Abstract
The Smc5/6 holocomplex executes key functions in genome maintenance that include ensuring the faithful segregation of chromosomes at mitosis and facilitating critical DNA repair pathways. Smc5/6 is essential for viability and therefore, dissecting its chromosome segregation and DNA repair roles has been challenging. We have identified distinct epigenetic and post-translational modifications that delineate roles for fission yeast Smc5/6 in centromere function, versus replication fork-associated DNA repair. We monitored Smc5/6 subnuclear and genomic localization in response to different replicative stresses, using fluorescence microscopy and chromatin immunoprecipitation (ChIP)-on-chip methods. Following hydroxyurea treatment, and during an unperturbed S phase, Smc5/6 is transiently enriched at the heterochromatic outer repeats of centromeres in an H3-K9 methylation-dependent manner. In contrast, methyl methanesulphonate treatment induces the accumulation of Smc5/6 at subtelomeres, in an Nse2 SUMO ligase-dependent, but H3-K9 methylation-independent manner. Finally, we determine that Smc5/6 loads at all genomic tDNAs, a phenomenon that requires intact consensus TFIIIC-binding sites in the tDNAs.
Keywords: heterochromatin, recombination, SMC5, SUMO, tDNA
Introduction
The evolutionarily conserved SMC (structural maintenance of chromosomes) family consists of three essential complexes, including cohesin (Smc1/3) that maintains sister chromatid cohesion until anaphase and condensin (Smc2/4) that facilitates chromosome compaction and decatenation during mitosis. The third member, Smc5/6, also has fundamental but mechanistically undefined functions in chromosome segregation and interestingly, all three SMC complexes facilitate DNA repair (Losada and Hirano, 2005; Nasmyth and Haering, 2005; Murray and Carr, 2008).
Yeast Smc5 and Smc6 interact stably with each other and with six core subunits called non-SMC elements (Nse1–6). Mutations in any Smc5/6 subunit render cells hypersensitive to DNA-damaging agents and cause severe DNA segregation defects in the absence of exogenous DNA damage (Fousteri and Lehmann, 2000; McDonald et al, 2003; Morikawa et al, 2004; Pebernard et al, 2004; Torres-Rosell et al, 2005; Zhao and Blobel, 2005; Pebernard et al, 2006). Smc5/6 is critical for repairing multiple types of DNA damage, including DNA double-strand breaks (DSBs) and collapsed replication forks (Lehmann et al, 1995; Fujioka et al, 2002; McDonald et al, 2003; Harvey et al, 2004; Pebernard et al, 2004; Potts and Yu, 2005; Torres-Rosell et al, 2005; Zhao and Blobel, 2005). Smc5/6 binds proximal to and promotes the repair of both lesions, apparently by stimulating homologous recombination (HR) between sister chromatids (De Piccoli et al, 2006; Lindroos et al, 2006; Potts et al, 2006). Interestingly, Smc5/6 also blocks pathological HR-dependent processes during replicative stress; as under such conditions, hypomorphic Smc5/6 mutants accumulate RAD51-dependent toxic recombination intermediates that impede chromosome segregation at mitosis (Branzei et al, 2006; Miyabe et al, 2006; Pebernard et al, 2006).
The Smc5/6 non-SMC subunit Nse2 is a SUMO E3 ligase conserved from yeast to man (Andrews et al, 2005; Potts and Yu, 2005; Zhao and Blobel, 2005). In fission yeast, the SUMO ligase-dead nse2-SA mutant supports cellular viability but renders cells sensitive to genotoxic agents, indicating the existence of critical Nse2 targets in DNA repair (Andrews et al, 2005).
The genomic localization of budding yeast Smc5/6 has been determined (Lindroos et al, 2006). However, the structure and regulation of budding yeast chromosomes are different from other eukaryotes. In particular, fission yeast and human centromeres are large repetitive heterochromatic structures maintained by a number of conserved proteins and mechanisms including RNAi, which are not found in budding yeast (Allshire, 2004; Zofall and Grewal, 2006). In fission yeast, heterochromatin establishment, spreading and maintenance depend on a succession of histone deacetylation and methylation events (Grewal and Elgin, 2007). A key step early in heterochromatin establishment is the di-methylation of histone H3 on lysine 9 (H3K9Me2) by the Clr4–Rik1–Cul4 complex. H3K9Me2 functions as a binding platform for chromodomain-containing proteins, for example, Swi6, which promote heterochromatin spreading and stable silencing of the modified loci (Grewal and Elgin, 2007). Swi6 at centromeric heterochromatin recruits cohesin, which is required both to maintain centromeric cohesion and to structurally organize centromeres for proper kinetochore–spindle interactions, and faithful chromosome transmission at mitosis (Bernard and Allshire, 2002).
We determined Smc5/6 localization (through the stable Nse4 subunit) and mechanisms of recruitment on the three fission yeast chromosomes, which has revealed important new information on the regulation and potential functions of the complex. Smc5/6 is transiently recruited to centromeres during early S phase (e.g. following hydroxyurea (HU) treatment), whereas exposure to methyl methanesulphonate (MMS) induces Nse4 accumulation at broad subtelomeric domains. Intriguingly, distinct signalling mechanisms promote the different genomic localizations of Smc5/6 following either HU or MMS treatment. Smc5/6 recruitment to centromeres depends on heterochromatin, whereas subtelomeric recruitment is enhanced by MMS-induced Nse2-dependent sumoylation. Finally, we show that Smc5/6 is localized at all transcriptionally active tRNA-coding sequences (tDNAs) throughout the genome. Overall, our results provide new insights on the genome-wide recruitment and thus, highlight potential functions of the Smc5/6 complex.
Results
Nse4 localizes to distinct subnuclear foci in response to HU and MMS treatment
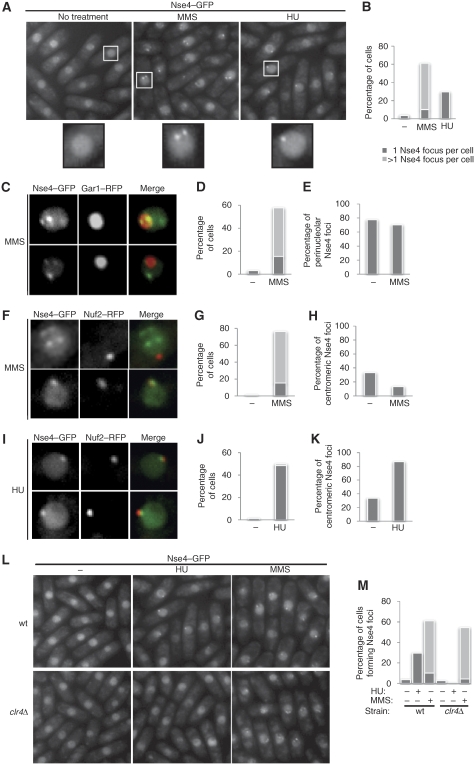
To determine whether Smc5/6 subnuclear localization is governed by genotoxic stress, we monitored endogenously tagged Nse4–GFP in live cells. MMS treatment triggered the formation of multiple Nse4 foci (Figure 1A and B). By contrast, HU-treated cells formed only one Nse4 focus (Figure 1A and B). MMS-induced Nse4 foci often formed doublets around the nucleolus; similar to the perinucleolar tethering of Chr3 telomeric rDNA repeats (Chikashige et al, 1997; Figure 1A). We hypothesized that the single HU-induced focus might correspond to the spindle pole body (SPB)/centromeric cluster (Chikashige et al, 1997; Figure 1A). Therefore, we tested for colocalization between Nse4–GFP and either RFP-tagged Gar1 (nucleolar) or Nuf2 (centromeric) markers (Asakawa et al, 2005; Dovey and Russell, 2007). In untreated cells, rare spontaneous foci were mostly perinucleolar (Figure 1D and E). Following MMS treatment, ∼70% of the visible Nse4 foci were perinucleolar, and 10% were centromeric (Figure 1C–H). The remaining MMS-induced foci are often non-nucleolar doublets, which likely correspond to Chr1/2 telomeric clusters (Figure 1F, upper panels). In HU-arrested cells, Nse4 colocalized almost exclusively with Nuf2 (Figure 1I–K). Therefore, Nse4 predominantly localizes to the centromeric/SPB cluster in HU, and to the telomeric clusters following MMS treatment.
Figure 1.
Nse4 differentially localizes to specific nuclear foci following replicative stress. Live cell microscopy of endogenous Nse4–GFP following MMS and HU treatments. (A) MMS induces formation of multiple Nse4 foci per cell, whereas HU induces only one focus per cell. (B) Quantification of (A). (C) Colocalization of Nse4–GFP and nucleolar Gar1-mRFP following MMS treatment. (D, E) Quantification of (C). (F) Colocalization of Nse4–GFP and centromeric Nuf2-mRFP in MMS-treated cells. (G, H) Quantification of (F). (I–K) Same as (F–H) but cells were exposed to HU instead of MMS. (L, M) Nse4 centromeric localization is Clr4 dependent. (L) Nse4 focus formation is abolished in HU-treated clr4Δ cells, but remains normal following MMS treatment. Wild-type and clr4Δ cells expressing Nse4–GFP were treated with HU or MMS, and analysed by live fluorescence microscopy. (M) Quantification of (L).
