Abstract
Rationale
3, 4-Methylenedioxymethamphetamine (MDMA or “ecstasy”) is a popular drug of abuse known to result in depletions of the serotonin (5-HT) system. A number of studies have reported that ecstasy users differ from controls on a variety of measures of cognitive function. However, the literature is not consistent and many negative findings were also reported. One reason for such inconsistency might be interindividual variance in vulnerability to the deleterious effects of ecstasy due to a number of factors, both genetic and environmental.
Objectives
To investigate the hypothesis that carriers of the s allele at the 5-HT transporter gene-linked polymorphic region (5-HTTLPR), which was associated with reduced serotonergic neurotransmission relative to the l allele, would be most vulnerable to the effects of ecstasy on cognitive function.
Methods
We assessed memory, decision-making, and executive function in ecstasy users and controls, stratifying by genotype at the 5-HTTLPR.
Results
We observed that the 5-HTTLPR genotype groups differed on a number of measures in both the ecstasy users and the controls. While performing a risky decision-making task, ss and ls controls attended to differences in the probability of winning chosen gambles to a greater extent than the ll controls. However, this difference was dramatically attenuated in the ss ecstasy users. Furthermore, independent of ecstasy use, volunteers of the ss genotype outperformed the ll genotype on a visual planning task.
Conclusions
The results are consistent with the hypothesis that cognitive impairment in ecstasy users may depend on genetic variation at the 5-HTTLPR.
Keywords: Ecstasy (MDMA), Decision-making, Serotonin transporter-linked polymorphic region (5-HTTLPR), Neuropsychology, Memory, Executive function
Introduction
3, 4-Methylenedioxymethamphetamine (MDMA or “ecstasy”) is a popular drug of abuse, taken for its ability to produce feelings of heightened mood, increased extroversion, derealization, and mild perceptual alterations with peak effects occurring approximately 2-4 h after ingestion (Gamma et al. 2000). However, MDMA also causes extensive serotonin (5-HT) depletion when administered in high doses to experimental animals (Green et al. 2003), and a number of studies have reported that human users of ecstasy show signs of serotonergic downregulation, including reduced 5-HT transporter (5-HTT) binding (Buchert et al. 2004; McCann et al. 2005; Reneman et al. 2001; Semple et al. 1999), attenuated response to 5-HT releasers such as fenfluramine and m-chlorophenylpiperazine (Gerra et al. 1998; McCann et al. 1999), and reduced concentration of 5-HT metabolites in cerebrospinal fluid (McCann et al. 1994).
Using the technique of acute tryptophan depletion, a number of studies have reported that a reduction in 5-HT synthesis results in changes in various cognitive domains. Performance on tests of episodic memory, particularly those paradigms that require subjects to learn lists of words or figures and then subsequently either recall or recognize the learned material, is frequently impaired after acute tryptophan depletion (Rubinsztein et al. 2001; Schmitt et al. 2000), as well as performance on gambling paradigms (Rogers et al. 1999a, 2003). By contrast, so-called “executive functions”, including indices of working memory and planning, are largely unaffected by acute tryptophan depletion (Gallagher et al. 2003; Murphy et al. 2002). Concordant with these findings, subclinical but statistically significant memory impairments were reported in ecstasy users by a number of investigators (Bhattachary and Powell 2001; Bolla et al. 1998; Fox et al. 2002; McCardle et al. 2004; Morgan 1999), though the few studies that have investigated decision-making in ecstasy users report conflicting results (Butler and Montgomery 2004; Fox et al. 2002; Morgan et al. 2005; Roiser et al. 2005b). Executive functions are less commonly affected in ecstasy users, though some studies have reported impairments (Alting Von Geusau et al. 2004;Gouzoulis-Mayfrank et al. 2003).
One potential factor underlying failures to replicate cognitive differences between ecstasy users and controls may be interindividual differences in vulnerability to the long-term effects of the MDMA. One such source of variability is genetic differences between individuals. We have previously reported that ecstasy users carrying the s allele at the 5-HTT linked polymorphic region (5-HTTLPR; Lesch et al. 1996) were most likely to exhibit depressive symptoms and perform abnormally on a test of emotional processing relative to genetically matched controls (Roiser et al. 2005a). The 5-HTTLPR is a 44-base pair insertion/deletion functional polymorphism upstream of the transcription initiator site. This polymorphism produces two alleles, designated l (“long”) and s (“short”), respectively. Cells with the l allele were shown to express more 5-HTT than cells with the s allele, and, concordant with this, reuptake of 5-HT in human lymphoblastoid cells homozygous for the l allele was shown to be approximately twice that of cells either heterozygous or homozygous for the s allele (Lesch et al. 1996).
While it might be expected that individuals carrying the s allele would have greater extracellular 5-HT availability due to reduced reuptake, a variety of evidence suggests that to the contrary, s carriers have impaired 5-HT function. For example, depressed s allele carriers show poor response to treatment with selective serotonin reuptake inhibitors (SSRIs; Smeraldi et al. 1998) and healthy volunteers carrying the s allele show attenuated prolactin response to fenfluramine (Reist et al. 2001) and SSRIs (Smith et al. 2004; Whale et al. 2000), and greater mood change after acute tryptophan depletion (Neumeister et al. 2002). These differences may be due to the long-term developmental effects of reduced 5-HT reuptake on cell number, firing rate, and postsynaptic receptor sensitivity (David et al. 2005; Li et al. 2000; Lira et al. 2003).
In the present study we compared chronic ecstasy users to control subjects on measures of decision-making, episodic memory, and working memory, stratifying the results by polymorphism at the 5-HTTLPR. We have previously reported the effect of 5-HTTLPR polymorphism on mood and personality measures in these groups (Roiser et al. 2005a). Based on findings that experimentally reducing 5-HT synthesis results in impairments in memory and decision-making, but not executive function (Harrison et al. 2004; Rogers et al. 1999b, 2003), that ecstasy users are more reliably impaired on tests of episodic memory than tests of executive function (Fox et al. 2002), and that s allele carrying healthy volunteers are vulnerable to acute tryptophan depletion (Neumeister et al. 2002), we hypothesized that chronic ecstasy users carrying the s allele, and particularly those of the ss genotype, would be vulnerable to impairments in decision-making and episodic memory, but not executive function. Conversely, it was predicted that neuropsychological function in ll ecstasy users would be relatively intact. Finally, it was predicted that 5-HTTLPR genotype would not affect neuropsychological function in the controls.
Materials and methods
Participants
Sixty-six ecstasy users, 30 polydrug users, and 28 healthy volunteers with no history of illicit drug use were recruited by advertisement from the community. Data from some of these subjects were included in previous publications (Roiser et al. 2005b; Roiser and Sahakian 2004). The ecstasy users had to have used ecstasy on at least 30 separate occasions and were required to abstain from use for at least 3 weeks before testing. Neither the polydrug users nor the healthy comparison subjects had used ecstasy. Participants with current or past diagnosed Axis I psychiatric disorders, other than substance abuse/dependence (which was not assessed) were excluded. Current or past Axis I diagnosis was assessed via a structured interview. Any participants who reported any drug use on the day of testing or who showed a positive plasma screen for MDMA/amphetamines were excluded (analysis carried out by enzyme assay followed by high-performance liquid chromatography/mass spectroscopy (Tricho-Tech, Cardiff, UK; http://www.tricho-tech.co.uk). All subjects provided written informed consent and the study was approved by the Cambridge Local Research Ethics Committee (REC number 02/076). Demographic and drug use statistics are presented in Tables 1, 2, and 3.
Table 1.
Demographic characteristics for each genetic subgroup
| Ecstasy users (N=66) | Polydrug controls (N=30) | Drug-naïve controls (N=28) | Combined controls (N=58) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | ll | ls | ss | ll | ls | ss | ll | ls | ss | ll | ls | ss |
| Number of patients/male patients | 20/13 | 31/23 | 15/12 | 9/4 | 16/8 | 5/3 | 12/4 | 10/6 | 6/5 | 21/8 | 26/14 | 11/8 |
| Age (years) | 22.4 (4.6) | 24.5 (6.4) | 26.1 (8.6) | 29.0 (12.5) | 24.4 (6.9) | 24.2 (6.4) | 23.8 (3.0) | 24.3 (4.5) | 24.2 (3.9) | 26.0 (8.7) | 24.3 (6.0) | 24.2 (4.9) |
| NART | 113.1 (6.5) | 112.7 (7.2) | 112.5 (7.3) | 108.3 (7.5) | 112.8 (8.1) | 114.4 (12.7) | 114.4 (5.3) | 113.9 (6.0) | 114.2 (5.5) | 111.8 (6.9) | 113.2 (7.3) | 114.3 (8.9) |
Numbers represent the mean (SD).
Table 2.
