Abstract
Apolipoprotein E receptor 2 (ApoER2), very low-density lipoprotein receptor (VLDLR), and Dab1 are the main components of the Reelin signalling cascade. Reelin is the sole ligand defined so far in signalling through this pathway. Postnatal migration of neuronal precursors from the subventricular zone (SVZ) to the olfactory bulb (OB), however, depends on ApoER2 and Dab1, but functions independently of Reelin. Here, we show that thrombospondin-1 (THBS-1) is a novel physiological ligand for ApoER2 and VLDLR. THBS-1 is present in the SVZ and along the entire rostral migratory stream (RMS). It binds to ApoER2 and VLDLR and induces phosphorylation of Dab1. In contrast to Reelin, it does not induce Dab1 degradation or Akt phosphorylation, but stabilizes neuronal precursor chains derived from subventricular explants. Lack of THBS-1 results in anatomical abnormalities of the RMS and leads to a reduction of postnatal neuronal precursors entering the OB.
Keywords: postnatal neuronal migration, Reelin receptors, rostral migratory stream, thrombospondin-1
Introduction
The Reelin signalling pathway is indispensable for the development of laminated structures in the brain (Tissir and Goffinet, 2003). The best studied role of Reelin is its function in radial neuronal migration during development of the neocortex and positioning of granule cells in the hippocampus (Forster et al, 2006). Key players of the Reelin signalling pathway are apolipoprotein E receptor 2 (ApoER2) and very low-density lipoprotein receptor (VLDLR) that relay the Reelin signal into radially migrating neurons. The molecular basis of this signalling cascade was recently summarized (Herz and Chen, 2006) and involves binding of oligomeric Reelin to ApoER2 and/or VLDLR, subsequent activation of Src family kinases, and phosphorylation of Disabled 1 (Dab1).
Reelin also has an important function in the integration of neuronal precursors into the olfactory bulb (OB) during postnatal development of the olfactory system in rodents. Neuronal precursors within the subventricular zone (SVZ) migrate tangentially through the rostral migratory stream (RMS) towards the OB forming chains ensheathed by glial cells (Lois et al, 1996). Reelin produced by mitral cells in the OB promotes the detachment of neuronal precursors from the incoming chains so as to allow proper radial migration of the precursors within the bulb (Hack et al, 2002). As recently discovered in our laboratory, however, ApoER2 and Dab1 within the RMS function independently of Reelin in postnatal tangential neuronal migration (Andrade et al, 2007). In mice lacking either ApoER2 or Dab1, chain formation is severely compromised, and neuronal precursors accumulate in the SVZ unable to migrate into the OB and thereby failing to form an RMS. As Reelin-producing cells are absent from both the SVZ and the RMS, it seems likely that ligands other than Reelin interact with ApoER2. The following observations prompted us to consider thrombospondin-1 (THBS-1) as such a ligand. THBSs are widely expressed in the developing central nervous system (O'Shea and Dixit, 1988; O'Shea et al, 1990). Cell culture experiments demonstrated that THBS-1 promotes neurite outgrowth from cultured neurons (O'Shea et al, 1991), and antibodies against THBSs block migration of cerebellar granule cells in vitro, suggesting that THBSs might be involved in neuronal migration (O'Shea et al, 1990). Despite these in vitro data, the function of THBS-1 in the central nervous system at the cellular level remained largely unknown mostly because mice lacking either THBS-1 (Lawler et al, 1998) or THBS-2 (Kyriakides et al, 1998) or both (Agah et al, 2002) did not exhibit an overt phenotype in the brain. THBS-1 belongs to a subfamily of THBSs that form trimers (Carlson et al, 2008) and are involved in cell-to-cell and cell-to-matrix communications (Lawler, 2000; Bornstein, 2001). They interact with membrane proteins such as integrins, CD47, CD36, proteoglycans, and low-density lipoprotein receptor-related protein 1 (LRP1) as well as with growth factors such as TGFβ and PDGF. The interaction of THBS-1 with LRP1 (Godyna et al, 1995) is of specific interest as ApoER2, VLDLR, and LRP1 belong to the same receptor family and share many ligands (Schneider and Nimpf, 2003). Here, we have identified THBS-1 as a component of the SVZ and RMS that binds to ApoER2 and VLDLR and stabilizes neuronal precursor chains without inducing the canonical Reelin signalling pathway.
Our findings demonstrate a hitherto unknown function of THBS-1 in the plasticity of neuronal precursor chains in the postnatal brain within the RMS. This function is mediated by ApoER2 and depends on the presence of Dab1, but stands in clear contrast to the Reelin signal that promotes disintegration of neuronal precursor chains.
Results
THBS-1 is expressed in the RMS of postnatal mice
ApoER2 and Dab1 knockout mice display phenotypes in the postnatal brain, where formation of neuronal precursor chains is severely compromised and the RMS is absent (Andrade et al, 2007). This effect cannot easily be ascribed to disruption of the Reelin signalling pathway, as Reelin, the only known physiological signalling ligand for ApoER2 and VLDLR to date, is absent from both the RMS and the SVZ. ApoER2 and VLDLR belong to the LDL receptor family and share many ligands with LRP1 (a comprehensive list of ligands of LRP1 can be found in Herz and Strickland, 2001). Screening the Allen Brain Atlas (Lein et al, 2007) for the expression of those candidate ligands revealed the presence of transcripts for THBS-1 in the RMS. THBS-1 belongs to a superfamily of proteins involved in neuronal development (Adams and Tucker, 2000) and has been shown to bind to LRP1 (Godyna et al, 1995).
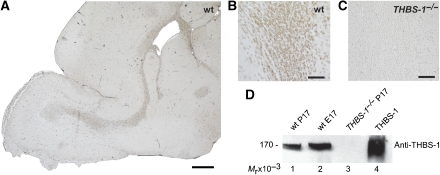
To confirm the presence of THBS-1 protein within the RMS, we performed immunohistochemical studies on sagittal sections of forebrains of P17 mice using a specific antibody against THBS-1. As demonstrated in Figure 1A, consistent staining for THBS-1 is present not only throughout the entire RMS but also in the SVZ. Higher magnification suggested that THBS-1 is associated with cells within the chains of migrating neurons forming the RMS (Figure 1B). Immunohistochemistry of a corresponding section derived from THBS-1−/− mice as a control (Figure 1C) verified the specificity of the staining shown in Figure 1A and B. It has been proposed that THBS-1 expression is downregulated after birth (Iruela-Arispe et al, 1993). Western blots performed using total brain extracts derived from postnatal brain and embryonic brain from wt mice, however, demonstrated that THBS-1 protein is present before and after birth (Figure 1D). A prominent band with an Mr (relative molecular mass) of 170 × 103 (under reducing conditions) is present in postnatal (P17, lane 1) and embryonic brain (E17, lane 2) from wt mice, but not in the brain of a THBS-1−/− mouse (P17, lane 3). This protein migrates with the same Mr as purified THBS-1 (lane 4).
Figure 1.
Thrombospondin-1 is expressed in the RMS and the SVZ of postnatal mice. Sagittal sections (6 μm) of the forebrains from (A, B) wt and (C) THBS-1−/− mice were immunostained with a monoclonal antibody against THBS-1. (D) Total protein extracts from wt P17 (lane 1), wt E17 (lane 2), and THBS-1−/− P17 brains (lane 3) were prepared. The samples (20 μg of total protein lysate per lane) and purified THBS-1 (0.5 μg, lane 4) were subjected to 6% SDS–PAGE under reducing conditions, and subsequent western blot analysis was performed using a monoclonal antibody against THBS-1 and an appropriate HRP-conjugated secondary antibody. Scale bars: (A) 500 μm; (B, C) 50 μm.