HU-induced Nse4 centromeric accumulation depends on heterochromatin establishment
Centromeres and telomeres are highly organized heterochromatic structures in fission yeast (Cam and Grewal, 2004). Thus, we tested the heterochromatin dependency of Nse4–GFP focus formation in cells unable to establish de novo heterochromatin, as they lack the H3K9 methyltransferase Clr4 (clr4Δ). Strikingly, in clr4Δ cells, HU-induced Nse4–GFP focus formation was abolished; however, MMS-induced Nse4–GFP foci were unaffected (Figure 1L and M). These data indicate that differential Nse4 subnuclear localization is promoted by Clr4-dependent and -independent mechanisms.
Identification of specific genomic loci occupied by Smc5/6 following exposure to HU and MMS
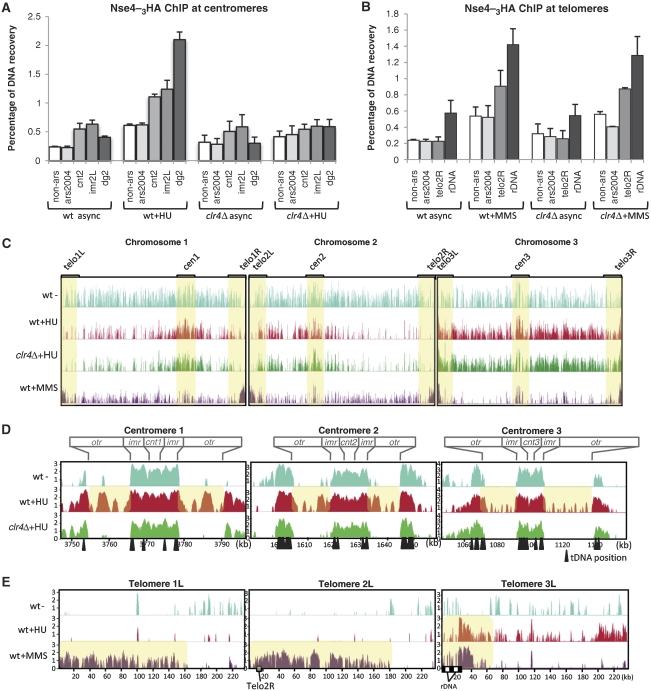
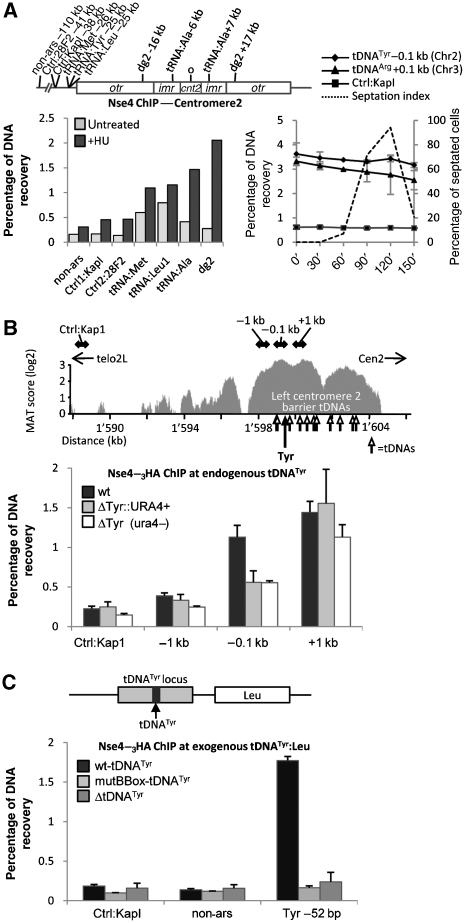
To confirm association of Nse4 with centromeric and telomeric chromatin, we performed chromatin immunoprecipitation (ChIP) of endogenously 3HA-tagged Nse4 in wild-type (wt) and clr4Δ cells, treated with HU or MMS. Nse4 enrichment at defined genomic loci was assessed by quantitative PCR (qPCR). Fission yeast centromeres consist of a unique central core domain (cnt), flanked by inverted inner (imr) and outer (otr) repeats, the latter of which contains multiple copies of dg/dh repeats (Cam and Grewal, 2004). Thus, to map Nse4 localization within centromeres, we measured its accumulation at cnt, otr (dg) and imr, as compared to the non-centromeric origin of replication ars2004 and a non-ars control located 30 kb upstream. In HU-arrested wild-type cells, Nse4 is enriched at otr, and to a lesser extent at cnt and imr (Figure 2A). Compared with untreated cells, Nse4 binding at otr increased up to six-fold. In HU-arrested clr4Δ cells, no Nse4 enrichment was observed at otr (Figure 2A). These data agree with our Nse4–GFP localization data and indicate that otr is the major centromeric domain occupied by Nse4 in HU.
Figure 2.
Genome-wide localization of Nse4–3HA in HU- and MMS-treated cells. (A) Nse4–3HA ChIP at centromeric loci. Nse4–3HA ChIP-enriched samples were compared with the total amount of DNA present in the input samples by qPCR using the indicated probes. (B) Nse4–3HA ChIP at telomeric loci. ChIP was performed as in (A). (C) Genome-wide mapping (ChIP-on-chip) of Nse4-binding sites in the indicated strains. Positions of centromeres (cen) and subtelomeres (telo) are marked with yellow boxes to highlight the differential Nse4 binding to these regions following HU and MMS treatment. Y axis: log2 (MATscore) from 0 to 4. (D, E) Detailed mapping of centromeric (D) and telomeric (E) domains bound by Nse4–3HA. ChIP-on-chip graphs from the indicated strains are enlarged to show the centromeric or telomeric domains on each chromosome. Boxes on top of (D) indicate the position of each centromeric domain (cnt, core domain; imr, inner repeats; otr, outer repeats). Location of Telo2R primers and rDNA repeats is indicated by grey and black boxes, respectively. Positions of tRNA-coding sequences (tDNAs) are pointed out by black arrows. Y axis: log2 (MAT score) representing Nse4–3HA enrichment. X axis: distance from the left chromosome end, in kilobases (kb).
To identify the telomeric domains bound by Nse4 following MMS treatment, we measured its enrichment at a Chr2 subtelomeric domain and at Chr3 rDNA loci (Figure 2B). MMS induced robust Clr4-independent accumulation of Nse4 at both Telo2R and rDNA loci, with a weaker increase at ars2004 and non-ars (Figure 2B). Although HU treatment predominantly induces otr enrichment of Nse4, MMS treatment induces Nse4 enrichment at telomeres and to a lesser extent at the otr, relative to asynchronous cells (Supplementary Figure 2A). As an additional control, we performed ChIP for 3HA-tagged Rad21, a cohesin subunit, which binds otr domains throughout most of the cell cycle (Tomonaga et al, 2000). In asynchronous cells, Rad21 binding to otr was strong relative to any other locus quantified, and did not change in HU- or MMS-treated cells (Supplementary Figure 2B). Overall, Nse4 binding at otr and telomeres is enhanced by HU and MMS treatment, respectively.