Drug use characteristics for each genetic subgroup within the ecstasy users
| ll (N=20) | ls (N=31) | ss (N=15) | Statistics | |
|---|---|---|---|---|
| Alcohol (n) | 20 | 31 | 15 | - |
| Units last month | 82.1 (80.1) | 73.1 (51.6) | 77.0 (53.8) | F2,63<1, p=NS |
| Tobacco (n) | 20 | 31 | 15 | - |
| Cigarettes last month | 132.7 (174.8) | 209.9 (221.6) | 174.0 (157.9) | F2,63<1, p=NS |
| Cannabis (n) | 20 | 31 | 15 | - |
| Joints last month | 30.4 (44.0) | 41.2 (51.2) | 56.8 (90.3) | F2,63<1, p=NS |
| Years of use | 3.9 (2.7) | 5.7 (4.5) | 4.2 (3.1) | F2,63=1.7, p=NS |
| Ecstasy (n) | 20 | 31 | 15 | - |
| Total tablets | 568.2 (850.5) | 470.5 (521.1) | 577.7 (520.0) | F2,63<1, p=NS |
| Ecstasy peak exposure (tablets in 12 h) | 8.1 (4.3) | 8.3 (4.9) | 9.6 (4.0) | F2,63<1, p=NS |
| Ecstasy usual dose (tablets) | 4.3 (2.6) | 4.1 (2.8) | 4.3 (2.1) | F2,63<1, p=NS |
| Ecstasy frequency (times/month) | 5.4 (4.8) | 4.9 (3.3) | 5.3 (3.2) | F2,63<1, p=NS |
| Time since last use (days) | 63.6 (64.5) | 72.4 (69.9) | 64.7 (66.5) | F2,63<1, p=NS |
| Psilocybin (n) | 15 | 24 | 14 | χ22<1, p=NS |
| Times life | 6.8 (7.2) | 11.4 (21.2) | 16.8 (20.3) | F2,50=1.7, p=NS |
| Lysergic acid (n) | 13 | 22 | 11 | χ22<1, p=NS |
| Times life | 10.1 (13.4) | 81.5 (193.9) | 73.6 (94.0) | χ22=2.8, p=NS |
| Amphetamine (n) | 19 | 28 | 12 | χ22<1, p=NS |
| Grams life | 469.4 (1927.0) | 244.7 (416.8) | 297.5 (547.4) | χ22=1.9, p=NS |
| Amyl nitrate (n) | 16 | 24 | 10 | χ22<1, p=NS |
| Times life | 164.3 (417.1) | 78.8 (251.6) | 15.2 (13.5) | χ22=1.8, p=NS |
| Ketamine (n) | 10 | 15 | 6 | χ22<1, p=NS |
| Grams life | 6.3 (8.0) | 5.1 (4.8) | 29.9 (34.2) | χ22<1, p=NS |
| Cocaine (n) | 17 | 27 | 14 | χ22<1, p=NS |
| Grams life | 67.0 (156.8) | 48.2 (89.8) | 44.4 (57.5) | χ22=4.3, p=NS |
| Opiates (n) | 4 | 10 | 5 | χ22<1, p=NS |
| Grams life | 0.13 (0.050) | 3.9 (8.0) | 18.5 (40.0)a | χ22=2.0, p=NS |
Numbers represent the mean (SD).
The mean opiate use for the ss genetic subgroup was inflated by one individual who reported very high use. Without this individual’s data, mean opiate use for the ss subgroup would be 0.6, SD 0.3.
Table 3.
Drug use characteristics for each genetic subgroup within the controls
| ll (N=21) | ls (N=26) | ss (N=11) | Statistics | |
|---|---|---|---|---|
| Alcohol (n) | 21 | 26 | 11 | - |
| Units last month | 26.0 (30.1) | 43.6 (34.2) | 39.5 (11.2) | F2,55=1.9, p=NS |
| Tobacco (n) | 4 | 11 | 2 | χ22=3.8, p=NS |
| Cigarettes last month | 525 (357) | 249 (217) | 173 (180) | χ22=3.0, p=NS |
| Cannabis (n) | 9 | 16 | 5 | χ22=1.8, p=NS |
| Joints last month | 30.4 (44.0) | 41.2 (51.2) | 56.8 (90.3) | χ22=1.1, p=NS |
| Years of use | 3.9 (2.7) | 5.7 (4.5) | 4.2 (3.1) | χ22=2.6, p=NS |
| Ecstasy (n) | 0 | 0 | 0 | - |
| Total tablets | - | - | - | - |
| Psilocybin (n) | 2 | 4 | 1 | χ22<1, p=NS |
| Times life | 5.8 (1.8) | 4.5 (4.0) | 1 (-) | χ22<1, p=NS |
| Lysergic acid (n) | 1 | 4 | 0 | χ22=2.9, p=NS |
| Times life | 2 (-) | 5.5 (6.5) | - | χ22=2.9, p=NS |
| Amphetamine (n) | 3 | 6 | 1 | χ22=1.3, p=NS |
| Grams life | 167.5 (210.6) | 59.6 (124.2) | 1 (-) | χ22=1.1, p=NS |
| Amyl nitrate (n) | 2 | 1 | 2 | χ22<1, p=NS |
| Times life | 85.5 (99.7) | 48.0 (-) | 5.5 (6.4) | χ22=1.8, p=NS |
| Ketamine (n) | 0 | 0 | 0 | - |
| Grams life | - | - | - | - |
| Cocaine (n) | 2 | 4 | 2 | χ22<1, p=NS |
| Grams life | 3.3 | 9.8 (11.4) | 3.0 (2.8) | χ22<1, p=NS |
| Opiates (n) | 0 | 0 | 0 | - |
| Grams life | - | - | - | - |
Numbers represent the mean (SD).
Procedure
Participants were tested at the Wellcome Trust Clinical Research Facility, Addenbrooke’s Hospital, Cambridge, UK. A 10-ml blood sample was taken, which was used to screen for recent use of ecstasy/amphetamines and to extract DNA. Verbal IQ was estimated using the National Adult Reading Test (NART; Nelson 1982), recent depressive symptomatology was assessed with the Beck Depression Inventory (BDI; Beck et al. 1961), and past drug use was assessed using a questionnaire. Participants were administered a battery of neuropsychological tests focusing on memory, executive function, and risky decision-making.
Psychological rating scales and neuropsychological assessment
All participants were assessed on the same battery of neuropsychological tests. Because each test used in this study has been described elsewhere, only a brief description is provided of each. All participants sat approximately 60 cm from a touch sensitive computer screen controlled by an Advantech Pentium personal computer (Model PPC-120T-RT) and carried out the tests in the order described below.
Beck depression inventory
The BDI (Beck et al. 1961) is a 21-item self-report measure of depressive symptomatology. For each item, the participant is asked to pick a statement that most closely corresponds to their experience over the last 2 weeks. Four statements are provided for each item, numbered 0-3 in increasing severity. The maximum possible score is 63.
Tile manipulation test
The tile manipulation test (Haaxma et al. 1993) is a computerized version of the Block Design subtest of the Wechsler Adult Intelligence Scale and depends upon working memory, planning, and visuospatial function (Haaxma et al. 1993). Participants are required to recreate a two-dimensional pattern made up of four smaller blocks, picking from four correct blocks and four incorrect distractors. Participants carry out the test by touching the screen and moving each block to its correct position. Participants are encouraged to plan their answer before making their first response. In the first stage (“copy”), participants simply have to identify and move a perfect match of each of the smaller blocks. In the second stage (“mirror”), the smaller target blocks are the mirror image of those in the two-dimensional pattern. In the third stage (“mental rotation”), the smaller target blocks are the 180° rotation of those in the two-dimensional pattern. If participants make five consecutive errors, a grid is added to the two-dimensional pattern to aid visualization of the smaller blocks. Four further errors are permitted before a participant is considered to have failed that problem. Measures arising from this test are the number of problems completed perfectly and thinking time, a measure of latency unconfounded by impulsive responding.
Mental rotation test
This test is based on the original investigation by Shepard and Metzler (1971). Participants are required to decide if a letter, either an “R” or an “F”, is presented as usually seen or in the mirror image. Letters are presented at various degrees of rotation (from 0 to 360° in 30° intervals), such that participants typically mentally rotate the image before pressing the appropriate button on a response box. Stimuli are presented at a fixed interval of 2 s with a failure to respond being considered an error. Measures arising from this task are number of errors and latency at each degree of rotation.
Risky choice task
This task was described previously (Rogers et al. 2003, 2004a,b). On each trial participants are asked to choose between playing one out of two simultaneously presented gambles. Each gamble is represented visually by a histogram in which the height of each indicates the probability of winning a number of experimenter-defined points. The possible gains are indicated in green text above the histogram, while the possible losses are indicated in red text underneath. One of the gambles is a “control” gamble, consisting of a 0.5 probability of winning 10 points and a 0.5 probability of losing 10 points. The alternative “experimental” gamble varies in the probability of winning, which can be either high or low (0.75 vs 0.25); possible gains, which can be either large or small (80 vs 20 points); and possible losses, which can be either large or small (80 vs 20 points). Orthogonal combination of these three factors produces eight trial types.
