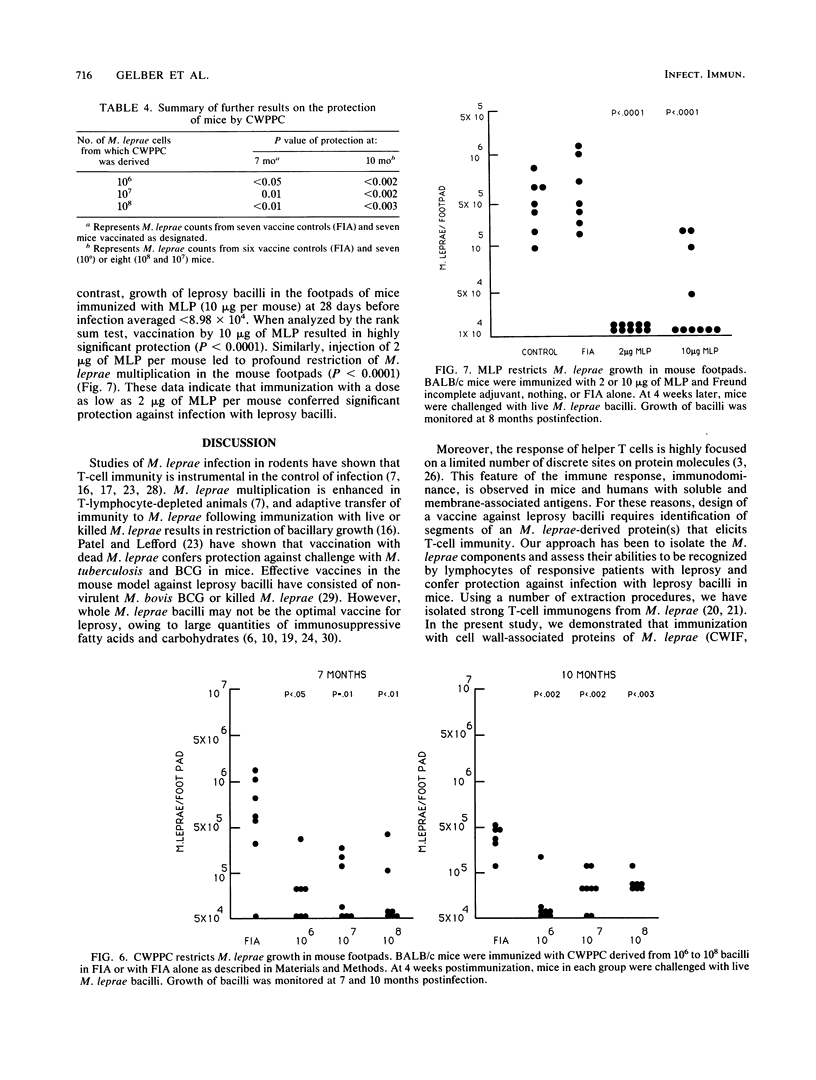
Abstract
Model vaccines against leprosy bacilli have consisted of nonvirulent, live, attenuated Mycobacterium bovis BCG and irradiated, heat-killed, or autoclaved intact M. leprae. We report that immunization with various cell wall fractions of M. leprae, progressively depleted of lipids, carbohydrates, and soluble proteins, as well as a partially purified protein(s) derived from a pellet fraction of sonicated M. leprae, conferred significant protection against subsequent infection with live leprosy bacilli. Moreover, lymphocytes from regional lymph nodes and spleens of mice immunized with these M. leprae-derived subunits responded by proliferation when stimulated with M. leprae in vitro. Our results provide the first evidence that vaccination with M. leprae-derived fractions protects mice against leprosy bacilli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Barclay W. R., Brehmer W., Goode G., List R. H., Ribi E., Tarmina D. F. Effectiveness of cell walls of Mycobacterium bovis strain BCG administered by various routes and in different adjuvants in protecting mice against airborne infection with Mycobacterium tuberculosis strain H37Rv. Am Rev Respir Dis. 1969 Feb;99(2):242–248. doi: 10.1164/arrd.1969.99.2.242. [DOI] [PubMed] [Google Scholar]
- Berkower I., Matis L. A., Buckenmeyer G. K., Gurd F. R., Longo D. L., Berzofsky J. A. Identification of distinct predominant epitopes recognized by myoglobin-specific T cells under the control of different Ir genes and characterization of representative T cell clones. J Immunol. 1984 Mar;132(3):1370–1378. [PubMed] [Google Scholar]
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rodríguez Ochoa G., Ulrich M., Avila J. L., Goihman M. Elimination of Mycobacterium leprae subsequent to local in vivo activation of macrophages in lepromatous leprosy by other mycobacteria. Clin Exp Immunol. 1974 Jun;17(2):261–265. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Fieldsteel A. H., Levy L. Neonatally thymectomized Lewis rats infected with Mycobacterium leprae: response to primary infection, secondary challenge, and large inocula. Infect Immun. 1976 Sep;14(3):736–741. doi: 10.1128/iai.14.3.736-741.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Ji B. H. Drug resistance in leprosy--a review. Lepr Rev. 1985 Dec;56(4):265–278. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Kirchheimer W. F., Storrs E. E. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of lepromatoid leprosy in an experimentally infected armadillo. Int J Lepr Other Mycobact Dis. 1971 Jul-Sep;39(3):693–702. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb F. I., Kingston A. E., Estrada I., Colston M. J. Heterologous expression of the 65-kilodalton antigen of Mycobacterium leprae and murine T-cell responses to the gene product. Infect Immun. 1988 May;56(5):1237–1241. doi: 10.1128/iai.56.5.1237-1241.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C., Brett S. J., Rees R. J. Adoptive cell transfer of resistance to Mycobacterium leprae infections in mice. Clin Exp Immunol. 1985 Aug;61(2):336–342. [PMC free article] [PubMed] [Google Scholar]