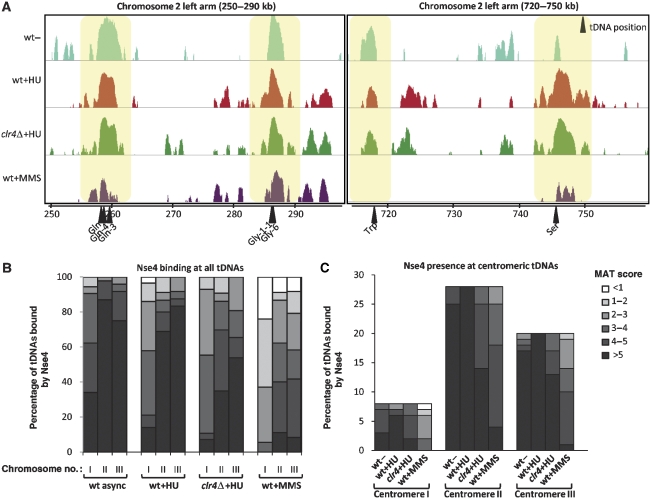
To map genome-wide Nse4-binding sites, Nse4–3HA ChIP samples were hybridized on Schizosaccharomyces pombe DNA tiling arrays for ‘ChIP-on-chip' analyses. Four cell populations were compared: wild-type asynchronous, wild-type HU and MMS-treated and HU-treated clr4Δ. Global Nse4-binding profiles reveal that it is widely spread along all three fission yeast chromosomes in asynchronous (mostly G2) wild-type cells, but is scarce at subtelomeres (Figure 2C). Apart from the tDNAs (see below), no clear patterns/preferences emerged for the loading of Nse4 on chromosomes of asynchronous fission yeast. Following HU treatment, Nse4 enriched at all centromeres, and disappeared from certain genomic regions (Figure 2C). In HU, Nse4 localized to the subtelomeric rDNA repeats on Chr3, but not to the subtelomeric regions of Chr1 and Chr2 (Figure 2C). The localization of Smc5/6 to Chr3 subtelomeres likely reflects the presence of rDNA at these loci, which is both repetitive and prone to potentially toxic recombination events (see Coulon et al, 2004; Murray and Carr, 2008). Given that Smc5/6 of both fission and budding yeasts suppress ‘illicit' recombination (e.g. Torres-Rosell et al, 2005; Miyabe et al, 2006; Pebernard et al, 2006) it seems likely that fission yeast Smc5/6 is loaded at the rDNA to suppress toxic recombination at this locus, similar to Smc5/6 of budding yeast (Torres-Rosell et al, 2005). The generally reduced binding of Nse4 to chromosomes in HU might reflect maximal loading of Smc5/6 on fully replicated chromosomes, and in HU, replication is incomplete. Indeed, in budding yeast and Xenopus (egg extracts) Smc5/6 loading is coupled to replication (Lindroos et al, 2006; Tsuyama et al, 2006). In HU, there is also de novo recruitment/enrichment of Nse4 at the subtelomeric regions of Chr3, which might marginally deplete other loci.
Globally, Nse4 localization appears similar in HU-treated wild-type and clr4Δ cells (Figure 2C). Strikingly, Nse4 preferentially bound to subtelomeric sequences on all three chromosomes following MMS treatment, but appeared somewhat depleted at other loci (Figure 2C). This global depletion might be caused by de novo Nse4 loading at the extensive subtelomeres, slowed replication and perhaps many more damage sites distributed across chromosomes, thus averaging out the MAT scores. This analysis confirms that HU treatment induces Nse4 accumulation at centromeres, whereas MMS treatment mainly mobilizes Nse4 to the subtelomeric regions of all three chromosomes.
We next analysed the detailed Nse4-binding pattern at centromeres using our ChIP-on-chip data (Figure 2D). In asynchronous cells, Nse4 is enriched at imr, cnt and the tDNAs localized at each centromeric barrier, but is absent from the otr regions (Figure 2D). Notably, HU treatment promotes Nse4 binding at the otr without affecting binding at other centromeric regions (Figure 2D). The otrs are the major heterochromatic regions of fission yeast centromeres (Partridge et al, 2000). Nse4 localization at otr follows a distinctive pattern, with peak binding occurring between dg/dh repeats (Figure 2D). When compared with published patterns of H3K9Me2 at the otr, Nse4 binding peaks at positions where H3K9Me2 is at a minimum (see Epigenome Home Page http://pombe.nci.nih.gov/ and Cam et al, 2005; Noma et al, 2006). This phenomenon is particularly obvious on Cen3, which contains the largest number of dg/dh repeats (Figure 2D). Notably, in HU-treated clr4Δ cells, Nse4 does not localize to otr but binds normally to the imr, cnt and tDNAs (Figure 2D). Therefore, increased Nse4 centromeric localization in HU-treated cells is mainly restricted to the otr and requires the H3K9Me2 heterochromatic mark. As the Smc5/6-related SMC complex cohesin is enriched at centromeres in a heterochromatin- and Swi6-dependent manner (Bernard and Allshire, 2002), we tested whether Swi6 is also required for Smc5/6 loading. ChIP-qPCR was performed on Nse4 in wild-type and swi6Δ cells treated with or without HU (Supplementary Figure 3). Nse4 was similarly enriched at the otr in both wild-type and swi6Δ cells in the presence of HU, suggesting that Smc5/6 loads at otr in a largely Swi6-independent manner. To determine whether Smc5/6 affects silencing at the imr and otr centromeric domains, we assayed silencing of a ura4+ marker inserted at either locus (see Allshire et al, 1995) and detected no differences between wild-type and Smc5/6 mutants (data not shown). Overall, these data indicate that Smc5/6 loads at otr independently of Swi6 and cohesin, and does not have a major impact on otr/imr transcriptional silencing.
Our ChIP-on-chip data also reveal specific enrichment of Nse4 at telomeres in MMS but not HU-treated cells (Figure 2E and not shown). Nse4 is enriched at large subtelomeric regions on Chrs1/2 in MMS but not HU-treated cells (Figure 2E). Interestingly, Chr3 telomeres that carry the rDNA repeats show a narrower distribution of Nse4 occupancy in close proximity to the rDNA repeats and local long terminal repeat (LTR) sequences, in both MMS- and HU-treated cells (Figure 2E). As the rDNA is a known challenge for replication and contains replication fork barriers (RFBs) to maintain co-directionality of transcription and replication, we determined more accurately the MMS-induced localization of Nse4 at the rDNA. Our ChIP-qPCR analyses show that Nse4 localizes proximal to RFBs but apparently peaks within the 17S coding sequence (Supplementary Figure 5). In conclusion, MMS triggers Nse4 recruitment to all six telomeres (in a Clr4-independent manner), suggesting that MMS-induced Nse4–GFP foci correspond to the telomeric clusters tethered at the nuclear membrane.
Smc5/6 is recruited to centromeres in an unperturbed S phase
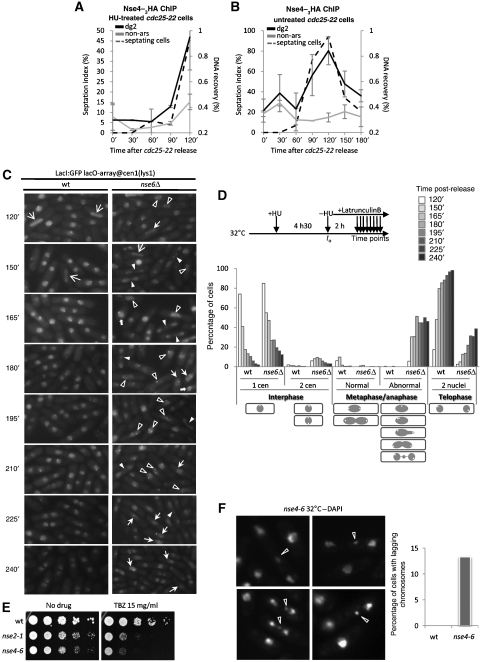
To discriminate between cell cycle and replicative stress-induced loading of Nse4 at centromeres, we monitored Nse4 recruitment to otr (dg2 primers), or the control non-ars site, throughout the cell cycle by ChIP-qPCR. We first arrested cells in G2 using the temperature-sensitive cdc25-22 allele and added HU prior to release from the arrest, to block S-phase progression and determine the kinetics of Nse4 centromeric loading (Figure 3A). Nse4 accumulated specifically at otr 90 min after release, as cells were septating and therefore, initiating S phase (Figure 3A). To determine whether Nse4 binds centromeres in an unperturbed S phase, we performed the same experiment in the absence of HU (Figure 3B). Nse4 binding at otr in G2/M phase was very low but increased specifically at the onset of S phase, reaching peak binding at maximal septation, and decreasing again in the subsequent G2 phase (Figure 3B). Nse4 protein levels do not fluctuate throughout the cell cycle or in response to replication stress (Palecek et al, 2006 and data not shown). Thus, Nse4 is transiently loaded at centromeric otr repeats during early S phase, indicating key functions for Smc5/6 at replicating centromeres.
Figure 3.
Smc5/6 participates in centromere structure establishment in S phase. (A, B) Nse4 accumulates at otr in S phase. Nse4–3HA ChIP in cdc25-22 cells synchronized at 36°C and released at 25°C in the presence (A) or absence (B) of HU, measured by qPCR. (C, D) The Smc5/6 complex is required for proper centromere segregation at anaphase. (C) Wild-type and nse6Δ cells containing an array of Lac operons (lacO) at Cen1 (lys1) and expressing a LacI–GFP fusion were blocked in HU and released in latrunculin B. Cen1 segregation was monitored between 2 and 4 h post-release. Arrows point out aberrant DNA structures as follows. White arrows  : abnormal interphase with two separated Cen1; White arrows →: normal anaphase, each centromere being located at each pole of the pulling spindle; White arrows ▹: aberrant anaphase with lagging chromosomes; Black arrows ▸: aberrant anaphase with two mis-oriented or mis-attached Cen1; White arrows
: abnormal interphase with two separated Cen1; White arrows →: normal anaphase, each centromere being located at each pole of the pulling spindle; White arrows ▹: aberrant anaphase with lagging chromosomes; Black arrows ▸: aberrant anaphase with two mis-oriented or mis-attached Cen1; White arrows  : aberrant anaphase with only one visible Cen1. (D) Experimental process and quantification of (C). Lower panel, drawings represent the types of events counted for each cell cycle phase described. Dark circle, nucleus; white spot, Cen1. Abnormal metaphase/anaphase regroups multiple abnormal Cen1 and DNA segregation events such as chromosome lagging, centromere missegregation, and so on. (E) Smc5/6 hypomorphic mutants nse2-1 and nse4-6 are hypersensitive to TBZ. Serial dilutions of the indicated strains were spotted on rich media untreated or treated with 15 mg/ml thiobendazole (TBZ), and incubated at 32°C. (F) nse4-6 mutants display an elevated number of chromosome lagging events at semi-permissive temperature (32°C) when stained with DAPI. Right panel, quantification of left panel.