The control and experimental gambles appear randomly on the left or right of the display. The participant presses the “1” or “2” key on the computer keyboard to indicate choice of the gamble presented on the left or right. Measures arising from this task are the proportion of choices of the experimental over the control gamble as a function of its probability of winning, size of the possible gains, size of the possible losses (“proportionate choice”), and the mean deliberation time for these choices.
As previously described (Rogers et al. 2003), we included two extra trial types that represented choices between gambles known to be subject to the nonnormative biases of risk-aversion and risk-seeking behavior (the “reflection effect”; see Kahneman and Tversky 1979). The first such type is a “gains only” trial in which volunteers are presented simultaneously with a guaranteed win of 40 points vs a 0.5 chance of winning 80 points and a 0.5 chance of losing 0 points. Neither option involves any losses. By contrast, on the “losses only” trials, the volunteers are presented simultaneously with a guaranteed loss of 40 points vs a 0.5 chance of losing 80 points and a 0.5 chance of losing 0 points. Neither option offers any gains. For both the gains only and losses only trials, measures arising are the proportion of trials on which the volunteers chose the risky option and the mean deliberation time associated with these choices.
CANTAB Pattern recognition memory (PRM)
In this memory test (Sahakian et al. 1989; see http://www.camcog.com), participants are shown a series of 12 abstract patterns and are instructed to remember them. After a 5-s delay, each pattern is then shown again to the participant in reverse order paired with a novel pattern. Participants are required to make a forced choice discrimination by touching the pattern they have seen previously. Feedback is provided to the participant by way of green ticks and red crosses. This procedure is then repeated with a further 12 patterns. After a delay of approximately 20 min, the recognition phase of the task is repeated with the same forced choice trials. Measures arising from this test are percent correct and latency.
CANTAB Delayed match to sample (DMTS)
In this test (Sahakian et al. 1988; see http://www.camcog.com), participants are presented with a complex abstract pattern, which they are required to remember. After a variable delay of 0, 4, or 12 s, four patterns are presented, one of which is identical to the pattern previously displayed. Participants are instructed to touch the pattern that they have seen before. As a control for motor speed, simultaneous match to sample trials are also included where the complex abstract pattern does not disappear. Measures arising from this task are percent correct and latency.
Genotyping
Genotyping was performed using the polymerase chain reaction protocol of Furlong et al. (1998), using the primers stpr5: 5′ ggc gtt gcc gct ctg aat gc 3′ and stpr3: 5′ gag gga ctg agc tgg aca acc ac 3′. Successful amplification was achieved using an initial denaturing step at 95°C for 4.5 min followed by 35 cycles of 95°C for 30 s, 61°C for 30 s, 72°C for 30 s, and a final extension of 72°C for 10 min. The 25-μl reaction mixture consisted of 1 μl DNA (approximately 50-200 ng), 10 mM Tris-HCl, 50 mM KCl, 0.1% Triton® X-100, 1.5 mM MgCl2, 5% DMSO, 200 μM deoxyribonucleotide triphosphates [including 2′-deoxyguanosine 5′-triphosphate (dGTP)/7-deaza dGTP 1:1], 100 ng of each primer, and 0.5 U Taq polymerase at pH 9.0. Products were analyzed on 2% agarose gels stained with ethidium bromide.
Statistical analysis
Data were analyzed using SPSS 13 (SPSS, Chicago, IL, USA). The polydrug controls and drug-naïve controls did not differ on any neuropsychological measure, or in terms of the frequencies of 5-HTTLPR genotypes (χ22=1.8, p>0.2) or alleles (χ21<1). Therefore the data from the two control groups were pooled to increase the power of the genetic analyses. Where appropriate, data were transformed before analysis as appropriate to reduce skew and stabilize variances. Proportionate choice data were arcsine-transformed, as is appropriate whenever the variance of a measure is proportional to its mean (Howell 2002); however, all of the data reported in the text and figures describe untransformed values.
Of the drug use variables, only alcohol, tobacco (in the ecstasy users), cannabis in the last month (in the ecstasy users), lifetime psilocybin (in the ecstasy users), and ecstasy use variables (other than days since last use) were normally distributed after transformation. For these variables, the statistics in Tables 2 and 3 include only those subjects who reported that they used the drug in question. For other drug use variables, the nonparametric Kruskal-Wallis test was used. For those variables, the statistics in Tables 2 and 3 include all subjects, regardless of whether they reported using the drug in question or not.
Where test assumptions were met, parametric analyses were used [i.e., t tests and analysis of variance (ANOVA)] with an appropriate correction where the homogeneity of variance assumption was violated. Post hoc comparisons among genotypes were conducted using Fisher’s Least Significant Difference test with a correction for inhomogeneity of variance where necessary (Howell 2002). Analysis of interaction effects was conducted by constructing appropriate linear contrasts. For tests where more than one condition or set of conditions was present, repeated-measures ANOVA was employed. In cases where there was a departure from the assumption of homogeneity of covariance in the repeated measures ANOVA, an epsilon (ε) factor was calculated and used to adjust degrees of freedom accordingly. The Greenhouse-Geisser procedure for adjusting degrees of freedom was used, unless the value calculated was near or above 0.75 in which case the Huynh-Felt procedure was used (Howell 2002). A p value of <0.05 was considered significant, while 0.1>p>0.05 was considered as a trend toward significance.
Proportionate choice and mean deliberation times on the risky choice task were analyzed using repeated measures ANOVAs with the between-subject factor of group, within-subject factors of probability of winning (high vs low), size of possible gains (large vs small), and size of possible losses (large vs small). The proportionate choices and mean deliberation times for the gains only and losses only trials were analyzed with group as the between-subject factor and trial type as the within-subject factor.
Results
Demographic measures
Age and NART IQ for each genetic subgroup are reported in Table 1. Age did not differ between the groups (F1,118<1) or the genotype groups (F2,118<1) and no group × genotype interaction was present (F2,118=1.6, p=0.2). Similarly, NART IQ did not differ between the groups (F1,118<1) or genotype groups (F2,118<1) and no group × genotype interaction was present (F2,118<1). No subject showed a positive plasma screen. Estimated drug use for each of the genotypes within the ecstasy users and controls are presented in Tables 2 and 3, respectively. The genetic subgroups did not differ significantly on any drug use measure in either group. However, the ecstasy users reported greater use of every substance in the questionnaire relative to the controls (p<0.001 for all).
Neuropsychological assessment
All behavioral data are presented in Table 4.
Table 4.
