Abstract
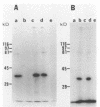
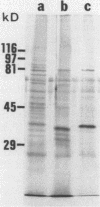
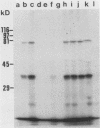
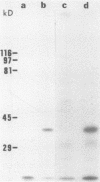
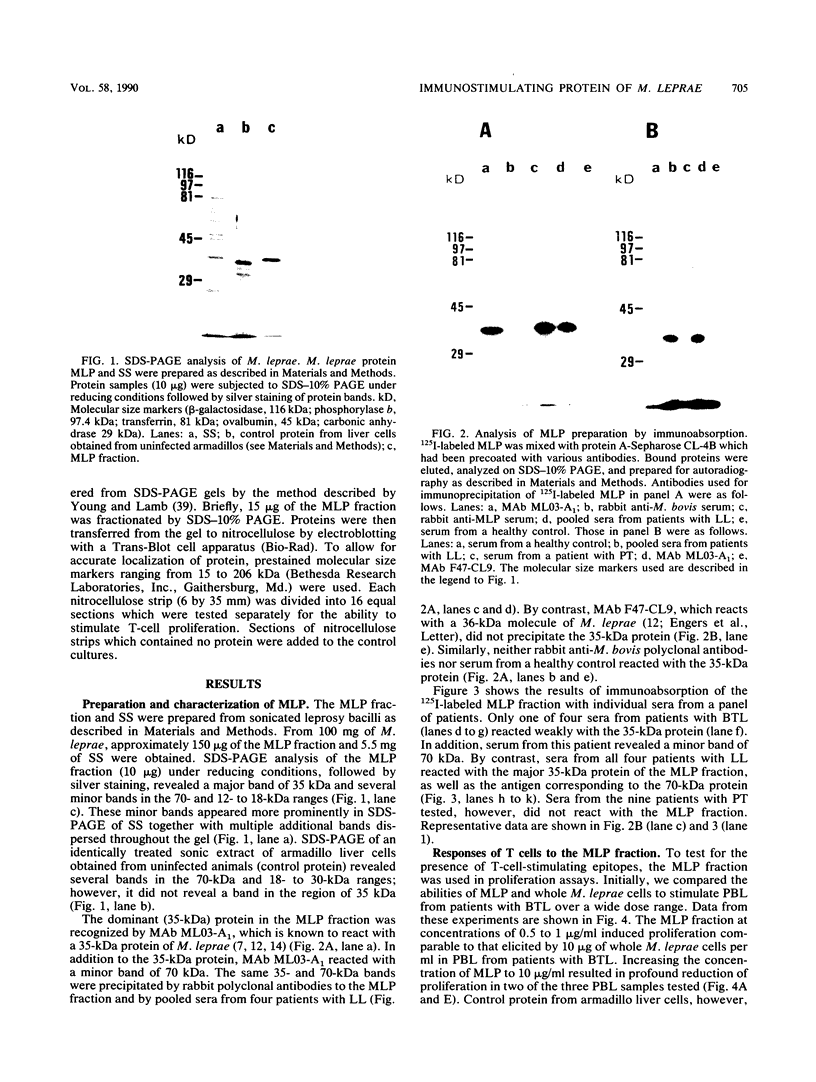
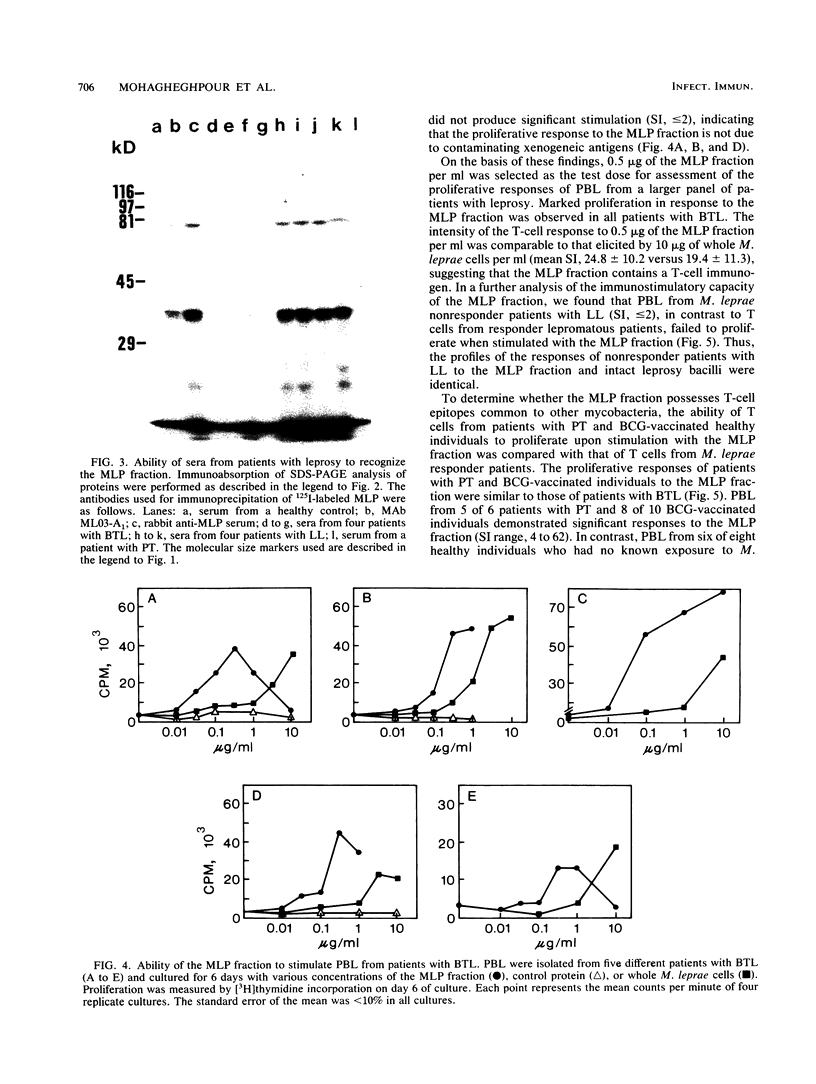
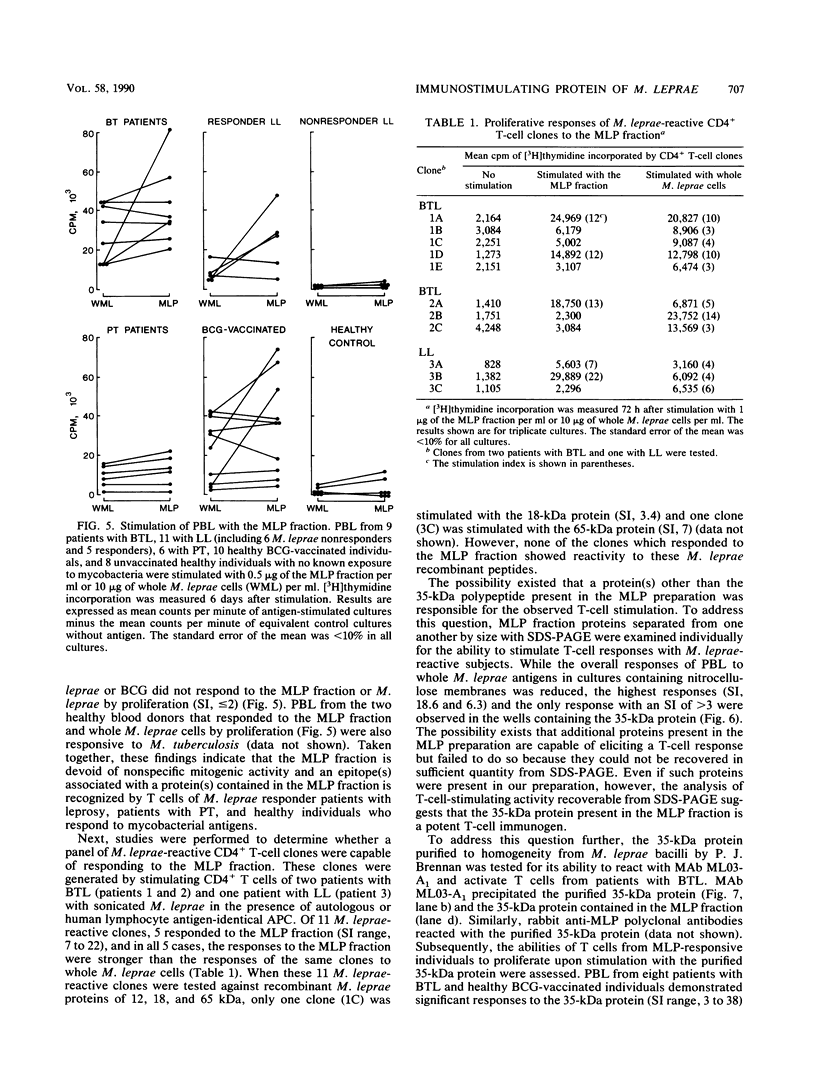
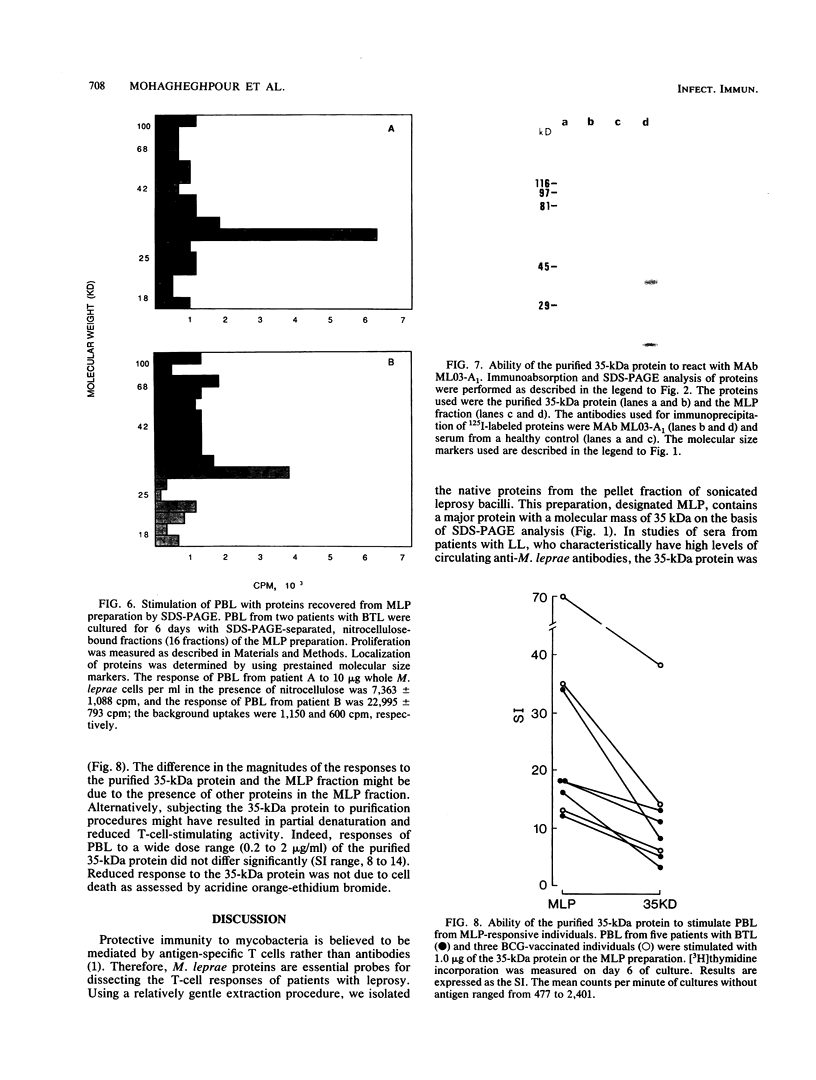
Despite the recent identification of a number of Mycobacterium leprae proteins, the major immunogenic determinants of this organism remain obscure. We isolated from M. leprae a potent immunostimulatory preparation, designated the MLP fraction, which contains a major protein of 35 kilodaltons (kDa). This protein was precipitated by monoclonal antibody ML03-A1, which recognizes a 35-kDa protein of M. leprae, and by sera obtained from patients with lepromatous leprosy. Neither sera from healthy controls nor sera from patients with pulmonary tuberculosis recognized the 35-kDa protein, and only one of four serum samples from patients with borderline tuberculoid leprosy reacted with this protein. The MLP fraction stimulated T-cell proliferation in patients with leprosy whose T cells proliferate in response to whole M. leprae cells. Apparently, the T-cell epitope associated with MLP is also expressed on M. tuberculosis and M. bovis BCG, since patients with pulmonary tuberculosis and BCG-vaccinated individuals demonstrated significant responses to the MLP fraction. The 35-kDa M. leprae protein, purified to homogeneity in the laboratory of P. J. Brennan, stimulated T-cell proliferative responses in all MLP-responsive subjects. These findings suggest that the 35-kDa protein present in MLP is an immunostimulatory component of M. leprae. In addition to serving as a useful probe for study of the T-cell anergy associated with lepromatous disease, this protein