THBS-1 binds to ApoER2 and VLDLR
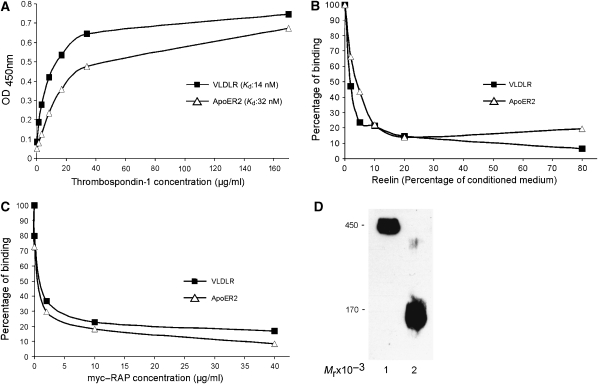
Having revealed the presence of a candidate ligand for ApoER2 and VLDLR within the RMS, we tested whether THBS-1 indeed binds to both receptors. We purified THBS-1 from human platelets and performed an ELISA-based binding assay using recombinant ligand-binding domains of ApoER2 and VLDLR (Koch et al, 2002) (Figure 2). THBS-1 is a homo-trimeric glycoprotein that migrates under non-reducing conditions with an Mr of 450 × 103 and with an Mr of 170 × 103 under reducing conditions (Figure 2D). As demonstrated in Figure 2A, purified THBS-1 binds with high affinity to both Reelin receptors. The affinities (Kd values) of THBS-1 were determined to be 32 nM for ApoER2 and 14 nM for VLDLR. Binding of THBS-1 to both receptors is inhibited by Reelin (Figure 2B) and receptor-associated protein (myc–RAP) (Figure 2C). RAP associates with most members of the LDL receptor family and inhibits the interaction with their cognate ligands (Willnow, 1998). These results demonstrate that the modes of interaction of THBS-1 and Reelin with ApoER2 and VLDLR are qualitatively and quantitatively similar.
Figure 2.
Thrombospondin-1 binds to ApoER2 and VLDLR. (A) Microtitre plates were coated with recombinant ligand-binding domains of ApoER2 and VLDLR (ApoER2Δ4-6-MBP/His and VLDLR1-8-MBP/His, respectively) and incubated with the indicated amounts of THBS-1. (B, C) Microtitre plates were coated with ApoER2Δ4-6-MBP/His or VLDLR1-8-MBP/His, and incubated with THBS-1 (15 μg/ml for ApoER2 and 6.5 μg/ml for VLDLR) in the presence of increasing amounts of Reelin-conditioned medium (B) or myc-tagged RAP (myc–RAP) (C). Bound THBS-1 was detected with a monoclonal anti-THBS-1 antibody and an appropriate HRP-conjugated secondary antibody. OD450, optical density at 450 nm. Kd, dissociation constant. In each case (A–C), the results of a representative experiment are shown. Kd values have been calculated from three independent experiments. (D) Western blot analysis of purified THBS-1. THBS-1 was purified from human plasma as described in Materials and methods and subjected to 6% SDS–PAGE (0.5 μg of protein per lane) under non-reducing (lane 1) and reducing conditions (lane 2) and subsequent western blot analysis using a monoclonal antibody against THBS-1 and an appropriate HRP-conjugated secondary antibody.
THBS-1 induces Dab1 phosphorylation in primary neurons, but does not stimulate other key events of the Reelin signalling cascade
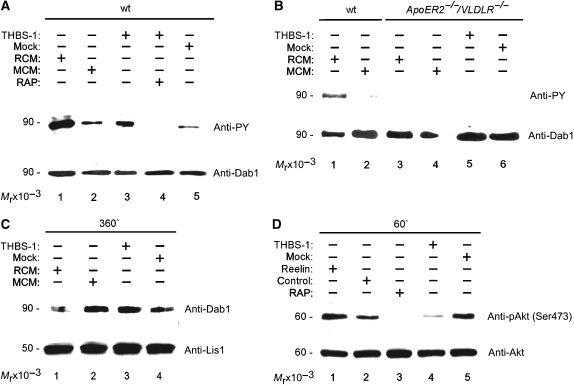
As recently demonstrated, receptor clustering by multivalent ligands, such as Reelin, is sufficient to promote Dab1 phosphorylation (Strasser et al, 2004). As THBS-1 forms homo-trimers, it is a prime candidate for signalling through ApoER2 and VLDLR. We tested whether purified THBS-1 triggers Dab1 phosphorylation in primary neurons. Primary mouse E16 neurons were treated with purified THBS-1 or Reelin-conditioned medium (RCM) as a control. As shown in Figure 3A (lane 3), neurons respond to THBS-1 (10 μg/ml) with a robust phosphorylation of Dab1 similar to that induced by RCM (lane 1). The effect of THBS-1 was dose dependent, leading to maximal Dab1 phosphorylation at 10–20 μg/ml with a decrease at higher concentrations (data not shown). Dab1 phosphorylation is most likely mediated by interaction of THBS-1 with ApoER2 and VLDLR, as it is abolished by the addition of myc–RAP (lane 4). myc–RAP even suppressed phosphorylation levels below the background (compare lane 4 to 2 and 5), which has been shown to be caused by small amounts of endogenous Reelin consistently present in the preparation of primary neurons (Koch et al, 2002). To further support the notion that the effect of THBS-1 is mediated by ApoER2/VLDLR, we used primary E16 neurons derived from mouse embryos lacking both receptor genes (ApoER2−/−/VLDLR−/−) (Trommsdorff et al, 1999). In sharp contrast to neurons derived from wt mice, these neurons neither responded to Reelin nor to THBS-1 with phosphorylation of Dab1 (Figure 3B, lanes 3–6). Again, due to the lack of the receptors, these neurons do not even exhibit background levels of Dab1 phosphorylation.
Figure 3.
Thrombospondin-1 induces Dab1 phosphorylation but not Dab1 degradation and Akt phosphorylation in primary embryonic neurons. (A) Primary mouse E16 wt neurons were incubated with Reelin-conditioned medium (RCM, lane 1), mock-conditioned medium (MCM, lane 2), purified THBS-1 (10 μg/ml, lane 3), THBS-1 in the presence of myc–RAP (lane 4), and mock medium (lane 5). Cells were processed for immunoprecipitation of Dab1, and western blotting was subsequently performed using antiphosphotyrosine (PY) or anti-Dab1 (Dab1) antibody as indicated. (B) Primary mouse E16 wt and primary E16 ApoER2−/−/VLDLR−/− neurons were incubated with RCM (lanes 1 and 3), MCM (lanes 2 and 4), purified THBS-1 (lane 5) and mock medium (lane 6). Dab1 phosphorylation was measured as indicated above. (C) Primary mouse E16 wt neurons were incubated with RCM (lane 1), MCM (lane 2), purified THBS-1 (10 μg/ml, lane 3), and mock medium (lane 4) for 6 h. Total cell extracts were prepared and western blotting was performed using anti-Dab1 and anti-Lis1 antibodies. (D) Primary mouse E16 wt neurons were incubated with Reelin (lane 1), control medium (lane 2), control medium and myc–RAP (lane 3), purified THBS-1 (10 μg/ml, lane 4), and mock medium (lane 5) for 1 h. Cell extracts were prepared and western blotting was performed using anti-phospho-Akt and anti-Akt antibodies.