Behavioral measures recorded in the study, stratified by genotype
| Test | Measure | Ecstasy |
Control |
||||
|---|---|---|---|---|---|---|---|
| ll (N=20) | ls (N=31) | ss (N=15) | ll (N=21) | ls (N=26) | Ss (N=11) | ||
| Tile manipulation | |||||||
| Copy | No. completed perfectly | 4.2 (1.1) | 4.3 (0.93) | 4.2 (1.0) | 4.0 (1.2) | 4.1 (1.1) | 4.5 (0.52) |
| Reaction time | 1,469.4 (565.2) | 1,811.3 (682.6) | 1,566.7 (769.4) | 2,220.5 (1,527.4) | 1,741.0 (1,025.3) | 1,745.1 (666.5) | |
| Mirror | No. completed perfectly | 2.4 (1.4) | 2.5 (1.2) | 2.9 (1.3) | 1.9 (1.5) | 2.7 (1.1) | 2.6 (1.1) |
| Reaction time | 2,655.2 (1,770.7) | 2,581.9 (1,083.7) | 2,728.6 (1,119.9) | 3,946.0 (2,803.6) | 2,874.0 (1,943.2) | 2,974.7 (1,301.7) | |
| Mental rotation | No. completed perfectly | 2.2 (1.2) | 2.5 (1.3) | 2.6 (1.1) | 1.9 (1.3) | 2.5 (1.3) | 2.6 (0.92) |
| Reaction time | 2,357.2 (1,150.8) | 2,392.7 (961.4) | 2,590.8 (1,420.1) | 3,626.6 (2,221.2) | 3,200.8 (2,161.8) | 3,022.5 (1,879.9) | |
| Mental rotation | |||||||
| Standard | Latency | 692.7 (78.4) | 687.7 (97.3) | 690.0 (111.4) | 745.4 (128.9) | 717.2 (104.6) | 683.2 (120.3) |
| Errors | 6.4 (5.9) | 5.1 (5.3) | 6.1 (4.5) | 6.5 (7.8) | 5.3 (4.5) | 5.3 (4.8) | |
| Mirror | Latency | 792.4 (89) | 793.6 (108.9) | 780.0 (103.0) | 866.5 (144.5) | 828.3 (120.7) | 776 (134.8.0) |
| Errors | 5.8 (5.8) | 5 (4.9) | 4.7 (4.4) | 5.6 (7.0) | 5.2 (4.3) | 4.4 (3.3) | |
| Risky-choice | |||||||
| Overall | Proportion choices | 0.53 (0.02) | 0.53 (0.02) | 0.56 (0.03) | 0.50 (0.03) | 0.49 (0.02) | 0.49 (0.02) |
| Latency | 2,449.2 (172.1) | 2,429.7 (134.7) | 2,459.4 (238.5) | 2,343.7 (181.0) | 2,535.2 (169.6) | 1,932.4 (177.1) | |
| High probability | Proportion choices | 0.75 (0.14) | 0.79 (0.18) | 0.74 (0.17) | 0.71 (0.19) | 0.77 (0.13) | 0.83 (0.16) |
| Latency | 2,275.0 (767.2) | 2,324.5 (851.9) | 2,386.2 (933.9) | 2,303.0 (860.0) | 2,400.3 (843.9) | 1,780.2 (557.7) | |
| Low probability | Proportion choices | 0.30 (0.17) | 0.24 (0.21) | 0.37 (0.22) | 0.30 (0.17) | 0.22 (0.17) | 0.16 (0.14) |
| Latency | 2,623.4 (858.1) | 2,657.4 (868.0) | 2,532.6 (1,008.6) | 2,384.3 (889.2) | 2,670.1 (1,027.9) | 2,084.7 (658.6) | |
| High win | Proportion choices | 0.64 (0.13) | 0.61 (0.13) | 0.68 (0.16) | 0.59 (0.15) | 0.59 (0.15) | 0.56 (0.09) |
| Latency | 2,358.7 (729.2) | 2,445.1 (846.5) | 2,410.6 (984.3) | 2,361.4 (807.3) | 2,528.2 (949.4) | 2,078.8 (827.0) | |
| Low win | Proportion choices | 0.41 (0.12) | 0.43 (0.13) | 0.44 (0.14) | 0.41 (0.12) | 0.40 (0.12) | 0.43 (0.10) |
| Latency | 2,539.7 (867.8) | 2,536.7 (809.8) | 2,508.3 v (923.2) | 2,326.0 (907.3) | 2,542.2 (974.3) | 1,786.1 (486.0) | |
| High loss | Proportion choices | 0.40 (0.16) | 0.43 (0.17) | 0.46 (0.18) | 0.39 (0.21) | 0.37 (0.18) | 0.41 (0.13) |
| Latency | 2,506.6 (713.6) | 2,540.1 (816.4) | 2,595.4 (913.6) | 2,362.5 (858.1) | 2,633.4 (979.6) | 1,872.8 (474.9) | |
| Low loss | Proportion choices | 0.65 (0.13) | 0.61 (0.12) | 0.66 (0.14) | 0.61 (0.12) | 0.61 (0.10) | 0.58 (0.07) |
| Latency | 2,391.8 (890.2) | 2,441.8 (860.8) | 2,323.5 (1,004.6) | 2,324.9 (822.6) | 2,437.1 (816.1) | 1,992.1 (754.4) | |
| Definite win | Proportion choices | 0.82 (0.23) | 0.77 (0.29) | 0.71 (0.30) | 0.74 (0.24) | 0.72 (0.33) | 0.97 (0.06) |
| Latency | 1,959.0 (902.4) | 2,324.6 (1,261.6) | 2,205.2 (1,156.0) | 2,271.2 (1,036.5) | 2,426.0 (1,549.3) | 1,416.0 (481.2) | |
| Definite loss | Proportion choices | 0.34 (0.28) | 0.30 (0.36) | 0.31 (0.29) | 0.48 (0.38) | 0.36 (0.29) | 0.19 (0.22) |
| Latency | 3,852.7 (2,679.2) | 3,807.3 (1,775.2) | 3,783 (1,604.5) | 3,089.3 (1,324.8) | 4,115.6 (2,190.9) | 3,208.2 (1,466.3) | |
| Pattern recognition memory | |||||||
| Immediate | Percent correct | 89.2 (9.6) | 89.9 (8.2) | 90.0 (11.6) | 88.5 (9.5) | 92.8 (9.2) | 95.1 (6.9) |
| Latency | 1,696.5 (361.5) | 1,776.4 (399.4) | 1,608.3 (276.8) | 1,846.2 (428.4) | 1,617.8 (290.4) | 1,651.0 (336.2) | |
| Delayed | Percent correct | 86.5 (9.4) | 83.5 (12.1) | 87.8 (11.0) | 84.1 (9.9) | 89.9 (12.6) | 92.4 (6.4) |
| Latency | 1,703.4 (329.2) | 1,719.7 (444.6) | 1,602.8 (335.4) | 1,926.6 (561.0) | 1,641.4 (272.9) | 1,578.1 (311.0) | |
| Delayed match to sample | |||||||
| Immediate | Percent correct | 96.5 (5.9) | 97.1 (5.3) | 98.0 (4.1) | 94.3 (8.1) | 94.2 (7.6) | 96.4 (5.0) |
| Latency | 2,866.2 (823.2) | 3,241.4 (937.4) | 3,290.7 (587.1) | 3,227.8 (694.5) | 3,172.1 (684.2) | 3,117.9 (492.1) | |
| Delayed | Percent correct 0 s | 86.0 (12.3) | 85.5 (13.6) | 92.0 (8.6) | 92.9 (9.0) | 86.9 (15.4) | 87.3 (9.0) |
| Percent correct 4 s | 90.0 (9.7) | 84.2 (18.8) | 86.7 (14.5) | 84.3 (14.3) | 89.6 (11.1) | 88.2 (9.8) | |
| Percent correct 12 s | 77.0 (17.5) | 81.6 (15.7) | 82.7 (16.7) | 76.7 (15.3) | 76.9 (18.3) | 79.1 (10.4) | |
| Latency | 2,383.4 (474.1) | 2,725.5 (623.7) | 2,524.0 (468.5) | 2,855.8 (770.6) | 2,642.5 (823.0) | 2,650.3 (487.9) | |
Numbers represent the mean (SD).
Tile manipulation
Copy stage
The groups and genotypes did not differ in terms of the number of problems completed perfectly with no group × genotype interaction (F<1 for all). Latency was also unaffected by group (F1,117=1.6, p=0.2) and genotype with no group × genotype interaction (F<1 for both).
Mirror stage
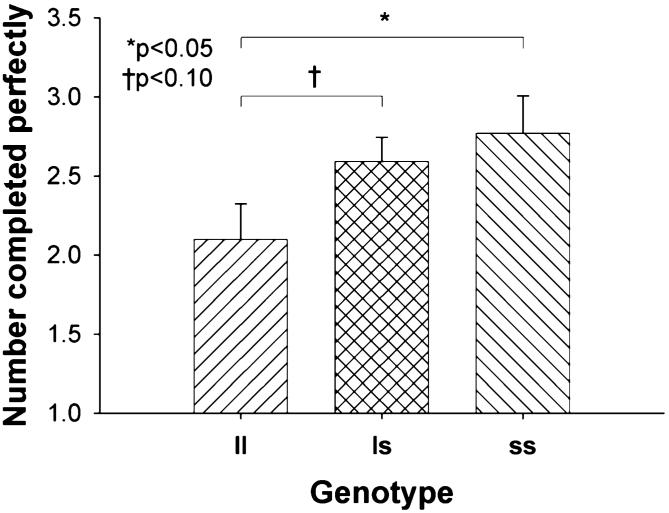
The number of problems completed perfectly was unaffected by group (F1,117<1), but differed between the genotypes (F2,117=3.5, p=0.034) with no group × genotype interaction (F2,117<1) (Fig. 1). The assumption of homogeneity of variance assumption was violated for the genotype comparison, but after correction for inhomogeneity of variance using the Brown-Forsythe procedure, the main effect of genotype remained significant (F2,93.6=3.5, p=0.033). Post hoc analysis revealed that the ll genotype responded significantly less accurately than the ss genotype (p=0.027), and showed a trend toward responding less accurately than the ls genotype (p=0.069). The ecstasy users showed a trend toward responding more quickly than the controls (F1,117=3.3, p=0.073), but latency was unaffected by genotype (F2,117<1) with no group × genotype interaction (F2,117=1.8, p=0.17).
Fig. 1.