: aberrant anaphase with only one visible Cen1. (D) Experimental process and quantification of (C). Lower panel, drawings represent the types of events counted for each cell cycle phase described. Dark circle, nucleus; white spot, Cen1. Abnormal metaphase/anaphase regroups multiple abnormal Cen1 and DNA segregation events such as chromosome lagging, centromere missegregation, and so on. (E) Smc5/6 hypomorphic mutants nse2-1 and nse4-6 are hypersensitive to TBZ. Serial dilutions of the indicated strains were spotted on rich media untreated or treated with 15 mg/ml thiobendazole (TBZ), and incubated at 32°C. (F) nse4-6 mutants display an elevated number of chromosome lagging events at semi-permissive temperature (32°C) when stained with DAPI. Right panel, quantification of left panel.
To test centromere function in Smc5/6 mutant cells, we first analysed mitosis in wild-type and nse6Δ cells following release from HU arrest into latrunculin B. Under these conditions, cells complete replication, undergo mitosis and arrest in telophase. This allows a more careful analysis of mitotic progression and accumulation of defects, as cells do not continue into a new cell cycle. We monitored segregation of centromere 1 (Cen1) in each strain through a Cen1 proximal LacO repeat array and LacI–GFP (Tatebe et al, 2001). Although wild-type cells rapidly progressed through mitosis and arrested in telophase, nse6Δ cells showed an extreme delay (2–3 h) in mitotic progression and accumulated aberrant mitotic figures (Figure 3C and D). A notable defect was the appearance and persistence of two Cen1 foci in nse6Δ nuclei. In addition, lagging chromosomes were abundant in nse6Δ cells, as also seen in nse4-6 mutant cells (Figure 3C and D; see below). We next treated Smc5/6 hypomorphic mutants, nse2-1 and nse4-6, with the microtubule-destabilizing agent thiabendazole (TBZ; Figure 3E). Both mutants are hypersensitive to TBZ, indicating centromere dysfunction and defective spindle–kinetochore interactions in these mutants (Figure 3E). When stained with DAPI, nse4-6 cells exhibited a high frequency of lagging chromosomes, another symptom of centromere dysfunction (Figure 3F). These phenotypes are similar to those of hypomorphic cohesin mutants, which also have defects in centromere structure and function (Bernard and Allshire, 2002).
Nse2 SUMO ligase activity is critical for MMS-induced subtelomeric recruitment of Nse4
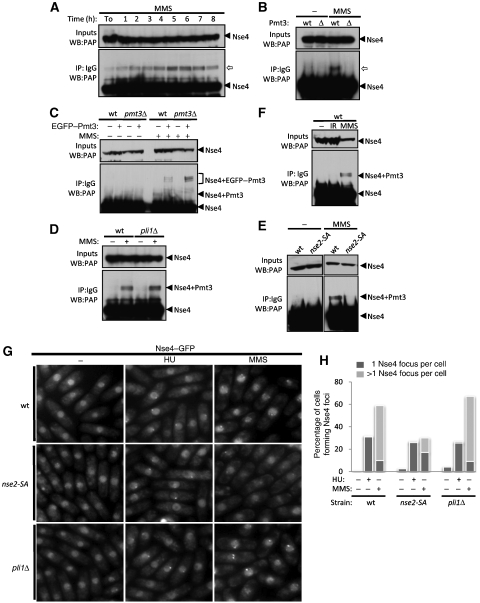
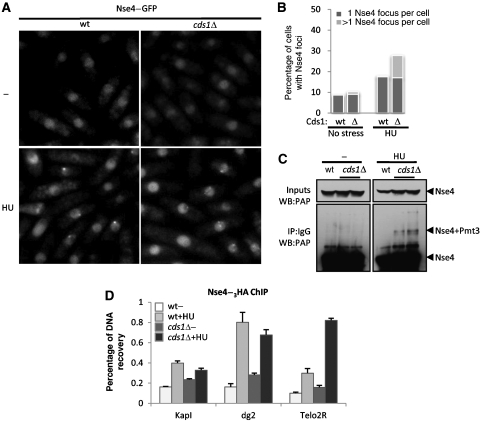
We observed an MMS-induced ∼15 kDa modification on TAP-tagged Nse4, which did not appear following HU treatment (Figures 4A and 5C). This modification was visible as early as 2 h post-MMS treatment and peaked at ∼5–6 h (Figure 4A). As the Smc5/6 subunit Nse2 is a SUMO E3 ligase, we tested whether Nse4 is a SUMO target. The MMS-induced Nse4 modification was absent in Pmt3 (SUMO)-deleted cells (Figure 4B; Tanaka et al, 1999). Therefore, either Nse4 is directly sumoylated in response to MMS treatment or indirectly modified in a SUMO-dependent manner. To discriminate these two possibilities, EGFP or EGFP–Pmt3 was ectopically expressed in wild-type or pmt3Δ backgrounds (Figure 4C). EGFP–Pmt3 expression in a pmt3Δ strain restored the MMS-induced Nse4 shift at the expected higher molecular weight (Figure 4C). To confirm these results at physiological levels of Pmt3, the pmt3 gene was replaced by 6His-tagged pmt3 at its endogenous locus (Xhemalce et al, 2004). This generates a slight change in molecular weight of modified Nse4 in MMS, reflecting the difference between 6His-Pmt3 and untagged Pmt3 (Supplementary Figure 4A). Therefore, Nse4 is directly sumoylated in response to MMS treatment. S. pombe has only two known E3 SUMO ligases, Pli1 and Nse2 (Xhemalce et al, 2004, 2007; Andrews et al, 2005). Pli1 is responsible for the majority of detectable sumoylation events (>95%) but is dispensable for resistance to genotoxic stress (Xhemalce et al, 2004, 2007; Prudden et al, 2007). In contrast, Nse2 mediates sumoylation of a small and largely undefined subset of target proteins involved in genomic stability. Catalytically inactive Nse2 (nse2-SA) mutant cells are viable but hypersensitive to a wide range of DNA-damaging agents but not spindle poisoning by TBZ (Andrews et al, 2005; Watts et al, 2007; Xhemalce et al, 2007). Nse4–TAP sumoylation levels were unchanged in pli1Δ cells (Figure 4D), but were abrogated in the nse2-SA mutant background (Figure 4E; Supplementary Figure 4B). Hence, Nse2 is responsible for Nse4 sumoylation, which appears to be specific to MMS treatment. For example, HU treatment does not result in Nse4 sumoylation (Figure 5C) and high doses of IR do not appear to induce Nse4 modification; even though damage is unrepaired at the time point assayed as monitored by persistent checkpoint activation (Figure 4F). However, we cannot exclude that transient or low levels of Nse4 sumoylation occur under these conditions.
Figure 4.
MMS exposure induces Nse2-dependent Nse4 sumoylation. (A–E) Immunoprecipitation (IP) and western blotting (WB) of endogenously TAP-tagged Nse4. (A) Time course showing Nse4–TAP modification ( ) appearance after MMS was added to an asynchronous culture. (B) MMS-induced Nse4–TAP shift depends on the presence of Pmt3 (SUMO). (C) MMS treatment triggers Nse4 sumoylation. EGFP-tagged Pmt3 was ectopically expressed from a pREP41 vector in the indicated genetic backgrounds. The triplet of bands observed is due to EGFP–Pmt3 partial degradation products (data not shown). (D) Pli1 is not responsible for Nse4 sumoylation. (E) Nse4 sumoylation depends on Nse2 E3 SUMO ligase activity. (F) Double-strand breaks do not trigger Nse4 sumoylation. (G) Nse4 foci formation in MMS-treated cells depends on Nse2 SUMO ligase activity. (H) Quantification of (G).
) appearance after MMS was added to an asynchronous culture. (B) MMS-induced Nse4–TAP shift depends on the presence of Pmt3 (SUMO). (C) MMS treatment triggers Nse4 sumoylation. EGFP-tagged Pmt3 was ectopically expressed from a pREP41 vector in the indicated genetic backgrounds. The triplet of bands observed is due to EGFP–Pmt3 partial degradation products (data not shown). (D) Pli1 is not responsible for Nse4 sumoylation. (E) Nse4 sumoylation depends on Nse2 E3 SUMO ligase activity. (F) Double-strand breaks do not trigger Nse4 sumoylation. (G) Nse4 foci formation in MMS-treated cells depends on Nse2 SUMO ligase activity. (H) Quantification of (G).
