Abstract
Androgens may regulate the male skeleton directly through a stimulation of androgen receptors or indirectly through aromatization of androgens into estrogen and, thereafter, through stimulation of estrogen receptors (ERs). The relative importance of ER subtypes in the regulation of the male skeleton was studied in ERα-knockout (ERKO), ERβ-knockout (BERKO), and double ERα/β-knockout (DERKO) mice. ERKO and DERKO, but not BERKO, demonstrated decreased longitudinal as well as radial skeletal growth associated with decreased serum levels of insulin-like growth factor I. Therefore, ERα, but not ERβ, mediates important effects of estrogen in the skeleton of male mice during growth and maturation.
Several studies demonstrate that androgens are important in the male skeleton. Orchidectomy decreases longitudinal growth and radial cortical growth in the long bones of rodents (1–3). Furthermore, androgen treatment stimulates growth in orchidectomized growing rats and mice (1, 3, 4), as well as in growing boys (5). These effects may either be direct through the stimulation of androgen receptors or indirect through aromatization of androgens into estrogen and, thereafter, through stimulation of estrogen receptors (ERs). Recently, it was demonstrated by Vanderschueren et al. (6) that the conversion of androgens into estrogen is required for normal body growth in male rats, indicating that indirect effects of androgens, mediated by estrogen, are important.
In addition to the growth-related effects of gonadal deficiency, orchidectomy also decreases bone mass in adult rodents (2, 6–8). This effect at least partly depends on the androgen receptor, because treatment with nonaromatizable androgens restores bone mass (7, 9). On the other hand, several clinical studies have demonstrated a strong relationship between serum estrogen levels and bone mineral density (BMD) in males (10, 11). Furthermore, aromatase deficiency in humans (12), as well as aromatase inhibition in rats (6, 13), is associated with osteopenia, suggesting that androgens also may regulate adult bone metabolism, either directly by stimulation of androgen receptors or indirectly by aromatization and subsequent stimulation of ERs.
Estrogen acts through the binding to, and activation of, two ERs. These receptors are commonly referred to as ERα and ERβ, and their expression has been described previously in skeletal cells (14–20). In humans, evidence for the importance of ERα for mediating effects of estrogen in the skeleton comes from a case report describing a young male with estrogen resistance caused by a mutation in the human ERα gene (21). This male was reported to suffer from osteoporosis at the age of 28 years. Mice lacking a functional ERα gene, ERα-knockout mice (ERKO), have been generated (22), and more recently ERβ-knockout mice (BERKO) also have been described (23). At present, the skeletal phenotype of male ERKO mice is unclear.**,‡‡,†† Furthermore, we recently demonstrated that male BERKO mice do not exhibit osteopenia (27). This finding has raised the question concerning the relative importance of ER subtypes in the skeleton of male mice. To investigate the ER specificity in the regulation of growth and adult bone metabolism in male mice, we have generated double ER-knockout mice (DERKO). In the present study, we have compared the skeletal phenotypes of male wild-type (WT), ERKO, BERKO, and DERKO mice.
Methods
Animals.
Male double heterozygous (ERα+/−β+/−) mice were mated with female double heterozygous (ERα+/−β+/−) mice, resulting in WT, ERKO, BERKO, and DERKO offspring. All mice were of mixed C57BL/6J/129 backgrounds. Genotyping of tail DNA was performed at 3 weeks of age. The ERα gene was analyzed with the following primer pairs. Primers AACTCGCCGGCTGCCACTTACCAT and CATCAGCGGGCTAGGCGACACG for the WT gene correspond to flanking regions in the targeted exon 2. They produce a fragment of ≈320 bp. Primers TGTGGCCGGCTGGGTGTG and GGCGCTGGGCTCGTTCTC for the knockout gene correspond to part of the NEO-cassette and the flanking exon 2. They produce a 700-bp fragment. Genotyping of the ERβ gene has been described previously (27). Animals were maintained in polycarbonate plastic cages (Scanbur, Køge, Denmark) containing wood chips. Animals had free access to fresh water and food pellets (B & K Universal, Sollentuna, Sweden) consisting of cereal products (76.9% barley, wheat feed, and wheat and maize germ), vegetable proteins (14.0% hipro soya), and vegetable oil (0.8% soya oil).
Dual X-Ray Absorptiometry (DXA).
Bone mineral content (BMC) and areal BMD (BMC/cm2) were measured with the Norland pDEXA Sabre and the sabre research software (Version 3.6; Norland Medical Systems, Fort Atkinson, WI) as previously described (27).
Peripheral Quantitative Computerized Tomography (pQCT).
Computerized tomography was performed with the stratec pqct xct (software version 5.4B; Norland Medical Systems) operating at a resolution of 70 μm as previously described (27).
Mid-diaphyseal pQCT scans of femora and tibiae were performed to determine the cortical volumetric BMD, cortical cross-sectional area, periosteal and endosteal circumferences, and the cross-sectional moment of inertia. The mid-diaphyseal region of femora and tibiae in mice contains mostly cortical bone.
Metaphyseal pQCT scans of left femora and tibiae were performed to measure trabecular volumetric BMD. The scan was positioned in the metaphysis at a distance from the distal growth plate corresponding to 4% of the total length of the femur (an area containing cortical as well as trabecular bone). The trabecular bone region was defined by setting an inner threshold to 45% of the total area. Although the cancellous bone density measurement at the metaphysis is not as precise as total bone density measurement at the mid-diaphysis, we have been successful in demonstrating significant changes in trabecular volumetric bone density at this site in rats and mice in response to estrogen deficiency. The interassay coefficients of variation for the pQCT measurements were <2%.