may ultimately be useful as a component of a vaccine designed to provide protection against infection with M. leprae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984 Aug;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Garsia R. J., Basten A. Dominant cell wall proteins of Mycobacterium leprae recognized by monoclonal antibodies. Clin Exp Immunol. 1987 Jan;67(1):31–42. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Jr, Fasal P. Studies of immune mechanisms in leprosy. 3. The role of cellular and humoral factors in impairment of the in vitro immune response. J Immunol. 1971 Apr;106(4):888–899. [PubMed] [Google Scholar]
- Daniel T. M., Hinz C. F., Jr Reactivity of purified proteins and polysaccharides from Mycobacterium tuberculosis in delayed skin test and cultured lymphocyte mitogenesis assays. Infect Immun. 1974 Jan;9(1):44–47. doi: 10.1128/iai.9.1.44-47.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gelber R. H., Brennan P. J., Hunter S. W., Munn M. W., Monson J. M., Murray L. P., Siu P., Tsang M., Engleman E. G., Mohagheghpour N. Effective vaccination of mice against leprosy bacilli with subunits of Mycobacterium leprae. Infect Immun. 1990 Mar;58(3):711–718. doi: 10.1128/iai.58.3.711-718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Buchanan T. M. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1982 Jul;37(1):172–178. doi: 10.1128/iai.37.1.172-178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T. Immunological aspects of leprosy--present status. Prog Allergy. 1978;25:211–242. [PubMed] [Google Scholar]
