflammatory pain (Price et al, 2001; Chen et al, 2002; Ikeuchi et al, 2008; and our own unpublished results) but have shown an effect on secondary mechanical hyperalgesia (related to central sensitization in the spinal cord) in injured or inflamed muscle and joint (Price et al, 2001; Sluka et al, 2003; Sluka et al, 2007; Ikeuchi et al, 2008). Therefore, the participation of peripheral ASICs to acid-induced cutaneous pain and primary inflammatory hyperalgesia remains an open question.
In this work, we show that rat cutaneous sensory neurons display a large amount of ASIC1a and ASIC3-like currents when stimulated by moderate acidosis (i.e., around pH 7.0). We then demonstrate the involvement of peripheral ASIC3 in sensing cutaneous acidic pain in normal and inflammatory conditions.
Results
DRG neurons innervating the skin exhibit a high level of ASIC3-like current
We have investigated native ASIC currents activated by moderate acidifications in rat skin dorsal root ganglion (DRG) neurons stained by retrograde labelling with the fluorescent dye DiI (Figure 1A). The very moderate pH value used in these experiments (pH 6.6) was chosen to mainly activate ASIC1-like and ASIC3-like currents, as ASIC2-like and TRPV1 currents have been described to be activated by more drastic acidifications (Tominaga et al, 1998; Lingueglia, 2007). These neurons have a resting membrane potential of −55.0±1.8 mV and a membrane capacitance of 39.6±1.8 pF (n=42 and 43, respectively, data from four different cultures), corresponding to estimated neuron diameters ranging from 20 to 45 μm. We found that 65.8±6.3% of these skin DRG neurons exhibit a transient pH 6.6-induced ASIC-like current (4 of 7, 8 of 11, 8 of 10 and 8 of 15 neurons; Figure 1A), with a mean amplitude of −60.3±16.0 pA/pF (n=28). Within the remaining skin DRG neurons, pH 6.6 induces either no current (n=11) or only a small sustained current (−1.2±0.5 pA/pF, n=4). The ratio of ASIC-like current is not dramatically changed by the culture conditions; 70% (7 of 10 neurons) and 65.2% (15 of 23 neurons) of DRG neurons have a pH 6.6-evoked ASIC-like current after 24–48 h or 24–72 h of culture, respectively. Molliver et al (2005) have previously found that 11% of the skin afferent neurons have a functional pH 6.8-evoked ASIC-like current after 24–48 h of culture, whereas 28% are positively stained for ASIC3 by immunohistochemistry. The discrepancy with our data is most probably explained by differences in experimental procedure (rat upper back skin versus dorsal face of the hind paw, neurons of diameter <25 μm versus neurons of diameter ranging from 20 to 45 μm, pH 6.8- versus pH 6.6-evoked ASIC-like currents and threshold >300 pA compared with 30 pA in our study).
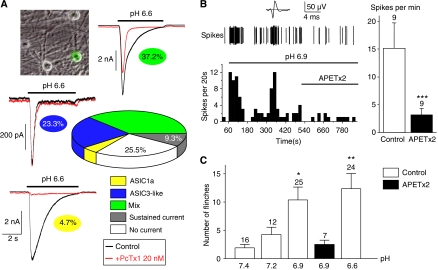
Figure 1.
ASIC3 senses cutaneous acidic pain in rat. (A) Quantification and analysis of pH 6.6-evoked ASIC currents recorded at −80 mV from rat skin DRG neurons using the PcTx1 toxin. Skin DRG neurons in primary culture were identified using fluorescence microscopy after retrograde labelling with DiI (see the image on the top left). The respective percentages of the different current types are highlighted under each current trace and on the graph (data obtained from a total of 43 neurons). (B) Exemplar response of a CM-fibre to pH 6.9 (spikes) with the corresponding time plot of the spike-frequency shown below. The firing of action potential is maintained at pH 6.9, and application of APETx2 10 μM (bar) inhibits the response. The top trace shows the average action potential. Average spike frequency at pH 6.9 and pH 6.9 with APETx2 10 μM (n=9) is represented on the left. (C) Effect of moderate subcutaneous acidification on pain behaviour in rat (determined as the number of flinches of the injected hind paw; see Materials and methods). The injected solution (NaCl 0.9%+20 mM HEPES) was buffered at pH values ranging from 7.4 to 6.6. Condition in which APETx2 10 μM was added to the injected solution is represented in black (*P<0.05 and **P<0.01, significantly different from pH 7.4, Kruskal–Wallis test followed by a Dunn's post hoc test).
To discriminate between the different pH 6.6-evoked ASIC-like currents within the skin DRG neurons, we used Psalmotoxin 1 (PcTx1), a potent and selective inhibitor of homomeric ASIC1a channels (Escoubas et al, 2000). We show in Figure 1A that 4.7% (2 of 43) of these neurons express a current that is largely inhibited by PcTx1 (inhibition >90%). This current is identified as flowing through native ASIC1a homomers, and it has the same inactivation kinetics as the recombinant ASIC1a current expressed in the F-11 cell line (τinactivation=1.6±0.4 s, n=2 versus τinactivation=1.7±0.09 s, n=10; see Supplementary Figure 1). Then, 23.3% of the neurons (10 of 43) exhibit a PcTx1-insensitive current (inhibition <10%). This current is identified as an ASIC3-like current, and its inactivation kinetics are similar to that of recombinant ASIC3 expressed in F-11 cells (τinactivation=0.2±0.01 s, n=14 versus τinactivation=0.4±0.09 s, n=9; see Supplementary Figure 1). Finally, 37.2% of the neurons (16 of 43) have partially PcTx1-sensitive currents (10%⩽inhibition⩽90%). This native mix current has an inactivation time constant (τinactivation=1.2±0.2 s, n=16) that is reduced by the treatment with PcTx1 to a value (τinactivation=0.5±0.1 s, n=16) not significantly different from that of recombinant ASIC3 and native ASIC3-like currents (see Supplementary Figure 1). This current is therefore considered as resulting from a mix of ASIC1a homomeric and ASIC3-like currents. Taken together, these data demonstrate that ASIC3-like currents are the most highly expressed ASIC currents activated by moderate acidifications in skin DRG neurons. They are present in 60.5% (26 of 43) of the skin sensory neurons.