Another important effect of the Reelin signalling pathway is the proteasome-dependent degradation of Dab1 (Arnaud et al, 2003; Bock et al, 2004). As recently demonstrated, this effect is an important regulator for correct positioning of radially migrating neurons during cortical development (Feng et al, 2007). Thus, we tested whether THBS-1 causes Dab1 degradation in primary neurons (Figure 3C). In contrast to Reelin (Figure 3C, lane 1), which causes significant loss of total Dab1 protein, THBS-1 treatment did not result in a detectable reduction of this protein even after 6 h (Figure 3C, lane 3). The surprising result that THBS-1 triggers Dab1 phosphorylation but not Dab1 degradation led us to examine whether Akt phosphorylation, another downstream event of Reelin-induced Dab1 phosphorylation, can be induced by THBS-1. Using primary neurons, Reelin-induced Akt phosphorylation is not as dramatic as can be seen using fibroblasts expressing ApoER2 and Dab1 (Mayer et al, 2006) (Figure 3D, lanes 1 and 2). Again, this is due to the presence of endogenous Reelin in such cultures, which results in detectable background phosphorylation of Akt. Addition of myc–RAP, which blocks the effect of Reelin by competing for the Reelin receptors, strongly reduced Akt phosphorylation (lane 3). Addition of THBS-1 also diminished Akt phosphorylation (lanes 4 and 5), demonstrating that THBS-1-induced Dab1 phosphorylation does not trigger Akt phosphorylation, but most likely inhibits background activation by competing with Reelin for the receptors.
Taken together, these results demonstrate that THBS-1 is present in the RMS, binds to both Reelin receptors, phosphorylates Dab1, but does not elicit cellular responses downstream of Dab1 similar to those of Reelin.
THBS-1 stabilizes neuronal chains produced by SVZ explants in vitro
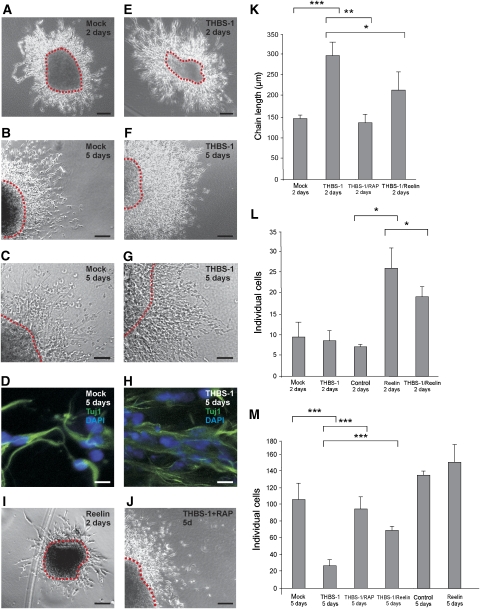
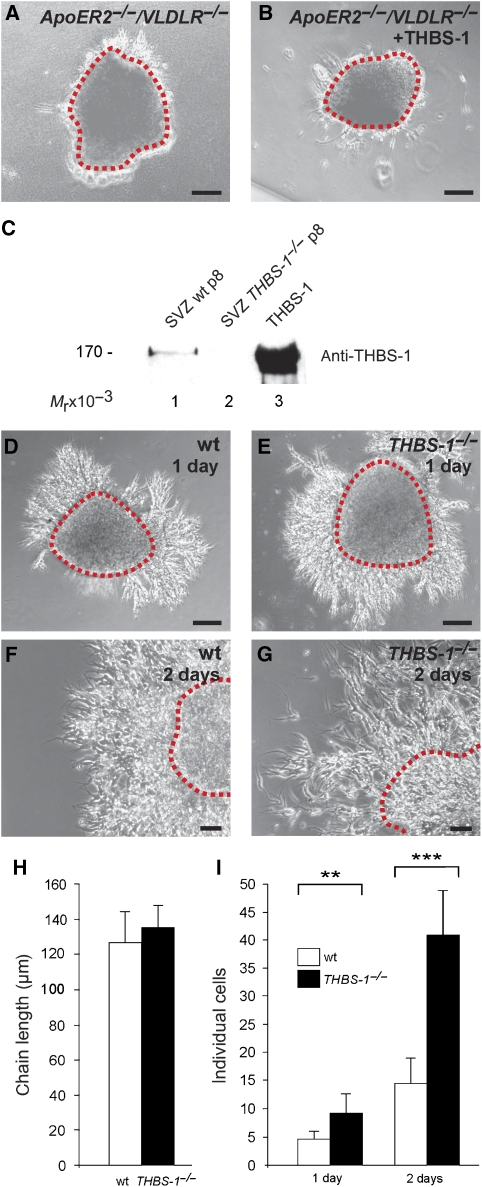
As neuronal precursors migrating along the RMS express ApoER2 (Andrade et al, 2007) and Dab1 (Hack et al, 2002), we next investigated the role of THBS-1 on migratory neuronal precursors. We used cultured SVZ explants in a three-dimensional matrix, a system where chain migration of neuronal precursors can be studied in vitro (Wichterle et al, 1997). SVZ explants were prepared from wt mice, and chain development was monitored with and without the addition of Reelin or THBS-1. In the absence of exogenous Reelin or THBS-1, typical chains were formed 2 days after the start of the experiment (Figure 4A). Addition of Reelin caused disassembly of the chains (Figure 4I) and a dramatic increase of individual cells (Figure 4L) as described (Hack et al, 2002). In sharp contrast, addition of THBS-1 did not dissolve the chains, but significantly increased the chain length (Figure 4K) when compared with the mock condition (Figure 4A and E) and there was no increase in the number of individual cells (Figure 4L). In accordance with previous experiments (Wichterle et al, 1997), longer incubation of the explants for 5 days led to disassembly of the chains (Figure 4B) and a dramatic increase in the number of dissociated cells even in the absence of Reelin (Figure 4M). Explants treated for 5 days with THBS-1 exhibited a significantly different behaviour. They retained the ability to form robust neuronal precursor chains (Figure 4F), and the number of detached cells was dramatically reduced in comparison to the mock situation (Figure 4M, control). Staining for Tuj1, a neuron-specific beta tubulin (Wichterle et al, 1997), demonstrated that the cells present within the chains of mock- and THBS-1-treated explants after 5 days are migrating neuronal precursors, which express the same marker as the precursors in vivo (Figure 4D and H). The effect of THBS-1 on the explants was completely abolished in the presence of myc–RAP (Figure 4J and M).
Figure 4.