Figure 5.
Replication fork collapse causes Nse4 relocalization to subtelomeres. (A) Nse4–GFP relocalizes to perinucleolar areas when replication forks are destabilized. (B) Quantification of (A). (C) Nse4 sumoylation is induced by replication fork collapse. (D) ChIP assay for Nse4–3HA shows that replication fork collapse in cds1Δ cells triggers Nse4 enrichment to subtelomeres.
To determine whether MMS-induced subtelomeric Nse4 foci were SUMO dependent, we monitored Nse4–GFP localization in pli1Δ and nse2-SA cells. MMS-induced Nse4–GFP foci formed normally in pli1Δ cells but were strongly attenuated in nse2-SA cells (Figure 4G and H). Notably, HU-induced Nse4–GFP foci were normal in both nse2-SA and pli1Δ cells (Figure 4G and H). Thus, sumoylation by Nse2 promotes maximal subtelomeric localization/retention of Nse4 in MMS, but not its centromeric localization in HU.
Replication fork collapse induces sumoylation and subtelomeric accumulation of Nse4
HU depletes cells of their nucleotide pool and induces a replication arrest, during which stalled forks are stabilized in a replication checkpoint kinase (Cds1)-dependent manner (Noguchi et al, 2003; Froget et al, 2008). Consistently, in HU-treated cells lacking Cds1 (cds1Δ), a high level of irreversible fork collapse is observed (Noguchi et al, 2003; Froget et al, 2008). Notably, multiple subtelomeric Nse4–GFP foci were detected in HU-treated cds1Δ cells, similar to those observed in MMS-treated cells (Figure 5A and B). In addition, Nse4 was sumoylated following HU treatment of cds1Δ but not wild-type cells (Figure 5C). To confirm that the Nse4 foci in HU-treated cds1Δ cells correspond to subtelomeric regions, we performed ChIP-qPCR analyses. As anticipated, we detected a robust enrichment of Nse4 at Telo2R in HU-treated cds1Δ cells as compared with wild type (Figure 5D). These results indicate that replication fork collapse stimulates Smc5/6 mobilization to subtelomeric DNA repeats and is accompanied by Nse2-dependent sumoylation of Nse4.
Novel loading sites of Nse4 revealed by ChIP-on-chip
In addition to the HU- and MMS-induced relocalization of Nse4, our ChIP-on-chip analyses revealed binding of Nse4 at all genomic tDNAs (Figures 2D and 6A). In contrast to the transient Nse4 centromeric and telomeric enrichments, Nse4 tDNA localization appears to be largely cell cycle- and DNA damage-independent (Figures 6A and 7A). We systematically analysed the MAT score value for Nse4 association at each known S. pombe tDNA and found that it was most elevated in asynchronous cells, but still remained high at every tDNA following HU treatment (Figure 6B). MMS treatment caused modestly decreased tDNA MAT scores, suggesting that in this context the presence of Nse4 at tDNAs is reduced (Figure 6B, left panel). On the basis of MAT score, centromeric tDNAs of all three chromosomes were preferential Nse4-binding sites (Figure 6B, right panel).
Figure 6.
Nse4 is constitutively associated with tDNAs. (A) Two examples of tDNA sequences bound by Nse4–3HA on Chr2 left arm. ChIP-on-chip results are enlarged to show Nse4 association with tDNA loci. Arrows below indicate the position of each tDNA. Distance from the left chromosome end is indicated in kilobases (kb). (B) All the genomic tDNAs are bound by Nse4. Nse4 ChIP-on-chip MAT score at each known tDNA locus was quantified and represented as a percentage of tDNAs bound by Nse4 for each MAT score category. Darker areas represent higher MAT scores, hence higher confidence for Nse4 enrichment. (C) Centromeric tDNAs preferentially recruit Nse4. All tDNAs localized within the centromeric and pericentromeric regions of each chromosome were quantified as in (B).
Figure 7.
A mechanism for Nse4 recruitment to tDNAs. (A) Nse4 is constitutively bound to tDNAs. Nse4-enriched ChIP samples were measured by qPCR using the indicated primer pairs. The location of primer sets used relative to Cen2 is schematized on the top panel. Left panel, Nse4 binding to tDNAs outside otr is not regulated by HU treatment. Right panel, cell cycle-ChIP experiment as described in Figure 3B. Cells released from cdc25-22 arrest show stable binding of Nse4–3HA to tDNATyr (chromosome2) and tDNAArg (chromosome3) throughout the cell cycle. (B) Nse4 recruitment to centromeric barriers is linked to the presence of tDNAs. Top panel, ChIP-on-chip signal for Nse4 binding at Chr2 left centromeric barrier in asynchronous cells. The position of qPCR primers used in bottom panel is represented by black double arrows. White arrows: position of all tDNAs at this locus; black arrow: position of tDNATyr, which is analysed in the bottom panel. Distance from left telomere is indicated in kilobases (kb). Bottom panel, ChIP-qPCR measuring Nse4–3HA recruitment around tDNATyr. Black bars: wild type; grey bars: tDNATyr deleted and replaced by a ura4+ cassette; white bars: tDNATyr deleted in a wild-type background (no ura4+ cassette). (C) Recruitment of Nse4 at tDNAs requires an intact RNA pol III promoter sequence. Upper panel, schematic description of the ectopic tDNATyr construct used. A ∼600 bp genomic fragment including and surrounding tDNATyr was inserted at the leu1+ locus. tDNATyr was mutated in its RNA pol III promoter B Box, or fully deleted, within this locus. Lower panel, Nse4–3HA binding around the ectopic tDNATyr locus with wild type (black bars), B Box mutant (light grey bars) or tDNATyr deletion mutant (dark grey bars) backgrounds was measured by ChIP-qPCR at the indicated sites.
Transcription-dependent Nse4 loading at tDNAs
To confirm that Nse4 binding to tDNAs is constitutive, we measured Nse4–3HA ChIP samples by qPCR at three tDNAs on Chr2 (Figure 7A). In untreated cells, Nse4 was associated with these tDNAs as compared with non-centromeric control loci (Figure 7A). HU treatment generally increased Nse4 binding to all loci, with the expected strong enrichment at otr (Figure 7A). However, there was no overt Nse4 enrichment at tDNAs located on the Cen2 boundaries (outside the otr), and only a slight enrichment at tDNAAla in the imr (Figure 7A). To determine whether Nse4 loading at tDNAs is sequence- or locus-dependent, we replaced a single tDNA-coding sequence (tDNATyr) in the pericentromeric region of Chr2 with a ura4+ cassette, and measured Nse4–3HA binding at adjacent sequences by ChIP-qPCR (Figure 7B). In wild-type cells, Nse4 binds tightly to this tDNA-rich region (Figure 7B, upper panel). Nse4 is recruited around tDNATyr, in particular at 100 bp upstream and 1 kb downstream of the locus (Figure 7B, lower panel). Replacing tDNATyr by a ura4+ cassette reduced Nse4 binding close to the tDNA ∼2-fold as compared with wild type, but had little effect 1 kb upstream or downstream of the deletion (Figure 7B). Similar results were obtained when deleting a pericentromeric tDNAArg gene on Chr3 (data not shown). To ensure that the absence of the tDNATyr and not the presence of the large ura4+ cassette reduced binding of Nse4, we removed the cassette by gene replacement and selection on 5-FOA. Nse4 binding 100 bp from the tDNATyr deletion was still reduced ∼2-fold in the absence of the ura4+ cassette, whereas no change in Nse4 levels could be detected 1 kb away from the deletion (Figure 7B). Therefore, Nse4 loading at tDNA-containing genomic loci is due to the presence of tDNA-coding sequences.
Genomic tDNAs are transcribed by RNA polymerase III (RNAPolIII) and can cause transcription-dependent replication fork pausing (Deshpande and Newlon, 1996). Sequential binding of TFIIIC, TFIIIB and RNAPolIII to conserved intragenic A and B Boxes, which constitute RNAPolIII-dependent promoters, is required for tDNA transcription (Paule and White, 2000). A single point mutation in the B Box abrogates TFIIIC binding, transcription of the tDNA and replication fork pausing activity (Kurjan and Hall, 1982; Allison et al, 1983; Deshpande and Newlon, 1996). To determine whether Nse4 only binds at transcriptionally active tDNAs, we measured Nse4 binding at a tDNATyr genomic locus integrated at an ectopic site (leu1+ locus) to remove it from the proximity of other active tDNAs. The ectopic tDNATyr genomic locus was wild type, B Box mutant or completely deleted for tDNATyr-encoding sequence. Nse4 binding at 52 bp upstream of the ectopic wild-type tDNATyr was similar to its binding at the endogenous tDNATyr locus, and no specific binding was detected at two control loci (compare Figure 7B and C). Strikingly, a single point mutation in the tDNATyr B Box eliminated Nse4 enrichment at this locus, comparable to the total absence of tDNATyr-encoding sequence (Figure 7C). Thus, Nse4 enrichment at tDNAs requires them to be transcriptionally active, which notably, correlates with their replication fork pausing activity.