Inhibition of ASIC3 removes cutaneous pain produced by moderate acidosis
To measure the contribution of the ASIC3 channel to nociceptor activation in response to moderate acidification, we have recorded unmyelinated single C-fibre activity with the nerve–skin preparation (Reeh, 1986; Alloui et al, 2006). When challenged with a moderate acidification to pH 6.9, a total of 51% of skin rat C-fibres show significant activation (n=17 of 33 fibres, P<0.001, Wilcoxon test) and 41% are activated by an exposure to pH 6.6 (n=9 of 22 fibres, P<0.01, Wilcoxon test). The spike discharge is irregular with bursts of activity, and some fibres show a delayed onset, but the activity is maintained as long as the pH is kept acidic (Figure 1B). In all the fibres tested (n=9), the sea anemone toxin APETx2 (a specific ASIC3 blocker; Diochot et al, 2004) at 10 μM suppresses the pH 6.9-induced spike activity (P<0.01, Wilcoxon test), confirming ASIC3 as the major pH-sensor of nociceptive fibres for moderate acidosis (Figure 1B).
Investigation of the pain behaviour of rats following subcutaneous injections of moderately acid solutions (pH 7.4, 7.2, 6.9 and 6.6) in one of the hind paws (Figure 1C) shows a significant pain behaviour at pH 6.9 (flinching score increasing from 1.9±0.6 at pH 7.4 to 10.4±2.3 at pH 6.9, n=16 and 25 respectively, P<0.05, Kruskal–Wallis test followed by a Dunn's post hoc test). The pain behaviour in rats fails to develop when APETx2 is co-injected together with the pH 6.9 solution (Figure 1C). Together, these results demonstrate that ASIC3 is a key sensor of cutaneous pain produced by moderate acidification.
Hypertonicity increases neuronal excitability in skin DRG neurons through an effect on ASIC3
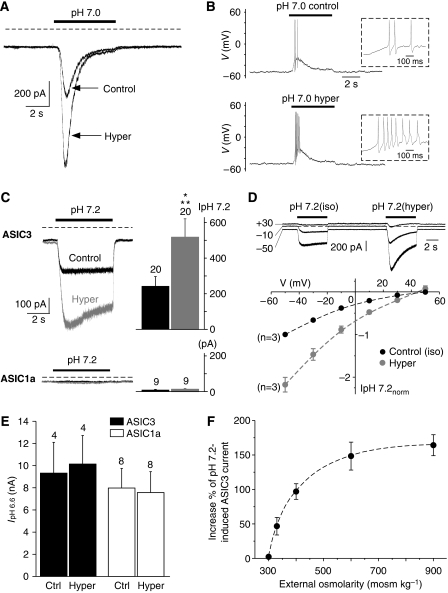
In inflamed or injured tissue, several potent mediators meet in the interstitial fluid and form an inflammatory exudate (Steen et al, 1995b), the content of which is acidic and hyperosmotic (Vakili et al, 1970). We have thus investigated the effect of hyperosmolarity on the ASIC currents in skin DRG neurons evoked by moderate acidification (i.e., pH 7.0). We show that hyperosmolarity (600 mosmol kg−1 with mannitol) strongly enhances the pH 7.0-evoked ASIC current in skin DRG neurons (increased by 95±35%, n=8, P<0.01, paired Student's t-test; Figure 2A). This leads to an increase of neuronal excitability by triggering more action potentials in current-clamped neurons (Figure 2B). The percentage of neurons in which APs are triggered following a pH 7.0 application is 37.5% (3 of 8 neurons), and all of them display an increase of the firing rate when hyperosmolarity or arachidonic acid (AA) was combined with pH 7.0. ASIC3 and ASIC1a are responsible for most of the currents activated by moderate acidification in rat cutaneous DRG neurons (Figure 1). To precisely determine which of these ASIC isoform(s) is involved in this effect, the ASIC3 and ASIC1a channels were heterologously expressed in the F-11 DRG cell line (Francel et al, 1987; Deval et al, 2006). Figure 2C shows that hyperosmolarity (600 mosmol kg−1 with mannitol) significantly potentiates the ASIC3 current evoked by a shift from pH 7.4 to 7.2 (increase of 148±20%, n=20, P<0.001, paired Student's t-test). Conversely, the same external acidification to pH 7.2 failed to produce any significant ASIC1a current from ASIC1a-transfected cells and hyperosmolarity was without effect (Figure 2C, lower panel). The control (measured in isotonic conditions) and the enhanced (measured in 600 mosmol kg−1 hypertonic conditions) pH 7.2-induced currents recorded from ASIC3-transfected cells have the same reversal potential (49.4±0.7 and 45.9±3.4 mV respectively, n=3, P=0.48, paired t-test; see Figure 2D), confirming the specificity of the effect on ASIC3. Interestingly, hyperosmolarity (600 mosmol kg−1) had no effect on pH 6.6-evoked ASIC3 current and was also without effect on the pH 6.6-evoked ASIC1a current (Figure 2E). Hyperosmolarity therefore seems to affect preferentially the persistent, non-inactivating ASIC3 window current (Yagi et al, 2006). The osmotic activation of pH 7.2-induced ASIC3 current was almost maximal when external osmolarity reached 600 mosmol kg−1 (Figure 2F). These results indicate that hyperosmolarity potentiates ASIC3 current, but not ASIC1a, at pH 7.2 probably through an effect on the window current.