It should be emphasized that the DXA technique gives the areal BMD, whereas the pQCT gives the true volumetric BMD. Therefore, a factor regulating the outer dimensions of a bone will affect the areal BMD (DXA) but not the volumetric BMD (pQCT).
Histological Examination and Histomorphometry.
Growth plate measurements.
Right and left tibiae were fixed in 4% formaldehyde, embedded in paraffin, and sectioned at a thickness of 4 μm. The width of the growth plate was measured, after staining with alcian blue/Van Gieson, by using an image-processing system (Easy Image; Bergströms Instrument, Stockholm) coupled to a microscope. The average of 20 growth-plate measurements (2 sections, 10 measurements per section) was calculated for each tibia.
Bone histomorphometry.
The areas of trabecular bone within a reference area of the proximal tibia were measured in sections stained with hematoxylin/eosin. Measurements were performed on printed copies by point counting using a square lattice (1 and 2 cm). Three fields of vision on three sections from each animal were used for the analysis. Data are presented as the ratio of trabecular bone volume to total volume.
Mechanical Testing.
The samples were applied to mechanical testing after pQCT measurements using Mechanical Tester 8841 (Instron, Canton, MA). Three-point bending strength was measured at mid-diaphysis. The bone was placed horizontally with the anterior surface upwards, centered on the supports, and the pressing force was directed vertically to the midshaft of the bone. Each bone was compressed with a constant speed of 2 mm/min until failure. Breaking force (maximal load) was defined as bending load at failure. Stress σ and elastic or Young's modulus E were calculated as previously described (28). Maximal stress was defined at breaking force.
RIA.
Serum insulin-like growth factor I (IGF-I) levels were measured by double-antibody IGF binding protein-blocked RIA (29). Serum osteocalcin levels were measured by RIA using rabbit anti-mouse osteocalcin antiserum and purified mouse osteocalcin as standard and tracer (30). The sensitivity of the mouse osteocalcin assay was 0.5 ng/ml, and intra- and interassay variations were <8%.
Statistical Procedure.
Dynamic measurements first were analyzed by a two-way analysis of variance (ANOVA), followed by Student–Newman–Keuls multiple range test. Static measurements (at the time of sacrifice) first were analyzed by one-way ANOVA, followed by Student–Newman–Keuls multiple range test.
Results
Body Growth.
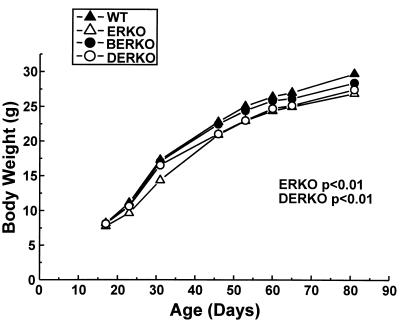
Body weight was unchanged in ERKO, BERKO, and DERKO at the prepubertal stage as compared with WT littermates (Fig. 1, day 17, one-way ANOVA). Late pubertal and young adult weight was decreased in ERKO and DERKO, but not in BERKO, as compared with WT mice (Fig. 1, days 46–81, two-way ANOVA).
Figure 1.
Body weight in wild type (WT), ERKO, BERKO, and DERKO mice at different ages. (n = 6 for WT; n = 9 for ERKO; n = 11 for BERKO; and n = 5 for DERKO). Values are given as means. The body weights in WT and ERKO mice at different ages first were analyzed by a two-way ANOVA, followed by Student–Newman–Keuls multiple range test. P values versus WT mice are indicated.
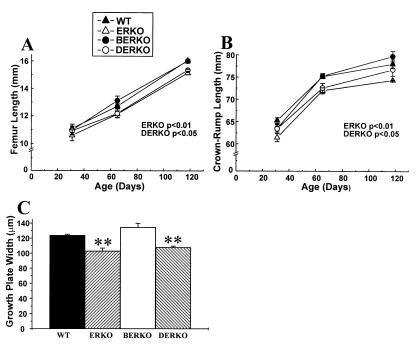
Growth of the appendicular as well as the axial skeleton was followed by using repeated x-ray measurements. The length of the femur was chosen as a measure of appendicular growth, whereas crown–rump length was used as a measure of axial growth. The length of the femur was unchanged at the early pubertal stage (Fig. 2A, day 31, one-way ANOVA). Thereafter, ERKO and DERKO demonstrated a gradual decrease in growth rate, resulting in a decreased femoral length at the adult stage (ERKO −5.7% and DERKO −4.4% versus WT, Figs. 2A and 4A). The decreased growth of the long bones in ERKO and DERKO was associated with a decreased growth plate width measured in the proximal tibia (Fig. 2C). The crown–rump length was also decreased in ERKO and DERKO as compared with WT (Fig. 2B).
Figure 2.
Length of femur (A) and crown–rump (B) and width of the proximal tibial growth plate (C) at 118 days of age in WT, ERKO, BERKO, and DERKO mice (n = 6 for WT; n = 9 for ERKO; n = 6 for BERKO; and n = 5 for DERKO). Values are given as means ± SEM. Data at different ages first were analyzed by a two-way ANOVA (A and B) or by a one-way ANOVA (C), followed by Student–Newman–Keuls multiple range test. P values versus WT mice are indicated in A and B. ∗∗, P < 0.01 versus WT (C).
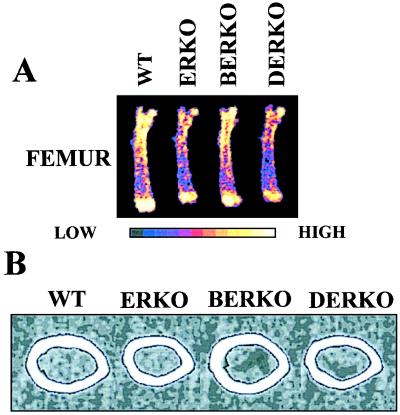
Figure 4.