Number completed perfectly at the “mirror” stage of the tile manipulation test in each genotype group. Bars represent the mean, error bars 1 SEM
Mental rotation stage
The number of problems completed perfectly was unaffected by group (F1,117<1), the main effect of genotype showed a trend toward significance (F2,117=2.8, p=0.062; ss accuracy>ls accuracy>ll accuracy), and there was no group × genotype interaction (F2,117<1). The ecstasy users responded more quickly than the controls (F1,117=5.8, p=0.017), while latency was unaffected by genotype (F2,117<1) with no group × genotype interaction (F2,117<1).
Mental rotation
Four ecstasy users and six controls failed to make at least one correct response at each angle, reducing the total number of participants included in the latency analyses to 114.
Participants were slower when responding to mirror image stimuli (F1,108=186.9, p<0.001) and at higher angles (F3.5,374.6=388.2, p<0.001, ε=0.58), and particularly slow when responding to mirror image stimuli at higher angles (reflection × angle interaction F3.7,398.3=20.7, p<0.001, ε=0.62). However, neither the groups (F1,108=1.9, p=0.2) nor the genotypes (F2,108=1.2, p=0.3) differed in terms of latency, and the group × genotype interaction was nonsignificant (F2,108<1). No other interactions approached significance.
Participants showed a trend toward making more errors when responding to mirror image stimuli (F1,118=3.2, p=0.075), made more errors at higher angles (F3.3,391.9=64.2, p<0.001, ε=0.55), and made a disproportionate number of errors when responding to mirror image stimuli at higher angles (reflection × angle interaction: F3.5,414.1=11.3, p<0.001, ε=0.59). However, the groups and genotypes did not differ on errors and the group × genotype interaction was nonsignificant (F<1 for all). No other interactions approached significance.
Pattern recognition memory
Participants were less accurate after the 20-min delay (F1,118=14.2, p<0.001) and the groups did not differ on accuracy (F1,118=2.5, p=0.12). The main effect of genotype showed a trend toward significance (F2,118=2.7, p=0.070; ss accuracy>ls accuracy>ll accuracy), but the group × genotype interaction was nonsignificant (F2,118=2.0, p=0.14). No other interactions approached significance.
Latency was unaffected by delay (F1,118<1), group (F1,118<1), or genotype (F2,118=2.0, p=0.13), and the group × genotype interaction was nonsignificant (F2,118=1.7, p=0.18). No other interactions approached significance.
Delayed match to sample
Participants became less accurate with increasing delay (F3,354=49.2, p<0.001), but the groups and genotypes did not differ on accuracy and the group × genotype interaction was nonsignificant (F<1 for all). No other interactions approached significance.
Participants were slower at the delayed stages (F1,118=79.2, p<0.001), but the groups and genotypes did not differ on latency (F<1 for both) and the group × genotype interaction was nonsignificant (F2,118=2.0, p=0.14). No other interactions approached significance.
Risky choice task
Data from one participant (an ls ecstasy user) were excluded due to a failure to understand test instructions. Therefore, the following analyses are based on data from 123 participants.
Proportionate choice
Overall, the ecstasy users chose the experimental gamble over the control gamble more often than the controls (F1,117=4.0, p=0.047), but the genotypes did not differ in terms of overall choice of the experimental gamble and the two-way interaction between group and genotype was nonsignificant (F<1 for both).
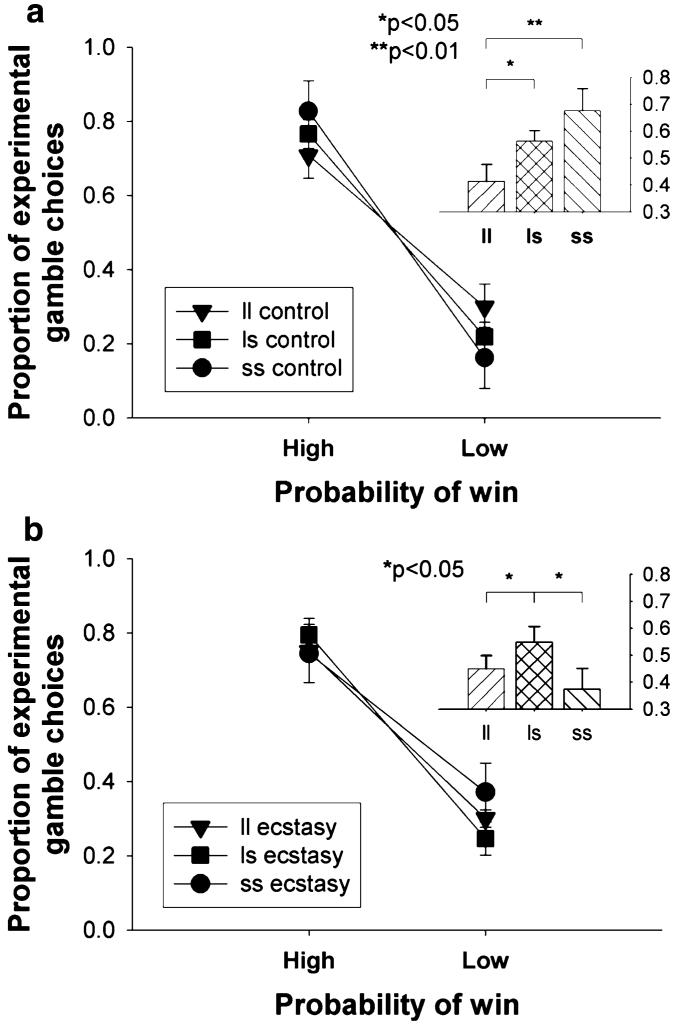
Analysis of the effect of probability of winning on proportionate choice of the experimental gamble revealed that participants chose the experimental gamble more often when the probability of winning was high compared to when it was low (F1,117=416.6, p<0.001). However, this pattern of response differed according to both group and genotype (probability × group × genotype interaction: F2,117=4.1, p=0.027). Analysis of the simple interaction effects revealed that the genetic subgroups differed in terms of their discrimination between high and low probabilities of winning both within the controls (control probability × genotype interaction: F2,55=4.4, p=0.017) and within the ecstasy users (ecstasy user probability × genotype interaction: F2,62=3.7, p=0.030). Further analysis of this interaction revealed that both the ss controls (p=0.006) and the ls controls (p=0.047) discriminated between high and low probabilities of winning to a greater extent than the ll controls (see Fig. 2a), while the ls ecstasy users discriminated between high and low probabilities of winning to a greater extent than both the ll (p=0.043) and the ss (p=0.019) ecstasy users (see Fig. 2b). We also analyzed this interaction by comparing the groups within each of the genetic subgroups. The ecstasy users and controls did not differ in terms of the discrimination between high and low probabilities of winning within the ll and ls genetic subgroups (t<1 for both), but the ss controls discriminated between high and low probabilities of winning to a greater extent than the ss ecstasy users (t24=2.8, p=0.011).
Fig. 2.
Effect of 5-HTTLPR polymorphism on proportion of choices of the “experimental” gamble over the “control” gamble at high vs low probability of winning for a ecstasy users and b controls (insets: the mean difference between the proportions of choices of the “experimental” gamble over the “control” gamble when probability of winning was high compared to when the probability of winning was low). Points and bars represent the mean, error bars 1 SED
Analysis of the effect of possible gains on proportionate choice of the experimental gamble revealed that participants chose the experimental gamble more often when possible gains were high than when they were low (F1,117=202.7, p<0.001). However, this pattern of response differed according to group (possible gains × group interaction: F1,117=5.5, p=0.020). Simple effects analysis revealed that the ecstasy users chose the experimental gamble significantly more often than the controls when possible gains were high (F1,121=7.0, p=0.009), but not when possible gains were low (F1,121<1). The genotypes did not differ in terms of the effect of possible gains, and the three-way interaction between possible wins, group, and genotype was nonsignificant (F<1 for both).
Analysis of the effect of possible losses on proportionate choice of the experimental gamble revealed that participants chose the experimental gamble more often when possible losses were low than when they were high (F1,117=142.2, p<0.001). However, the interactions between possible losses, group, and genotype were all nonsignificant (F<1 for all).
Latency
Overall latency did not differ between the groups (F1,117=1.2, p=0.3) or the genotypes (F2,117=1.5, p=0.2), and the group × genotype interaction was nonsignificant (F2,117<1). Analysis of the effect of probability of winning on latency revealed that participants were quicker when the probability of winning was high (F1,117=26.5, p<0.001), but the interactions between probability of winning, group, and genotype were all nonsignificant (p>0.1 for all).
Analysis of the effect of possible gains on latency revealed a significant possible gains × group interaction (F1,117=6.3, p=0.014). Simple effects analysis revealed that the ecstasy users responded more quickly when possible gains were high than when possible gains were low (F1,64=7.4, p=0.008), while in the controls possible gains did not affect latency (F1,57<1). The main effect of possible gains on latency, the two-way interaction between possible gains and genotype, and the three-way interaction between possible gains, group, and genotype were all nonsignificant (p>0.1 for all).