Figure 2.
The potentiating effect of hypertonicity on native pH 7.0-evoked ASIC current is mainly mediated by ASIC3. (A) Typical ASIC current, recorded from a skin DRG neuron under voltage clamp at −80 mV, induced by a shift from pH 7.4 to 7.0. This current was strongly potentiated when a hypertonic solution (600 mosmol kg−1 with mannitol; see Materials and methods) was co-applied with pH 7.0. (B) Current clamp experiment performed on the same neuron as in A. The depolarization induced by the pH 7.0-evoked ASIC current was sufficient to trigger three action potentials (APs). Co-application of the hypertonic solution together with the pH drop led to an increase of the number of APs triggered. Time-scale magnifications of these APs are shown within the dotted rectangles. (C) Effect of hyperosmolarity (600 mosmol kg−1 with mannitol; see Materials and methods) on pH 7.2-induced ASIC1a and ASIC3 currents recorded at −50 mV from F-11-transfected cells. The number of experiments (n) is indicated above each bar (***P<0.001, paired Student's t-test). (D) Current–voltage relationship of the pH 7.2-induced ASIC3 current obtained from F-11-transfected cells before (control, isotonic) and during hyperosmotic shocks. Typical current traces are shown above the I/V curve. (E) Hyperosmolarity (600 mosmol kg−1 with mannitol) was without effect on both pH 6.6-induced ASIC3 and ASIC1a currents. (F) The increase in the percentage of the pH 7.2-induced ASIC3 current is represented as a function of external osmolarity. The hyperosmotic solutions were obtained by the addition of mannitol (n=5–20).
Moderate acidosis, hypertonicity and AA synergistically affect ASIC3 to produce cutaneous pain
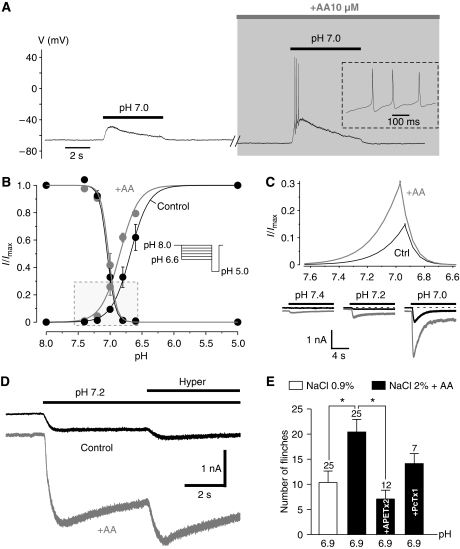
Arachidonic acid is known to positively affect ASIC currents (Allen and Attwell, 2002; Smith et al, 2007). We show here that AA increases the native ASIC current induced by a shift from pH 7.4 to 7.0 (+172±65%, n=6, P=0.06, paired Student's t-test). This effect leads to an increased excitability of the skin DRG neurons by increasing the triggering of AP (Figure 3A). We have further explored the pH sensitivity of the AA effect on ASIC1a and ASIC3 channels transfected in F-11 cells. As observed for the effect of hypertonicity, AA potentiates the pH 7.2-evoked ASIC3 current (+547±103%, P<0.0001, n=23, Wilcoxon test), whereas it has no effect on ASIC1a at the same pH (Supplementary Figure 2A). However, the kinetics of the two effects (i.e., hypertonicity and AA) are different. The effect of hypertonicity (co-application with the pH drop) is immediate, whereas the effect of AA needs a few minutes to be fully established (Supplementary Figure 2A). We have observed that AA also increases ASIC1a. The activation is larger at pH 7.0 (+183±54%, n=5, P=0.06, Wilcoxon test) than at pH 6.6 (+21±15%, n=2), but remains much lower than that observed for ASIC3 (increase of 493±84%, n=9, P<0.001, Wilcoxon test, and of 40±22%, n=7, P=0.45, paired Student's t-test at pH 7.0 and 6.6, respectively; see Supplementary Figure 2B). The potent effect of AA on the ASIC3 current essentially results from a shift of the pH dependence of activation towards less acidic values (pH1/2 for activation shifted from 6.68±0.01 to 6.84±0.01, n=5 and 3 cells, respectively, Figure 3B). No significant effect of AA is observed on the pH dependence of the inactivation curve. As a consequence of these differential effects of AA on the pH dependence of activation and inactivation, the non-inactivating ASIC3 window current is strongly enhanced in the presence of AA (Figure 3C, upper panel). This leads to an activation of the ASIC3 channel at resting physiological pH (i.e., pH 7.4; see Figure 3C, lower panel). Figure 3D shows that the effects of AA and hypertonicity on ASIC3 currents are synergistic, demonstrating that this channel is built to integrate different signals such as moderate acidification, hypertonicity and AA that are found in inflammatory conditions.
Figure 3.