Representative DXA scans (A) and mid-diaphyseal pQCT scans (B) of femora in adult WT, ERKO, BERKO, and DERKO mice. High, high bone mineral density; Low, low bone mineral density.
Bone Mineral Status as Determined by DXA.
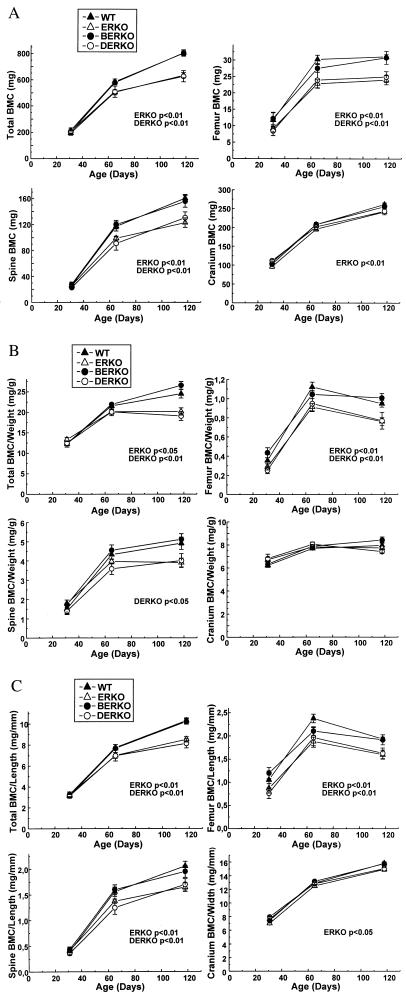
BMC (mg) and areal BMD (mg/mm2) were measured with DXA. BERKO demonstrated unchanged BMC and areal BMD (Fig. 3A; Table 1). Furthermore, in ERKO and DERKO, no effect was seen on BMC and areal BMD at the early pubertal stage (day 31, one-way ANOVA). However, later on ERKO and DERKO presented a marked decrease in total body BMC (Fig. 3A). In addition, regional measurements of BMC in the femur and spine also showed a significant decrease (day 118; total body: ERKO −21%, DERKO −22%; femur: ERKO −23%, DERKO −20%; spine: ERKO −23%, DERKO −19%, versus WT; Figs. 3A and 4A). Only a small effect was seen in the cranium (ERKO −7% versus WT; Fig. 3A). Total body areal BMD was decreased slightly in ERKO at the adult stage. Both ERKO and DERKO displayed a decreased adult areal BMD in the femur (Table 1).
Figure 3.
DXA measurements of bone parameters in WT, ERKO, BERKO, and DERKO mice (n = 6 for WT; n = 9 for ERKO; n = 6 for BERKO; and n = 5 for DERKO). The BMC (A), BMC/body weight (B), and BMC/length (C) of the whole skeleton (total), femur, spine, and cranium were measured by using DXA technique as described in Methods. Values are given as means ± SEM. Data at different ages first were analyzed by a two-way ANOVA, followed by Student–Newman–Keuls multiple range test. P values versus WT mice are indicated.
Table 1.
Areal BMD as measured by using DXA
| Genotype | n | Day | BMD, mg/cm2
|
||
|---|---|---|---|---|---|
| Total body | Femur | Spine | |||
| WT | 6 | 31 | 48.7 ± 0.8 | 35.1 ± 0.9 | 36.6 ± 0.9 |
| 65 | 59.0 ± 0.7 | 58.2 ± 1.3 | 53.0 ± 0.8 | ||
| 118 | 66.5 ± 0.2 | 64.3 ± 1.6 | 61.2 ± 0.8 | ||
| ERKO | 9 | 31 | 47.2 ± 0.3 | 33.3 ± 0.5 | 36.1 ± 0.6 |
| 65 | 58.2 ± 0.6 | 52.9 ± 1.8 | 51.5 ± 0.8 | ||
| 118 | 65.2 ± 0.7 | 58.9 ± 1.3 | 56.8 ± 1.1 | ||
| Two-way | |||||
| ANOVA | P < 0.05 | P < 0.01 | NS | ||
| BERKO | 6 | 31 | 48.4 ± 0.7 | 34.5 ± 0.7 | 35.7 ± 1.1 |
| 65 | 59.5 ± 0.6 | 55.0 ± 1.7 | 53.4 ± 1.0 | ||
| 118 | 66.4 ± 0.3 | 64.0 ± 1.9 | 61.2 ± 1.5 | ||
| Two-way | |||||
| ANOVA | NS | NS | NS | ||
| DERKO | 5 | 31 | 50.6 ± 0.5 | 35.5 ± 1.2 | 37.2 ± 0.7 |
| 65 | 58.6 ± 0.2 | 51.0 ± 2.6 | 49.5 ± 1.9 | ||
| 118 | 65.8 ± 0.8 | 60.8 ± 2.1 | 60.8 ± 3.0 | ||
| Two-way | |||||
| ANOVA | NS | P < 0.05 | NS | ||
Values are presented as mean ± SEM. Data at different ages first were analyzed by a two-way ANOVA, followed by Student–Newman–Keuls multiple range test. P values versus WT mice are as indicated. NS, not significant.
To determine whether the decrease in BMC in ERKO and DERKO males was greater than that associated with retarded body growth, BMC/body weight was calculated for the whole skeleton and for individual bones. Interestingly, in adult mice, total body BMC/body weight was decreased in ERKO (−18%) and DERKO (−22%) as compared with WT. This finding was also the case for femur (ERKO −20; DERKO −19%) and spine (ERKO −21%; DERKO −18%; Fig. 3B). Similar results were seen when the BMC was divided by the length of the individual bones (Fig. 3C).