- Harboe M., Ivanyi J. Analysis of monoclonal antibodies to Mycobacterium leprae by crossed immunoelectrophoresis. Scand J Immunol. 1987 Feb;25(2):133–138. doi: 10.1111/j.1365-3083.1987.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- Kansas G. S., Wood G. S., Fishwild D. M., Engleman E. G. Functional characterization of human T lymphocyte subsets distinguished by monoclonal anti-leu-8. J Immunol. 1985 May;134(5):2995–3002. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Kingston A. E., Colston M. J. Concentration-dependent effects of mycobacteria on the stimulation of murine T-cell clones. Acta Leprol. 1984 Oct-Dec;2(2-4):369–377. [PubMed] [Google Scholar]
- Klatser P. R., Hartskeerl R. A., van Schooten W. C., Kolk A. H., van Rens M. M., de Wit M. Y. Characterization of the 36 K antigen of Mycobacterium leprae. Lepr Rev. 1986 Dec;57 (Suppl 2):77–81. doi: 10.5935/0305-7518.19860057. [DOI] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Torigian V. K., Mandich D., Reichel M., Young S. M., Salgame P., Convit J., Hunter S. W., McNeil M. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J Immunol. 1989 Apr 15;142(8):2873–2878. [PubMed] [Google Scholar]
- Melancon-Kaplan J., Hunter S. W., McNeil M., Stewart C., Modlin R. L., Rea T. H., Convit J., Salgame P., Mehra V., Bloom B. R. Immunological significance of Mycobacterium leprae cell walls. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1917–1921. doi: 10.1073/pnas.85.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohagheghpour N., Damle N. K., Moonka D. K., Terrell C. P., Engleman E. G. A human alloreactive inducer T cell clone that selectively activates antigen-specific suppressor T cells. J Immunol. 1984 Jul;133(1):133–136. [PubMed] [Google Scholar]
- Mohagheghpour N., Gelber R. H., Larrick J. W., Sasaki D. T., Brennan P. J., Engleman E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by interleukin 2. J Immunol. 1985 Aug;135(2):1443–1449. [PubMed] [Google Scholar]