Analysis of the effect of possible losses on latency revealed a significant possible loss × group interaction (F1,117=4.6, p=0.035). Simple effects analysis revealed that the ecstasy users were quicker when the possible losses were low (F1,64=11.9, p=0.001) while in the controls possible losses did not affect latency (F1,57=2.3, p=0.14). The main effect of possible losses on latency was also significant (F1,117=10.7, p=0.001), but the two-way interaction between possible losses and genotype and the three-way interaction between possible losses, group, and genotype were both nonsignificant (p>0.1 for both).
Gains only/losses only trials
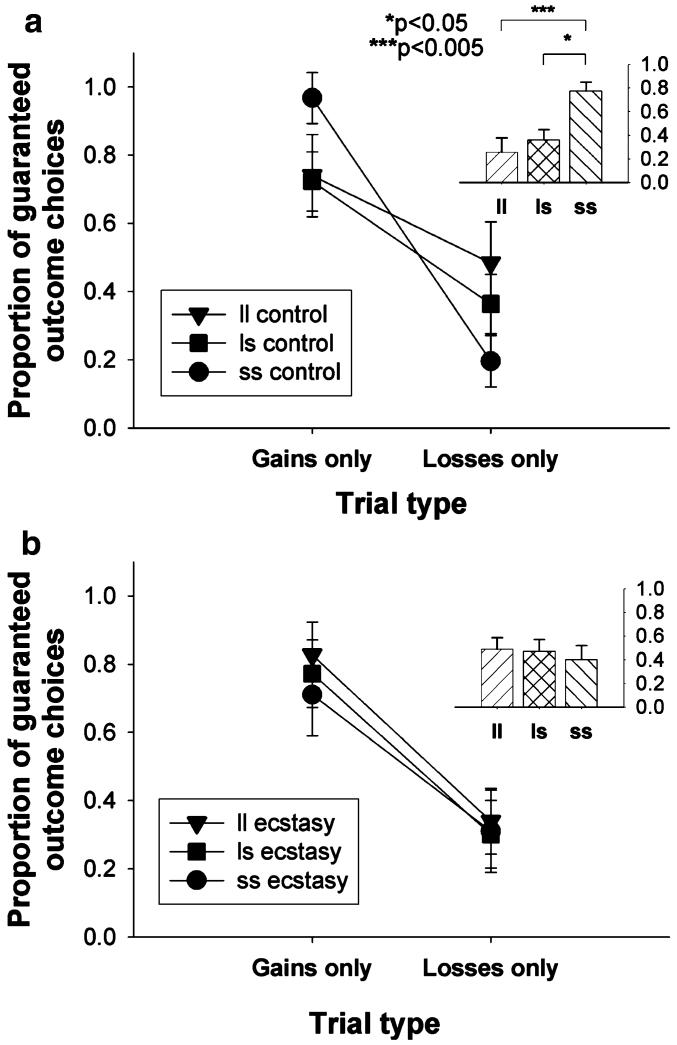
Analysis of proportionate choice data on the gains only and losses only trials revealed that as expected, participants showed the usual reflection effect, taking significantly fewer risks when offered a choice between a sure gain (of 40 points) and a gamble with a 0.5 chance of winning a larger gain (80 points) or winning nothing (gains only trials) compared to when offered a choice between a certain loss (of 40 points) and a gamble with a 0.5 chance of losing nothing or losing a larger amount (80 points) (losses only trials) (F1,117=95.6, p<0.001; see Fig. 3).
Fig. 3.
Effect of 5-HTTLPR polymorphism on proportion of choices of the guaranteed outcome for the “gains only” and “losses only” trials for a ecstasy users and b controls (insets: the mean difference between the proportions of choices of the “definite win” option and the “definite loss” option—the “reflection effect”). Points and bars represent the mean, error bars 1 SED
A significant trial type × group × genotype interaction emerged (F2,117=3.7, p=0.028). Analysis of the simple interaction effects revealed that within the ecstasy users, the genetic subgroups did not differ with respect to the reflection effect (trial type × genotype interaction: F2,64<1; see Fig. 3b). In the controls, however, a significant trial type × genotype interaction was present (F2,57=5.0, p=0.010; see Fig. 3a); the ss controls showed a significantly greater reflection effect than both the ll (p=0.003) and the ls (p=0.014) controls. We also analyzed this interaction by comparing the groups within each of the genetic subgroups. The ecstasy users and controls did not differ within the ll and ls genotype groups (t36.4=1.3, p=0.19 and t54=1.3, p=0.2, respectively), but the ss controls exhibited a greater reflection effect than the ss ecstasy users (t24=2.5, p=0.022). All other main effects and interactions were nonsignificant (p>0.1 for all).
Analysis of the effect of trial type on latency revealed that all participants were significantly quicker to make their decisions on gains only trials compared to the losses only trials (F1,117=170.1, p<0.001). The three-way interaction between trial type, group, and genotype narrowly missed significance (F2,117=3.0, p=0.056), and was not analyzed further. All other main effects and interactions were nonsignificant (p>0.1 for all).
Controlling for polydrug use
Because all ecstasy users in this study also used other drugs, we conducted further analyses to control for the possible effects of nonecstasy drug use. In particular, we sought to clarify whether the interactions between ecstasy use and 5-HTTLPR polymorphism on the risky choice task, i.e., the probability × group × genotype interaction for proportionate choice and the trial-type × group × genotype interaction on the gains only/losses only trials, might be due to the effect of nonecstasy illicit substance use.
First, we repeated the ANOVAs on proportionate choice data and gains only/losses only data from the risky choice task within the ecstasy users only, including nonecstasy illicit substance use (classified as either regular or rarely/never) as an additional between-subjects factor in separate analyses for psilocybin, LSD, amyl nitrate, ketamine, cocaine, and heroin. Neither the probability × drug use group × genotype interaction nor the trial type × drug use group × genotype interaction approached significance in any of these analyses. When amphetamine use was included as the between-subjects factor, the amphetamine use × possible gains interaction was significant (F1,59=7.4, p=0.009). However, simple effects analyses revealed that ecstasy users who also regularly used amphetamine exhibited reduced discrimination relative to ecstasy users who did not regularly use amphetamine and, hence, amphetamine use cannot account for the increased discrimination between possible gains in ecstasy users relative to controls.
Second, we repeated the same ANOVAs including both ecstasy users and controls, but excluding regular users of psilocybin, LSD, amphetamine, amyl nitrate, ketamine, cocaine, and heroin in separate analyses. The probability × group × genotype interaction and trial type × group × genotype interaction either remained significant or were at trend (0.05<p<0.1) for all of these analyses, other than when regular amphetamine users were excluded for the gains only/losses only trials (trial type × group × genotype interaction: p=0.11).
The above methods were not appropriate to control for cannabis use because all ecstasy users also used cannabis on a regular basis. Instead, we conducted the analyses of proportionate choice data and gains only/losses only data in the controls alone, including group (polydrug control or drug-naïve control) and genotype as between-subjects factors. The probability × control group × genotype interaction was nonsignificant.
Finally, because we previously reported that s allele carrying ecstasy users have increased BDI scores relative to s-allele-carrying controls, we included BDI as a covariate in the analysis; both the probability × group × genotype and trial type × group × genotype interactions remained significant or were at trend (0.05<p<0.1), and BDI score did not account for a significant proportion of the variance in either analysis (p>0.1 for both).
Discussion
In this study, we investigated the effect of 5-HTTLPR polymorphism on cognitive function in ecstasy users and controls, assessing memory, executive function, and decision-making. To our surprise, we found some preliminary evidence that polymorphism at the 5-HTTLPR mediated cognitive function in both ecstasy users and controls. Specifically, on the risky choice task, the ss controls and ls controls attended to differences in probabilities of winning to a greater extent than the ll controls. The ss controls also showed a greater reflection effect on gains only/losses only trials relative to the ls and ll controls. However, in the ecstasy users, these effects were attenuated, such that the ss ecstasy users did not differ either in terms of their attention toward differences in probabilities of winning or in terms of the reflection effect compared to the ll ecstasy users. Independent of ecstasy use, the ss volunteers were also more accurate than the ll volunteers at the mirror stage of the tile manipulation test, and showed a trend toward better accuracy on the PRM.
Though these results should be treated with caution until independently replicated, the differences in accuracy between the ss and ll volunteers at the mirror stage of the tile manipulation test and the delayed stage of the PRM were surprisingly large; both were approximately 0.55 in the range of a medium effect size (Cohen 1988) and were comparable to the size of the effect of the catechol-O-methyltransferase val158met polymorphism on working memory (Goldberg et al. 2003). Polymorphism at the 5-HTTLPR may play an important role in mediating individual differences in planning and visual episodic memory ability, and we suggest that these findings warrant further investigation.