Cancellous Bone Density.
The pQCT technique was used to measure trabecular volumetric BMD in the metaphysis of the distal femur and in the proximal tibia. In addition, histomorphometry was performed in the metaphysis of the proximal tibia, where trabecular bone volume/total volume was measured. Neither the pQCT technique (Table 2 and data not shown) nor bone histomorphometry (trabecular bone volume/total volume: WT, 0.32 ± 0.05; ERKO, 0.32 ± 0.02; BERKO, 0.33 ± 0.02; DERKO, 0.34 ± 0.02; one-way ANOVA) detected any significant changes in cancellous bone density.
Table 2.
Trabecular volumetric BMD and cortical bone parameters of femur as measured with pQCT
| Genotype | n | Trabecular density, mg/mm3 | Cortical density, mg/mm3 | Cortical area, mm2 | Cortical BMC, mg/mm | Cortical periosteal circumference, mm | Cortical endosteal circumference, mm |
|---|---|---|---|---|---|---|---|
| WT | 6 | 0.312 ± 0.021 | 1.188 ± 0.016 | 1.06 ± 0.02 | 1.26 ± 0.04 | 5.65 ± 0.08 | 4.31 ± 0.13 |
| ERKO | 9 | 0.293 ± 0.011 | 1.189 ± 0.006 | 0.91 ± 0.02** | 1.08 ± 0.03* | 5.15 ± 0.06** | 3.89 ± 0.05* |
| BERKO | 6 | 0.268 ± 0.021 | 1.193 ± 0.011 | 1.01 ± 0.04 | 1.21 ± 0.06 | 5.57 ± 0.11 | 4.28 ± 0.09 |
| DERKO | 5 | 0.285 ± 0.019 | 1.184 ± 0.011 | 0.92 ± 0.04* | 1.09 ± 0.05* | 5.26 ± 0.12* | 4.02 ± 0.11 |
Values are presented as mean ± SEM. Data first were analyzed by a one-way ANOVA, followed by Student–Newman–Keuls multiple range test. *, P < 0.05; **, P < 0.01, both versus WT.
Cortical Bone Parameters.
Cortical bone parameters were studied in detail in mid-diaphyseal pQCT scans of femora and tibiae (Table 2; Fig. 4B; and data not shown). The cortical BMC in the mid-diaphyseal section of femur was decreased in ERKO (−14%) and DERKO (−14%) mice as compared with WT, and this decrease was mainly caused by a decreased cross-sectional bone area, whereas cortical volumetric density was unchanged (Table 2). The decrease in cross-sectional area in ERKO and DERKO was associated with decreased periosteal and endosteal circumference (Fig. 4B and Table 2).
Mechanical Testing of the Femur Diaphysis.
The size and position of the cortical cross-sectional bone area in ERKO and DERKO resulted in a pronounced decrease of cortical cross-sectional moment of inertia (ERKO, −29%, and DERKO, −24% versus WT; Table 3). Changes in area moment of inertia often are directly correlated to changes in the mechanical strength of the bone. Therefore, mechanical strength was tested by three-point-bending at the mid-diaphyseal region of the femur. ERKO demonstrated a significantly decreased maximal load, whereas a tendency to decrease was seen in DERKO (ERKO −18%; DERKO −15%) as compared with WT (Table 3). Other bone parameters, including maximal stress and elastic modulus, reflecting the quality of the bone, were not significantly different in the four groups (Table 3).
Table 3.
Mechanical testing of femur diaphysis
| Genotype | n | Area moment of inertia, mm4 | Maximal load, N | Elastic modulus, MPa | Maximal stress, GPa |
|---|---|---|---|---|---|
| WT | 6 | 0.34 ± 0.01 | 28.4 ± 1.9 | 3.8 ± 0.2 | 132 ± 8 |
| ERKO | 9 | 0.24 ± 0.01** | 23.2 ± 0.7* | 4.4 ± 0.3 | 140 ± 5 |
| BERKO | 5 | 0.32 ± 0.03 | 26.5 ± 2.1 | 3.5 ± 0.3 | 122 ± 10 |
| DERKO | 5 | 0.26 ± 0.02** | 24.3 ± 1.6 | 3.5 ± 0.1 | 140 ± 6 |
Values are presented as mean ± SEM. Data first were analyzed by a one-way ANOVA, followed by Student–Newman–Keuls multiple range test. *, P < 0.05; **, P < 0.01, both versus WT.
Biochemical Bone Markers and IGF-I in Serum.
Osteocalcin, a marker of bone formation, was measured in serum at 110 days of age. Osteocalcin was decreased by 25% (P < 0.05) and 9%, respectively, in ERKO and DERKO mice (Table 4).
Table 4.
Serum levels of osteocalcin and IGF-I
| Genotype | n | Age, days | Osteocalcin, ng/ml | IGF-I, ng/ml |
|---|---|---|---|---|
| WT | 6 | 45 | — | 369 ± 11 |
| 110 | 94 ± 3 | 337 ± 36 | ||
| ERKO | 9 | 45 | — | 259 ± 11** |
| 110 | 71 ± 3* | 250 ± 8* | ||
| BERKO | 6 | 45 | — | 384 ± 9 |
| 110 | 93 ± 6 | 313 ± 12 | ||
| DERKO | 5 | 45 | — | 298 ± 23* |
| 110 | 86 ± 11 | 264 ± 6 |
Values are presented as mean ± SEM. Data first were analyzed by a one-way ANOVA, followed by Student–Newman–Keuls multiple range test. *, P < 0.05; **, P < 0.01, both versus WT.