Thrombospondin-1 stabilizes chains of migratory neuronal precursors from explants of the SVZ. (A–D) SVZ explants were prepared from P7 wt mice and treated with mock medium, or (E–H) cultivated in the presence of 4 μg/ml purified THBS-1, (I) Reelin or (J) THBS-1 in the presence of myc–RAP for 2 days (A, E, I) or 5 days (B, C, D, F, G, H, J), respectively. (D, H) Explants were immunostained using antibodies against Tuj1 with the appropriate secondary Alexa-Fluor antibody and DAPI. Representative explants are shown. (K) Explants were analysed by measuring the length of migratory chains after 2 days in culture and the number of individual cells per field after (L) 2 days and after (M) 5 days in culture (chain length 2 days: mock: n=8, THBS-1: n=13, THBS-1/myc–RAP: n=7, THBS-1/Reelin: n=6; dissociated neurons 2 days: mock: n=5, THBS-1: n=7, control: n=2, Reelin: n=2, THBS-1/Reelin: n=4; dissociated neurons 5 days: mock: n=6, THBS-1: n=28, THBS-1/myc–RAP: n=6, THBS-1/Reelin: n=6, control: n=4, Reelin: n=4; individual explants were derived from at least four different wt mice). Plots show average+s.e.m.; ***P<0.001; **P<0.01; *P<0.05 (Student's t-test); scale bars: (A, B, E, F, I, J) 100 μm; (C, G) 50 μm; (D, H) 10 μm.
The results from these experiments, together with the finding that THBS-1 does not induce Dab1 degradation and Akt phosphorylation, strongly suggest that THBS-1 and Reelin have different physiological effects on migrating neuronal precursors. As Reelin binds with higher affinity to the receptors than THBS-1 does, we expected the effect of Reelin to be dominant over THBS-1. When SVZ explants were treated with a combination of both ligands, Reelin-promoted destabilization of the chains at day 2 was only partially inhibited by the addition of an excess of THBS-1 (Figure 4L). On the other hand, THBS-1-induced stabilization after 5 days of incubation was significantly reduced in the presence of Reelin (Figure 4M). It is to be noted here that Reelin was added as a component of RCM, and its estimated concentration was below that of THBS-1.
Next, we tested whether the effect of THBS-1 is indeed mediated by ApoER2/VLDLR as suggested by the RAP experiments described above. Explants from ApoER2−/−/VLDLR−/− mice were treated with THBS-1 and chain formation was monitored. As demonstrated in Figure 5A and B, addition of THBS-1 did not rescue the inability of those explants to form neuronal precursor chains.
Figure 5.
Stability of SVZ explants is dependent on THBS-1. (A) P7 ApoER2−/−/VLDLR−/− SVZ explants were treated with control medium or (B) cultivated in the presence of 4 μg/ml purified THBS-1 for 2 days. (C) Protein extracts (45 μg) from SVZ explants from wt (lane 1), THBS-1−/− (lane 2) mice, and purified THBS-1 (0.5 μg) were subjected to 6% SDS–PAGE under reducing conditions. Subsequent western blot analysis was performed using a monoclonal antibody against THBS-1 and an appropriate HRP-conjugated secondary antibody. (D, F) SVZ explants were prepared from P7 wt and (E, G) THBS-1−/− mice and kept in culture for 1 day (D, E) and 2 days (F,G). Representative explants are shown. (H) Explants were analysed by measuring the length of migratory chains after 2 days in culture and (I) the number of individual cells per field after 1 and 2 days (chain length 2 days: wt: n=5, THBS-1−/−: n=6; individual neurons 1 day: wt: n=7, THBS-1−/−: n=7; individual neurons 2 days: wt: n=17, THBS-1−/−: n=10; individual explants were derived from at least five different wt and THBS-1−/− mice). Plots show average+s.e.m.; ***P<0.001; **P<0.01; (Student's t-test); scale bars: (A, B, D, E) 100 μm; (F, G) 50 μm.
To confirm an intrinsic function of THBS-1 in chain formation and stabilization, we first confirmed the presence of THBS-1 in SVZ explants and tested whether it is involved in the initial formation of neuronal precursor chains. As demonstrated by western blot analysis (Figure 5C), THBS-1 is indeed expressed by explants used for the assays. Comparing chain formation in explants derived from THBS-1−/− mice (Figure 5E) with those from wt mice (Figure 5D) clearly showed that initial chain formation as judged by chain length (Figure 5H) appeared normal. Counting individual cells, however, revealed a significant increase in explants from THBS-1−/− mice (Figure 5I). This phenotype became even more evident after 2 days in culture, when a dramatic increase in individual cells was apparent in THBS-1−/− explants (Figure 5I). After 3 days, chains derived from THBS-1−/− explants were completely disintegrated and closely resembled wt explants after 5 days in culture (not shown). To test whether other THBSs might be upregulated in the THBS-1−/− situation, expression of THBS-2 was evaluated by western blotting. THBS-2 could be detected neither in explants from wt nor from THBS-1−/− mice (data not shown). In total brain extracts and extracts derived from OBs, THBS-2 expression was not different between wt and THBS-1−/− mice. q-PCR experiments using mRNA derived from total brain demonstrated that levels of THBS-2, -3, and -4 transcripts were not upregulated in THBS-1−/− mice (data not shown).
Lack of THBS-1 leads to a widening of the RMS and to a reduction of neuronal precursors integrated into the postnatal OB
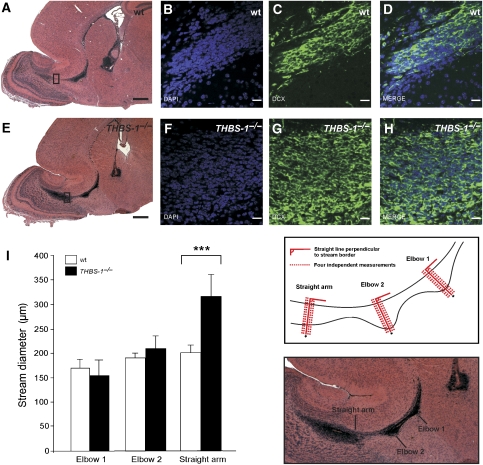
To assess the effect of THBS-1 in vivo, we examined the RMS of THBS-1−/− mice (Figure 6). Haematoxylin and eosin staining of sagittal sections derived from THBS-1−/− mice showed that the RMS is present, but exhibits an altered morphology at the entrance point to the OB (Figure 6A and E). In this area, the stream appears much wider and less focused. Closer examination of the RMS using immunofluorescence to stain for cells present in this area (DAPI) and for migrating neuronal precursors (doublecortin, DCX) demonstrated that the widening of the stream is due to less densely packed cells forming the RMS in THBS-1−/− mice (Figure 6F–H) when compared with cells forming the wt RMS (Figure 6B–D).
Figure 6.
The architecture of the RMS of THBS-1−/− mice is altered. (A–D) Matched sagittal sections (6 μm) of the forebrains from wt mice and (E–H) THBS-1−/− mice were stained with (A, E) haematoxylin and eosin, (B, F) DAPI, or (C, G) immunostained with anti-doublecortin (DCX). (I) Measurement of the width of the RMS from wt mice (empty bars) and THBS-1−/− mice (black bars). Matched serial sections from (P17) wt (n=4) and THBS-1−/− mice (n=8) were used to measure the width of the RMS at three defined sites as described in Materials and methods. Plots show average+s.e.m., ***P<0.001 (Student's t-test); scale bars: (A, E) 500 μm; (B–D, F–H) 20 μm.