The finding of altered decision-making between 5-HTTLPR genotype groups is, to our knowledge, novel, and the risky choice task was the only measure on which the ss ecstasy users differed from the ss controls. Because the latter group showed greater attention to differences between probabilities of winning, it would appear that ss controls placed greater weight on this kind of information when making decisions compared to the ll controls. Furthermore, the ss controls also exhibited a significantly greater reflection effect than the ll controls on the gains only/losses only trials, suggesting that this nonnormative behavior is more marked in this genotype.
The reason behind this genotypic difference in risk evaluation strategy is unclear. However, it may be that the affective responses elicited by cues signaling either high probability or low probability of receiving reward or punishment were greater in ss than ll controls. This is consistent with reports that the amygdala response to fearful faces is greater in s carriers than ll homozygotes (Hariri et al. 2002, 2005), and that s carriers have greater coupling between amygdala and ventromedial prefrontal cortex (Heinz et al. 2005). The neural mechanisms underlying this difference in decision-making behavior await clarification in future studies combining molecular genetics with function brain imaging.
By contrast, the ss ecstasy users exhibited a dramatically reduced discrimination between high and low probabilities of winning and a reduced reflection effect on gains only/losses only trials compared to the ss controls. At this stage, the significance of this difference remains unclear. However, we hypothesize that the changes observed on the risky choice task in the ss ecstasy users may reflect attenuated responses elicited by the cues signaling high and low probabilities of winning, and that this attenuation is a result of the 5-HT depletion caused by chronic ecstasy use. Consistent with this hypothesis, it was previously reported that acute tryptophan depletion reduces and tryptophan loading increases fear perception in a facial expression recognition task (Attenburrow et al. 2003; Harmer et al. 2003), and that acute tryptophan depletion results in impaired quality of decision-making (Rogers et al. 1999b).
Though consistent with our hypothesis, it is not clear why only the ss ecstasy users exhibited altered decision-making behavior relative to their genotype-matched controls. Previous research has suggested that ss individuals are particularly vulnerable to 5-HT depletion via acute tryptophan depletion (Neumeister et al. 2002), and we have previously reported that the s-carrying ecstasy users in this group reported higher depressive symptomatology and exhibited abnormal emotional processing (Roiser et al. 2005a). Recently, it was reported that s-allele-carrying healthy volunteers have reduced 5-HT1A binding (David et al. 2005), and that 5-HTT knockout mice have both reduced tissue 5-HT concentration (Kim et al. 2005) and reduced 5-HT1A binding (Li et al. 2000). However, further research is needed to understand the neurodevelopmental impact of the s allele of the 5-HTTLPR, and how the resulting changes may confer vulnerability to the effects of 5-HT depletion.
Some caveats to these findings must be acknowledged. First, the number of ss individuals included in this study was low. Furthermore, because the s allele was associated with reduced serotonergic synaptic transmission, the finding that ss volunteers outperformed ll volunteers on a number of indices is somewhat surprising. These unexpected differences in cognitive performance between 5-HTTLPR genotypes should therefore be treated with caution until replicated independently. A recent publication from Reneman et al. (2006) did not find any effect of 5-HTTLPR polymorphism on verbal memory, though their sample size was considerably smaller than in the present study, with only eight ecstasy users in the ss genotype group. Future studies might consider preselecting volunteers by genotype to prevent unequal recruitment of volunteers between genotype groups and hence improve statistical power.
Second, the increased discrimination between possible gains in the ecstasy users is unexpected given a recent finding of Morgan et al. (2005) who reported decreased discrimination between both possible gains and possible losses in an independent sample of ecstasy users. However, in that study a slightly different version of the risky choice task was used (with 0.66 and 0.33 chances of winning and losing as opposed to 0.75 and 0.25 in the present study). Furthermore, the mean abstention period for the ecstasy users tested by Morgan et al. (2005) was shorter than ours with one third of their sample having taken ecstasy in the week before testing. Both of these factors may have altered behavior on the risky choice task.
Third, though the genetic subgroups within the ecstasy users did not differ in terms of illicit drug use, the ecstasy users and controls were not well-matched in terms of illicit drug use. Though we attempted to control for this confound using additional analyses, it remains possible that the results of the risky choice task may, in part, at least be due to the effects of other illegal drugs. Furthermore, because many of our ecstasy users were regular cannabis users, it is possible that cannabis withdrawal might have contributed to the effects we observed, or even masked differences between the ecstasy users and controls. It should also be noted that we did not test plasma samples for drugs other than amphetamines and ecstasy. Polydrug use is a notoriously difficult problem to overcome in cross-sectional studies of this nature, and the strategies used to reduce this confound, for example, by excluding ecstasy users with regular illicit drug use other than cannabis and ecstasy, may recruit a sample that is not typical of the general ecstasy user population (Roiser et al. 2005b).
Furthermore, we did not assess whether our volunteers met the criteria for substance abuse/dependence. Future studies of ecstasy users should consider assessing the presence of substance abuse/dependence because it was reported that cognitive impairment after ecstasy use is particularly marked in individuals who show clinically significant patterns of use (Hanson and Luciana 2004).
Finally, although we did not find any differences between ss ecstasy users and ss controls on the mental rotation or tile manipulation tests, in accordance with our hypotheses and previous reports (Peppas et al. 2001), we also did not find any differences between these genetic subgroups on the PRM test of episodic memory. This is somewhat surprising because this test is sensitive to acute tryptophan depletion (Rubinsztein et al. 2001) and performance on the same test was previously found to be impaired in chronic ecstasy users (Fox et al. 2002). However, most of the studies reporting deficits in episodic memory in ecstasy users have employed tests of verbal recall, not visual recognition (Morgan 2000), and therefore future studies should include both verbal and visual tests to further assess this question.
In conclusion, we have provided preliminary evidence of differences in risky decision-making, executive function, and episodic memory in both controls and ecstasy users according to polymorphism at the 5-HTTLPR. However, in the ss ecstasy users the effects of the polymorphism on decision-making were markedly attenuated. The differences in cognitive function between 5-HTTLPR genotype groups appear to be large and are worthy of further investigation. Further research is needed to characterize the mechanism by which the s allele affects cognitive function in controls and also confers vulnerability to alterations in decision-making in chronic ecstasy users.
Acknowledgements
We would like to thank Professor David C. Rubinsztein for supervising the genotyping and Caroline Humphries and all the staff at the Wellcome Trust Clinical Research Facility, Addenbrooke’s Hospital, for their help and support. We would also like to thank the Cambridge Evening News for their support in recruiting the participants. Barbara Sahakian holds the F. C. Donders Chair in Psychopharmacology at Utrecht University and is a consultant for Cambridge Cognition. This study was funded by a Wellcome Trust Program Grant (number 019407) to Trevor Robbins, Barbara Sahakian, Barry Everitt, and Angela Roberts, and was completed within the MRC Centre for Behavioural and Clinical Neuroscience. Jonathan Roiser was funded by a Medical Research Council Studentship.