Overall size and cortical radial growth are parameters that are highly sensitive to changes in the growth hormone (GH)/IGF-I axis. Because these parameters were altered in ERKO and DERKO males, serum IGF-I levels were measured to investigate whether the GH/IGF-I axis was affected in the ERKO and DERKO mice. Serum IGF-I levels were significantly decreased in ERKO and DERKO (Table 4). Furthermore, serum IGF-I levels were statistically correlated with length, BMC, BMC/weight, cortical cross-sectional area, periosteal circumference, moment of inertia, and ultimate load of femur (Table 5).
Table 5.
Correlation with serum IGF-I
| Organ | Measurement | r |
|---|---|---|
| Femur | Length | 0.60** |
| BMC | 0.70*** | |
| BMC/weight | 0.59** | |
| Trabecular volume BMD | 0.22 | |
| Cortical cross-sectional area | 0.55** | |
| Endosteal circumference | 0.47* | |
| Periosteal circumference | 0.54** | |
| Moment of inertia | 0.55** | |
| Ultimate load | 0.50* | |
| Liver | Weight | 0.18 |
| Kidney | Weight | 0.04 |
| Heart | Weight | 0.39 |
| Lung | Weight | 0.07 |
| Brain | Weight | −0.17 |
Correlations of all animals included in the study were calculated using Pearson's correlation coefficient (r). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
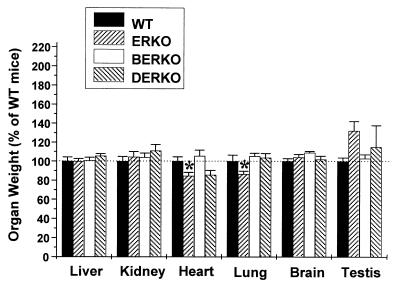
Organ Weights.
The weights of several other organs were measured to see whether the effect on the skeleton in ERKO and DERKO was tissue-specific. To compare the relative growth of different organs, the individual organ weights were divided by the total body weight. The weights of the liver, kidney, and brain were not significantly changed in any group. However, the weights of heart and lung were decreased in ERKO mice as compared with WT (heart, −15%; lung, −17%; Fig. 5). A tendency to increase in testis weight was seen in ERKO and DERKO. Previous studies have shown that testicular weight is increased in male ERKO until ≈80 days of life, after which testes atrophy and decrease in weight (31).
Figure 5.
Organ weights/body weight expressed as percentage of WT mice at 4 months of age in WT, ERKO, BERKO, and DERKO (n = 6 for WT; n = 9 for ERKO; n = 5 for BERKO; and n = 5 for DERKO). Values are given as means ± SEM. Data were first analyzed by a one-way ANOVA, followed by Student–Newman–Keuls multiple range test. *, P < 0.05 versus WT.
Discussion
The effect of androgens on the male skeleton may be either direct through a stimulation of androgen receptors or indirect through aromatization of androgens into estrogen and, thereafter, through stimulation of ERs. Possible direct effects of androgens are illustrated by skeletal abnormalities in androgen-resistant humans and rodents (32, 33). However, several studies have clearly demonstrated that the effect of androgens on the male skeleton, at least partly, depends on the conversion of androgens into estrogen. In the present study, we demonstrate that estrogen resistance in the male mouse, because of loss of all known ERs, results in decreased skeletal growth. ERKO and DERKO but not BERKO mice display similar growth phenotypes, demonstrating that ERα, but not ERβ, is the ER mediating the effects of estrogen on skeletal growth in the male mouse. The shortening of the long bones in ERKO and DERKO mice was associated with decreased growth plate width in the proximal tibia. Similar findings have also been reported in orchidectomized mice and rats (2, 34). Furthermore, Ornoy et al. (3) showed that orchidectomy in mice decreases growth plate area measured in the proximal tibia and that low-dose estrogen treatment increases the same parameter. These findings demonstrate that physiological levels of estrogen have a stimulatory effect on longitudinal growth in male rodents. Similarly, estrogens are required for the pubertal growth spurt in boys (35). Estrogen regulates final height in humans by a stimulatory effect on the pubertal growth spurt, followed by closure of the epiphyseal growth plates at the end of puberty.
It is a well established fact that orchidectomized rodents, as well as hypogonadal humans, develop osteopenia (36–38). Although androgen replacement restores bone mass in gonadectomized male rats (9), it has also been demonstrated that estrogen, at least partly, reverses bone loss caused by orchidectomy (3, 7). The present study, with estrogen insensitivity caused by inactivation of both ERα and ERβ, supports the notion that estrogen exerts important effects on the male skeleton. The phenotype of the male DERKO mouse is similar to what has been described earlier for aromatase-inhibited male rats (6). Both male DERKO mice and aromatase-inhibited male rats demonstrate decreased femoral BMC and areal BMD, as well as decreased cortical dimensions and moment of inertia, without any effect on cortical volumetric BMD. These two studies demonstrate that the conversion of androgens into estrogen is important in male rodents and that skeletal maturation in such species is estrogen-dependent. ERKO and DERKO, but not BERKO, demonstrated, in the present study, a decreased longitudinal as well as radial skeletal growth, This finding clearly demonstrates that ERα alone, and not ERβ, is the mediator of estrogenic effects on growth and maturation of the skeleton in male mice. The changes, seen in the present study, are predominantly growth-related and are not consistent with effects previously described for sex steroids in the adult skeleton. Therefore, we believe that inducible gene knockouts of the ERs are needed to fully clarify the role of ERs in the adult male skeleton. Such an animal model would make it possible to induce the deletion of ERs after normal postnatal and pubertal growth, thus permitting effects in adult animals to be investigated. Our present finding that young adult BERKO males have no skeletal phenotype is similar to what we previously have described (27). However, in the previous study, an increase in the size of the skeleton was observed in female BERKO mice.