ASIC3 senses different inflammatory signals to produce cutaneous pain. (A) Current clamp experiment showing that arachidonic acid (AA) increased the excitability of a skin DRG neuron in response to a depolarization induced by a pH 7.0-evoked ASIC current. Time-scale magnifications of the APs triggered are shown within the dotted rectangle. Note that pH 7.0-induced ASIC current leads to a membrane depolarization near the AP threshold, which is not always sufficient to produce firing (left panel). (B) pH-dependent activation and inactivation curves were obtained from F-11 cells transfected with ASIC3, at −80 mV, according to the protocol shown in inset (n=3–5). The framed rectangle indicates a part of the graph where the activation and inactivation curves overlap (window current). (C) Magnification of the framed zone shown in B indicating that the non-inactivating ASIC3 window current is strongly enhanced by AA (upper panel). The effect of AA on ASIC3 current induced by moderate acidifications is also represented (lower panel). (D) Representative whole-cell recording from transfected F-11 cells of a pH 7.2-induced ASIC3 current at −80 mV showing the synergistic effect of AA (10 μM) and hypertonicity (600 mosmol kg−1 with sucrose). (E) The effect on pain behaviour in rat of subcutaneous injections of acid (pH 6.9), hyperosmolarity and AA 10 μM together are compared with the effect of pH 6.9 alone. Conditions in which APETx2 10 μM or PcTx1 60 nM were added to the injected inflammatory cocktail are indicated on the bargraph. The number of experiments (n) is indicated above each bar (*P<0.05, Kruskal–Wallis test followed by a Dunn's post hoc test).
In good agreement with the latter results, combining hypertonicity (NaCl 2%, ∼600 mOsm kg−1) and AA (10 μM) significantly increases the flinching score of rats (Figure 3E) induced by moderate acidosis (i.e., pH 6.9; from 10.4±2.3, n=25 to 20.4±2.5, n=25, P<0.05, Kruskal–Wallis test followed by a Dunn's post hoc test). This increase in pain behaviour is largely prevented by co-injection of the ASIC3 blocker APETx2, whereas the homomeric ASIC1a blocker PcTx1 has no significant effect (Figure 3E). Taken together, all these results strongly suggest that the activation of peripheral ASIC3, but not homomeric ASIC1a, by inflammatory mediators contributes to inflammatory pain.
ASIC3 contributes to the development of CFA-induced primary inflammatory pain
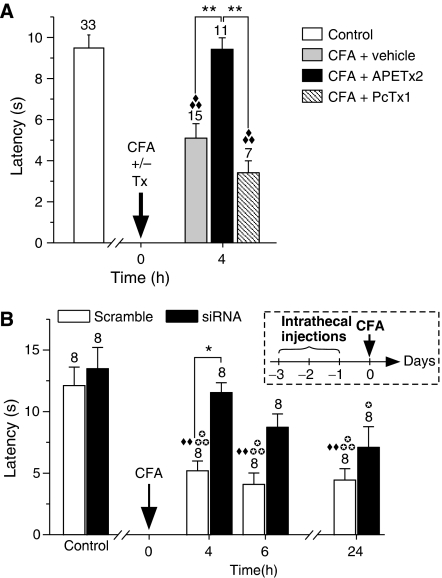
To demonstrate more directly the function of ASIC3 in cutaneous inflammatory pain, the effect of APETx2 and PcTx1 were tested on a rat model of inflammatory pain. Four hours after the induction of inflammation by CFA injection in the hind paw, a significant heat hyperalgesia appears (Figure 4A). The heat hyperalgesia does not develop when the ASIC3 blocker APETx2 is co-injected with CFA, whereas PcTx1 has no significant effect. The implication of ASIC3 in inflammatory thermal hyperalgesia is further confirmed by intrathecal injections of an siRNA targeting the ASIC3 channel before the induction of inflammation. A marked and specific knockdown of ASIC3 expression at the mRNA level has been demonstrated in lumbar DRGs after intrathecal injections of this siRNA (Supplementary Figure 3). In pain behaviour experiments, these injections prevent CFA-induced heat hyperalgesia, whereas the corresponding scramble siRNA used as a control is without effect (Figure 4B). These results directly demonstrate that ASIC3, but not homomeric ASIC1a, has an important function in primary inflammatory heat hyperalgesia at the peripheral level in rats.
Figure 4.
ASIC3 is a detector of cutaneous inflammatory pain in rat. (A) Effect of APETx2 20 μM and PcTx1 120 nM on CFA-induced thermal hyperalgesia in rat. Hind paw withdrawal latencies were measured at 50°C (see Materials and methods), and the time at which inflammation was induced (t0) is indicated by the arrow (**P<0.01; ♦♦♦P<0.001, significantly different from control, Kruskal–Wallis test followed by a Dunn's post hoc test). (B) Effect of intrathecal injections (see the inset for the protocol) of ASIC3 siRNA on the CFA-induced heat hyperalgesia described in A (* and  P<0.05; ♦♦P<0.01;
P<0.05; ♦♦P<0.01;  P<0.001, significantly different from scramble and siRNA control, respectively; one-way ANOVA followed by a Tukey's post hoc test).
P<0.001, significantly different from scramble and siRNA control, respectively; one-way ANOVA followed by a Tukey's post hoc test).