- Lwin K., Sundaresan T., Gyi M. M., Bechelli L. M., Tamondong C., Garbajosa P. G., Sansarricq H., Noordeen S. K. BCG vaccination of children against leprosy: fourteen-year findings of the trial in Burma. Bull World Health Organ. 1985;63(6):1069–1078. [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R. Induction of cell-mediated immunity to Mycobacterium leprae in guinea pigs. Infect Immun. 1979 Mar;23(3):787–794. doi: 10.1128/iai.23.3.787-794.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Torigian V. K., Mandich D., Reichel M., Young S. M., Salgame P., Convit J., Hunter S. W., McNeil M. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J Immunol. 1989 Apr 15;142(8):2873–2878. [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Mohagheghpour N., Munn M. W., Gelber R. H., Engleman E. G. Identification of an immunostimulating protein from Mycobacterium leprae. Infect Immun. 1990 Mar;58(3):703–710. doi: 10.1128/iai.58.3.703-710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen S. K. Vaccination against leprosy; recent advances and practical implications. Lepr Rev. 1985 Mar;56(1):1–3. doi: 10.5935/0305-7518.19850001. [DOI] [PubMed] [Google Scholar]
- Patel P. J., Lefford M. J. Induction of cell-mediated immunity to Mycobacterium leprae in mice. Infect Immun. 1978 Jan;19(1):87–93. doi: 10.1128/iai.19.1.87-93.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad H. K., Mishra R. S., Nath I. Phenolic glycolipid-I of Mycobacterium leprae induces general suppression of in vitro concanavalin A responses unrelated to leprosy type. J Exp Med. 1987 Jan 1;165(1):239–244. doi: 10.1084/jem.165.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E., Anacker R. L., Brehmer W., Goode G., Larson C. L., List R. H., Milner K. C., Wicht W. C. Factors influencing protection against experimental tuberculosis in mice by heat-stable cell wall vaccines. J Bacteriol. 1966 Oct;92(4):869–879. doi: 10.1128/jb.92.4.869-879.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri N., Oki A., Miller A., Sercarz E. E. Distinct recognition phenotypes exist for T cell clones specific for small peptide regions of proteins. Implications for the mechanisms underlying major histocompatibility complex-restricted antigen recognition and clonal deletion models of immune response gene defects. J Exp Med. 1985 Jul 1;162(1):332–345. doi: 10.1084/jem.162.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., Van Landingham R., Walker L. L. Immunity to Mycobacterium leprae infections in mice stimulated by M. leprae, BCG, and graft-versus-host reactions. Infect Immun. 1976 Oct;14(4):919–928. doi: 10.1128/iai.14.4.919-928.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., van Landingham R. M., Walker L. L., Ye S. Z. Comparison of the immunogenicity of vaccines prepared from viable Mycobacterium bovis BCG, heat-killed Mycobacterium leprae, and a mixture of the two for normal and M. leprae-tolerant mice. Infect Immun. 1983 Jun;40(3):1096–1103. doi: 10.1128/iai.40.3.1096-1103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S. J., Howland C., Stone M. M., Sutherland I. BCG vaccination of children against leprosy in Uganda: final results. J Hyg (Lond) 1981 Oct;87(2):233–248. doi: 10.1017/s002217240006945x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toman K. Bacterial persistence in leprosy. Int J Lepr Other Mycobact Dis. 1981 Jun;49(2):205–217. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]