Interestingly, a slight decrease in the relative weights of the heart and lung was also seen in ERKO but not in BERKO mice, indicating that ERα may exert specific effects in these two organs as well. In contrast, the relative weights of liver, kidney, and brain were unchanged in ERKO, BERKO, and DERKO males.
ERKO and DERKO mice demonstrated a decreased diaphyseal cross-sectional area and periosteal circumference of femur, resulting in a pronounced decrease of the area moment of inertia. When the quality of the bone is unchanged, the area moment of inertia is normally proportional to the mechanical strength of the bone determined by three-point-bending (39). The maximal load was decreased in male ERKO mice but it was not decreased more than suggested by the changes in area moment of inertia. Because the size of the bone is a major determinant of its strength, further studies using bones of similar size from control mice are needed to evaluate whether other parameters such as bone quality contribute to decreased bone strength in the ERKO and DERKO mice.
Aromatase inhibition of male rats resulted in a small decrease in trabecular BMD (6). In the present study, neither the pQCT technique nor bone histomorphometry detected any significant changes in cancellous bone density in male ERKO, BERKO, or DERKO mice. Thus, our experiments indicate that neither ERα nor ERβ is essential for the maintenance of cancellous bone mass in the male mouse. This finding raises the question of whether other ER subtypes exist (40, 41) or whether other hormones may compensate for estrogen resistance in the skeleton of male DERKO mice. Androgens prevent cancellous osteopenia in orchidectomized rats. Therefore, androgens could, in theory, compensate for loss of ER activity in ERKO, BERKO, and DERKO males. Interestingly, ERKO males have somewhat increased serum levels of testosterone (42).
Bone loss after gonadal deficiency normally is associated with increased bone turnover. Surprisingly, osteocalcin, a marker for bone formation, was decreased in ERKO males. This finding and the decreased longitudinal as well as radial skeletal growth seen in ERKO and DERKO males led us to seek other explanations to the skeletal phenotype in these mice. Overall size and cortical radial growth are parameters that are highly sensitive to changes in the GH/IGF-I axis (43). Because these parameters were altered in ERKO and DERKO males, serum IGF-I was measured to investigate whether the GH/IGF-I axis was affected in ERKO and DERKO males. Serum IGF-I levels were decreased in ERKO and DERKO mice. We also found a strong correlation between serum IGF-I levels and affected skeletal parameters in the ERKO and DERKO mice, including length, BMC of femur, periosteal circumference, and maximal load in the femur diaphysis. These findings do not prove but do suggest that changes in the GH/IGF-I axis could partly explain the skeletal phenotype seen in male ERKO and DERKO mice. GH and IGF-I are known to increase serum osteocalcin (43). Therefore, the decreased serum osteocalcin levels in male ERKO mice may be caused by reduced serum IGF-I levels. This result also is supported by the finding that aromatase-inhibited male rats have decreased serum IGF-I levels and reduced levels of serum osteocalcin (6). An effect of estrogen on the GH/IGF-I axis in males also is supported by several clinical as well as experimental studies. Circulating GH and IGF-I concentrations increase during normal male puberty (44–47). These changes seem to be secondary to the pubertal rise in testosterone concentrations, because they are also observed in prepubertal and hypogonadal boys undergoing induction of puberty with exogenous testosterone (44, 48). The mechanism whereby testosterone interacts with the somatotropic axis may be either direct, mediated by androgen receptors, or indirect through the action of estrogen on ERs. The possibility that estrogen mediates the effects of testosterone on the somatotropic axis has been suggested in a previous study showing a significant correlation between circulating levels of estrogen, but not testosterone, and GH secretion in men (24). Furthermore, it has also been demonstrated that testosterone plays an important role in the modulation of the somatotropic axis in adulthood, and this effect, at least partly, depends on the conversion of testosterone to estrogen (25).
Our present results demonstrate that loss of ERα in mice is associated with a significant shortening of the long bones and decreased serum levels of osteocalcin. On the other hand, recent clinical case reports have shown that aromatase-deficient and estrogen-resistant humans display an increased adult stature and increased serum levels of osteocalcin (12, 21). Therefore, it is obvious that species differences between mice and humans regarding the regulation of skeletal changes do exist. Some of these changes may be explained by the fact that the human growth plate normally is closed after puberty, whereas growth plate closure occurs at a later stage in life in mice.
It is well known that peak bone density differs among inbred strains of mice (26). The mice used in this study were of mixed genetic background, as the strains C57BL/6 and 129 were used for generation of ER-knockout animals. Although siblings from the same mixed genetic background were used as control, it is possible that differences in genetic background could have confounded the results. It is therefore necessary to confirm our results with ER-knockout mice that have been bred successively in C57BL/6 background for 10 or more generations or with ER-knockout mice generated by using different mixed genetic background.
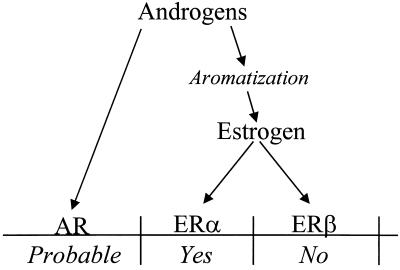
The effects of androgens in the skeleton of the male mouse are summarized in Fig. 6. Others have presented studies indicating that androgens, directly through interaction with the androgen receptor, exert effects on the male skeleton. In the present study, we have confirmed that part of the effect of androgens depends on aromatization. Furthermore, the present study clearly demonstrates that ERα but not ERβ mediates the effect of estrogen on the skeleton in the male mouse. In conclusion, we have generated DERKO mice, which are fully viable, despite the fact that they are devoid of all known ERs. Male ERKO and DERKO mice have decreased body weight, reduced longitudinal bone growth, and a pronounced cortical osteopenia. Our findings demonstrate that ERα but not ERβ mediates the effect of estrogen in the male skeleton. We propose that some of the skeletal effects seen in ERα-inactivated male mice may be caused by an inhibition of the GH/IGF-I axis.