Discussion
Protons are direct activators of nociceptors (Steen et al, 1992). Studies conducted both in humans and animals have shown a positive correlation between pain and tissue acidity. Perfusion of acidic solutions or iontophoresis of protons into the skin produces pain in humans (Steen et al, 1995a; Ugawa et al, 2002; Jones et al, 2004), and ASIC channels seem to be the best candidates to sense this cutaneous acidic pain (Ugawa et al, 2002; Jones et al, 2004). Recent results with transcutaneous iontophoresis, a non-invasive method, are particularly illustrative (Jones et al, 2004). They have shown that amiloride, a blocker of ASIC channels (Waldmann et al, 1997b), and NSAIDs, which also inhibit this class of channels (Voilley et al, 2001), significantly decrease acidic pain. They have also demonstrated that skin desensitized by repeated capsaicin application shows no significant reduction in acid-induced pain. This latter result strongly suggests that the acid detection is not through the capsaicin receptor TRPV1 (Jones et al, 2004). This conclusion is fully consistent with previous observations using direct perfusion of acidic solutions into human skin (Ugawa et al, 2002), which strongly suggested that acidic pain elicited by pH values between 7.4 and 6 was not significantly associated with the TRPV1 channel but was blockable by amiloride. The only limit of this interesting series of papers is that neither amiloride nor NSAIDs are specific inhibitors of ASIC channels, and that besides ASIC and TRPV1 channels, other types of ionic channels could be involved.
Results obtained in humans have yet had no parallel in mice. Deletion of ASIC3 channels in this animal species has failed to indicate a clear function of this channel in pain behaviour associated with cutaneous acidosis or inflammation (Price et al, 2001; Chen et al, 2002). Silencing of ASIC3 using a dominant-negative subunit has even led to an increased sensitivity to inflammatory stimuli (Mogil et al, 2005). Interpretation of these results is complicated by the fact that (i) mice express relatively low levels of ASIC channels in their DRGs (Leffler et al, 2006; Lin et al, 2008; and unpublished data from this laboratory) as compared with other animal species such as rats (this article), (ii) deleting or silencing ASIC genes might be associated with the appearance of compensatory mechanisms.
We report here that subcutaneous injections of moderately acidic solutions elicit pain in rats within the same pH range (pH ∼6.9) as in humans (Steen et al, 1995a), and that this effect depends on ASIC3, but not homomeric ASIC1a. Consistent with this in vivo observation, we have shown that DRG neurons innervating rat skin display a high level of ASIC-like currents, which, when they are activated by slight acidification (pH 7.0), depolarize the neurons and trigger APs. We also show that very moderate acidifications also induce a significant increase in skin C-fibres firing, which is totally inhibited by the ASIC3-specific toxin APETx2. This demonstrates that ASIC3 is the leading receptor for moderate acidosis in skin nociceptors and participates in the signalling of acidic pain in rat.
Inflammation is one of the pain conditions that produce local tissue acidosis which, in principle, can be detected by ASIC channels. Inflammation also induces a large increase of ASIC channel expression in rat sensory neurons, particularly ASIC3. The nociceptor level of ASIC3 mRNA is increased by more than 15 times in CFA-evoked inflammation (Voilley et al, 2001). In addition, in primary cultures of DRG neurons, a mixture of the proinflammatory mediators NGF, serotonin, interleukin-1 and bradykinin increases the number of ASIC-expressing neurons as well as ASIC-like current amplitude in these neurons (Mamet et al, 2002). NGF has a particularly important function in regulating ASIC3 gene expression (Mamet et al, 2002, 2003). Besides this transcriptional regulation, post-translational modulation of ASIC3 by proinflammatory mediators also takes place. ASIC3 channel activity is increased, for instance, through the PKC pathway by compounds such as serotonin and bradykinin (Deval et al, 2004; Lingueglia, 2007). ASIC activity is increased by nitric oxide (NO), a mediator that reaches high levels in inflammation (Cadiou et al, 2007). Inflammation is also associated with hypertonicity (Vakili et al, 1970; Hamamoto et al, 2000), which modulates the activity of several ionic channels involved in pain perception, such as TRPV4 and TREK-1 (Alessandri-Haber et al, 2005; Alloui et al, 2006). We show here that hypertonicity increases the ASIC3 channel activity. Another important property of inflammation is that it dramatically increases levels of expression of phospholipases A2 (Vadas et al, 1993) that catalyses phospholipid hydrolysis with an increased production of AA. AA was shown previously to directly activate ASIC channels (Smith et al, 2007), and this work shows that ASIC3 is more sensitive than ASIC1a to AA. The very important observation about the effects of hypertonicity and AA is that they develop for very slight acidifications not far from the physiological pH (pH 7.4). In the presence of a hypertonic medium or AA, acidifications of only 0.2–0.4 pH units, which are easily attained in many pain-related situations such as inflammation, ischaemia, haematomas, fractures and lesions as well as in post-operative states (Issberner et al, 1996; Reeh and Steen, 1996; Woo et al, 2004), suffice to generate relatively large ASIC3-like currents and trigger action potential generation in cultured DRG neurons. The mechanism of this effect has been analysed here in detail for AA. AA produces an alkaline shift of the pH dependence of the activation process and, by doing so, enhances a non-inactivating window current. In fact, in the presence of AA, the ASIC3 channel gains a small activity at physiological pH (pH 7.4).
The other interesting observation is that the effects of AA and hypertonicity on ASIC3 current are synergistic. In agreement with this in vitro effect, we have observed that injection of a hypertonic solution together with AA significantly increases pain produced by moderate acidosis. This pain enhancement is dramatically decreased by APETx2, the selective blocker of ASIC3, and not by PcTx1, the selective blocker of homomeric ASIC1a, indicating the important function of the ASIC3 channel in such a process. At this stage, it became important to investigate how these data could be extrapolated to real conditions of inflammation. Inflammation was produced by CFA injection, which led to primary heat hyperalgesia, and this hyperalgesia was drastically reduced by the ASIC3 blocker APETx2 injected subcutaneously, which only access cutaneous nociceptors. It was also drastically reduced when, before triggering the inflammation state, intrathecal injections of an siRNA against ASIC3 had induced a knockdown of ASIC3 expression in lumbar DRGs.