Discussion
Key functions for Smc5/6 at the heterochromatic centromeres of fission yeast
Heterochromatin is found at centromeres in most eukaryotes where it promotes optimal kinetochore structure/function and thus, high-fidelity chromosome segregation (Allshire, 2004; Zofall and Grewal, 2006; Folco et al, 2008). For example, heterochromatin directs cohesin loading at otrs and consistently, cells defective for either cohesin or pericentric heterochromatin display chromosome segregation errors such as lagging chromosomes and hypersensitivity to the microtubule poison TBZ (Bernard and Allshire, 2002). Notably, Smc5/6 also localizes to otrs in a heterochromatin-dependent manner and cells defective for Smc5/6 display similar hallmarks of centromere dysfunction. Therefore, an intimate functional relationship could exist between cohesin and Smc5/6 at the centromeres. Earlier studies in budding yeast and mammalian cells have suggested a dependency of cohesin loading/function on Smc5/6 in response to DNA DSBs (Potts et al, 2006; Strom et al, 2007). Our results show that Smc5/6 is transiently loaded at centromeres when they are replicated in early S phase (Kim and Huberman, 2001; Hayashi et al, 2007), which is at or before the establishment of full inter-sister cohesion. Therefore, Smc5/6 might be spatially and temporally placed to modulate cohesin function at centromeres.
A second and not necessarily exclusive role for Smc5/6 at centromeres might involve the known anti-recombinogenic functions of Smc5/6. In response to replication stress and at the rDNA loci in the absence of exogenous damage, Smc5/6 mutant cells accumulate toxic HR-dependent structures, causing severe chromosome segregation defects and cell death at mitosis (Torres-Rosell et al, 2005; Ampatzidou et al, 2006; Miyabe et al, 2006; Pebernard et al, 2006). On the basis of the enrichment of Nse4 at rDNA, fission yeast Smc5/6 likely functions similarly to the budding yeast complex at these loci, inhibiting deleterious recombination that otherwise impedes normal chromosome segregation (Torres-Rosell et al, 2005). Interestingly, transcription of the centromeric otr repeats is maximal during early S phase, at which time siRNA precursors are generated to nucleate heterochromatin establishment (Chen et al, 2008; Kloc et al, 2008). As otr repeats are bi-directionally transcribed, collisions between the transcription machinery and replication forks arising from early replicating pericentric origins appear inevitable. Collision between transcription and replication machineries induces recombination that can be deleterious in repeat rich genomic loci (Prado and Aguilera, 2005) and therefore, Smc5/6 might limit such recombination at otrs to maintain optimal centromere function and chromosome segregation.
Potential genome-wide protective function for Smc5/6 at tDNA loci
The constitutive genome-wide loading of Smc5/6 at tDNAs is remarkably similar to that of the Ino80 chromatin remodelling and DNA repair complex (Shimada et al, 2008). The Ino80 complex is critical for recovery from replication arrest/fork pausing, which is known to occur at transcribed tDNAs (Deshpande and Newlon, 1996; Azvolinsky et al, 2006). Likewise, the Smc5/6 complex is pivotal for recovery from replication fork arrest (Ampatzidou et al, 2006; Miyabe et al, 2006). Transcription-associated recombination and resultant genomic instability constitute a well-known phenomenon (Aguilera, 2002), whereby collision of the RNAPolII transcription and replication fork machineries promotes extraneous recombination (Prado and Aguilera, 2005). The same principle almost certainly applies to the highly transcribed tDNAs. Notably, ‘breakpoints' of gross chromosomal rearrangements in Smc5/6 mutants of budding yeast cluster strongly around tDNAs and some other repeated sequences in the genome (Hwang et al, 2008). Thus, Smc5/6 loading at tDNAs genome-wide likely reflects the need to protect replication-sensitive and repetitive chromosome loci from spontaneous rearrangement. Although this is our favoured model for Smc5/6 function at tDNAs, we do not exclude other possibilities such as tDNAs being cis-acting loading sites for Smc5/6, as they are for the related condensin complex (see D'Ambrosio et al, 2008 and below).
Comparison of genome-wide Smc5/6 loading between the budding and fission yeasts
The global chromosomal localization of budding yeast Smc5/6 has been described (Lindroos et al, 2006) and our studies of the fission yeast Smc5/6 complex identify some interesting similarities and differences in chromosomal loading of the complex. Comparison of the loading sites of Smc5/6 on the structurally distinct chromosomes of these highly divergent yeasts indicates functions that are likely to be conserved to higher eukaryotes.
In fission yeast, Smc5/6 transiently localizes to centromeres as they are replicated, whereas in budding yeast, centromere occupancy of Smc5/6 is maximal at G2/M. Despite these differences, Smc5/6 function is required during replication in both yeasts to promote faithful and timely centromere separation at the metaphase–anaphase transition (this study and Lindroos et al, 2006). This indicates that Smc5/6 will also have critical functions at the regional heterochromatic centromeres of higher eukaryotes, which are similar to those of fission yeast.
Smc5/6 in both yeasts is enriched around the rDNA loci; however, in budding yeast loading is apparently reduced in HU-arrested cells, whereas in fission yeast, it is strongly increased. Irrespective of this difference, it is likely that Smc5/6 has similar replication-coupled functions at these loci in both yeasts, that is, suppressing recombination in replication-sensitive repetitive chromosomal regions. In budding yeast replication checkpoint-defective rad53Δ cells, HU treatment resulted in a genome-wide enrichment of Smc5/6 around what are presumably sites of fork collapse (Lindroos et al, 2006). We did not perform ChIP-on-chip analyses of Smc5/6 in replication checkpoint-deficient cells; however, using immunofluorescence and ChIP-qPCR we found that Smc5/6 is enriched at subtelomeric regions in HU-treated cds1Δ (ScRad53) cells. Thus, Smc5/6 in both yeasts is recruited to chromatin when forks collapse, presumably reflecting the requirement for the complex in restarting collapsed replication forks (Ampatzidou et al, 2006). Interestingly, MMS treatment of fission yeast provoked a similar redistribution of Smc5/6 to subtelomeres as seen in HU-treated cds1Δ cells, indicating that Smc5/6 is also important for replication on damaged DNA templates. A similar genome-wide effect has not been determined for MMS-treated budding yeast.
An intriguing finding is that fission yeast Smc5/6 loads at all tDNAs across the genome in a TFIIIC and transcription-dependent manner (see above). Such tDNA loading was not reported for the Smc5/6 complex in budding yeast (Lindroos et al, 2006). However, a very recent study demonstrated that the Smc5/6-related SMC complex, condensin, loads at tDNAs in both budding and fission yeasts (D'Ambrosio et al, 2008). Furthermore, the loading of condensin at tDNAs requires the Scc2/4 cohesin loader complex, which also localizes at tDNAs across the genome (D'Ambrosio et al, 2008). As Scc2/4 was previously shown to load budding yeast Smc5/6 on undamaged chromosomes (Lindroos et al, 2006), it is possible that Smc5/6 will also load at tDNAs in budding yeast, but this remains to be confirmed. In this regard, it is interesting to note that Smc5/6 mutant budding yeast undergo gross chromosomal rearrangements, which in many cases are initiated proximal to tDNAs (Hwang et al, 2008). It is now clear that Scc2/4 has roles in certain aspects of loading for all three SMC complexes, thereby confounding straightforward assignment of Scc2/4 mutant phenotypes, requiring confirmation that observed defects are also associated with specific SMC complex mutants.
Sumoylation by Nse2 promotes Nse4 recruitment to damaged telomeres
Nse2-dependent sumoylation is crucial for cell survival following MMS treatment (Andrews et al, 2005; Zhao and Blobel, 2005) and we now show that Nse2 promotes the sumoylation and enhanced subtelomeric recruitment of Nse4 in response to MMS. Thus, recruitment of Smc5/6 to damaged telomeres might be a crucial function of Nse2-dependent sumoylation in response to MMS; however, the critical targets of Nse2-dependent sumoylation required for this phenomenon are currently undefined. Both sumoylation and ubiquitination can modify protein function by generating new protein–protein interactions that are mediated by SUMO and ubiquitin-binding motifs, respectively (Kerscher et al, 2006). For example, PML subnuclear body formation requires both auto-sumoylation of PML and non-covalent PML–SUMO interactions mediated by SUMO-interacting motifs in PML (Shen et al, 2006). In yeast, Nse2-mediated sumoylation inhibits the formation of pathological recombination structures at damaged replication forks, and similar results have been observed with other hypomorphic mutants of the Smc5/6 holocomplex (Ampatzidou et al, 2006; Branzei et al, 2006; Miyabe et al, 2006; Pebernard et al, 2006). Thus, the SUMO-mediated recruitment/accumulation of Smc5/6 at damaged/collapsed forks is likely a critical function of Nse2-dependent sumoylation.