To further investigate and quantify the observation that THBS-1−/− mice have a wider and less compact stream architecture at the area proximal to the entrance point into the OB, serial sagittal sections derived from wt and THBS-1−/− mice were prepared, and the width of the RMS was measured at three defined positions in every section (Figure 6I; all measurements were performed blind to the genotype, see Materials and methods). These points are situated at the beginning of the stream (elbow 1), in the middle of the stream (elbow 2), and at the entrance point to the OB (straight arm). Comparison of the mean values derived from four wt and eight THBS-1−/− mice in the same genetic background showed a significant widening (34%) of the stream in the area between elbow 2 and the entrance point to the OB in THBS-1−/− mice (Figure 6I). Measuring the actual size of the respective OBs did not reveal a significant difference between wt and THBS-1−/− mice.
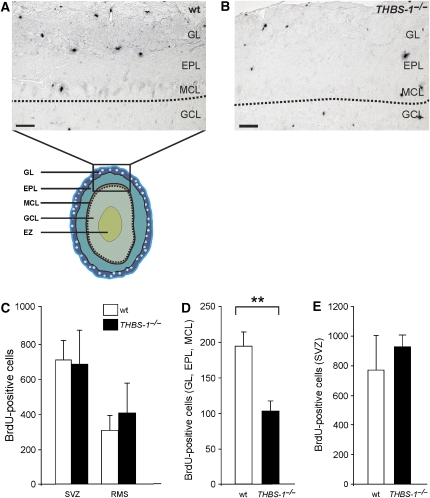
To test whether the widening of the RMS at the entrance point to the OB leads to fewer postnatal neuronal precursors reaching the OB, BrdU labelling experiments were performed. wt and THBS-1−/− animals were injected at P12, and the number of BrdU-positive cells present in the SVZ, along the RMS and in the layers of the OB were determined 5 days after BrdU administration (P17). As demonstrated in Figure 7C, there was no significant difference between wt and THBS-1−/− animals in the number of newly generated neuronal precursors present in the SVZ and along the RMS. Within the OB, however, significantly fewer BrdU-positive cells (47%) were detected in the mitral cell layer, the ependymal layer, and glomerular layer of THBS-1−/− animals than in the corresponding structures of wt animals (Figure 7A, B and D). To assess the possibility that the observed difference in the number of newly arrived precursors within the OB might be caused by an interference of THBS-1 with neuronal precursor production in the SVZ, a very short BrdU pulse (2 h) was performed to label only actively proliferating cells in the SVZ. As demonstrated in Figure 7E, there is no overt effect of THBS-1 on neurogenesis that could account for fewer neuronal precursors reaching the OB. In addition, the number of apoptotic cells in these structures (assessed by TUNEL stainings) was indistinguishable between the two genotypes (data not shown).
Figure 7.
Number of newly generated neuronal precursors derived from the RMS is reduced in the OB of THBS-1−/− mice. (A) wt and (B) THBS-1−/− mice received a BrdU pulse at P12, and brains were collected at P17. Matched sagittal sections of the forebrains were stained for BrdU-positive cells. Borders between granule cell layer (GCL) and mitral cell layer (MCL) are marked by dotted lines. (C) Quantification of BrdU-positive cells in the SVZ and RMS. (D) Quantification of BrdU-positive cells in the glomerular layer (GL), ependymal layer (EPL), and MCL of wt (empty bar) and THBS-1−/− mice (black bar). (E) Quantification of BrdU-positive cells in the SVZ after a 2 h BrdU pulse. Representative sections are shown (wt mice: empty bars, THBS-1−/− mice: black bars; BrdU 5 days, SVZ and RMS: wt: n=4, THBS-1−/− n=4; BrdU 5 days, GL, EPL, and MCL: wt: n=3, THBS-1−/− n=6; BrdU 2 h, SVZ: wt: n=4, THBS-1−/−:n=3). Plots show average+s.e.m., **P<0.01 (Student's t-test); scale bars: 50 μm.
Taken together, these results strongly suggest that lack of THBS-1 leads to a widening of the RMS and consequently allows fewer neuronal precursors to reach the OB. In turn, this reduction might have an impact on the fate determination and/or life expectancy of these neuronal precursors.
Discussion
THBS-1 is expressed in the SVZ and RMS of postnatal mice and binds to ApoER2 and VLDLR receptor
The finding that ApoER2 functions independently of Reelin in the postnatal RMS (Andrade et al, 2007) prompted us to search for alternative ligands for ApoER2 and VLDLR. ApoER2 belongs to the LDL receptor family and is expected to share many of the ligands that bind to VLDLR or LRP1. From a list of potential ligands, THBS-1 was identified as a very interesting candidate present in the entire RMS and the SVZ, but not in the OB. THBS-1 has been described to bind to LRP1 (Godyna et al, 1995) and belongs to a family of proteins, the members of which are known to be involved in neuronal development (Adams and Tucker, 2000).
As demonstrated here, THBS-1 binds to ApoER2 and VLDLR and competes for binding with both Reelin and myc–RAP, demonstrating that similar or overlapping binding sites are involved. The affinity of THBS-1 to both receptors is lower than that of Reelin (Koch et al, 2002; Strasser et al, 2004), but still in the range to be considered high affinity. Although the local concentration of THBS-1 present within the RMS and the SVZ cannot be assessed, functional in vitro analysis of THBS-1 demonstrated that Dab1 phosphorylation in primary neurons is triggered by THBS-1. This is in agreement with previous observations that receptor clustering is the key event in inducing Dab1 phosphorylation (Strasser et al, 2004). As THBS-1 forms homo-trimers in vivo, it is possible that this complex binds up to three receptor molecules simultaneously. The effect of THBS-1 was dose dependent with maximal Dab1 phosphorylation at concentrations between 10 and 20 μg/ml, the range at which according to our studies considerable ligand occupation of the receptors is achieved. At higher concentrations (five times higher or more), the effect decreased. Such kinetics are typical for a multivalent ligand that functions through forming higher order complexes with the interacting partner (receptor) (Strasser et al, 2004). At non-stoichiometric concentrations, the effect decreases by dilution. Several observations suggest that the effect of THBS-1 is indeed mediated by specific binding to ApoER2 and/or VLDLR. First, the maximal effect occurred at concentrations in the range between the Kd values and maximal saturation of both receptors. Second, THBS-1-mediated Dab1 phosphorylation does not occur in primary neurons lacking both receptors (Figure 3B), and third, the effect is inhibited by myc–RAP (Figure 3A). Dab1 phosphorylation in primary neurons triggered with THBS-1, however, does not lead to Dab1 degradation and Akt phosphorylation, both intricate events of the Reelin signalling pathway. These results further strengthen the hypothesis that Dab1 phosphorylation is necessary but not sufficient to trigger the complete Reelin response in neurons (Jossin et al, 2004). In summary, these experiments demonstrate that we have found the first alternative physiological ligand for ApoER2 and VLDLR capable of elucidating Dab1 phosphorylation but not other key events of the Reelin signalling pathway.