All these results taken together demonstrate the important function of ASIC3 in primary pain generation associated with moderate acidosis and inflammation. Therefore, blocking ASIC channels at different levels of the sensory system would clearly be beneficial in relieving pain. Central inhibition of ASIC1a acting upstream of the opiate system (Mazzuca et al, 2007) is well adapted for treating all types of pain and, in particular, chronic pain such as neuropathic pain. Local inhibition of the ASIC3 channel that has an important function downstream of the multiple stimuli associated with inflammation is clearly another option to treat inflammatory pain.
Materials and methods
F-11 cell line culture and transfection
The F-11 cell line (Francel et al, 1987; Deval et al, 2006) was cultured at 37°C under 5% CO2 at a density of 50 000 cells per 35-mm-diameter Petri dish. The culture medium contained Ham's F-12 medium (Invitrogen) supplemented with 15% fetal bovine serum (ICN Biomedicals), 1 × HAT (sodium hypoxanthine, aminopterin and thymidine), 200 μg ml−1 allo-4-hydroxy-L-proline (Sigma-Aldrich) and 1% antibiotics (penicillin and spreptomycin, Gibco). One day after plating, cells were transfected with ASIC1a or ASIC3 cDNAs using Lipofectamin 2000 (Invitrogen) according to the manufacturer's instructions, with a mix of the pCI-ASIC1a and pIRES2-EGFP vectors (1:2 ratio) or the pCI-ASIC3 and pIRES2-EGFP vectors (1:10 ratio). Cells were used for patch-clamp experiment 2–4 days after transfection. F-11 cells natively express low levels of ASIC1a homomeric channels. However, this level of endogenous current recorded at −50 mV is very low (IpH 6.6=1.20±0.15 pA/pF, n=7) compared with the level of the ASIC3 or ASIC1a currents recorded here after transfection (IpH 6.6=322.01±78.44 pA/pF, n=7 and 310.63±49.80 pA/pF, n=4, respectively).
Retrograde labelling of skin afferents
Dorsal root ganglion neurons innervating skin from Wistar rats (8–11 weeks) were labelled by subcutaneous injections of 5 × 1 μl of the fluorescent dye DiI (5% in DMSO, Molecular Probes) into the dorsal faces of hind paws. Dye was injected 2 weeks before killing the rat to prepare DRG primary culture.
Primary culture of labelled dorsal root ganglia neurons
Lumbar DRG L3–L6 were dissected bilaterally from the previously DiI-injected rats and enzymatically dissociated with 0.1% collagenase. Cells were then plated on collagen-coated 35-mm Petri dishes and maintained in culture at 37°C (95% air/5% CO2) in DMEM containing 5% fetal calf serum. Electrophysiological experiments were carried out 1–8 days after plating.
Electrophysiology
We used the whole-cell configuration of the patch clamp technique to measure membrane currents (voltage clamp) or membrane potentials (current clamp). Recordings were made at room temperature using an RK-400 amplifier (Bio-Logic Science Instruments) with a 3-kHz low-pass filter (Krohn-Hite). Data were sampled at 10 kHz, digitized by a Digidata 1322A A-D/D-A converter (Axon Instruments) and recorded on a hard disk using pClamp software (version 9; Axon Instruments). Patch pipettes (1–4 MΩ) contained (in mM) the following: 135 KCl, 2.5 Na2-ATP, 2 MgCl2, 2.1 CaCl2, 5 EGTA, 10 HEPES (pH 7.25 with KOH). Various pH-buffered solutions and drugs of interest were applied to individual patch-clamped cells using a homemade microperfusion system driven by microsolenoid valves (Sirai, Italy) allowing rapid solution changes. The control bath solution contained (in mM) the following: 145 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES (pH 7.4 with NaOH). MES was used instead of HEPES to buffer solution pH ranging from 6 to 5 and ASIC currents were induced by shifting one out of eight outlets of the microperfusion system from the pH 7.4 control solution to an acidic test solution. For the experiments performed on cultured DRG neurons, glucose (10 mM) was added to the control bath solution. Hypertonic conditions were obtained by adding mannitol or sucrose to the external bathing solutions as indicating in the text.
Single C fibre recording
The isolated skin–saphenous nerve preparation and single C-fibre recording technique was used as described previously (Alloui et al, 2006). The skin of the hind paw of 8- to 14-weeks-old male rat was dissected with the saphenous nerve. The skin was superfused with warm (∼30°C) synthetic interstitial fluid, in mM: NaCl 107, KCl 3.48, NaHCO3 26.2, NaH2PO4 1.67, CaCl2 1.53, MgSO4 0.69, Na-gluconate 9.64, glucose 5.5, sucrose 7.6, HEPES 10, pH adjusted to 7.4, 6.9 and 6.6 with NaOH, saturated with O2/CO2—95%/5%. The receptive field of an identified C-fibre was searched by mechanical probing of the skin and further characterized for mechanosensitivity with calibrated von Frey filaments. This protocol implies that all C-fibres were mechanosensitive. Conduction velocity was <1.3 ms. C-fibres receptive fields were isolated with a thick-walled elrin ring (diameter 400 μm) inside which solutions and toxin were applied through local perfusion pipes of a CL-100 bipolar temperature controller (Warner Instrument). Recordings were band-pass-filtered between 60 Hz and 2 kHz and sampled at 10 kHz on computer with pClamp 9 software (Axon Instrument). Action potential were analysed with Clampfit software (Axon Instrument).