Conclusion
Similar to cohesin, the localization of Smc5/6 to otrs is heterochromatin dependent. In addition, Smc5/6 mutant cells exhibit chromosome segregation defects similar to cells hypomorphic for cohesin and heterochromatin establishment factors. In a distinct pathway, Nse2-mediated sumoylation promotes accumulation of Smc5/6 at collapsed replication forks and damaged subtelomeric DNA, but is dispensable for Smc5/6 localization to centromeres. Thus, the fission yeast Smc5/6 holocomplex responds to different signals to promote faithful chromosome segregation during normal or perturbed cell cycles.
Materials and methods
Yeast strains and methods
Standard fission yeast culture methods were used as described earlier (Moreno et al, 1991). A complete list of the strains used in this study is provided in Supplementary Table 1.
Microscopy
Microscopy techniques were described before (Pebernard et al, 2004). Cells were treated with 0.03% MMS (v/v) or 15–25 mM HU (Sigma-Aldrich) and grown for 6 h at 30°C or 7 h at 25°C. For DAPI staining experiments, cells were fixed for 1 min in 70% EtOH, washed in 1 × PBS, and mixed with 500 μg/ml DAPI prior to analysis. To monitor centromeric segregation, wild-type and nse6Δ cells fluorescently marked at Cen1 were synchronized in early S phase by a 4.5 h treatment with 12 mM HU at 32°C. After full arrest was observed, cells were released into fresh media containing 10 μM latrunculin B (AG Scientific). This treatment allows wild-type cells to go through mitosis and arrest in telophase. Time points were collected 2–4 h post-release and cells were scored for nuclei and Cen1 content. In total, 200–300 cells were counted in 2–3 independent experiments and a representative experiment is shown.
ChIP assays
ChIP experiments were performed as described (Ogawa et al, 1999). Cleared lysates were incubated with protein G dynabeads (Invitrogen, CA) pre-bound to anti-HA (12CA5; Babco) or anti-myc (9E10; Babco) antibodies. DNA samples were purified using the PureLink PCR purification kit (Invitrogen). qPCR was performed on inputs and ChIP-enriched samples using a Chromo-4 four-colour detector system (Bio-Rad) using the iQ SYBR Green Supermix (Bio-Rad) and indicated primer pairs. Primer sequences are detailed in Supplementary data. Data are represented as the % DNA recovery in ChIP samples relative to the initial amount of DNA in the inputs, or in fold increase normalized to the background signal at non-ars site. All error bars represent the standard error between experimental duplicates, averaged between duplicate or triplicate qPCR measurements.
ChIP-on-chip
Input and ChIP samples were amplified and hybridized on Affymetrix S. pombe tiling arrays FR1.0. See Supplementary data.
Detection of sumoylated Nse4
Nse4–TAP-tagged strains were grown in YES at 32°C and treated with 0.03% MMS (v/v) or 12 mM HU for 5 h at 32°C. For gamma-irradiation, cells were treated with 200 Gy and recovered at 32°C for 2 h. Cells were lysed in SpLysis buffer (50 mM Tris–HCl pH 8.0, 300 mM NaCl, 0.2% NP-40, 0.5 mM EDTA, 20 mM N-ethyl maleimide, 50 mM NaF, 0.05 mM Na3VO4, Complete Protease Inhibitors EDTA-free (Roche, IN), 4 mM PMSF) bound to IgG-Sepharose beads (GE Healthcare), washed and directly boiled in sample buffer. Eluates were loaded on 3–8% Tris-acetate midi gels (Invitrogen). Nse4–TAP was detected by immunoblotting with the peroxidase–anti-peroxidase (PAP) antibody (Sigma-Aldrich) and developed using Super signal west dura substrate (Pierce, IL).
Supplementary Material
Supplementary data
Acknowledgments
We thank Benoit Arcangioli, Robin Allshire, Yasushi Hiraoka, Katsuhiro Tanaka, Jessica Williams and Paul Russell for generously providing us with fission yeast strains, John Prudden for generating strains, Sophie Rozenzhak and Eva Mejia for providing rDNA primers and mapping information, and The Scripps Cell Cycle Group for support and encouragement. This study was funded in part by NIH grant GM068608 awarded to MNB. This paper is dedicated to the loving memory of J-M de Crescenzo.
References
- Aguilera A (2002) The connection between transcription and genomic instability. EMBO J 21: 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DS, Goh SH, Hall BD (1983) The promoter sequence of a yeast tRNAtyr gene. Cell 34: 655–664 [DOI] [PubMed] [Google Scholar]
- Allshire RC (2004) RNA interference, heterochromatin, and centromere function. Cold Spring Harb Symp Quant Biol 69: 389–395 [DOI] [PubMed] [Google Scholar]
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G (1995) Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev 9: 218–233 [DOI] [PubMed] [Google Scholar]
- Ampatzidou E, Irmisch A, O'Connell MJ, Murray JM (2006) Smc5/6 is required for repair at collapsed replication forks. Mol Cell Biol 26: 9387–9401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews EA, Palecek J, Sergeant J, Taylor E, Lehmann AR, Watts FZ (2005) Nse2, a component of the Smc5–6 complex, is a SUMO ligase required for the response to DNA damage. Mol Cell Biol 25: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa H, Hayashi A, Haraguchi T, Hiraoka Y (2005) Dissociation of the Nuf2–Ndc80 complex releases centromeres from the spindle-pole body during meiotic prophase in fission yeast. Mol Biol Cell 16: 2325–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azvolinsky A, Dunaway S, Torres JZ, Bessler JB, Zakian VA (2006) The S.cerevisiae Rrm3p DNA helicase moves with the replication fork and affects replication of all yeast chromosomes. Genes Dev 20: 3104–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Allshire R (2002) Centromeres become unstuck without heterochromatin. Trends Cell Biol 12: 419–424 [DOI] [PubMed] [Google Scholar]
- Branzei D, Sollier J, Liberi G, Zhao X, Maeda D, Seki M, Enomoto T, Ohta K, Foiani M (2006) Ubc9- and mms21-mediated sumoylation counteracts recombinogenic events at damaged replication forks. Cell 127: 509–522 [DOI] [PubMed] [Google Scholar]
- Cam H, Grewal SI (2004) RNA interference and epigenetic control of heterochromatin assembly in fission yeast. Cold Spring Harb Symp Quant Biol 69: 419–427 [DOI] [PubMed] [Google Scholar]
- Cam HP, Sugiyama T, Chen ES, Chen X, FitzGerald PC, Grewal SI (2005) Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat Genet 37: 809–819 [DOI] [PubMed] [Google Scholar]
- Chen ES, Zhang K, Nicolas E, Cam HP, Zofall M, Grewal SI (2008) Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 451: 734–737 [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Ding DQ, Imai Y, Yamamoto M, Haraguchi T, Hiraoka Y (1997) Meiotic nuclear reorganization: switching the position of centromeres and telomeres in the fission yeast Schizosaccharomyces pombe. EMBO J 16: 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon S, Gaillard PH, Chahwan C, McDonald WH, Yates JR III, Russell P (2004) Slx1–Slx4 are subunits of a structure-specific endonuclease that maintains ribosomal DNA in fission yeast. Mol Biol Cell 15: 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio C, Schmidt CK, Katou Y, Kelly G, Itoh T, Shirahige K, Uhlmann F (2008) Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev 22: 2215–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Piccoli G, Cortes-Ledesma F, Ira G, Torres-Rosell J, Uhle S, Farmer S, Hwang JY, Machin F, Ceschia A, McAleenan A, Cordon-Preciado V, Clemente-Blanco A, Vilella-Mitjana F, Ullal P, Jarmuz A, Leitao B, Bressan D, Dotiwala F, Papusha A, Zhao X et al. (2006) Smc5–Smc6 mediate DNA double-strand-break repair by promoting sister-chromatid recombination. Nat Cell Biol 8: 1032–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande AM, Newlon CS (1996) DNA replication fork pause sites dependent on transcription. Science 272: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Dovey CL, Russell P (2007) Mms22 preserves genomic integrity during DNA replication in Schizosaccharomyces pombe. Genetics 177: 47–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco HD, Pidoux AL, Urano T, Allshire RC (2008) Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science 319: 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fousteri MI, Lehmann AR (2000) A novel SMC protein complex in Schizosaccharomyces pombe contains the Rad18 DNA repair protein. EMBO J 19: 1691–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froget B, Blaisonneau J, Lambert S, Baldacci G (2008) Cleavage of stalled forks by fission yeast mus81/eme1 in absence of DNA replication checkpoint. Mol Biol Cell 19: 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y, Kimata Y, Nomaguchi K, Watanabe K, Kohno K (2002) Identification of a novel non-structural maintenance of chromosomes (SMC) component of the SMC5–SMC6 complex involved in DNA repair. J Biol Chem 277: 21585–21591 [DOI] [PubMed] [Google Scholar]