Disassembly of neuronal precursor chains in vitro is prevented by THBS-1
In addition to Reelin's role to orchestrate radial migration of neuronal precursors during cortical development, it was also shown to be important for the development of the OB (Hack et al, 2002). Reelin was reported to induce the switch from tangential to radial migration of neuronal precursors in the postnatal OB. Reelin is produced by mitral cells and is believed to function as detachment signal for neuronal precursors entering the OB within chains moving along the RMS. This effect was corroborated in vitro, as Reelin was shown to dissolve neuronal precursor chains produced by SVZ explants in three-dimensional Matrigel cultures (Hack et al, 2002). Here, we show that THBS-1, however, has the opposite effect on such cultures. It increases chain length and stabilizes the structure of established chains. Incubation of SVZ explants in the presence of THBS-1 prevents the disassembly of neuronal precursor chains after 5 days in culture and increases chain stability with significantly fewer dissociated cells than in the mock situation. This is in accordance with previous findings (Wichterle et al, 1997). Apparently, after an initial period during which neuronal precursors form chains and migrate away from the explant, after 4–5 days in culture the chains disintegrate. Furthermore, explants from THBS-1−/− mice initially form neuronal precursor chains, which are not significantly different from those produced from wt explants. These chains, however, start to disintegrate significantly earlier (2 days after starting the experiment, a significant portion of the chains is already dispersed). As revealed by competition experiments, even small amounts of Reelin were able to partially revert the effect of THBS-1, in agreement with our binding data, which demonstrate that Reelin has higher affinity for the receptors than THBS-1. Considering that the initial molecular event produced by Reelin and THBS-1 is identical (i.e. Dab1 phosphorylation), this finding is very intriguing. Most likely, this observation can be explained by one or more parallel pathways for the two signalling molecules. One possibility might be the co-receptor concept originally put forward for the action of Reelin, which is based on the finding that Reelin also binds to CNR (Senzaki et al, 1999) and α3β1 integrin (Dulabon et al, 2000). The interaction of Reelin with α3β1 integrin is of particular interest as it might also serve as intracellular binding site for Dab1 (Calderwood et al, 2003) and, under some circumstances, might interact with THBS-1 (Chandrasekaran et al, 2000). Although the interaction of Reelin with CNRs or α3β1 integrin or an hitherto unknown receptor is not required for promoting Dab1 phosphorylation, such interaction might promote a ‘full-blown' Reelin effect, in which Dab1 phosphorylation is necessary but not sufficient (Jossin et al, 2004). In such a scenario, THBS-1 could interact either with another co-receptor not involved in the Reelin action or with ApoER2 and/or VLDLR only, which although promoting Dab1 phosphorylation would elicit a different overall cellular response. On the basis of the results of our competition experiments, we envision an alternate mode of action of THBS-1 and Reelin along the RMS. THBS-1 binding to ApoER2 stabilizes the neuronal precursor chains along the RMS without activation of the canonical Reelin signalling pathway. Reelin present in the OB does not dissolve the chains actively by triggering the Reelin signalling cascade, but in displacing THBS-1 from the receptor it would dissolve the chains and consequently promote correct positioning achieved by radial migration within the OB. Although Dab1 is present in the RMS (Hack et al, 2002) and becomes phosphorylated in vitro by THBS-1, it is not clear yet whether Dab1 is phosphorylated in vivo within the RMS and whether it is involved in the chain stabilizing effect of THBS-1.
Postnatal THBS-1−/− mice display an altered morphology of the RMS and a decrease of postnatal neurons in the OB
The concept of THBS-1 stabilizing neuronal precursor chains within the RMS is in agreement with previous findings that THBS-1 promotes adhesion of central and peripheral neurons and PC12 cells (O'Shea et al, 1991). As demonstrated here, lack of THBS-1 leads to an altered morphology of the stream and to a decrease of postnatal neuronal precursors derived from the SVZ reaching the OB. Although the SVZ and the proximal part of the RMS appear unchanged (which is in agreement with the results obtained using SVZ explants, where lack of THBS-1 alters neither neurogenesis nor the primary formation of chains), the distal part of the RMS is significantly wider at the entrance point to the OB. This is most likely due to the fact that in the absence of THBS-1 the neuronal precursors present at this point of the RMS are significantly less densely packed. In addition, and possibly as a result of this altered structure of the RMS, fewer precursors and subsequently interneurons become integrated into the peripheral layers of the OB. This phenotype is less severe than that of mice lacking both ApoER2 and VLDLR, where chain formation is virtually absent. Most likely, ApoER2 and VLDLR integrate the signals from other signalling molecules in addition to THBS-1, which might also be present in the SVZ and RMS, like F-Spondin (Andrade et al, 2007).
Materials and methods
Animals
wt mice, ApoER2−/−/VLDLR−/− mice and THBS-1−/− mice on a C57BL6/J background were housed under standard conditions.
Antibodies
Polyclonal anti-Dab1 antibody (anti-54) was raised in rabbits against a glutathione S-transferase fusion protein containing the first 180 amino acids of the short splice variant of murine Dab1. Monoclonal mouse anti-Dab1 (D4) was obtained from Andre Goffinet (University of Louvain, Belgium) and monoclonal mouse anti-Lis1 was obtained from Orly Reiner (Weizmann Institute of Science, Israel). The following antibodies were purchased from the indicated sources: anti-THBS-1 (mouse monoclonal; Abcam), anti-THBS-2 (mouse monoclonal; BD Bioscience), anti-phosphotyrosine (mouse monoclonal; Santa Cruz Biotechnology), anti-Akt (rabbit polyclonal; Cell Signaling), anti-phospho Akt (rabbit polyclonal; Cell Signaling), anti-doublecortin C18 (goat polyclonal; Santa Cruz Biotechnology), anti-Tuj1 (mouse monoclonal; Santa Cruz Biotechnology), and anti-BrdU (mouse monoclonal; Becton Dickinson, Mountain View).
THBS purification
THBS-1 purification was performed as described (Roberts et al, 1994) with some minor modifications. The THBS-1-containing solution was brought to 20% (w/v) with ultrapure sucrose. Before use, sucrose was removed by dialysis against TBS by means of a Dialysis Cassette with 20 kDa molecular weight cutoff (Pierce). The purity of THBS-1 following dialysis was checked by Coomassie staining after SDS–PAGE.
Expression of recombinant proteins, preparation of cell extracts, electrophoresis, and western blotting
Reelin was expressed in stably transfected 293 cells, and conditioned medium was prepared as described earlier (Brandes et al, 2001). RCM was concentrated by ultra-centrifugation for 2 h. The resulting pellet was dissolved and enrichment estimated on a Coomassie gel to be 20-fold.
Preparation of MBP fusion proteins containing the ligand-binding domain of ApoER2 (ApoER2Δ4-6-MBP/His), VLDLR (VLDLR 1-8-MBP/His), and of myc–RAP were performed as described earlier (Koch et al, 2002).
Total cell extracts from primary neuronal cultures, isolated SVZ, and OBs were obtained after washing twice with PBS and scraping or homogenizing in Hunt buffer with protease inhibitor cocktail (Roche), 50 mM NaF and 10 mM Na3VO4 and centrifugation for 15 min at 20 000 g.
SDS–PAGE was performed according to the method of Laemmli (1970), and proteins were transferred onto nitrocellulose membranes by semidry blotting. For western blotting, nitrocellulose membranes were blocked for 1 h in PBS–0.1% Tween-20 containing 5% bovine serum albumin. Primary antibodies were used at the following dilutions: D4 1:7500, PY99 1:750, Akt 1:1000, phospho-Akt 1:1000, Lis1 1:15000, THBS-1 1:1000, and THBS-2 1:250. Appropriate horseradish peroxidase-conjugated antibodies (1:10 000; Jackson ImmunoResearch) were used for detection with enhanced chemiluminescence (Pierce).