Contributor Information
Jonathan P. Roiser, Wellcome Department of Imaging Neuroscience, Institute of Neurology, Queen Square, London WC1N 3BG, UK & Department of Psychiatry, University of Cambridge, School of Clinical Medicine, Addenbrooke’s Hospital, Box 189, Hills Road, Cambridge CB2 2QQ, UK
Robert D. Rogers, Department of Psychiatry, University of Oxford, Warneford Hospital, Oxford OX3 7XJ, UK
Lynnette J. Cook, Department of Medical Genetics, Cambridge Institute for Medical Research, Wellcome Trust/MRC Building, Addenbrooke’s Hospital, Cambridge CB2 2XY, UK
Barbara J. Sahakian, Department of Psychiatry, University of Cambridge, School of Clinical Medicine, Addenbrooke’s Hospital, Box 189, Hills Road, Cambridge CB2 2QQ, UK
References
- Alting Von Geusau N, Stalenhoef P, Huizinga M, Snel J, Ridderinkhof KR. Impaired executive function in male MDMA (“ecstasy”) users. Psychopharmacology (Berl) 2004;175:331–341. doi: 10.1007/s00213-004-1832-8. [DOI] [PubMed] [Google Scholar]
- Attenburrow MJ, Williams C, Odontiadis J, Reed A, Powell J, Cowen PJ, Harmer CJ. Acute administration of nutritionally sourced tryptophan increases fear recognition. Psychopharmacology (Berl) 2003;169:104–107. doi: 10.1007/s00213-003-1479-x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Medelson M, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bhattachary S, Powell JH. Recreational use of 3,4-methylenedioxymethamphetamine (MDMA) or ‘ecstasy’: evidence for cognitive impairment. Psychol Med. 2001;31:647–658. doi: 10.1017/s0033291701003828. [DOI] [PubMed] [Google Scholar]
- Bolla KI, McCann UD, Ricaurte GA. Memory impairment in abstinent MDMA (“ecstasy”) users. Neurology. 1998;51:1532–1537. doi: 10.1212/wnl.51.6.1532. [DOI] [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Wilke F, Petersen K, Nebeling B, Obrocki J, Schulze O, Schmidt U, Clausen M. A voxel-based PET investigation of the long-term effects of “ecstasy” consumption on brain serotonin transporters. Am J Psychiatry. 2004;161:1181–1189. doi: 10.1176/appi.ajp.161.7.1181. [DOI] [PubMed] [Google Scholar]
- Butler GK, Montgomery AM. Impulsivity, risk taking and recreational ‘ecstasy’ (MDMA) use. Drug Alcohol Depend. 2004;76:55–62. doi: 10.1016/j.drugalcdep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. 2nd edn. Lawrence Earlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R, Walton RT, Grasby PM. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci. 2005;25:2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, McLean A, Turner JJ, Parrott AC, Rogers RD, Sahakian BJ. Neuropsychological evidence of a relatively selective profile of temporal dysfunction in drug-free MDMA (“ecstasy”) polydrug users. Psychopharmacology (Berl) 2002;162:203–214. doi: 10.1007/s00213-002-1071-9. [DOI] [PubMed] [Google Scholar]
- Furlong RA, Ho L, Walsh C, Rubinsztein JS, Jain S, Paykel ES, Easton DF, Rubinsztein DC. Analysis and meta-analysis of two serotonin transporter gene polymorphisms in bipolar and unipolar affective disorders. Am J Med Genet. 1998;81:58–63. [PubMed] [Google Scholar]
- Gallagher P, Massey AE, Young AH, McAllister-Williams RH. Effects of acute tryptophan depletion on executive function in healthy male volunteers. BMC Psychiatry. 2003;3:10. doi: 10.1186/1471-244X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamma A, Buck A, Berthold T, Liechti ME, Vollenweider FX. 3,4-Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by [H(2)(15)O]-PET in healthy humans. Neuropsychopharmacology. 2000;23:388–395. doi: 10.1016/S0893-133X(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Giucastro G, Maestri D, Monica C, Sartori R, Caccavari R, Delsignore R. Serotonergic function after (+/-)3,4-methylenedioxymethamphetamine (‘ecstasy’) in humans. Int Clin Psychopharmacol. 1998;13:1–9. doi: 10.1097/00004850-199801000-00001. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thimm B, Rezk M, Hensen G, Daumann J. Memory impairment suggests hippocampal dysfunction in abstinent ecstasy users. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:819–827. doi: 10.1016/S0278-5846(03)00114-3. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxy-methamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Haaxma R, Robbins TW, James M, Browuer WH, Colebatch J, Marsden CD. Neurobehavioural changes in a patient with bilateral globus pallidal lesions. Behav Neurol. 1993;6:229–237. doi: 10.3233/BEN-1993-6410. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Luciana M. Neurocognitive function in users of MDMA: the importance of clinically significant patterns of use. Psychol Med. 2004;34:229–246. doi: 10.1017/s0033291703001132. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology (Berl) 2003;167:411–417. doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Olver JS, Norman TR, Burrows GD, Wesnes KA, Nathan PJ. Selective effects of acute serotonin and catecholamine depletion on memory in healthy women. J Psychopharmacol. 2004;18:32–40. doi: 10.1177/0269881104040225. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. 5th edn. Duxbury; London: 2002. [Google Scholar]
- Kahneman D, Tversky A. Prospect: an analysis of decision-making. Econometrica. 1979;47:263–291. [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- McCann UD, Ridenour A, Shaham Y, Ricaurte GA. Serotonin neurotoxicity after (+/-)3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”): a controlled study in humans. Neuropsychopharmacology. 1994;10:129–138. doi: 10.1038/npp.1994.15. [DOI] [PubMed] [Google Scholar]
- McCann UD, Eligulashvili V, Mertl M, Murphy DL, Ricaurte GA. Altered neuroendocrine and behavioral responses to m-chlorophenylpiperazine in 3,4-methylenedioxymethamphetamine (MDMA) users. Psychopharmacology (Berl) 1999;147:56–65. doi: 10.1007/s002130051142. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [(11)C]McN5652 and [(11)C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCardle K, Luebbers S, Carter JD, Croft RJ, Stough C. Chronic MDMA (ecstasy) use, cognition and mood. Psychopharmacology (Berl) 2004;173:434–439. doi: 10.1007/s00213-004-1791-0. [DOI] [PubMed] [Google Scholar]
- Morgan MJ. Memory deficits associated with recreational use of “ecstasy” (MDMA) Psychopharmacology (Berl) 1999;141:30–36. doi: 10.1007/s002130050803. [DOI] [PubMed] [Google Scholar]
- Morgan MJ. Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology (Berl) 2000;152:230–248. doi: 10.1007/s002130000545. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Impallomeni LC, Pirona A, Rogers RD. Elevated impulsivity and impaired decision-making in abstinent Ecstasy (MDMA) users compared to polydrug and drug-naive controls. Neuropsychopharmacology. 2005;31:1562–1573. doi: 10.1038/sj.npp.1300953. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test (NART): test manual. NFER-Nelson; Windsor: 1982. [Google Scholar]
- Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, Praschak-Rieder N, Zach J, de Zwaan M, Bondy B, Ackenheil M, Kasper S. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- Peppas E, Turner JJD, Parrot AC. Memory decrements but trends towards faster mental rotation in abstinent ecstasy/MDMA users. J Psychopharmacol. 2001;15S:a37. [Google Scholar]
- Reist C, Mazzanti C, Vu R, Tran D, Goldman D. Serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. Am J Med Gene t. 2001;105:363–368. doi: 10.1002/ajmg.1360. [DOI] [PubMed] [Google Scholar]
- Reneman L, Lavalaye J, Schmand B, de Wolff FA, van den Brink W, den Heeten GJ, Booij J. Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”): preliminary findings. Arch Gen Psychiatry. 2001;58:901–906. doi: 10.1001/archpsyc.58.10.901. [DOI] [PubMed] [Google Scholar]
- Reneman L, Schilt T, de Win MM, Booij J, Schmand B, van den Brink W, Bakker O. Memory function and serotonin transporter promoter gene polymorphism in ecstasy (MDMA) users. J Psychopharmacol. 2006;20:389–399. doi: 10.1177/0269881106063266. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, Hopwood A, Wallace C, Deakin JF, Sahakian BJ, Robbins TW. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology (Berl) 1999a;146:482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999b;20:322–329. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28:153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Lancaster M, Wakeley J, Bhagwagar Z. Effects of beta-adrenoceptor blockade on components of human decision-making. Psychopharmacology (Berl) 2004a;172:157–164. doi: 10.1007/s00213-003-1641-5. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004b;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Sahakian BJ. Relationship between ecstasy use and depression: a study controlling for poly-drug use. Psychopharmacology (Berl) 2004;173:411–417. doi: 10.1007/s00213-003-1705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Cook LJ, Cooper JD, Rubinsztein DC, Sahakian BJ. Association of a functional polymorphism in the serotonin transporter gene with abnormal emotional processing in ecstasy users. Am J Psychiatry. 2005a;162:609–612. doi: 10.1176/appi.ajp.162.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Rogers RD, Sahakian BJ. Neuropsychological function in ecstasy users: a study controlling for polydrug use. Psychopharmacology (Berl) 2005b;15:1–12. doi: 10.1007/s00213-005-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein JS, Rogers RD, Riedel WJ, Mehta MA, Robbins TW, Sahakian BJ. Acute dietary tryptophan depletion impairs maintenance of “affective set” and delayed visual recognition in healthy volunteers. Psychopharmacology (Berl) 2001;154:319–326. doi: 10.1007/s002130000655. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111(Pt 3):695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry. 1989;154:797–800. doi: 10.1192/bjp.154.6.797. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Jorissen BL, Sobczak S, van Boxtel MP, Hogervorst E, Deutz NE, Riedel WJ. Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J Psychopharmacol. 2000;14:21–29. doi: 10.1177/026988110001400102. [DOI] [PubMed] [Google Scholar]
- Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br J Psychiatry. 1999;175:63–69. doi: 10.1192/bjp.175.1.63. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171:701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- Smith GS, Lotrich FE, Malhotra AK, Lee AT, Ma Y, Kramer E, Gregersen PK, Eidelberg D, Pollock BG. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29:2226–2234. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- Whale R, Quested DJ, Laver D, Harrison PJ, Cowen PJ. Serotonin transporter (5-HTT) promoter genotype may influence the prolactin response to clomipramine. Psychopharmacology (Berl) 2000;150:120–122. doi: 10.1007/s002130000432. [DOI] [PubMed] [Google Scholar]