Figure 6.
Effects of androgens in the male skeleton during growth and maturation. AR, androgen receptor.
Acknowledgments
Excellent technical assistance was provided by Maud Pettersson and Lotta Uggla at the Department of Clinical Pharmacology, University of Gothenburg, Sweden, and Rongqing Guo at the Jerry L. Pettis Veterans Administration Medical Center in Loma Linda, CA. This study was supported by the Swedish Medical Research Council, the Swedish Foundation for Strategic Research, the Swedish Cancer Society, and the Lundberg Foundation, and by National Institutes of Health Grant AR31062.
Abbreviations
- ER
estrogen receptor
- BMD
bone mineral density
- ERKO
ERα-knockout
- BERKO
ERβ-knockout
- DERKO
double ERα/β-knockout
- WT
wild type: DXA, dual x-ray absorptiometry
- BMC
bone mineral content
- pQCT
peripheral quantitative computerized tomography
- IGF
insulin-like growth factor
- GH
growth hormone
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Kimbro, K., Migiliaccio, S. & Korach, S. (1996) J. Bone Miner. Res. 11, S125 (abstr.).
Schmidt, A., Seedor, J., Gentile, M., Pennypacker, B., Rodan, G. & Kimmel, D. (1999) J. Bone Miner. Res. 14, S456 (abstr.).
Ederveen, A. & Kloosterboer, H. (1999) J. Bone Miner. Res. 14, S170 (abstr.).
References
- 1.Turner R T, Wakley G K, Hannon K S. J Orthop Res. 1990;8:612–617. doi: 10.1002/jor.1100080418. [DOI] [PubMed] [Google Scholar]
- 2.Turner R T, Hannon K S, Demers L M, Buchanan J, Bell N H. J Bone Miner Res. 1989;4:557–563. doi: 10.1002/jbmr.5650040415. [DOI] [PubMed] [Google Scholar]
- 3.Ornoy A, Giron S, Aner R, Goldstein M, Boyan B D, Schwartz Z. Bone Miner. 1994;24:43–58. doi: 10.1016/s0169-6009(08)80130-4. [DOI] [PubMed] [Google Scholar]
- 4.Jansson J O, Ekberg S, Isaksson O, Mode A, Gustafsson J A. Endocrinology. 1985;117:1881–1889. doi: 10.1210/endo-117-5-1881. [DOI] [PubMed] [Google Scholar]
- 5.Richman R A, Kirsch L R. N Engl J Med. 1988;319:1563–1567. doi: 10.1056/NEJM198812153192402. [DOI] [PubMed] [Google Scholar]
- 6.Vanderschueren D, Van Herck E, Nijs J, Ederveen A G, De Coster R, Bouillon R. Endocrinology. 1997;138:2301–2307. doi: 10.1210/endo.138.6.5216. [DOI] [PubMed] [Google Scholar]
- 7.Vanderschueren D, Van Herck E, Suiker A M, Visser W J, Schot L P, Bouillon R. Endocrinology. 1992;130:2906–2916. doi: 10.1210/endo.130.5.1572302. [DOI] [PubMed] [Google Scholar]
- 8.Koh E T, Yeh J K, Bourdeau J E, Chen M M, Om A S. Magnes Res. 1996;9:13–21. [PubMed] [Google Scholar]
- 9.Wakley G K, Schutte H D, Jr, Hannon K S, Turner R T. J Bone Miner Res. 1991;6:325–330. doi: 10.1002/jbmr.5650060403. [DOI] [PubMed] [Google Scholar]
- 10.Slemenda C W, Longcope C, Zhou L, Hui S L, Peacock M, Johnston C C. J Clin Invest. 1997;100:1755–1759. doi: 10.1172/JCI119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillberg P, Johansson A G, Ljunghall S. Calcif Tissue Int. 1999;64:209–213. doi: 10.1007/s002239900604. [DOI] [PubMed] [Google Scholar]
- 12.Morishima A, Grumbach M M, Simpson E R, Fisher C, Qin K. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 13.Vanderschueren D, Van Herck E, De Coster R, Bouillon R. Calcif Tissue Int. 1996;59:179–183. doi: 10.1007/s002239900106. [DOI] [PubMed] [Google Scholar]
- 14.Komm B S, Terpening C M, Benz D J, Graeme K A, Gallegos A, Korc M, Greene G L, O'Malley B W, Haussler M R. Science. 1988;241:81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Hur H, Mor G, Blickstein I, Likhman I, Kohen F, Dgani R, Insler V, Yaffe P, Ornoy A. Calcif Tissue Int. 1993;53:91–96. doi: 10.1007/BF01321885. [DOI] [PubMed] [Google Scholar]
- 16.Eriksen E F, Colvard D S, Berg N J, Graham M L, Mann K G, Spelsberg T C, Riggs B L. Science. 1988;241:84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- 17.Onoe Y, Miyaura C, Ohta H, Nozawa S, Suda T. Endocrinology. 1997;138:4509–4512. doi: 10.1210/endo.138.10.5575. [DOI] [PubMed] [Google Scholar]
- 18.Arts J, Kuiper G G, Janssen J M, Gustafsson J A, Lowik C W, Pols H A, van Leeuwen J P. Endocrinology. 1997;138:5067–5070. doi: 10.1210/endo.138.11.5652. [DOI] [PubMed] [Google Scholar]