Solid-phase binding assay
The solid-phase binding assay was essentially performed as described in Koch et al (2002) and Bajari et al (2005). Here, 100 μl TBS-C (2 mM CaCl2) containing 10 μg/ml of ApoER2Δ4-6-MBP/His or VLDLR 1-8-MBP/His were incubated on a 96-well plate overnight at 4°C. All further incubations were carried out at room temperature for 1 h, and ligands or antibodies were diluted in blocking solution (2% BSA in TBS-C and 0.05% Tween). After blocking and binding of THBS-1, anti-THBS-1 antibody followed by HRP-conjugated secondary antibody was used for the detection of bound THBS-1. For the colour reaction, 0.1 mg/ml 3,3′,5,5′-tetramethylbenzidine in 0.1 M sodium acetate, pH 6.0 containing 10 mM H2O2 was used. The reaction was stopped after 5 min by the addition of 0.3 M H2SO4, and the resulting yellow product was measured at 450 nm. For the competition assay, plates were coated with ApoER2Δ4-6-MBP/His or VLDLR 1-8-MBP/His as described above. Plates were overlaid with 14 or 32 nM THBS-1 for VLDLR or ApoER2, respectively, in the presence of increasing amounts of either RCM or myc–RAP. Bound THBS-1 was detected by the addition of an antibody against THBS-1 followed by HRP-conjugated secondary antibody.
Dab1 phosphorylation and degradation assay
The Dab1 phosphorylation assay was essentially performed as described (Hiesberger et al, 1999). Briefly, brains from embryonic day 16 (E16) wt mouse embryos or E16 ApoER2−/−/VLDLR−/− mouse embryos were homogenized in HBSS, centrifuged (1200 r.p.m., 2 min), resuspended in Dulbecco's modified Eagle's medium F-12 (DMEM-F12; Gibco) containing B27 supplement (Gibco), 2 mM L-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin sulphate, and subsequently plated on tissue culture dishes coated with 150 μg/ml poly-L-ornithine. Cells were grown at 37°C in a humidified environment and 5% CO2. After 3 days in culture, the cells were washed with PBS and subsequently incubated with different media containing the indicated ligands (see Figure 3A and B). After 20 min at 37°C, cells were lysed in Hunt buffer and the supernatants were immediately used for immunoprecipitation of Dab1 using 5 μl of anti-Dab1 antiserum. After overnight incubation at 4°C, 40 μl of a suspension containing protein A beads (Amersham) was added, and the mixture was incubated for 2 h at 4°C. The beads were washed with Hunt buffer and boiled in reducing Laemmli buffer prior to SDS–PAGE and western blotting.
To analyse Dab1 degradation, primary neuronal cultures were washed with PBS and subsequently incubated with different media containing the indicated ligands (see Figure 3C) for 360 min and total cell extracts were prepared as described earlier.
Akt phosphorylation
Phosphorylation of Akt was measured directly in total cell extracts derived from stimulated E16 neurons. After 3 days in culture, the cells were starved overnight using plain DMEM-F12 to reduce background phosphorylation of Akt, washed with PBS, and subsequently incubated with different media containing the indicated ligands (see Figure 3D). Equal amounts of protein from cell lysates were separated by SDS–PAGE and immunoblotted using an antibody directed against phospho-Akt and Akt.
Histology, immunohistochemistry, and immunofluorescence
Postnatal day 17 (P17) animals were anaesthetized with a combination of xylazine–ketamine (10 and 75 mg/kg respectively) in 0.9% NaCl, and immediately perfused with 4% paraformaldehyde (PFA) in PBS at 4°C. Brains were dehydrated and embedded in paraffin according to standard protocols. Serial sagittal paraffin sections (6 μm) were obtained. For immunohistochemistry, dehydrated paraffin sections were boiled with citrate buffer (10 mM sodium citrate, 0.05% Tween-20, pH 6.0) for 20 min to unmask antigenic epitopes. Endogenous peroxidase activity was blocked by adding 3% H2O2 for 10 min. Anti-THBS-1 (mouse monoclonal; Abcam) was used to detect endogenous THBS-1 in tissue sections. Primary antibody was visualized using the Vecta Stain Elite ABC Kit and Peroxidase Substrate Kit DAB (both from Vector Laboratories).
For immunofluorescence, tissue was prepared as described above. Dehydrated paraffin sections were incubated with anti-doublecortin antibody and DAPI and visualized using the TSA Plus Kit (Perkin Elmer).
SVZ explants
SVZ explants were prepared as reported (Andrade et al, 2007). Briefly, newborn pups were killed at P7 by decapitation. Brains were dissected and placed in cold OptiMEM medium (Gibco). Here, 250 μm slices were obtained using a Vibratome (Leica). The SVZ was dissected from the lateral wall of the anterior horn of the lateral ventricle and cut into pieces of 250–350 μm in diameter. The explants were mixed with Matrigel (BD Bioscience) and cultured in four-well dishes. After polymerization (20 min), 500 μl of serum-free neurobasal A medium (Gibco), supplemented with B-27 (Gibco-Invitrogen), insulin (50 ng/ml; Novo Nordisk), putrescine (100 μM; Sigma), progesterone (1 nM; Sigma), glutamine and penicillin/streptomycin (Gibco) containing either purified THBS-1 (3 μg/ml in TBS), TBS alone (mock), concentrated RCM (Reelin), concentrated MCM (control), or 50 μg/ml myc–RAP. Cultures were maintained in a humidified, 5% CO2, 37°C incubator. After 2 and 5 days, the explants were monitored and the length of migratory chains and the number of individual neurons per field were determined using AxioVision software (Zeiss).
Migratory chain length was determined by dividing each explant into quadrants, with each quadrant receiving two measurements. All measurements from one explant were averaged and the resulting chain length was referred to as the mean chain length of a given explant. The mean chain length of all explants from one experimental group was then averaged for n explants. All measurements and subsequent evaluation were performed blind.
For immunofluorescence analysis, tissue explants were treated as described (Lois and Alvarez-Buylla, 1993). Briefly, explants were fixed in 4% PFA for 10 min, rinsed in PBS and cells were permeabilized with 0.25% Triton X-100 for 5 min. After incubation with a blocking solution containing 10% BSA/PBS for 20 min, explants were incubated for 24 h at 4°C with primary antibodies against Tuj1 (dilution 1:50) diluted in 3% BSA/PBS. After rinsing in PBS, samples were incubated for 1 h at room temperature with the appropriate secondary Alexa-Fluor antibody and DAPI diluted in 3% BSA/PBS.
For western blot analysis, tissue explants were homogenized in Hunt buffer using a 26 G needle immediately after dissection and total cell extracts were prepared as described earlier.
Measurement of RMS width
To determine the width of the RMS in THBS-1−/− and control mice, three characteristic morphological features (elbow 1, elbow 2, and straight arm shown in Figure 6I) of the stream were defined and used to measure the width of the RMS. ‘Elbow 1' was defined as the characteristic elbow-like prominence situated at the base of the RMS. ‘Elbow 2' was defined as the second characteristic elbow-like prominence situated in the central part of the RMS and ‘straight arm' as the site where the stream widens at the entrance point to the OB at the end of the RMS. Serial sagittal sections of P17 THBS-1−/− and P17 control mice were stained for haematoxylin and eosin to visualize nuclei. The diameter of the stream was measured in every consecutive section as follows (see cartoon in Figure 6I): through each of the characteristic morphological features, a straight line perpendicular to the clearly defined stream border was set. Two measurements on either side of each straight line, in total four measurements per straight line and feature, were averaged and used for analysis. Consecutive slices were taken from each brain. Measurements were performed on each slice, and the biggest average width value from each feature was used for statistical analysis. All measurements and subsequent evaluation were performed blind to the mouse genotype. To exclude strain-specific phenotypes, THBS-1−/− mice on a C57BL6/J background were backcrossed for at least four generations with wt C57BL6/J mice.