Nociceptive behaviour in rats
Adult (7–8 weeks) male Wistar rats (Charles River) were kept with a 12-h light/dark cycle and with ad libitum access to food and water. Rats were acclimated for at least 1 week before experiments. For nociceptive behaviour experiments, rats were placed in a transparent observation chamber where they were acclimated for 20–30 min. They were then gently restrained, while 20 μl of a saline solution (0.9 or 2% NaCl+20 mM HEPES, 7.4⩽pH⩽6.6 supplemented or not with 10 μM AA and/or ASIC-specific toxins; see figure legends) was administered subcutaneously into the dorsal face of the hind paw using a 30-gauge needle connected to a 100-μl Hamilton syringe. Nociceptive behaviour (i.e., number of flinches) was counted over a 5-min period starting immediately after the injection (Alessandri-Haber et al, 2005).
Inflammation-induced heat-hyperalgesia in rats
Heat sensitivity of adult male Wistar rats (Charles River) was assessed by measuring hind paw withdrawal latency from a hot plate at 50°C (Bioseb, France). Rats were acclimated to the experience room for at least 30 min, and each measurement was made in duplicate. A first measure was performed before the induction of inflammation. Rats were then anesthetized (isoflurane 2.5%) and 150 μl of complete Freund's adjuvant (CFA), diluted 1:1 with saline, and containing either toxins (PcTx1 120 nM or APETx2 20 μM final concentration) or water was injected (26-gauge needle) subcutaneously into the plantar face of one hind paw. The withdrawal latency of the injected hind paw was then measured on the hot plate at 50°C, 4, 6 and 24 h after CFA injection.
In vitro evaluation and in vivo injection and validation of the siRNA
The siRNA sequence targeting rat ASIC3 (no. 1121, CTACACGCTATGCCAAGGA) has been inserted into the siRNA expression vector pCi-U6-eCMV-neo as short hairpin RNA. This sequence showed no significant homology with other known rat sequences according to the Genbank database. This vector has been locally developed from the pCI neo vector (Promega) by replacing the SgfI/NotI fragment (which includes the CMV promoter) with a cassette containing the U6 promoter that was amplified by PCR from the vector developed by Sui et al (2002). The pCi-U6-eCMV-neo vector contains a U6 promoter, a CMV enhancer and a neomycin resistance gene for stable transfection. shRNAs were inserted into the ApaI and EcoRI sites downstream of the U6 promoter. COS cells were transfected by the DEAE-dextran method (Deval et al, 2006), with the shRNA constructs together with a vector coding for N-terminal Myc-tagged ASIC3 at a 20:1 ratio. Cells were lysed between 48 and 72 h after transfection and processed for western blot analysis to assess the level of ASIC3 protein. The blots were probed with the anti-Myc A14 antibody (1:500; Santa Cruz Biotechnology) and a monoclonal antibody against actin (clone AC-40; 1:1000; Sigma) as a loading control.
Cy3-labelled siRNA no. 1121 and its corresponding scramble (no. 1121S; GCTCACACTACGCAGAGAT) synthesized by MWG Biotech (Germany) were injected in rats by intrathecal bolus to the lumbar region of the spinal cord once a day for 3 days before the induction of inflammation with CFA. Each 10-μl injection corresponded to 2 μg of siRNA complexed with i-Fect siRNA transfection reagent (Neuromics) at a ratio of 1:4 (w:v) (Luo et al, 2005), following the supplier's suggested protocol. siRNA uptake in lumbar DRGs was monitored by fluorescence microscopy on cryostat sections 24 h after a single intrathecal injection. The specificity of the effect was evaluated in lumbar dorsal root ganglia by quantitative reverse-transcription PCR 24 h after the last injection. L5 and L6 ganglia were removed, RNA was extracted with the RNeasy micro kit (Qiagen) and cDNA was prepared with cloned AMV First-Strand cDNA synthesis kit (Invitrogen) and used for qPCR in a LightCycler480 (Roche Products). The primers used are as follows: 18S aagtccctgccctttgtacaca/gatccgagggcctcactaaac; ASIC1a ctatcaccacgtcaccaagc/agtgtgacagcagggaaggt; ASIC1b ggagttggatgagggtgatg/tggagggtacagctgttgg; ASIC2a gaccctctgcaacctcaatg/cagcgtggtacaagtcgttg; ASIC2b agtggttccgcaaactgg/ctgatgccctcgaagtgg; ASIC3 cacccaatgacttgcactgg/taggcagcatgttcagcagg; TRPV1 agcagcagtgagacccctaa/gaagtagaagatgcgcttgaca. Results were normalized against 18S and converted to fold induction relative to vehicle controls.
Statistical analysis
Data analysis was performed using Microcal Origin 6.0 and GraphPad Prism 4.03 softwares. Data are presented as mean±s.e.m. and statistical differences between sets of data were assessed using either parametric or non-parametric tests followed by post hoc tests when appropriate.
Supplementary Material
Supplementary Figures 1–3
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Agence Nationale de la Recherche (ANR), the Association Française contre les Myopathies (AFM) and the Association pour la Recherche sur le Cancer (ARC-INCa).