- Mohagheghpour N., Gelber R. R., Engleman E. G. T cell defect in lepromatous leprosy is reversible in vitro in the absence of exogenous growth factors. J Immunol. 1987 Jan 15;138(2):570–574. [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Mwatha J., Moreno C., Sengupta U., Sinha S., Ivanyi J. A comparative evaluation of serological assays for lepromatous leprosy. Lepr Rev. 1988 Sep;59(3):195–199. doi: 10.5935/0305-7518.19880024. [DOI] [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenhoff T. H., Klatser P. R., Ivanyi J., Elferink D. G., de Wit M. Y., de Vries R. R. Mycobacterium leprae-specific protein antigens defined by cloned human helper T cells. Nature. 1986 Jan 2;319(6048):66–68. doi: 10.1038/319066a0. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Root R. K., Wolff S. M., Baer H. Skin reactivity of human subjects to a polysaccharide component of BCG culture filtrates. Proc Soc Exp Biol Med. 1971 Feb;136(2):403–406. doi: 10.3181/00379727-136-35274. [DOI] [PubMed] [Google Scholar]
- Sasaki D. T., Dumas S. E., Engleman E. G. Discrimination of viable and non-viable cells using propidium iodide in two color immunofluorescence. Cytometry. 1987 Jul;8(4):413–420. doi: 10.1002/cyto.990080411. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., van Landingham R. M., Walker L. L., Ye S. Z. Comparison of the immunogenicity of vaccines prepared from viable Mycobacterium bovis BCG, heat-killed Mycobacterium leprae, and a mixture of the two for normal and M. leprae-tolerant mice. Infect Immun. 1983 Jun;40(3):1096–1103. doi: 10.1128/iai.40.3.1096-1103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. B., Lowe A. C., Rees R. J., Colston M. J. Vaccination of mice against Mycobacterium leprae infection. Infect Immun. 1989 Feb;57(2):653–655. doi: 10.1128/iai.57.2.653-655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]