BrdU experiments
Animals were injected i.p. with BrdU (50 mg/kg of body weight; Sigma) dissolved in 0.9% NaCl at P12 or P17 and were killed 5 days or 2 h later, respectively. BrdU-positive cells were quantified in three distinct areas: The SVZ, defined as the area between ventricle and elbow 1 and the RMS defined as the area between elbow 1 and elbow 2. In the OB, only BrdU-positive cells within the mitral cell layer, ependymal layer, and glomerular layer were quantified. BrdU stainings were performed on paraffin sections (6 μm) after 20 min of boiling in citrate buffer (pH 6) for antigen retrieval using anti-BrdU antibodies diluted 1:50 in 1% BSA/0.1% gelatine in PBS. Quantification of BrdU-positive cells was carried out blind to the mouse genotype.
Microscopy
Confocal images were acquired using a Zeiss LSM 5 system and LSM 5 software (Zeiss). DIC and phase contrast images were acquired using an Apotome system or an Axiovert 135 system and AxioVision software (Zeiss).
Acknowledgments
This study was supported by the ‘Fonds zur Förderung der Wissenschaftlichen Forschung, FWF', Austria, grants P16872-B09, and P19611-B09, and the Herzfelder'sche Familienstiftung. We thank Christoph Daniel for THBS-1−/− mice. We appreciate the technical assistance of Harald Rumpler, Vukoslav Komnenovic, Thomas Sauer and Stephan Weinhart and thank Stefan Stricker for critical reading of the paper.
References
- Adams JC, Tucker RP (2000) The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn 218: 280–299 [DOI] [PubMed] [Google Scholar]
- Agah A, Kyriakides TR, Lawler J, Bornstein P (2002) The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol 161: 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade N, Komnenovic V, Blake SM, Jossin Y, Howell B, Goffinet A, Schneider WJ, Nimpf J (2007) ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin. Proc Natl Acad Sci USA 104: 8508–8513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Forster E, Cooper JA (2003) Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr Biol 13: 9–17 [DOI] [PubMed] [Google Scholar]
- Bajari TM, Strasser V, Nimpf J, Schneider WJ (2005) LDL receptor family: isolation, production, and ligand binding analysis. Methods 36: 109–116 [DOI] [PubMed] [Google Scholar]
- Bock HH, Jossin Y, May P, Bergner O, Herz J (2004) Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J Biol Chem 279: 33471–33479 [DOI] [PubMed] [Google Scholar]
- Bornstein P (2001) Thrombospondins as matricellular modulators of cell function. J Clin Invest 107: 929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes C, Kahr L, Stockinger W, Hiesberger T, Schneider WJ, Nimpf J (2001) Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not alpha2-macroglobulin. J Biol Chem 276: 22160–22169 [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH (2003) Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA 100: 2272–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CB, Lawler J, Mosher DF (2008) Structures of thrombospondins. Cell Mol Life Sci 65: 672–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran L, He CZ, Al-Barazi H, Krutzsch HC, Iruela-Arispe ML, Roberts DD (2000) Cell contact-dependent activation of alpha3beta1 integrin modulates endothelial cell responses to thrombospondin-1. Mol Biol Cell 11: 2885–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES (2000) Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron 27: 33–44 [DOI] [PubMed] [Google Scholar]
- Feng L, Allen NS, Simo S, Cooper JA (2007) Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev 21: 2717–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster E, Zhao S, Frotscher M (2006) Laminating the hippocampus. Nat Rev Neurosci 7: 259–267 [DOI] [PubMed] [Google Scholar]
- Godyna S, Liau G, Popa I, Stefansson S, Argraves WS (1995) Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol 129: 1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Bancila M, Loulier K, Carroll P, Cremer H (2002) Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci 5: 939–945 [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y (2006) Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci 7: 850–859 [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland DK (2001) LRP: a multifunctional scavenger and signaling receptor. J Clin Invest 108: 779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J (1999) Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosin phosphorylation of the adaptor protein disabled-1 and modulates tau phosphorylation. Neuron 24: 481–489 [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P (1993) Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn 197: 40–56 [DOI] [PubMed] [Google Scholar]
- Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM (2004) The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci 24: 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Strasser V, Hauser C, Fasching D, Brandes C, Bajari TM, Schneider WJ, Nimpf J (2002) A secreted soluble form of ApoE receptor 2 acts as dominant negative receptor and inhibits reelin signaling. EMBO J 21: 5996–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P (1998) Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol 140: 419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lawler J (2000) The functions of thrombospondin-1 and-2. Curr Opin Cell Biol 12: 634–640 [DOI] [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO (1998) Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 101: 982–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR et al. (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176 [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A (1993) Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA 90: 2074–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A (1996) Chain migration of neuronal precursors. Science 271: 978–981 [DOI] [PubMed] [Google Scholar]
- Mayer H, Duit S, Hauser C, Schneider WJ, Nimpf J (2006) Reconstitution of the Reelin signaling pathway in fibroblasts demonstrates that Dab1 phosphorylation is independent of receptor localization in lipid rafts. Mol Cell Biol 26: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea KS, Dixit VM (1988) Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol 107 (6 Part 2): 2737–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea KS, Liu LH, Dixit VM (1991) Thrombospondin and a 140 kD fragment promote adhesion and neurite outgrowth from embryonic central and peripheral neurons and from PC12 cells. Neuron 7: 231–237 [DOI] [PubMed] [Google Scholar]
- O'Shea KS, Rheinheimer JS, Dixit VM (1990) Deposition and role of thrombospondin in the histogenesis of the cerebellar cortex. J Cell Biol 110: 1275–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DR, Cashel J, Guo N (1994) Purification of thrombospondin from human platelets. J Tissue Culture Meth 16: 217–222 [Google Scholar]
- Schneider WJ, Nimpf J (2003) LDL receptor relatives at the crossroad of endocytosis and signaling. Cell Mol Life Sci 60: 892–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzaki K, Ogawa M, Yagi T (1999) Proteins of the CNR family are multiple receptors for Reelin. Cell 99: 635–647 [DOI] [PubMed] [Google Scholar]
- Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, Howell B, Schneider WJ, Nimpf J (2004) Receptor clustering is involved in Reelin signaling. Mol Cell Biol 24: 1378–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM (2003) Reelin and brain development. Nat Rev Neurosci 4: 496–505 [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer R, Richardson JA, Herz J (1999) Reeler/Disabled-like disruption of neuronal migration in knock out mice lacking the VLDL receptor and apoE receptor-2. Cell 97: 689–701 [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A (1997) Direct evidence for homotypic, glia-independent neuronal migration. Neuron 18: 779–791 [DOI] [PubMed] [Google Scholar]
- Willnow TE (1998) Receptor-associated protein (RAP): a specialized chaperone for endocytic receptors. Biol Chem 379: 1025–1031 [PubMed] [Google Scholar]