References
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD (2005) TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 118: 70–79 [DOI] [PubMed] [Google Scholar]
- Allen NJ, Attwell D (2002) Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischaemia-related signals. J Physiol 543: 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, Guy N, Blondeau N, Voilley N, Rubat-Coudert C, Borsotto M, Romey G, Heurteaux C, Reeh P, Eschalier A, Lazdunski M (2006) TREK-1, a K+ channel involved in polymodal pain perception. EMBO J 25: 2368–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM (2002) Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA 99: 2326–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Grunder S (2001) Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem 276: 33782–33787 [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM (2002) Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadiou H, Studer M, Jones NG, Smith ES, Ballard A, McMahon SB, McNaughton PA (2007) Modulation of acid-sensing ion channel activity by nitric oxide. J Neurosci 27: 13251–13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN (1998) A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA 95: 10240–10245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ (2002) A role for ASIC3 in the modulation of high-intensity pain stimuli.Proc Natl Acad Sci USA 99: 8992–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Friend V, Thirant C, Salinas M, Jodar M, Lazdunski M, Lingueglia E (2006) Regulation of sensory neuron-specific acid-sensing ion channel 3 by the adaptor protein Na+/H+ exchanger regulatory factor-1. J Biol Chem 281: 1796–1807 [DOI] [PubMed] [Google Scholar]
- Deval E, Salinas M, Baron A, Lingueglia E, Lazdunski M (2004) ASIC2b-dependent regulation of ASIC3, an essential acid-sensing ion channel subunit in sensory neurons via the partner protein PICK-1. J Biol Chem 279: 19531–19539 [DOI] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M (2004) A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J 23: 1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD (2005) Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 117: 88–96 [DOI] [PubMed] [Google Scholar]
- Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M (2000) Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 275: 25116–25121 [DOI] [PubMed] [Google Scholar]
- Francel PC, Harris K, Smith M, Fishman MC, Dawson G, Miller RJ (1987) Neurochemical characteristics of a novel dorsal root ganglion X neuroblastoma hybrid cell line, F-11. J Neurochem 48: 1624–1631 [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Forkey MW, Davis WL, Kajander KC, Simone DA (2000) The role of pH and osmolarity in evoking the acetic acid-induced wiping response in a model of nociception in frogs. Brain Res 862: 217–229 [DOI] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, Ahring PK (2004) pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem 279: 11006–11015 [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA (2008) Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain 137: 662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issberner U, Reeh PW, Steen KH (1996) Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett 208: 191–194 [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E (2007) Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323 [DOI] [PubMed] [Google Scholar]
- Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB (2004) Acid-induced pain and its modulation in humans. J Neurosci 24: 10974–10979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI (1981) A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience 6: 2599–2601 [DOI] [PubMed] [Google Scholar]
- Leffler A, Monter B, Koltzenburg M (2006) The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 139: 699–709 [DOI] [PubMed] [Google Scholar]
- Lin YW, Min MY, Lin CC, Chen WN, Wu WL, Yu HM, Chen CC (2008) Identification and characterization of a subset of mouse sensory neurons that express acid-sensing ion channel 3. Neuroscience 151: 544–557 [DOI] [PubMed] [Google Scholar]
- Lingueglia E (2007) Acid-sensing ion channels in sensory perception. J Biol Chem 282: 17325–17329 [DOI] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M (1997) A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem 272: 29778–29783 [DOI] [PubMed] [Google Scholar]
- Luo MC, Zhang DQ, Ma SW, Huang YY, Shuster SJ, Porreca F, Lai J (2005) An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol Pain 1: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamet J, Baron A, Lazdunski M, Voilley N (2002) Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamet J, Lazdunski M, Voilley N (2003) How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem 278: 48907–48913 [DOI] [PubMed] [Google Scholar]
- Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gelot A, Cupo A, Zimmer A, Zimmer AM, Eschalier A, Lazdunski M (2007) A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci 10: 943–945 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P (2005) Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci 25: 9893–9901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW (2005) ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ (2001) The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083 [DOI] [PubMed] [Google Scholar]
- Reeh PW (1986) Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett 66: 141–146 [DOI] [PubMed] [Google Scholar]
- Reeh PW, Steen KH (1996) Tissue acidosis in nociception and pain. Prog Brain Res 113: 143–151 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ (2003) Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP (2007) ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain 129: 102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Cadiou H, McNaughton PA (2007) Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. Neuroscience 145: 686–698 [DOI] [PubMed] [Google Scholar]
- Steen KH, Issberner U, Reeh PW (1995a) Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neurosci Lett 199: 29–32 [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO (1992) Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci 12: 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen KH, Steen AE, Reeh PW (1995b) A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J Neurosci 15: 3982–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC, Shi Y (2002) A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA 99: 5515–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543 [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S (2002) Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest 110: 1185–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas P, Browning J, Edelson J, Pruzanski W (1993) Extracellular phospholipase A2 expression and inflammation: the relationship with associated disease states. J Lipid Mediat 8: 1–30 [PubMed] [Google Scholar]
- Vakili C, Ruiz-Ortiz F, Burke JF (1970) Chemical and osmolar changes of interstitial fluid in acute inflammatory states. Surg Forum 21: 227–228 [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, Lazdunski M (2001) Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci 21: 8026–8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M (1997a) Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978 [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M (1997b) A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177 [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M (1998) H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol 8: 418–424 [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ (2006) Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586 [DOI] [PubMed] [Google Scholar]
- Woo YC, Park SS, Subieta AR, Brennan TJ (2004) Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 101: 468–475 [DOI] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, McCleskey EW (2006) Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 99: 501–509 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1–3