- Grewal SI, Elgin SC (2007) Transcription and RNA interference in the formation of heterochromatin. Nature 447: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SH, Sheedy DM, Cuddihy AR, O'Connell MJ (2004) Coordination of DNA damage responses via the Smc5/Smc6 complex. Mol Cell Biol 24: 662–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Katou Y, Itoh T, Tazumi A, Yamada Y, Takahashi T, Nakagawa T, Shirahige K, Masukata H (2007) Genome-wide localization of pre-RC sites and identification of replication origins in fission yeast. EMBO J 26: 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JY, Smith S, Ceschia A, Torres-Rosell J, Aragon L, Myung K (2008) Smc5–Smc6 complex suppresses gross chromosomal rearrangements mediated by break-induced replications. DNA Repair (Amst) 7: 1426–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M (2006) Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 22: 159–180 [DOI] [PubMed] [Google Scholar]
- Kim SM, Huberman JA (2001) Regulation of replication timing in fission yeast. EMBO J 20: 6115–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc A, Zaratiegui M, Nora E, Martienssen R (2008) RNA interference guides histone modification during the S phase of chromosomal replication. Curr Biol 18: 490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J, Hall BD (1982) Mutations at the Saccharomyces cerevisiae SUP4 tRNA(Tyr) locus: isolation, genetic fine-structure mapping, and correlation with physical structure. Mol Cell Biol 2: 1501–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann AR, Walicka M, Griffiths DJ, Murray JM, Watts FZ, McCready S, Carr AM (1995) The rad18 gene of Schizosaccharomyces pombe defines a new subgroup of the SMC superfamily involved in DNA repair. Mol Cell Biol 15: 7067–7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroos HB, Strom L, Itoh T, Katou Y, Shirahige K, Sjogren C (2006) Chromosomal association of the Smc5/6 complex reveals that it functions in differently regulated pathways. Mol Cell 22: 755–767 [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano T (2005) Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev 19: 1269–1287 [DOI] [PubMed] [Google Scholar]
- McDonald WH, Pavlova Y, Yates JR III, Boddy MN (2003) Novel essential DNA repair proteins Nse1 and Nse2 are subunits of the fission yeast Smc5–Smc6 complex. J Biol Chem 278: 45460–45467 [DOI] [PubMed] [Google Scholar]
- Miyabe I, Morishita T, Hishida T, Yonei S, Shinagawa H (2006) Rhp51-dependent recombination intermediates that do not generate checkpoint signal are accumulated in Schizosaccharomyces pombe rad60 and smc5/6 mutants after release from replication arrest. Mol Cell Biol 26: 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morishita T, Kawane S, Iwasaki H, Carr AM, Shinagawa H (2004) Rad62 protein functionally and physically associates with the smc5/smc6 protein complex and is required for chromosome integrity and recombination repair in fission yeast. Mol Cell Biol 24: 9401–9413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Carr AM (2008) Smc5/6: a link between DNA repair and unidirectional replication? Nat Rev Mol Cell Biol 9: 177–182 [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH (2005) The structure and function of SMC and kleisin complexes. Annu Rev Biochem 74: 595–648 [DOI] [PubMed] [Google Scholar]
- Noguchi E, Noguchi C, Du LL, Russell P (2003) Swi1 prevents replication fork collapse and controls checkpoint kinase Cds1. Mol Cell Biol 23: 7861–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Cam HP, Maraia RJ, Grewal SI (2006) A role for TFIIIC transcription factor complex in genome organization. Cell 125: 859–872 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Takahashi T, Masukata H (1999) Association of fission yeast Orp1 and Mcm6 proteins with chromosomal replication origins. Mol Cell Biol 19: 7228–7236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek J, Vidot S, Feng M, Doherty AJ, Lehmann AR (2006) The Smc5–Smc6 DNA repair complex. bridging of the Smc5–Smc6 heads by the KLEISIN, Nse4, and non-Kleisin subunits. J Biol Chem 281: 36952–36959 [DOI] [PubMed] [Google Scholar]
- Partridge JF, Borgstrom B, Allshire RC (2000) Distinct protein interaction domains and protein spreading in a complex centromere. Genes Dev 14: 783–791 [PMC free article] [PubMed] [Google Scholar]
- Paule MR, White RJ (2000) Survey and summary: transcription by RNA polymerases I and III. Nucleic Acids Res 28: 1283–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebernard S, McDonald WH, Pavlova Y, Yates JR III, Boddy MN (2004) Nse1, Nse2, and a novel subunit of the Smc5–Smc6 complex, Nse3, play a crucial role in meiosis. Mol Biol Cell 15: 4866–4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebernard S, Wohlschlegel J, McDonald WH, Yates JR III, Boddy MN (2006) The Nse5–Nse6 dimer mediates DNA repair roles of the Smc5–Smc6 complex. Mol Cell Biol 26: 1617–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts PR, Porteus MH, Yu H (2006) Human SMC5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. EMBO J 25: 3377–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts PR, Yu H (2005) Human MMS21/NSE2 is a SUMO ligase required for DNA repair. Mol Cell Biol 25: 7021–7032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F, Aguilera A (2005) Impairment of replication fork progression mediates RNA polII transcription-associated recombination. EMBO J 24: 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudden J, Pebernard S, Raffa G, Slavin DA, Perry JJ, Tainer JA, McGowan CH, Boddy MN (2007) SUMO-targeted ubiquitin ligases in genome stability. EMBO J 26: 4089–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TH, Lin HK, Scaglioni PP, Yung TM, Pandolfi PP (2006) The mechanisms of PML-nuclear body formation. Mol Cell 24: 331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Oma Y, Schleker T, Kugou K, Ohta K, Harata M, Gasser SM (2008) Ino80 chromatin remodeling complex promotes recovery of stalled replication forks. Curr Biol 18: 566–575 [DOI] [PubMed] [Google Scholar]
- Strom L, Karlsson C, Lindroos HB, Wedahl S, Katou Y, Shirahige K, Sjogren C (2007) Postreplicative formation of cohesion is required for repair and induced by a single DNA break. Science 317: 242–245 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nishide J, Okazaki K, Kato H, Niwa O, Nakagawa T, Matsuda H, Kawamukai M, Murakami Y (1999) Characterization of a fission yeast SUMO-1 homologue, pmt3p, required for multiple nuclear events, including the control of telomere length and chromosome segregation. Mol Cell Biol 19: 8660–8672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebe H, Goshima G, Takeda K, Nakagawa T, Kinoshita K, Yanagida M (2001) Fission yeast living mitosis visualized by GFP-tagged gene products. Micron 32: 67–74 [DOI] [PubMed] [Google Scholar]
- Tomonaga T, Nagao K, Kawasaki Y, Furuya K, Murakami A, Morishita J, Yuasa T, Sutani T, Kearsey SE, Uhlmann F, Nasmyth K, Yanagida M (2000) Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev 14: 2757–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rosell J, Machin F, Farmer S, Jarmuz A, Eydmann T, Dalgaard JZ, Aragon L (2005) SMC5 and SMC6 genes are required for the segregation of repetitive chromosome regions. Nat Cell Biol 7: 412–419 [DOI] [PubMed] [Google Scholar]
- Tsuyama T, Inou K, Seki M, Seki T, Kumata Y, Kobayashi T, Kimura K, Hanaoka F, Enomoto T, Tada S (2006) Chromatin loading of Smc5/6 is induced by DNA replication but not by DNA double-strand breaks. Biochem Biophys Res Commun 351: 935–939 [DOI] [PubMed] [Google Scholar]
- Watts FZ, Skilton A, Ho JC, Boyd LK, Trickey MA, Gardner L, Ogi FX, Outwin EA (2007) The role of Schizosaccharomyces pombe SUMO ligases in genome stability. Biochem Soc Trans 35 (Part 6): 1379–1384 [DOI] [PubMed] [Google Scholar]
- Xhemalce B, Riising EM, Baumann P, Dejean A, Arcangioli B, Seeler JS (2007) Role of SUMO in the dynamics of telomere maintenance in fission yeast. Proc Natl Acad Sci USA 104: 893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xhemalce B, Seeler JS, Thon G, Dejean A, Arcangioli B (2004) Role of the fission yeast SUMO E3 ligase Pli1p in centromere and telomere maintenance. EMBO J 23: 3844–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Blobel G (2005) A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc Natl Acad Sci USA 102: 4777–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zofall M, Grewal SI (2006) RNAi-mediated heterochromatin assembly in fission yeast. Cold Spring Harb Symp Quant Biol 71: 487–496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data