- 19.Vidal O, Kindblom L G, Ohlsson C. J Bone Miner Res. 1999;14:923–929. doi: 10.1359/jbmr.1999.14.6.923. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson L O, Boman A, Savendahl L, Grigelioniene G, Ohlsson C, Ritzen E M, Wroblewski J. J Clin Endocrinol Metab. 1999;84:370–373. doi: 10.1210/jcem.84.1.5531. [DOI] [PubMed] [Google Scholar]
- 21.Smith E P, Boyd J, Frank G R, Takahashi H, Cohen R M, Specker B, Williams T C, Lubahn D B, Korach K S. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 22.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 23.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J A, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho K Y, Evans W S, Blizzard R M, Veldhuis J D, Merriam G R, Samojlik E, Furlanetto R, Rogol A D, Kaiser D L, Thorner M O. J Clin Endocrinol Metab. 1987;64:51–58. doi: 10.1210/jcem-64-1-51. [DOI] [PubMed] [Google Scholar]
- 25.Weissberger A J, Ho K K. J Clin Endocrinol Metab. 1993;76:1407–1412. doi: 10.1210/jcem.76.6.8501143. [DOI] [PubMed] [Google Scholar]
- 26.Beamer W G, Donahue L R, Rosen C J, Baylink D J. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 27.Windahl S H, Vidal O, Andersson G, Gustafsson J A, Ohlsson C. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamsa T, Jalovaara P, Peng Z, Vaananen H K, Tuukkanen J. Bone. 1998;23:155–161. doi: 10.1016/s8756-3282(98)00076-3. [DOI] [PubMed] [Google Scholar]
- 29.Blum W F, Breier B H. Growth Regul. 1994;4, Suppl. 1:11–19. [PubMed] [Google Scholar]
- 30.Richman C, Baylink D J, Lang K, Dony C, Mohan S. Endocrinology. 1999;140:4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]
- 31.Hess R A, Bunick D, Lee K H, Bahr J, Taylor J A, Korach K S, Lubahn D B. Nature (London) 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertelloni S, Baroncelli G I, Federico G, Cappa M, Lala R, Saggese G. Horm Res. 1998;50:309–314. doi: 10.1159/000023296. [DOI] [PubMed] [Google Scholar]
- 33.Vanderschueren D, Van Herck E, Suiker A M, Visser W J, Schot L P, Chung K, Lucas R S, Einhorn T A, Bouillon R. J Bone Miner Res. 1993;8:801–809. doi: 10.1002/jbmr.5650080705. [DOI] [PubMed] [Google Scholar]
- 34.Sandstedt J, Ohlsson C, Norjavaara E, Nilsson J, Tornell J. Endocrinology. 1994;135:2574–2580. doi: 10.1210/endo.135.6.7988445. [DOI] [PubMed] [Google Scholar]
- 35.MacGillivray M H, Morishima A, Conte F, Grumbach M, Smith E P. Horm Res. 1998;49, Suppl. 1:2–8. doi: 10.1159/000053061. [DOI] [PubMed] [Google Scholar]
- 36.Vanderschueren D. Horm Res. 1996;46:95–98. doi: 10.1159/000185003. [DOI] [PubMed] [Google Scholar]
- 37.Seeman E, Melton L J, III, O'Fallon W M, Riggs B L. Am J Med. 1983;75:977–983. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- 38.Stanley H L, Schmitt B P, Poses R M, Deiss W P. J Am Geriatr Soc. 1991;39:766–771. doi: 10.1111/j.1532-5415.1991.tb02698.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferretti J L, Capozza R F, Zanchetta J R. Bone. 1996;18:97–102. doi: 10.1016/8756-3282(95)00438-6. [DOI] [PubMed] [Google Scholar]
- 40.Das S K, Taylor J A, Korach K S, Paria B C, Dey S K, Lubahn D B. Proc Natl Acad Sci USA. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh D, Taylor J A, Green J A, Lubahn D B. Endocrinology. 1999;140:3526–3533. doi: 10.1210/endo.140.8.6877. [DOI] [PubMed] [Google Scholar]
- 42.Eddy E M, Washburn T F, Bunch D O, Goulding E H, Gladen B C, Lubahn D B, Korach K S. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 43.Ohlsson C, Bengtsson B A, Isaksson O G, Andreassen T T, Slootweg M C. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 44.Miller J D, Tannenbaum G S, Colle E, Guyda H J. J Clin Endocrinol Metab. 1982;55:989–994. doi: 10.1210/jcem-55-5-989. [DOI] [PubMed] [Google Scholar]
- 45.Mauras N, Blizzard R M, Link K, Johnson M L, Rogol A D, Veldhuis J D. J Clin Endocrinol Metab. 1987;64:596–601. doi: 10.1210/jcem-64-3-596. [DOI] [PubMed] [Google Scholar]
- 46.Martha P M, Jr, Rogol A D, Veldhuis J D, Kerrigan J R, Goodman D W, Blizzard R M. J Clin Endocrinol Metab. 1989;69:563–570. doi: 10.1210/jcem-69-3-563. [DOI] [PubMed] [Google Scholar]
- 47.Weissberger A J, Ho K Y, Lazarus L. Horm Res. 1989;32:148–150. doi: 10.1159/000181278. [DOI] [PubMed] [Google Scholar]
- 48.Link K, Blizzard R M, Evans W S, Kaiser D L, Parker M W, Rogol A D. J Clin Endocrinol Metab. 1986;62:159–164. doi: 10.1210/jcem-62-1-159. [DOI] [PubMed] [Google Scholar]